Abstract
The tenth annual international G-CAN research symposium was held in Alexandria, VA on the 13th and 14th of November 2024. This hybrid meeting, a live face-to-face and virtual live symposium, was attended by 201 participants. Over 20 research abstract submissions were received from early career investigators, for plenary oral and poster presentations. Here, we present the 22 accepted, lightly edited abstracts from the early career presenters consenting to have their materials published. We thank and congratulate the presenters for their work and contributions to the meeting.
1. Prognostic Value of Hyperuricemia in Developing Cardiovascular Events in Patients with Chronic Inflammatory Arthritis: A 10-Year Prospective Study
Antonio Avilés *, Zulema Plaza, Fernando Sánchez–Alonso, Santos Castañeda, Benjamin Fernandez–Gutierrez, Cesar Diaz–Torne, Pilar Font, Olga Martinez, Emilio Giner, José Miguel Senabre, Amalia Rueda, Ana Perez, Gines Sanchez Nievas, Carlos Gonzalez, Javier García, Javier Llorca, Miguel Angel Gonzalez–Gay and Mariano Andres
- Rheumatology Department, Hospital General Universitario Doctor Balmis, 03010 Alicante, Spain
- * Correspondence: antonioaviles1997@gmail.com
- Abstract: Background: The role of hyperuricemia as a cardiovascular risk factor in chronic inflammatory arthritis (CIA) is unclear. We investigated whether hyperuricemia increases cardiovascular events in rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) patients in the Spanish CARMA prospective registry. Methods: Post hoc analysis of the CARMA project, a 10-year prospective study of cardiovascular events in patients with CIAs (RA, AS, PsA) and controls with non-inflammatory rheumatic diseases (osteoarthritis, osteoporosis, soft tissue injuries) from 67 Spanish hospitals. The study spanned 2010–2022, with two-year recruitment and four visits at 2.5, 5, 7.5, and 10 years. Baseline blood tests included serum urate (SU) levels; hyperuricemia was defined as SU > 6.8 mg/dL. Both were primary explanatory variables. The 10-year cumulative incidence of major cardiovascular events (MACEs) was recorded, including angina, ischemic heart disease, stroke, transient ischemic attacks, atherosclerotic renal failure, heart failure, and peripheral arterial disease. A multiple logistic regression model was built, adjusted for age, sex, traditional cardiovascular risk factors, renal disease, and established cardiovascular disease. Results were reported as odds ratios (ORs) with 95% confidence intervals (95% CI). Results were stratified by CIA type and non-inflammatory group. Results: Out of 1552 participants with CIA, 174 (11.2%) were classified as hyperuricemic, 39 (6.9%) with RA, 78 (15.5%) with AS, and 57 (11.8%) with PsA. Mean SU levels (SD) of the CIA cohort were 5.0 (2.3) mg/dL (4.6 (3.1), 5.3 (1.5), and 5.1 (2.0), in RA, AS, and PsA, respectively). Out of 349 non-inflammatory controls, 39 were classified as hyperuricemic (11.2%), with a mean SU level (SD) of 5.0 (1.4) mg/dL. After ten years, 233 MACEs were recorded in the CIAs, 49 (21.0%) occurring in patients with hyperuricemia. By type of arthritis, 14 events (28.6%) occurred in the RA population with hyperuricemia, 23 (46.9%) in AS, and 12 (24.5%) in PsA. Within the control group, 41 MACEs were recorded, 9 (23.1%) occurring in patients with hyperuricemia. Table 1 and Table 2 show baseline urate variables’ association with MACEs in CIA patients. Hyperuricemia, as a dichotomous variable, independently increased MACE risk (OR 2.06) and trended towards higher vascular kidney disease risk. SU levels were not linked to MACEs despite bivariate associations. By arthritis type, hyperuricemia risk persisted only in AS (OR 4.02, 95% CI 1.96–8.24), not in RA (OR 1.85, 95% CI 0.76–4.49) or PsA (OR 0.94, 95% CI 0.39–2.26). Neither hyperuricemia (OR 1.79, 95% CI 0.89–3.60) or SU levels (OR 1.10, 95% CI 0.98–1.23) were cardiovascular prognostic factors in non-inflammatory participants. Conclusions: In a 10-year prospective cohort, hyperuricemia at baseline and non-contiguous SU levels showed a prognostic impact on developing cardiovascular events in patients with CIAs, especially in those suffering from AS, in contrast to non-inflammatory patients. Our findings reinforce the need to adequately address comorbidities in this setting.
 Table 1. Logistic regression model assessing serum urate levels as a cardiovascular risk factor.
Table 1. Logistic regression model assessing serum urate levels as a cardiovascular risk factor. Table 2. Logistic regression model assessing hyperuricemia (>6.8 mg/dL) as cardiovascular risk factor.
Table 2. Logistic regression model assessing hyperuricemia (>6.8 mg/dL) as cardiovascular risk factor.
2. