Abstract
The quest for reliable techniques to predict Cheddar cheese maturity has gained momentum to ensure quality and consistency in large-scale production. Given the complexity of cheese ripening and the industry’s need for fast and reliable evaluation methods, this review addresses the challenge by scrutinising the application of spectroscopic techniques such as Fourier transform infrared (FT-IR), near-infrared (NIR), and nuclear magnetic resonance (NMR). These methods are evaluated for their noninvasive and rapid on-site analysis capabilities, which are essential for ensuring quality in cheese production. This review synthesises current research findings, discusses the potential and limitations of each technique, and highlights future research directions. Overall, NIR spectroscopy emerges as the most promising, offering quick, nondestructive assessments and reasonably accurate compositional predictions, crucial for real-time maturation monitoring. It provides rapid results within minutes, making it significantly faster than FT-IR and NMR. While FT-IR also offers high accuracy, it typically requires longer analysis times due to extensive calibration and can be sensitive to sample conditions, while NMR, although highly accurate, involves complex and time-consuming procedures. Nonetheless, further studies are necessary to refine these spectroscopic techniques, enhance their predictive accuracy, and deepen the understanding of the correlations between chemical attributes and sensory qualities in Cheddar cheese.
1. Introduction
Cheddar cheese, renowned for its unique flavour and texture, undergoes a complex ripening process involving biochemical and microbiological transformations. The quality and composition of cheese milk, variations in cheesemaking processes, and ripening conditions significantly influence the quality of a final product. As the cheese matures, proteolysis, glycolysis, and lipolysis play crucial roles in shaping its texture, appearance, and sensory profile.
Traditionally, the assessment of cheese maturation has relied on human sensory analysis, a method that, although valuable, is subjective, time-consuming, and requires a high level of expertise [1]. These factors pose significant challenges for consistent quality control in large-scale production, which has led to a growing demand for efficient, reliable methods to predict Cheddar cheese maturity, aiming to reduce human labour dependence and enhance product quality. Early quality prediction is vital, especially for cheeses with extended maturation periods, allowing for proactive quality control. Accurate sensory quality prediction aligns closely with consumer experience.
Furthermore, conventional methods for analysing cheese involve substantial sample handling and laboratory processing, including potentially hazardous chemicals. These procedures, although precise, are labour-intensive, destructive, and generate chemical waste, posing environmental and health risks. The necessity for efficient routine cheese analyses has led to the development of swift, noninvasive, and cost-effective techniques to assess cheese composition, quality, and maturation, addressing both sustainability and efficiency concerns.
Numerous studies have widely explored factors influencing cheese quality [1,2,3,4]. Moreover, the challenge of assessing cheese composition and monitoring ripening using chemical and instrumental approaches has been the subject of comprehensive reviews [5,6]. Numerous efforts have been dedicated to predicting the chemical and sensory properties of cheese through spectroscopic methods [7,8,9]. For these methods to be effective in industrial quality control, they must demonstrate sensitivity to even subtle deviations from the standard composition.
Spectroscopic methods like Fourier transform infrared (FT-IR), near-infrared (NIR), and nuclear magnetic resonance (NMR) spectroscopy have been investigated for their potential to predict cheese maturation. FT-IR provides detailed molecular information and high accuracy with rapid measurements, although it requires extensive calibration and can be sensitive to sample conditions [10,11,12]. NIR spectroscopy offers the fastest data acquisition and excellent repeatability, making it ideal for real-time monitoring, with portable options enhancing its industrial applicability [13,14,15]. NMR spectroscopy is a powerful technique that can quickly analyse complex mixtures at the molecular level without the need for separation or purification, making it highly suitable for food science applications. However, due to its high cost, relatively low sensitivity, and lack of expertise, it remains underutilised in the food industry [16,17].
Current Cheddar cheese quality assessment relies on subjective and time-consuming sensory analysis and costly outsourced testing, necessitating a shift to rapid, nondestructive, and cost-effective on-site methods for better efficiency and quality control. This review aims to examine the applicability and effectiveness of FT-IR, NIR, and NMR spectroscopy in predicting cheese maturity and identify the most promising technique for enhancing quality control in cheese production, with particular emphasis on Cheddar cheese.
2. Context and Scope
The ripening of Cheddar cheese is a complex process characterised by a series of biochemical transformations that are pivotal in the formation of the final product’s flavour and texture. The duration of ripening for rennet-coagulated cheese can vary significantly, ranging from as short as two weeks to as long as two years. During this ripening period, substantial alterations in both texture and flavour occur, driven by a series of interconnected biochemical reactions. Several significant changes occur during the cheese ripening process, each playing a vital role in developing the final product quality. Initially, the starter lactic acid bacterial cells in cheese undergo cell death and lysis. This is followed by the proliferation of non-starter lactic acid bacteria as the cheese ages. Concurrently, the cheese texture is softened, primarily due to the breakdown of casein. Alongside these processes, the pH of the cheese undergoes alterations, influencing various aspects of flavour and texture. Finally, the ripening process involves the breakdown of proteins, fats, and lactose, contributing significantly to developing the characteristic flavour of cheese. These changes highlight the dynamic nature of cheese ripening, where a variety of biochemical processes play a crucial role [18].
The biochemical changes during Cheddar cheese ripening can generally be grouped into two categories: Primary processes, which involve the metabolism of residual lactose, lactate, and citrate, along with breakdown of proteins and fats, processes known as glycolysis, proteolysis and lipolysis, respectively, and Secondary processes, which include the metabolism of amino acids and fatty acids, culminating in the formation of volatile and flavour compounds. As Cheddar cheese matures, substantial biochemical changes are crucial for developing the characteristic taste and texture of cheese [18].
Understanding the complexity of cheese ripening is vital for the dairy industry, as it influences the quality and consistency of the final product. The industry has long relied on sensory analysis to evaluate cheese quality and predict shelf life, a method that, while invaluable, is subjective, time-consuming, and requires a high level of expertise [19]. These limitations highlight the need for more efficient, objective, and scalable methods to assess cheese maturation, particularly in large-scale production.
Given these complexities, there is a growing interest in leveraging advanced technologies to monitor cheese ripening more effectively. Spectroscopic techniques like FT-IR, NIR, and NMR spectroscopy have emerged as promising tools in this regard. These methods offer rapid and reliable means to assess the chemical and physical changes occurring in cheese during ripening, providing valuable insights that can aid in predicting the final product’s quality [20].
NIR spectroscopy, for instance, is known for its rapid and noninvasive analysis, making it an attractive option for real-time monitoring of cheese maturation [6]. NIR’s ability to correlate spectral data with physicochemical properties and sensory evaluation scores renders it a potent tool for real-time on-site monitoring of cheese production [8].
FT-IR spectroscopy, with its applications predominantly in the mid-infrared region, offers detailed insights into the molecular composition of cheese, aiding in understanding maturation-related chemical transformations [21,22]. Despite its sensitivity to environmental factors and the complexity of biological samples, FT-IR’s ability to identify specific molecular bonds and functional groups makes it invaluable for studying cheese ripening processes [23].
Although less commonly employed in routine cheese analysis due to cost and operational complexity, NMR spectroscopy provides incomparable molecular-level insights, facilitating detailed assessments of cheese composition and structure [16]. This technique’s ability to elucidate the dynamics of water and fat within cheese offers a deeper understanding of textural and flavour development during ripening [24].
In this regard, applying these spectroscopic methods to predict Cheddar cheese maturity represents a significant advancement in dairy science. It offers the potential to enhance quality control processes, reduce dependency on subjective assessments, and improve the efficiency and consistency of cheese production. The following sections will delve deeper into these spectroscopic techniques, examining their principles, applications, and research findings, highlighting their potential in evaluating cheese quality and predictive maturation analysis.
3. Materials and Methods
Cheddar cheese quality assessment and maturation prediction are critical for ensuring consistent product quality in the dairy industry. Rapid, nondestructive spectroscopic techniques offer the potential for real-time, on-site monitoring, which is essential for industrial applications.
This review critically examines existing spectroscopic methods to enhance the understanding and methodology of Cheddar cheese quality assessment, with a particular emphasis on FT-IR, NIR, and NMR techniques. The review addresses two primary research questions:
- Research Question 1 (RQ1): What are the most prevalent spectroscopic methods in the literature for rapid and noninvasive assessment of Cheddar cheese quality and prediction of maturation length?
- Research Question 2 (RQ2): Based on the literature review, which of these methods is shown to be the most effective?
3.1. Spectroscopy Methods for Cheese Quality and Ripening Prediction
Spectroscopy examines how light interacts with matter. Light is a type of electromagnetic radiation carrying a specific energy level. This energy depends on the light’s frequency; as the frequency increases or the wavelength decreases, the light energy rises [25].
The electromagnetic spectrum is categorised into various regions based on wavelength or frequency, as illustrated in Figure 1.
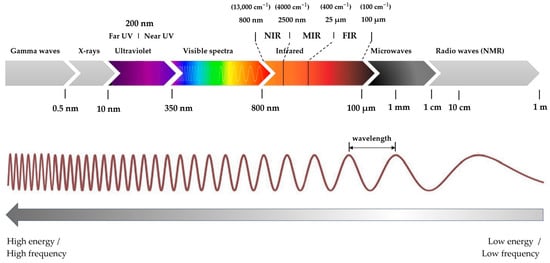
Figure 1.
Spectral regions of the electromagnetic spectrum.
- Gamma rays, with the shortest wavelengths and highest frequencies, are utilised in medical fields.
- X-rays, found next in the spectrum, are used primarily for medical imaging and materials analysis.
- Ultraviolet (UV) light, spanning from wavelengths of about 10 nm to 350 nm, is invisible to the human eye and has applications in sterilisation and scientific research.
- Visible light, ranging from violet (shorter wavelength of approx. 350 nm) to red light (longer wavelength of up to 800 nm), is employed in numerous applications, including lighting, communication, and analysis.
- Infrared (IR) light, ranging from 800 nm to 100 µm and located beyond visible light, is split into near-infrared (NIR), mid-infrared (MIR), and far-infrared (FIR) regions. It is used in thermal imaging, communication, and chemical analysis.
- Microwaves facilitate communication, cooking, and radar technology.
- Lastly, radio waves, with the longest wavelengths, support various wireless communication forms, such as radio and TV.
Each spectral region is characterised by its wavelength, frequency, and energy and is utilised in various scientific, industrial, and technological applications [23,26].
Figure 1 presents the electromagnetic spectrum segmented into various spectral zones described by Stuart [23] and Dufour [26]. As the wavelength increases from gamma rays to radio waves, the energy of the light decreases [25].
3.2. Implementation of Spectroscopy Techniques in Cheddar Cheese Quality Assessment and Maturation Prediction
This review scrutinises the effectiveness and precision of various spectroscopic approaches to outline the most reliable method for predicting cheese maturation. This is paramount for optimising production processes and ensuring quality in the dairy industry. Additionally, reducing external chemical analysis by employing rapid spectroscopic techniques results in potential savings of tens of thousands of euros annually.
The application of spectroscopic techniques such as NIR, FT-IR, and NMR in assessing Cheddar cheese maturation illustrates the merging of advanced technology and food science. These methods provide a noninvasive, efficient, and reliable means to monitor the chemical changes during cheese ripening, offering valuable insights for quality control and production optimisation.
Incorporating these spectroscopic methods in the cheese industry requires integrating the instruments into the production line or quality control labs, training personnel to operate the equipment and interpret the data, and developing calibration models specific to the cheese types and maturation stages being analysed. The calibration process is critical, involving the collection of spectral data from cheese samples of known maturity levels and correlating these with standard quality measures to build predictive models.
The success of implementing these techniques in a production environment depends on the ability to provide reliable, accurate predictions that can guide decision-making in cheese maturation and quality control. As such, ongoing research and development are essential to refine these methods, enhance their predictive accuracy, and expand their applications in the dairy industry.
3.2.1. NIR Spectroscopy Implementation
NIR spectroscopy stands out for its rapid, nondestructive analysis, allowing for real-time monitoring of cheese maturation and quality. This technique is particularly advantageous because of its minimal sample preparation requirements and the ability to provide immediate results, making it suitable for on-site analysis in cheese production [27]. NIR spectrometers typically comprise a light source, beam splitter, sample detector, optical detector, and data processing unit, as schematically illustrated in Figure 2. It utilises various wavelength selection technologies to cater to different analytical needs [28].
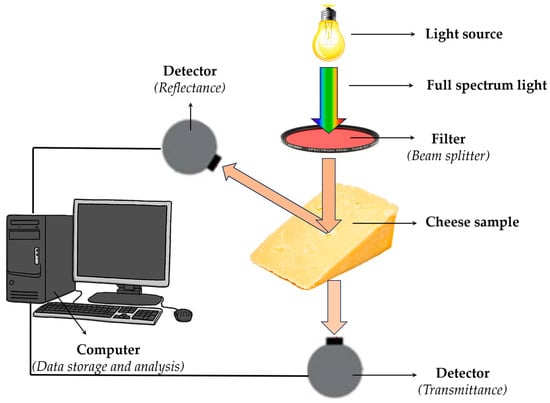
Figure 2.
Schematic illustration of reflected spectra measurement of cheese.
Reflectance and transmittance detector options are commonly used in these systems, each with specific advantages. Transmittance spectroscopy achieves the highest accuracy levels for cheese compositional analysis. However, the ease of collecting reflectance spectra offers a significant advantage for industrial applications. Reflectance measurements, which utilise a broader wavelength range (750–2498 nm), provide more detailed information than the restricted range (850–1050 nm) typically used in transmission through opaque samples like cheese. This broader range is particularly beneficial when analysing complex parameters, such as sensory measurements, rather than simply compositional features [13].
Portable NIR instruments have been developed to analyse the cheese surface directly, simplifying the process and reducing the time from sample collection to analysis. This method’s practicality is demonstrated in its ability to accurately predict various cheese compositional parameters, providing a foundation for quality control decisions [8].
3.2.2. FT-IR Spectroscopy Implementation
FT-IR spectroscopy, encompassing a wide range of the infrared spectrum, offers detailed chemical information by detecting specific bonds and functional groups within the cheese matrix [29]. While it is highly informative, the technique requires more controlled sample preparation compared to NIR, mainly due to the interference of water in cheese, particularly younger cheeses [30,31,32]. However, its application in cheese ripening studies has provided critical insights into the chemical transformations during maturation, contributing to a deeper understanding of the ripening process [12].
3.2.3. NMR Spectroscopy Implementation
Although NMR is less commonly employed in routine cheese quality control due to its higher operational costs and specialised knowledge requirements, its potential has to be considered. NMR offers unique molecular-level insights, allowing for the detailed characterisation of cheese composition and structure. The technique has been utilised to investigate the distribution and mobility of water and fat within the cheese, providing indicators of maturation and texture [33].
3.3. Chemometrics Techniques in Predictive Modelling for Cheese Maturation Assessment
Chemometrics involves the application of mathematical and statistical techniques to analyse chemical data, enabling the extraction of meaningful information from complex datasets [34]. This field plays a crucial role in predictive modelling for cheese maturation assessment, facilitating the correlation of spectral data with cheese quality parameters. Various chemometric techniques, including principal component analysis (PCA), partial least squares (PLS) regression, and artificial neural networks (ANNs), are employed to develop robust predictive models.
3.3.1. Principal Component Analysis (PCA)
PCA is a multivariate statistical technique used to reduce the dimensionality of large datasets while retaining most of the variance present in the data. This technique identifies principal components, which are linear combinations of the original variables, and projects the data into a new coordinate system [35,36]. PCA helps visualise clustering patterns and distinguish chemical changes in cheese due to ageing. It is widely used in cheese analysis to interpret complex spectral data and identify critical factors affecting cheese quality.
The innovative application of PCA is a pivotal analytical tool, facilitating the reduction of complex spectroscopic data for enhanced cheese quality assessment and prediction of the maturation period [9,29]. In Curto et al.’s study [9], PCA transforms NIR spectral data into principal components, forming the basis for an artificial neural network model predicting cheese’s sensory attributes. Similarly, Dewantier et al. [29] applied PCA to FT-IR and Raman spectroscopy data, distinguishing Cheddar cheese samples by maturity and cheese manufacturer. These applications demonstrate PCA’s effectiveness in extracting essential information from high-dimensional data, offering a robust framework for correlating spectroscopic measurements with the sensory and chemical properties of cheese, thus providing valuable insights for the dairy industry.
3.3.2. Partial Least Squares Regression (PLS-R)
PLS regression is a powerful tool in cheese analysis, used to correlate spectral data with cheese maturity and quality parameters. It works by projecting the predictor and response variables into a new space, maximising the covariance between them. PLS is highly effective in dealing with collinear and noisy data, making it suitable for complex biochemical datasets in cheese maturation studies. It has been successfully applied in predicting various compositional and sensory attributes of cheese. Downey et al. [13] utilised PLS regression to predict the maturity and sensory attributes of Cheddar cheese, achieving accurate results within the spectral range of 750–1098 nm. Priyashantha et al. [37] developed a PLS-based model using NIR-HS imaging to predict cheese maturation end dates with 69.6% accuracy, while Reis et al. [14] applied PLS regression to estimate fatty acids in various cheese types, demonstrating its effectiveness in rapid, nondestructive compositional analysis.
PLS regression, combined with spectral data from infrared and other spectroscopic methods, offers a robust framework for predicting cheese quality, aiding in the development of precise, noninvasive quality control tools in the dairy industry.
3.3.3. Artificial Neural Networks (ANNs)
Accurate prediction models are essential to replace human sensory panels with instrumental measurements successfully. These models are built by calibrating instrumental measurements with sensory data [38]. However, traditional regression methods based on multivariate statistics often fail with non-linear sensory data. One promising alternative is the application of ANNs, which are well-suited for handling non-linear data. ANNs are trained using the spectral data obtained from NIR and FT-MIR spectroscopy. The network learns to identify patterns and correlations in the spectral data corresponding to the different cheese sample categories. The use of ANNs shows high accuracy in classification, demonstrating the capability to handle complex, non-linear data relationships inherent in spectral data. ANNs, such as multi-layer perceptron (MLP) and radial basis function, offer powerful learning capabilities, making them suitable for complex dataset analysis [9,39,40].
ANNs consist of interconnected processing elements that mimic the human brain’s neurons and synapses, allowing for dynamic information processing and adaptability through learning processes. These capabilities make ANNs particularly useful for non-linear data in food safety and quality analysis [39]. The results from the ANN models are compared with other statistical methods, highlighting the superior performance of ANNs in terms of accuracy and reliability for this specific application. Combined with NIR and FT-MIR spectroscopy, ANNs are practical tools for cheese authentication and quality control [40].
4. Literature Review
In order to enhance the understanding and methodology around Cheddar cheese quality assessment and maturation prediction, this literature review critically examines existing spectroscopic methods utilised within the field.
Extensive research has been conducted to quickly and cost-effectively predict cheese composition and age, particularly in the visible and near-infrared range. Recent advancements, such as the use of portable spectrometers on cheese surfaces, have eliminated the need for time-consuming sample collection and processing.
Understanding the intricate interactions among different components in cheese and their transformations during ripening is pivotal for both process optimisation and product development.
4.1. Determining the Most Prevalent Spectroscopy Techniques for Cheddar Cheese Quality Assessment and Maturation Prediction (Answer to RQ1)
Table 1 encapsulates the key aspects of various spectroscopic methods used in cheese analysis, highlighting the differences in sample preparation, spectral ranges, and analytical approaches. The diversity in techniques and preparation methods underscores the adaptability of spectroscopic tools in addressing the complex cheese matrix. These tools provide comprehensive insights into the composition and quality of cheese, making them highly effective in the field of food science and spectroscopy.
FT-IR, NIR, and NMR are the three fundamental spectroscopic methods predominantly used in food analysis, including cheese. Each technique offers unique advantages and has specific applications depending on the tested parameters and the type of cheese being analysed. The following section will examine these three spectroscopic techniques, discussing their principles, applications, and the distinctive features that make them suitable for different aspects of cheese quality assessment.
4.1.1. Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectroscopy is renowned for its broad application spectrum, ranging from about 0.7 to 500 μm (14,285 cm−1 to 20 cm−1), with particular emphasis on the mid-infrared region from 2.5 to 25 μm (4000 cm−1 to 400 cm−1) for chemical analysis due to the rich molecular information it provides [20,41]. In Cheddar cheese ripening, FT-IR’s capability to detect changes in protein, lipid, and moisture content offers a detailed understanding of maturation processes [20]. Notably, FT-IR, coupled with chemometric models, has been instrumental in predicting cheese maturation stages, showcasing its utility in the dairy industry [42].
In FT-MIR spectroscopy, molecules interact with mid-infrared energy, leading to various vibrational motions like stretching, bending, twisting, rocking, and scissoring. These motions occur at specific locations in the spectrum, determined by factors such as bond configuration and its location. FT-MIR spectroscopy provides rich information for analysing chemical composition and determining molecular structures [5,22].
Key spectral regions and functional groups are highlighted in an example FT-MIR spectrum of a Cheddar cheese water-soluble fraction (WSF) extract captured in the mid-infrared range of 2.5–14.3 μm (4000–700 cm−1) with a resolution of 1.25 mm. For instance, the region from 2.5 to 3.2 μm (4000–3125 cm−1) corresponds to absorbance from O-H and N-H stretching vibrations of hydroxyl groups and amide bonds of proteins [22].
Complementing these studies, Dewantier et al. [29] employed vibrational FT-IR spectroscopy and Raman, combined with PCA, to differentiate Cheddar cheese samples based on their maturity and manufacturer, as shown in Table 1 and Figure 3. PCA of the IR spectra from cheeses of four different brands was conducted to identify if the spectra could distinguish chemical changes due to ageing. Their analysis revealed that changes in the amide I and II bands in FT-IR spectra are crucial in distinguishing cheeses by ripening time and manufacturer, demonstrating the applicability of these techniques in cheese quality assessment.
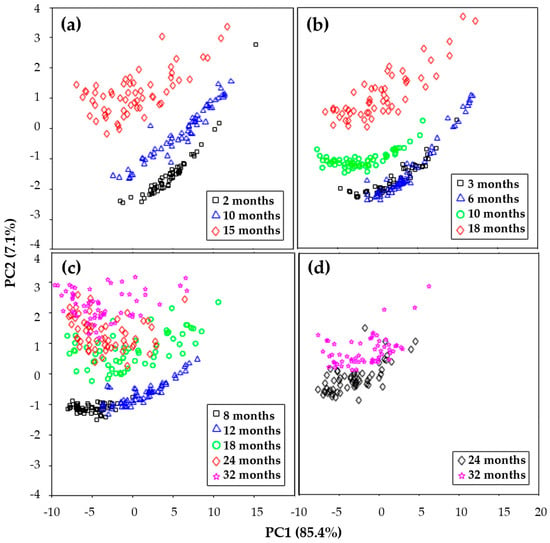
Figure 3.
Principal component analysis score plots using FT-IR spectra for Cheddar cheese from various brands: (a) Bega, (b) Great Ocean Road, (c) Mainland, and (d) Cracker Barrel [29].
FT-IR’s usefulness is further exemplified in studies focusing on the “fingerprint region” (8–16 μm/12,500–6250 cm⁻1), where unique spectral signals provide detailed compositional insights, especially regarding phosphodiesters and carbohydrates in Cheddar cheese water-soluble fractions [21,22]. Despite its advantages, including simplicity and sensitivity, FT-IR’s limitations, such as its sensitivity to environmental variations and the complexity of biological samples, necessitate meticulous sample preparation to mitigate interference, particularly from water content [5,23].

Table 1.
Literature data on spectroscopic methods used in cheese analysis.
Table 1.
Literature data on spectroscopic methods used in cheese analysis.
| Method | Instrument Brand | Tested Parameter | Mode (T/R) | Spectral Range | Best Results Spectral Range/Peak Wavelengths (nm) | Analysed Cheese Type (s) | Sample Preparation for Spectroscopy | Reference Method | Number of Samples | Cheese Age | Data Analysis | Software | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT-IR | Perkin Elmer FT-IR | Molecular changes (amide I and II bands) | R | 4000–400 cm−1 (2500–25,000 nm) | 1720–1134 cm−1 | Cheddar brands: Bega, Great Ocean Road (GOD), Cracker Barrel (CB), Mainland | Flat slices cut near the centre of the block | None | 42 samples | 2–32 months | PCA | MATLAB R2021b (Mathworks Inc., Natick, MA, USA) | [29] |
| Raman | PerkinElmer Raman Station 400 | Lipid-associated bands, Phenylalanine residues | R | 3200–400 cm−1 (3125–25,000 nm) | 1800–990 cm−1 | None | 42 samples | 2–32 months | PCA | ||||
| FT-IR | Varian 3100 (Varian Inc., Palo Alto, CA, USA) | Amino acids, organic acids | R | 4000–700 cm−1 (2500–14,285 nm) | 1800–900 cm−1 | Cheddar | Water-soluble extract (WSE) of powdered cheese sample | GC-FID for amino acids, HPLC for organic acids | 12 samples | 7, 15, 32, 46, 73 additional days (commercial samples) | PLSR, SIMCA; ANOVA | Pirouette v3.11 (Infometrix Inc., Woodville, WA, USA); Minitab v15 (Minitab Inc., State College, PA, USA) | [20] |
| FT-IR | ATI Mattson Infinity Series FT-IR Spectrophotometer (Madison, WI, USA) | Sensory and texture attributes | R | 4000–640 cm⁻1 (2500–15,625 nm) | 930–1767 cm⁻1 2839–4000 cm⁻1 | Processed cheese | None, sample equilibrated to room temperature | Descriptive Sensory Analysis (10 experts) | 32 samples | 2–4 weeks | PLS regression, PCA | Unscrambler v. 8.0 (Camo A/S, Oslo, Norway) | [7] |
| FT-IR | FT-MIR spectrophotometer (Bruker, model Vertex70, Billerica, MA, USA) | Detection of adulterations in butter cheese with soybean oil | R | 4000–650 cm⁻1 (2500–15,385 nm) | 3100–2800 cm⁻1 1800–960 cm⁻1 | Butter cheese | Cuts of the core and surface area | AOAC standard methods of chemical analysis | 12 samples | Young cheese, unspecified age | PCA, PLS-R | OriginPro 2015 Software | [10] |
| FT-IR | Portable spectrometer FT-IR 4500a (Agilent Technologies, Santa Clara, CA, USA) | Organic acids, Amino acids, Fatty acids, | R | 4000–650 cm−1 (2500–14,285 nm) | 1800–900 cm⁻1 3700–2850 cm⁻1; 1700–1000 cm⁻1 | Turkish white cheese | Water-soluble extract (WSE) of powdered cheese sample | HPLC (organic acids); GC-MS (amino acids); GC-FID (fatty acids) | 12 samples | Various stages of ripening (100 days) | SIMCA; PLS-R; ANOVA | Pirouette v. 4.5 (Infometrix Inc., Bothell, WA, USA); SPSS v.25 (IBM Corp., Armonk, NY, USA) | [12] |
| FT-MIR | Nicolet iS50 (Thermo Fisher Scientific, Madison Wis., USA) | Chemical composition (moisture, fat, protein, salt) and physical properties (pH, acidity, texture) | R | 4000–700 cm⁻1 (2500–14,286 nm) | 1800–650 cm⁻1 (all features) | Turkish Ezine cheese from bovine, caprine, and ovine milk mixtures | Hand-crushed cheese | Traditional gold standard methods (e.g., Gerber, Soxhlet, Kjeldahl); Texture profile analysis | 81 samples | Various stages of ripening | PCA, PLS-R | Pirouette v. 4.5 (Infometrix, Inc., Bothell, WA, USA) | [11] |
| NIRS | Nicolet iS50 (Thermo Fisher Scientific) with InGaAs (Indium Gallium Arsenide) detector | R | 1000–2500 nm (10,000–4000 cm⁻1) | 8695–4000 cm⁻1 (Protein); 5827–4000 cm⁻¹ (WSN); 10,000–4000 cm⁻¹ (composition, pH, titr. acidity) | Cheese cubes | PCA, PLS-R | |||||||
| Vis/ NIRS | Portable VisNIRS-R (LabSpec 2500, Boulder, CO, USA) | Chemical composition (moisture, fat, protein, salt) and physical properties (pH, texture, colour) | R | 350–1830 nm | Various ranges specified per instrument | 37 different cheese types | Different sites of a freshly cut cheese surface | AOAC methods (Chemical components) Kjeldahl (for protein) Texture analysis; Colour measurements | 197 samples | Various (from 20 days to >20 months), depending on a cheese type | PLS-R; GLM procedure | Not specified | [8] |
| Benchtop NIRS-R (NIRSystem 5000, Foss Electric A/S, Hillerod, Denmark) | R | 1100–2498 nm | Grated cheese in a small cup | Not specified | |||||||||
| Benchtop NIRS-T (FoodScan, Foss Electric A/S, DK) | T | 850–1048 nm | Ground cheese in a petri-dish | Not specified | |||||||||
| NIRS | 6500 Scanning Monochromator (FOSS NIR Systems, Silversprings, MD, USA) | Maturity age and 11 sensory attributes | R | 400–2498 nm | 750–1098 nm (sensory, age); 1100–2498 nm (age) | Experimental Cheddar made using 5 renneting enzymes | Sliced | Descriptive Sensory Analysis (10 experts) | 24 samples | 2, 6, 4, 9 months | PCA, PLS-R | WINISI II software (v. 1.04a; Infrasoft International, Port Matilda, MD, USA). | [13] |
| NIRS | Fox NFoss NIR 5000 with 1.5 m 210/7210- bundle fibre-optic probe | 9 sensory attributes | R | 1100–2000 nm | Not specified | Raw cow’s, goat’s and ewe’s milk pilot-plant cheese from winter and summer milk | 1 cm thick slices | Quantitative Descriptive Sensory Analysis (8 experts) | 64 samples | 4, 6 months | PCA; Modified Partial least squares (MPLS) | WinISI II version 1.50 (Intrasoft International, LLC) | [43] |
| NIRS | Pocket-sized handheld NIR device (SCiO, Consumer Physics, Tel Aviv, Israel) | Moisture, Fat | R | 800–1070 nm | 900–1100 nm | Variety of cheese | Whole and grated | N/A | 46 samples | N/A | PLS-R; Multivariate data analysis | The Unscrambler X version 10.5; Cloud-based web-application SCiO Lab (Consumer Physics, Tel Aviv, Israel) | [15] |
| Benchtop NIRFlex N-500 i | R | 1000–2500 nm | 1100–2100 nm | The Unscrambler X version 10.5 (Camo Software, Oslo, Norway) | |||||||||
| NIRS | Foss NIR 5000 with 1.5 m 210/7210- bundle fibre-optic probe | 19 sensory attributes | R | 1100–2000 nm | 1210, 1450, 1730, 1930 nm | Variety of cheese from winter and summer milk | Sliced | Quantitative Descriptive Sensory Analysis (8 experts) | 64 samples | 2, 4 months | PCA | Not specified | [9] |
| ANN | Java NNS application within the Multi-Layer Perception network (MLP) | ||||||||||||
| NIRS | Handheld NIR’ (Trek-ASD Inc., Boulder, CO, USA) | Fatty acids | R | (1) 350–2500 nm (2) 900–1700 nm, | Not specified | 36 types (cow, goat, ewe, buffalo) Feta, Camembert, Blue, Cottage, Colby Gouda, Parmesan | No sample preparation | Gas Chromatography-Flame Ionization Detection (GC-FID) | 68 samples | Various ages (not specified) | PLS-R, SVM | Not specified | [14] |
| Miniaturised NIR (NIRscan Nano-Texas Instruments Inc., Texas, USA) | Fatty acids | R | 900–1700 nm | No sample preparation | 68 samples | Not specified | |||||||
| Vis/NIRS | FieldSpec® 3 FR Spectroradiometer Devices (ASD, Inc. Boulder, CO, USA) | Protein content | R | 350–2500 nm | Not specified | Bayerische brotzeit, Anchor, Milkana cheese | None | Dumas combustion method | 92 samples | Various | DOSC-KPLS, SVM, BP-ANN, MSC, SNV | Not specified | [44] |
| NMR | Bruker 800 MHz Avance III spectrometer using a 5 mm QCI Cryoprobe | Amino acids, organic acids, ripening markers | N/A | N/A | N/A | Cheddar | Cheese extract | Descriptive sensory analysis | 36 samples | 56, 90, 180, 270, 360, 450 days | PCA, PLS; ANOVA | Unscrambler X (CAMO ASA, Trondheim, Norway); XLSTAT (Addinsoft, France) | [33] |
| NMR | Bruker DMX 500 MHz | Free amino acids, fatty acids, organic acids | N/A | 0.4 to 10.5 ppm | N/A | Italian Parmigiano Reggiano, East-Europe “Grana type” | Supernatant from dissolved in D2O and centrifuged | None | 33 samples (25 Parmigiano Reggiano, 8 Grana type) | 14, 24, 30 months | PCA, PLS-DA, O-PLS | ACD/Spec Manager v. 8.12 (ACD Labs), SIMCA-P V. 11 (Umetrics, Umea, Sweden) | [17] |
R—Reflectance; ATR—Attenuated total reflectance; PCA—Principal component analysis, PLS-R—Partial least squares regression; PLS-DA—PLS discriminant analysis; ANN—Artificial neural network; SIMCA—Soft independent modelling of class analogy; SNV—Standard normal variate; SVM—Support vector machine; GC-FID—The gas chromatography with flame ionization detection; WSN—Water soluble nitrogen.
4.1.2. Near-Infrared Spectroscopy (NIR)
NIR spectroscopy offers a nondestructive approach, gaining popularity for its speed and suitability for online monitoring in food quality assessment, particularly in tracking the cheese ripening process. The analysis of over 70 studies by Bittante et al. [6] underscores high predictive accuracy (86–90%) for moisture, fat, and protein content in cheese but lower accuracy for salt content and pH (79% and 56%, respectively) using visible and NIR spectrometry. However, the results varied based on the cheese type and the sample preparation technique employed.
Reis et al. [14] conducted a comparative evaluation of a miniaturised and handheld Vis-NIR spectrometer for estimating fatty acids in various cheese types made from cow, goat, ewe, and buffalo milk, namely Feta, Camembert, Blue, Cottage, Colby, Gouda, and Parmesan. The study involved 68 cheese samples and used no sample preparation. Critical absorption bands observed included those for fat (1200 nm, 1730 nm, 2310 nm, 2350 nm), water (1450 nm, 1850 nm), and protein (1760 nm, 2000 nm, 2175 nm). Both partial least squares regression (PLS-R) and support vector machine (SVM) modelling approaches were applied. The study highlighted the effectiveness of NIR technology in nondestructive, rapid compositional analysis of cheeses, with the handheld device showing a higher signal-to-noise ratio compared to the miniaturised NIR at the extremes of the 900–1700 nm range. Fatty acid analysis was compared using gas chromatography-flame ionization detection (GC-FID) and the authors found a strong correlation between the spectrometry results and the GC-FID analysis, indicating that NIR spectroscopy can reliably estimate fatty acid content in cheeses.
Lin et al. [44] investigated the application of non-linear multivariate methods combined with visible near-infrared spectroscopy (Vis/NIRS) to detect protein content in cheese. The research addresses the limitations of traditional methods, such as the Kjeldahl and Dumas combustion methods, which are labour-intensive, environmentally unfriendly, and unsuitable for real-time measurement. The authors propose using Vis/NIRS techniques with advanced chemometric methods to provide a rapid, nondestructive, and accurate protein content determination in cheese. The study analysed three varieties of semi-hard cheese from the following producers: Bayerische Brotzeit (Germany), Anchor (New Zealand), and Milkana (China), with a total of 92 samples (Table 1). The study explored several preprocessing methods and non-linear modelling techniques. The combination of direct orthogonal signal correction (DOSC) preprocessing with kernel partial least squares (KPLS) modelling and the Vis/NIRS technique provided a robust and accurate method for quantifying protein content in cheese. This approach offers significant improvements over traditional methods, being faster, nondestructive, and more suitable for real-time applications in cheese quality control.
The studies conducted by Abbas and Baeten [28], Priyashantha et al. [37,45], and Downey et al. [13] underscore the pivotal role of NIR spectroscopy in the analysis and prediction of cheese maturation and composition. Abbas and Baeten [28] detail the technical distinctions of NIR spectrometers, which are instrumental in analysing cheese through different wavelength selection technologies, encompassing IR-A (740–1400 nm) and IR-B (1400–2500 nm) spectrums as defined by CIE [46], with most research incorporating the IR-B section to study cheese, but with some authors using the IR-A.
Downey et al. [13] utilised NIR spectroscopy to predict the maturity and sensory attributes of Cheddar cheese. This process involved a detailed analysis of 24 experimental Cheddar cheeses during 9 months of storage. A trained panel assessed the sensory attributes, such as “crumbly”, “rubbery”, “chewy”, “mouthcoating”, and “mass-forming”, and NIR spectroscopy provided contemporaneous reflectance spectra. The results demonstrated that NIR spectroscopy could effectively predict both the age and several sensory attributes of Cheddar cheese. As presented in Table 1, the predictive models were refined using various spectral data treatments, with the most accurate results obtained within the IR-A spectral range of 750–1098 nm after secondary derivatisation. The study concluded that NIR spectroscopy offers a viable, noninvasive technique for rapidly determining cheese maturity and sensory characteristics, presenting an industrially applicable method for cheese quality assessment. The authors found that water and lipid bands significantly contributed to the spectra, while proteins, identified between 1000 and 1100 nm, did not show significant features in the spectral data until the fourth and fifth principal components (PC4 and PC5) were analysed.
The study by González-Martín et al. [43] evaluated the use of NIRS to predict the sensory attributes of cheese, including visual, taste, texture, and other sensory characteristics. Using a remote reflectance fibre-optic probe, NIR spectra were collected directly from cheese samples without pre-treatment. Sensory evaluations by a trained panel were correlated with NIR data through modified partial least squares (MPLS) regression. The study found that NIR spectroscopy, combined with chemometric techniques, effectively predicts sensory attributes, demonstrating good calibration and validation, offering significant advantages for real-time quality control in cheese production.
Priyashantha et al. [45] utilised near-infrared hyperspectral (NIR-HS) imaging to visualise and model the maturity of Swedish hard cheeses, with the findings illustrated in Figure 4 and Figure 5. They employed PLS regression to correlate spectral data with cheese maturity, revealing significant variation in the M-index for cheeses at each scanning occasion (chronological age), as shown in Figure 4. This spatial heterogeneity of cheese ripening within cheese wheels is further visually depicted in Figure 5, showcasing how different regions within a single cheese wheel mature at varying rates, with distinct maturation patterns emerging at different stages of maturity.
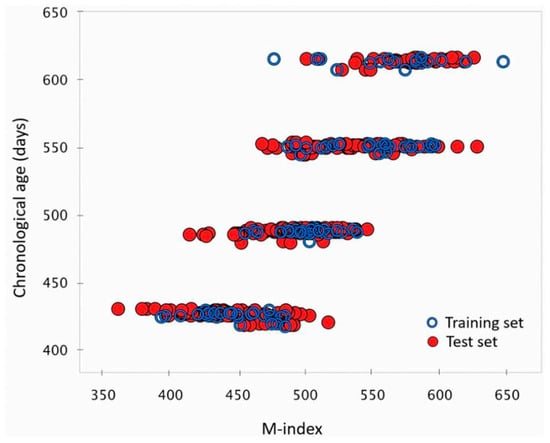
Figure 4.
Performance of the best prediction PLS model in determining cheese maturity levels * [45]. * This figure illustrates the model’s accuracy in quantifying the maturity of cheeses at different ages (423, 487, 550, and 614 days, or 14, 16, 18, and 20 months, respectively), calculated as the number of days from production to imaging. The maturity index (M-index) is established through NIR-hyperspectral imaging.
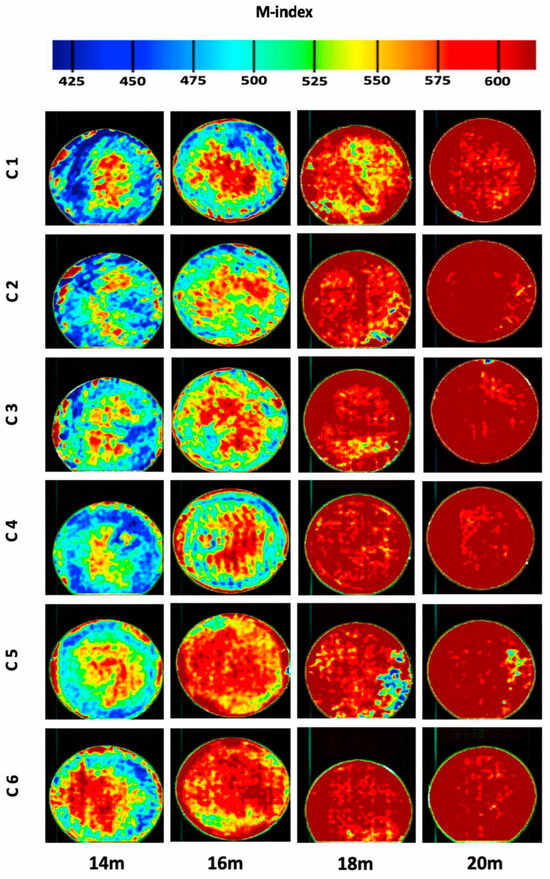
Figure 5.
Mapping of cheese maturity (M-index) in six cheeses (C1-C6) over time * [45]. * Using the PLS model, this figure shows the distribution of the maturity index (M-index) across six cheeses at four ripening stages (14, 16, 18, and 20 months, or 423, 487, 550, and 614 days).
Subsequently, Priyashantha et al. [37] advanced their work by developing a predictive model for determining the end date of cheese maturation, as illustrated in Figure 6. This model, underpinned by NIR-HS imaging and PLS regression, achieved a 69.6% accuracy in predicting maturation end dates, offering a noninvasive tool to enhance the overall prediction efficiency of cheese ageing duration. The overall study demonstrates the effectiveness of the PLS model with SNV pre-processing in predicting the end date of cheese ripening. However, the significant variation observed in the 14-month-old cheese samples, leading to a 69% prediction accuracy, underscores the need for further model refinement. The substantial prediction deviation, particularly values above the red line in Figure 6, indicates the model’s reduced reliability for these earlier-stage ripening cheeses. This conclusion reinforces the potential for future research to improve prediction reliability, especially for mature cheeses at the earlier stages of ripening.
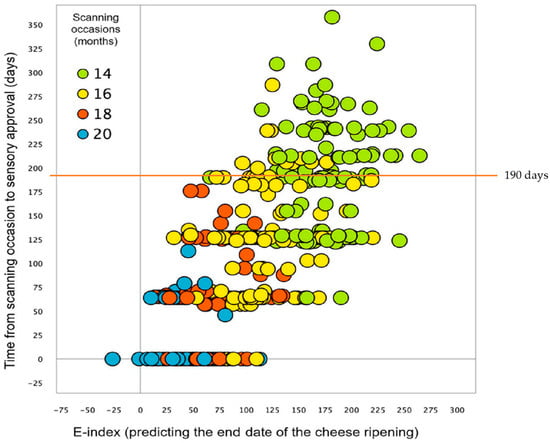
Figure 6.
The optimal predictive model using PLS analysis (enhanced with SNV pre-processing) for predicting the cheese ripening end date * [37]. * The model calculated the time from each scanning session to the sensory approval date, with intervals of approximately 0, 64, 127, and 190 days, aligning with scanning at 20, 18, 16, and 14 months, respectively. This duration was determined by the difference in days between the sensory approval and the image acquisition date. Points above the 190−day line represent samples where the model’s prediction deviated significantly from the actual maturation readiness time, implying prediction errors.
The study by Alinaghi et al. [47] used NIR-HS image analysis to track and predict cheese composition and quality changes during maturation. By analysing the spectral data, the authors aimed to correlate specific wavelengths with chemical and physical changes occurring during cheese ripening. Their findings indicate that NIR hyperspectral imaging is a promising technique for real-time monitoring, offering detailed insights into the ripening process and enabling better quality control in cheese production.
Stocco et al. [8] differentiated the impact of spectral range versus technical features of spectrometers, analysing three distinct types: Vis/NIRS-R, NIRS-R, and NIRS-T. They illustrated how each performs in predicting cheese composition, which is shown in Figure 7. The comparison indicates that spectrometers’ technical specifications, rather than spectral range, predominantly influence predictive accuracy.
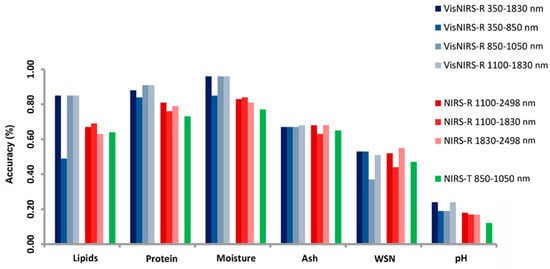
Figure 7.
Impact of infrared spectrometry techniques on chemical predictive validity across various cheese categories * [8]. * A dataset of 97 cheese samples across 37 distinct cheese varieties was analysed using different spectrometry methods: Vis/NIRS-R, a portable visible near-infrared spectrometer in reflectance mode; NIRS-R, a laboratory-based near-infra-red spectrometer also in reflectance mode; and NIRS-T, a laboratory near-infrared spectrometer in transmittance mode. WSN stands for water-soluble nitrogen [8].
Curto et al. [9] successfully developed ANN models to predict 19 sensory attributes of cheese using NIR spectroscopy data, as presented in Figure 8. Principal component analysis reduced the NIRS data dimensionality, identifying critical components used as inputs for the ANNs. The models demonstrated high accuracy in sensory attribute predictions, with root mean squared error of prediction (RMSEP) values ranging from 0.34 to 0.59 and R2 values between 0.73 and 0.87, indicating strong alignment with human sensory evaluations (Figure 8). Scatter and residual plots confirmed the models’ predictive reliability, showcasing their potential as cost-effective, objective tools for sensory analysis in the food industry.
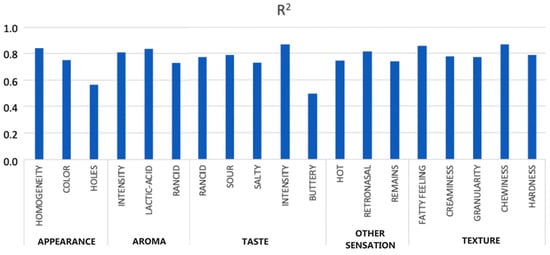
Figure 8.
Predictive squared correlation coefficient (R2) values for predictive accuracy across all sensory attributes for unknown samples [9].
International Dairy Federation guidelines [48] recommend sample preparation, particularly for non-homogeneous solid or semi-solid samples, to ensure sample consistency. This step typically involves grinding or mixing the sample to create a uniform sample for analysis. However, the effectiveness of sample preparation methods varied in scientific articles. For instance, Cattaneo and Barzaghi [49] compared NIR spectra obtained from the surface of a cheese block and grated samples of a hard cheese. They found no systematic superiority of one method over the other for predicting moisture, fat, protein content, and pH. Conversely, Wiedemair et al. [15] achieved better predictions with ground cheese samples. These discrepancies might be attributed to cheese body characteristics, such as the presence of holes or a crystalline structure, with better results observed when grinding more heterogeneous cheeses. Then again, grinding might introduce heterogeneity due to varying particle sizes, compression levels, and air distribution within the sample.
Cheese sample preparation was minimal in cases where portable spectrometers were used, with spectra collected directly from freshly cut cheese surfaces. This eliminated the need for extensive sample preparation beyond reference analyses using gold-standard methods [8].
Additionally, the incorporation of ANNs and PCA in NIR methodologies illustrates the technique’s evolution. These techniques enable more accurate predictions of cheese sensory characteristics, a leap forward from traditional quality descriptive analysis [9]. These advancements underscore NIR’s potential in refining sensory analysis and quality control within the dairy sector.
Furthermore, the importance of data treatment in prediction results must also be considered. Factors such as the mathematical pre-treatment of spectral data, the chemometric approach, statistical models, and validation strategies significantly impact the final prediction accuracy. While individual studies have examined the effects of specific steps in this process, there is a need for comprehensive analyses that consider all these factors together for improved prediction accuracy in NIRS [6].
Lastly, the accuracy of predictions using near-infrared spectroscopy depends on the repeatability of the reference methods. While, theoretically, prediction accuracy could reach 100%, the repeatability and reproducibility of the reference analyses constrain it. The expected maximum coefficients of determination for NIRS predictions can be calculated based on the standard deviation (SD) and standard error of the laboratory method (SEL) as [(SD2 − SEL2)/SD2]. Therefore, neither the calibration nor validation of NIRS models can achieve coefficients of determination of 100%, as they encompass errors from reference analyses and the NIRS system [6,50].
4.1.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR technology has emerged as a valuable tool for ensuring the authenticity of dairy products and quality control throughout processing and storage. It proves effective in measuring milk and dairy product fat content and assessing the geographical origin, molecular water mobility, and water retention capacity of cheese. Moreover, NMR enables the evaluation of dairy products’ physicochemical and sensory parameters [16,24]. It involves the application of an external magnetic field to nuclei in a sample, causing the nuclei to absorb and re-emit electromagnetic radiation at specific frequencies. This absorption is measured and used to obtain detailed information about the atomic and molecular structure of the sample. NMR is widely used in various fields, including chemistry, physics, biochemistry, and medicine, to determine the structure of organic compounds, study molecular dynamics, and even for medical imaging in the form of magnetic resonance imaging (MRI) [16].
Chen et al. [33] utilised NMR spectroscopy to identify key metabolites in Cheddar cheese that correlate with its ripening and quality. They found that the normalised intensity of citrulline and arginine significantly decreases over a 450-day ripening period, serving as markers for maturation. Additionally, tyrosine, tyramine, and lysine levels are closely associated with mature cheese sensory attributes, while β-galactose and glycerol are linked to younger cheese. This investigation highlights the potential of NMR spectroscopy as a robust and noninvasive approach to predict cheese quality and maturation, offering valuable insights for the cheese manufacturing industry. Figure 9 presents the PCA score plot showing the metabolic trajectories of Cheddar cheese samples at various ripening stages based on NMR spectral data. The plot highlights the unique ripening trajectory of batches B and C, showcasing their distinct paths through the PCA space. Premium quality Batch B samples are positioned at lower PC2 scores, indicating a different maturation profile than downgraded low-quality Batch C that remained on a different trajectory from the rest of the batches.
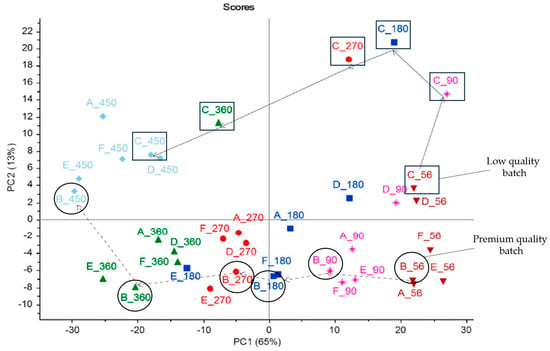
Figure 9.
PCA score plot showing maturation trajectories of Cheddar cheese batches * [33]. * This plot visualises the progression of Cheddar cheese ripening over 56 to 450 days, using 1H NMR spectral data of aqueous cheese extracts. Different symbols and colours represent various ripening periods, illustrating distinct maturation paths for batches A to F (inverted brown triangle (56 days); pink star (90 days); □ dark blue (180 days); o red (270 days); Δ green triangle (360 days), ◊ light blue rhombus (450 days)).
The study by Consonni and Cagliani [17] focused on the ripening and geographical characterisation of Italian Parmigiano Reggiano cheese using 1H NMR spectroscopy. The authors analysed Parmigiano Reggiano cheese samples of different ripening stages (14, 24, and 30 months) and compared them with “Grana type” cheese samples from Eastern Europe. Multivariate statistical protocols, including PCA and PLS-DA, assess the ripening process and discriminate between geographical origins (Table 1). The results indicate significant differences in metabolite content corresponding to both the ripening stages and geographical origins of cheese, with specific compounds like leucine, isoleucine, and threonine being key differentiators. East-European “Grana type” samples were distinctly different, characterised by high levels of leucine and isoleucine—compounds typical of young Italian samples (14 months)—along with lactate, butanoate, and acetate, indicating shorter ripening times. In contrast, Parmigiano Reggiano samples exhibited higher levels of threonine, typical of older samples (30 months), and other amino acids and volatile flavour compounds. The NMR and statistical approaches employed in this study were shown to be reliable tools in food analysis.
Brescia et al. [51] conducted a study to characterise the geographical origin of buffalo milk and Mozzarella cheese using NMR with a combination of analytical and spectroscopic methods, including high-performance ion chromatography (HPIC), inductively coupled plasma emission spectroscopy (ICP-AES), and isotope ratio mass spectrometry (IRMS). The study utilised these techniques, alongside chemometric methods, to distinguish buffalo Mozzarella cheese samples from different regions in Southern Italy based on specific chemical and isotopic parameters.
Despite its detailed molecular insights, NMR remains underutilised in the food industry due to its high operational costs and the need for specialised analytical knowledge [16]. Nevertheless, its applications in assessing the quality and authenticity of dairy products, including Cheddar cheese, by evaluating fat content, water mobility, and retention, underscore its potential to provide comprehensive insights into cheese maturation [16,24].
4.2. Comparative Analysis of Spectroscopic Techniques in Predicting Cheese Maturity (Answer to RQ2)
To determine the most effective spectroscopic method for predicting Cheddar cheese maturity, we evaluated FT-IR, NIR, and NMR techniques based on the literature review and existing comparative studies.
The comparative study by Stocco et al. [8] provides an in-depth analysis of the predictive accuracy of various spectroscopic techniques in the Vis/NIR region, emphasising the impact of the spectral range and technical features of the spectrometers on prediction outcomes. Their findings reveal that while technical specifications often outweigh the spectral range in determining predictive accuracy, NIR spectroscopy stands out for its effectiveness in predicting the composition of a wide variety of cheeses. However, the study also highlights that NIR is less successful in evaluating cheese pH and texture characteristics, underscoring the need for complementary methods when these parameters are of interest. This insight is crucial for designing future studies and selecting appropriate spectrometric equipment.
Ayvaz et al. [11] compared FT-NIR and FT-MIR spectroscopy for analysing compositional and physicochemical parameters in cheese. Their findings underscore the practical implications of sample preparation in enhancing the predictive power of NIR and FT-IR models. They found that FT-NIR spectroscopy was significantly superior to FT-MIR for most tested parameters, such as protein, fat, salt, dry matter, moisture, and ash content. However, for pH and titratable acidity, FT-MIR spectroscopy showed better performance. Notably, NIR accuracy was generally high regardless of whether spectra were collected from hand-crushed cheese or cheese cubes, and although hand-crushed samples yielded slightly better results for protein content, the spectra collected from cheese cubes gave better results for NIR models. Therefore, the authors recommended using cheese cubes for future applications. This highlights the importance of sample preparation in enhancing the predictive power of NIR models.
Cevoli et al. [40] conducted a study to evaluate the effectiveness of FT-NIR and FT-MIR spectroscopy in conjunction with statistical methods to authenticate grated Parmigiano Reggiano cheese. Their research focused on comparing these spectroscopic techniques’ predictive abilities and classification accuracy. The results demonstrated that both FT-NIR and FT-MIR spectroscopy were effective for rapid, nondestructive, and accurate screening of cheese authenticity. Specifically, FT-NIR showed a slightly higher correct classification probability, averaging around 0.974, compared to 0.969 for FT-MIR. This indicates that while both techniques are valuable for cheese authentication, FT-NIR may offer a marginally better performance in terms of predictive accuracy. The study highlights the potential of these spectroscopic methods in ensuring the quality and authenticity of grated cheese products, with FT-NIR having a slight superiority in classification capabilities.
Brescia et al. [51] explored various analytical and spectroscopic techniques, including NMR, to characterise the geographical origin of buffalo milk and its finished product, Mozzarella cheese. Their findings highlighted the effectiveness of these methods, particularly NMR, in distinguishing samples based on their geographical origin. While NMR provided detailed molecular insights, the study emphasised its utility in combination with other techniques like isotope ratio mass spectrometry (IRMS) for accurate geographical discrimination. However, due to the high operational costs and the specialised expertise required, NMR is less commonly used in routine quality control, making it more suitable for in-depth research applications rather than everyday industry practice. This comparative analysis underscored the importance of combining techniques to achieve reliable results in food authentication, particularly for products like Mozzarella cheese that are closely tied to specific geographical origins.
Most Effective Spectroscopic Method in Cheddar Cheese Analysis
NIR spectroscopy has shown significant superiority in cheese analysis due to its ease of sample handling, rapid analysis, and accuracy in predicting key parameters like maturity and sensory attributes, with models accurate enough for industrial use [13]. Miniaturised NIR devices can accurately monitor cheese fat composition without sample preparation, making it accessible for small producers [14]. Studies also demonstrated that pocket-sized NIR devices offer performance comparable to larger, more expensive benchtop devices, making them accessible for small businesses [15]. Additionally, NIRS provides precise, real-time process control in cheese production, reducing delays associated with conventional methods [27]. Moreover, NIR-HS imaging offers rapid monitoring of cheese ripening, underscoring its versatility and efficiency in dairy product analysis [37,44,45].
Downey et al. [13] demonstrated NIR’s effectiveness in predicting Cheddar cheese maturity and sensory attributes, reinforcing its industrial applicability. Dewantier et al. [29] emphasised the potential of spectroscopic techniques like FT-IR and Raman in assessing Cheddar cheese maturation, highlighting their ability to classify cheeses based on ripening time and manufacturer. Subramanian et al. [11] supported the use of FT-IR in monitoring amino acids and organic acids during Cheddar ripening, demonstrating its efficacy in predicting cheese age and composition efficiently. Chen and Irudayaraj [31] used FT-IR with a microtome sampling technique to achieve precise compositional analysis of Cheddar cheese, while Margolies and Barbano [52] found that mid-infrared (MIR) transmittance analysis provided superior accuracy in measuring fat, protein, moisture, and salt content.
Overall, the reviewed studies indicate that while FT-IR and NIR spectroscopy each offer valuable tools for predicting Cheddar cheese maturation, NIR emerges as the most effective method for routine analysis. The effectiveness of NIR, with its combination of speed, nondestructive analysis, and high predictive accuracy, reassures industry professionals about the reliability of this method for real-time monitoring in industrial settings. Despite providing detailed molecular information, FT-IR faces challenges in wet food systems like cheese, where sample heterogeneity and environmental conditions can impact its reproducibility. NMR, although offering incomparable depth of analysis, remains less practical for routine use due to its complexity and cost. The continued development and integration of these spectroscopic techniques promise to enhance the precision and efficiency of cheese quality assessment, contributing to the industry’s advancement toward more scientifically informed practices.
5. Discussion
The core of this discussion revolves around synthesising the insights gathered from implementing these techniques, addressing the distinctions, effectiveness, and potential future directions in the field, particularly concerning Cheddar cheese.
5.1. Efficacy and Comparison of Spectroscopic Methods
The studies reviewed in this paper, which are at the forefront of dairy science, underscore the potential of NIR, MIR and NMR spectroscopy in providing rapid, nondestructive assessments of cheese quality and maturation [17,28,52,53]. NIR spectroscopy, with its categorisation into IR-A and IR-B segments, has been pivotal in capturing detailed compositional and maturation-related data in cheese samples [27,54].
The work of Bittante et al. [6] and Stocco et al. [8] outlines the importance of selecting appropriate spectrometric techniques based on the intended analytical outcomes. These studies reveal that the choice of spectrometer, considering aspects like wavelength range and technical features, significantly influences the accuracy and reliability of compositional predictions in cheese analysis.
Bittante et al. [6] demonstrated the efficacy of different spectrometers in capturing the absorbance spectra of diverse cheese samples, revealing the distinctions between transmittance and reflectance spectra. Similarly, Priyashantha et al.’s studies [37,45] showcased NIR-HS imaging’s potential to visualise and model cheese maturation, utilising PLS regression to correlate spectral data with maturity levels, as depicted in Figure 4 and Figure 5. NIR-HS imaging technique’s ability to provide a comprehensive visualisation and modelling of cheese maturation stages signifies a leap forward in understanding the structural and chronological dynamics of cheese ripening.
Stocco et al. [8] presented a critical analysis of spectroscopic techniques, highlighting the impact of a spectrometer’s technical features over spectral range on predictive accuracy. Dewantier et al. [29] further explored this through PCA combined with FT-IR and Raman spectroscopies, effectively distinguishing Cheddar cheese samples based on ripening times and manufacturing differences. Their study also emphasised the importance of targeted spectral ranges to enhance sample differentiation (Table 1).
Curto et al. [9] applied PCA in conjunction with ANNs to predict sensory attributes of cheese from NIR spectra, illustrating a high correlation coefficient for texture-related attributes and an effective model for predicting sensory characteristics (Figure 8). This approach indicates the potential of combining PCA with ANN machine learning for detailed food quality analysis.
The study by Chen et al. [33] offers significant insights into the maturation process of Cheddar cheese using NMR spectroscopy, highlighting the potential of metabolomics in predicting cheese quality. The PCA plot presented in Figure 9 describes the maturation paths of different cheese batches, with premium Batch B and lower-quality Batch C showcasing divergent trajectories. This variance underscores the detailed relationship between the metabolomic profile and the sensory attributes of cheese as it matures. Moreover, the study underscores the importance of specific metabolites in defining the sensory attributes of mature Cheddar cheese. For instance, it highlights the association of tyrosine, tyramine, and lysine with these attributes, as well as the correlation of β-galactose and glycerol with the attributes of younger cheese. These findings underscore the interpretive value of the PCA plot in Figure 9, providing a visual and analytical representation of how cheese evolves over time, a key insight from the study. Moreover, Chen et al.’s study [33] emphasises the role of metabolites in the maturation process, and showcases how NMR spectroscopy, coupled with PCA, can offer profound insights into the ripening process, aiding in the development of predictive models for cheese quality. This could significantly benefit cheese producers by allowing more informed decisions regarding the ageing process, potentially leading to more consistent and optimised production outcomes.
The collective findings from these studies highlight the significant potential of spectroscopic techniques in improving the cheese industry’s quality control and maturation prediction processes. They illustrate a shift towards more objective, detailed, and noninvasive methods for assessing cheese maturation, emphasising the critical role of advanced imaging and chemometric techniques in modern food science.
Consequently, based on the literature review, NIR spectroscopy emerges as the most effective method for predicting Cheddar cheese maturity. Its rapid data acquisition, high repeatability, robust reproducibility, and high accuracy make it highly suitable for real-time, on-site industrial applications. Portable and miniaturised NIR instruments further enhance its applicability in the cheese industry.
5.2. Integration of PCA, PLS, and Machine Learning
The integration of PCA and PLS with ANNs represents a cutting-edge approach to sensory analysis and quality prediction [9,29,44,55]. By reducing dimensionality and enhancing data interpretability through PCA and PLS, coupled with the predictive ability of ANNs, researchers are equipped to derive more profound insights from spectroscopic data. This synergy surpasses the limitations of conventional analytical techniques, facilitating a more comprehensive understanding of the data’s underlying structure and its relationship with quality metrics.
5.3. Implications for Cheese Quality Control, Challenges, and Considerations
The methodologies discussed offer significant implications for the dairy industry, especially in quality control and assurance. The ability to predict sensory attributes and maturation stages with high precision can revolutionise quality monitoring, enabling more efficient production processes and ensuring product consistency and consumer satisfaction.
While the reviewed studies highlight the potential of spectroscopic techniques, challenges such as the complexity of cheese matrices, variability in cheese production processes, and the need for extensive calibration and validation of models are noted. The complex interaction between cheese composition, maturation processes, and sensory attributes necessitates a comprehensive and detailed analytical approach.
5.4. Future Directions
Looking forward, the integration of spectroscopic techniques with other analytical modalities, such as metabolomics or proteomics, could offer a more holistic assessment of cheese maturation and quality. This collaborative approach to research could further refine the predictive capabilities and enhance the applicability of these techniques across different cheese varieties and production methods.
Incorporating spectroscopic techniques, particularly with advanced data analysis tools like PCA, PLS, and ANNs, holds promise for transforming cheese quality assessment and maturation prediction. The body of work reviewed herein highlights the current state of the art and lays the groundwork for future research endeavours aimed at optimising and expanding the usage of these techniques within dairy science and beyond.
6. Conclusions
While the dairy industry has long relied on traditional methods for assessing Cheddar cheese quality and predicting maturation, spectroscopic techniques offer a new frontier in quality control and production efficiency. Techniques like NIR, FT-MIR, and NMR spectroscopy have demonstrated the potential to provide deep insights into the compositional changes and maturation dynamics of cheese.
NIR spectroscopy stands out due to its practicality and ease when considering the requirements for an efficient, nondestructive, cost-effective, and preferably portable method for replacing human sensory grading. It provides a rapid and noninvasive means to assess cheese quality, aligning perfectly with the industry’s need for swift, online, or at-line monitoring systems. NIR’s portability and ease of integration into the production line make it a practical choice for real-time quality control, offering a significant advantage over more complex and stationary methods like NMR, which, despite its comprehensive analytical depth, may be more suited to detailed laboratory studies rather than routine production monitoring.
The effectiveness of NIR in the cheese industry is not a single endeavour but a collective effort that hinges on developing robust calibration models. These models must accurately correlate spectral data with sensory and quality parameters. This requires a strong and united collaborative effort between researchers, industry practitioners, and technology developers to collect extensive spectral data across various cheese batches and maturation stages. This ensures the models’ reliability and predictive accuracy, fostering a sense of shared responsibility and unity in pursuing excellence.
Using portable NIR technology for cheese quality assessment can lead to significant cost savings, reducing outsourced testing expenses and providing a rapid return on investment. However, to implement these spectroscopic techniques effectively, the industry must invest in training personnel to operate the equipment and interpret data, integrating the technology seamlessly into existing workflows. The goal is to establish a system where spectroscopic analysis complements traditional sensory evaluation, providing a more comprehensive and objective assessment of cheese quality.
Future research should optimise NIR and MIR spectroscopy’s predictive capabilities, exploring their integration with other analytical methods and data analytics tools to create a holistic quality assessment framework. Such advancements will enhance quality control and enable more informed decisions in cheese production and maturation assessment, ultimately contributing to the industry’s sustainability and growth.
Author Contributions
All authors have contributed to this paper. S.S. is the primary author, responsible for drafting, literature review, and concept development. B.G. and S.M. are co-authors of the article and are responsible for proofreading, peer-reviewing the draft, and preparing the article for submission. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The primary author would like to acknowledge the contributions made by the co-authors, who helped the article reach its current state by sharing their valuable time and insights on the subject matter.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kraggerud, H.; Solem, S.; Abrahamsen, R.K. Quality scoring—A tool for sensory evaluation of cheese? Food Qual. Prefer. 2012, 26, 221–230. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Drake, M.A. Sensory character of cheese and its evaluation. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2004; pp. 455–487. [Google Scholar]
- Fox, P.F.; Cogan, T.M. Factors that affect the quality of cheese. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2004; pp. 583–608. [Google Scholar]
- Subramanian, A.; Rodriguez-Saona, L.E. Chemical and instrumental approaches to cheese analysis. In Advances in Food and Nutrition Research; Taylor, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 59, pp. 167–213. [Google Scholar]
- Bittante, G.; Patel, N.; Cecchinato, A.; Berzaghi, P. Invited review: A comprehensive review of visible and near-infrared spectroscopy for predicting the chemical composition of cheese. J. Dairy Sci. 2022, 105, 1817–1836. [Google Scholar] [CrossRef]
- Fagan, C.; Everard, C.; O’donnell, C.; Downey, G.; Sheehan, E.; Delahunty, C.; O’callaghan, D. Evaluating mid-infrared spectroscopy as a new technique for predicting sensory texture attributes of processed cheese. J. Dairy Sci. 2007, 90, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Cipolat-Gotet, C.; Ferragina, A.; Berzaghi, P.; Bittante, G. Accuracy and biases in predicting the chemical and physical traits of many types of cheeses using different visible and near-infrared spectroscopic techniques and spectrum intervals. J. Dairy Sci. 2019, 102, 9622–9638. [Google Scholar] [CrossRef]
- Curto, B.; Moreno, V.; García-Esteban, J.A.; Blanco, F.J.; González, I.; Vivar, A.; Revilla, I. Accurate prediction of sensory attributes of cheese using near-infrared spectroscopy based on artificial neural network. Sensors 2020, 20, 3566. [Google Scholar] [CrossRef]
- Nunes-Leite, S.; Goncalves, C.; Pinheiro, A.C.; Silva, S.; Madureira, A.R. Application of FTIR-ATR spectroscopy combined with chemometrics for the detection of adulteration in cheese with soy oil. J. Food Sci. Technol. 2019, 56, 3016–3024. [Google Scholar]
- Ayvaz, H.; Mortas, M.; Dogan, M.A.; Atan, M.; Tiryaki, G.Y.; Yuceer, Y.K. Near- and mid-infrared determination of some quality parameters of cheese manufactured from the mixture of different milk species. J. Food Sci. Technol. 2020, 58, 3981–3992. [Google Scholar] [CrossRef]
- Yaman, H.; Aykas, D.P.; Rodriguez-Saona, L.E. Monitoring Turkish white cheese ripening by portable FT-IR spectroscopy. Front. Nutr. 2023, 10, 1107491. [Google Scholar] [CrossRef]
- Downey, G.; Sheehan, E.; Delahunty, C.; O’callaghan, D.; Guinee, T.; Howard, V. Prediction of maturity and sensory attributes of Cheddar cheese using near-infrared spectroscopy. Int. Dairy J. 2005, 15, 701–709. [Google Scholar] [CrossRef]
- Reis, N.; Silva, S.; Pinheiro, A.C.; Gonçalves, C.; Martins, G.; Madureira, A.R. Comparative evaluation of miniaturized and conventional NIR spectrophotometer for estimation of fatty acids in cheeses. Food Chem. 2022, 383, 132377. [Google Scholar] [CrossRef] [PubMed]
- Wiedemair, V.; Langore, D.; Garsleitner, R.; Dillinger, K.; Huck, C. Investigations into the performance of a novel pocket-sized near-infrared spectrometer for cheese analysis. Molecules 2019, 24, 428. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, E. Nuclear magnetic resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 18, 189–220. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L. Ripening and geographical characterization of Parmigiano Reggiano cheese by 1H NMR spectroscopy. Talanta 2008, 76, 200–205. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 4th ed.; Springer: Boston, MA, USA, 2017; pp. 185–229. [Google Scholar] [CrossRef]
- Bodyfelt, F.; Drake, M.; Rankin, S. Developments in dairy foods sensory science and education: From student contests to impact on product quality. Int. Dairy J. 2008, 18, 729–734. [Google Scholar] [CrossRef]
- Subramanian, A.; Alvarez, V.B.; Harper, W.J.; Rodriguez-Saona, L.E. Monitoring amino acids, organic acids, and ripening changes in Cheddar cheese using Fourier-transform infrared spectroscopy. Int. Dairy J. 2011, 21, 434–440. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Subramanian, A.; Rodriguez-Saona, L.E. Rapid Extraction Method for Analysis of Cheese Flavour Using Infrared Spectroscopy; Provisional Appln. Ser. No. 61/059,890; The Ohio State University: Columbus, OH, USA, 2008. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley and Sons, Ltd.: Chichester, UK, 2004; ISBN 0470854278. [Google Scholar]
- Balthazar, C.F.; Guimarães, J.T.; Rocha, R.S.; Pimentel, T.C.; Neto, R.P.; Tavares, M.I.B.; Graça, J.S.; Filho, E.G.A.; Freitas, M.Q.; Esmerino, E.A.; et al. Nuclear magnetic resonance as an analytical tool for monitoring the quality and authenticity of dairy foods. Trends Food Sci. Technol. 2020, 108, 84–91. [Google Scholar] [CrossRef]
- Pu, Y.-Y.; O’Donnell, C.; Tobin, J.T.; O’Shea, N. Review of near-infrared spectroscopy as a process analytical technology for real-time product monitoring in dairy processing. Int. Dairy J. 2019, 103, 104623. [Google Scholar] [CrossRef]
- Dufour, E. Principles of infrared spectroscopy. In Infrared Spectroscopy for Food Quality Analysis and Control; Sun, D.W., Ed.; Elsevier Science: San Diego, CA, USA, 2009; Chapter 1; pp. 1–27. [Google Scholar]
- Adamopoulos, K.G.; Goula, A.M.; Petropakis, H.J. Quality control during processing of feta cheese—NIR application. J. Food Compos. Anal. 2001, 14, 431–440. [Google Scholar] [CrossRef]
- Abbas, O.; Baeten, V. Near-infrared spectroscopy. In Spectroscopic Methods in food Analysis, 1st ed.; Franca, A.S., Nollet, L.M.L., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2018; pp. 80–87. [Google Scholar]
- Dewantier, G.R.; Torley, P.J.; Blanch, E.W. Identifying chemical differences in cheddar cheese based on maturity level and manufacturer using vibrational spectroscopy and chemometrics. Molecules 2023, 28, 8051. [Google Scholar] [CrossRef]
- Chen, M.; Irudayaraj, J. Sampling technique for cheese analysis by ftir spectroscopy. J. Food Sci. 1998, 63, 96–99. [Google Scholar] [CrossRef]
- Chen, M.; Irudayaraj, J.; McMahon, D.J. Examination of full fat and reduced fat cheddar cheese during ripening by fourier transform infrared spectroscopy. J. Dairy Sci. 1998, 81, 2791–2797. [Google Scholar] [CrossRef]
- Bittante, G.; Cecchinato, A. Genetic analysis of the Fourier-transform infrared spectra of bovine milk with emphasis on individual wavelengths related to specific chemical bonds. J. Dairy Sci. 2013, 96, 5991–6006. [Google Scholar] [CrossRef]
- Chen, Y.; MacNaughtan, W.; Jones, P.; Yang, Q.; Williams, H.; Foster, T. Selection of potential molecular markers for cheese ripening and quality prediction by NMR spectroscopy. LWT 2020, 136, 110306. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier: Amsterdam, The Netherlands, 1997; pp. 1–12. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Priyashantha, H.; Höjer, A.; Saedén, K.H.; Lundh, Å.; Johansson, M.; Bernes, G.; Geladi, P.; Hetta, M. Determining the end-date of long-ripening cheese maturation using NIR hyperspectral image modelling: A feasibility study. Food Control. 2021, 130, 108316. [Google Scholar] [CrossRef]
- Wilkinson, C.; Yuksel, D. Using artificial neural networks to develop prediction models for sensory-instrumental relationships; an overview. Food Qual. Prefer. 1997, 8, 439–445. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Minim, V.P.R.; Silva, R.d.C.d.S.N.; Della Lucia, S.M.; Minim, L.A. Artificial neural networks (ANN): Prediction of sensory measurements from instrumental data. Food Sci. Technol. 2013, 33, 722–729. [Google Scholar] [CrossRef]
- Cevoli, C.; Gori, A.; Nocetti, M.; Cuibus, L.; Caboni, M.F.; Fabbri, A. FT-NIR and FT-MIR spectroscopy to discriminate competitors, non-compliance and compliance grated Parmigiano Reggiano cheese. Food Res. Int. 2013, 52, 214–220. [Google Scholar] [CrossRef]
- Jaggi, N.; Vij, D. Fourier transform infrared spectroscopy. In Handbook of Applied Solid State Spectroscopy; Vij, D., Ed.; Springer: Boston, MA, USA, 2006; pp. 411–450. [Google Scholar]
- Kraggerud, H.; Næs, T.; Abrahamsen, R.K. Prediction of sensory quality of cheese during ripening from chemical and spectroscopy measurements. Int. Dairy J. 2013, 34, 6–18. [Google Scholar] [CrossRef]
- González-Martín, M.; Severiano-Pérez, P.; Revilla, I.; Vivar-Quintana, A.; Hernández-Hierro, J.; González-Pérez, C.; Lobos-Ortega, I. Prediction of sensory attributes of cheese by near-infrared spectroscopy. Food Chem. 2011, 127, 256–263. [Google Scholar] [CrossRef]
- Lin, L.; He, Y.; Wang, L.; Liu, F.; Pan, J.; Wang, R. Study on nonlinear multivariate methods combined with the visible near-infrared spectroscopy of cheese. Food Chem. 2014, 152, 416–422. [Google Scholar]
- Priyashantha, H.; Höjer, A.; Saedén, K.H.; Lundh, Å.; Johansson, M.; Bernes, G.; Geladi, P.; Hetta, M. Use of near-infrared hyperspectral (NIR-HS) imaging to visualize and model the maturity of long-ripening hard cheeses. J. Food Eng. 2019, 264, 109687. [Google Scholar] [CrossRef]
- CIE (International Commission on Illumination). Colorimetry, 3rd ed.; CIE 015; Commission Internationale de l’Éclairage: Vienna, Austria, 2004; Available online: https://cie.co.at/publications/colorimetry-3rd-edition (accessed on 30 September 2024).
- Alinaghi, M.; Nilsson, D.; Singh, N.; Höjer, A.; Saedén, K.H.; Trygg, J. Near-infrared hyperspectral image analysis for monitoring the cheese-ripening process. J. Dairy Sci. 2023, 106, 7407–7418. [Google Scholar] [CrossRef]
- ISO 21543:2020; Milk and Milk Products—Guidelines for the Application of Near-Infrared Spectrometry, Updated Guideline. ISO: Geneva, Switzerland, 2020.
- Cattaneo, T.M.; Barzaghi, S. Outer product analysis applied to near infrared and mid infrared spectra to study a spanish protected denomination of origin cheese. J. Near Infrared Spectrosc. 2009, 17, 135–140. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be included in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Brescia, M.; Monfreda, M.; Buccolieri, A.; Carrino, C. Characterisation of the geographical origin of buffalo milk and mozzarella cheese by means of analytical and spectroscopic determinations. Food Chem. 2005, 89, 139–147. [Google Scholar] [CrossRef]
- Margolies, B.J.; Barbano, D.M. Determination of fat, protein, moisture, and salt content of Cheddar cheese using mid-infrared transmittance spectroscopy. J. Dairy Sci. 2018, 101, 924–933. [Google Scholar] [CrossRef]
- Martín-Del-Campo, S.; Picque, D.; Cosío-Ramírez, R.; Corrieu, G. Evaluation of chemical parameters in soft mold-ripened cheese during ripening by mid-infrared spectroscopy. J. Dairy Sci. 2007, 90, 3018–3027. [Google Scholar] [CrossRef]
- McQueen, D.H.; Wilson, R.; Kinnunen, A.; Jensen, E.P. Comparison of two infrared spectroscopic methods for cheese analysis. Talanta 1995, 42, 2007–2015. [Google Scholar] [CrossRef]
- Mayr, S.; Beć, K.B.; Grabska, J.; Wiedemair, V.; Pürgy, V.; Popp, M.A.; Bonn, G.K.; Huck, C.W. Challenging handheld NIR spectrometers with moisture analysis in plant matrices: Performance of PLSR vs. GPR vs. ANN modelling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 249, 119342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).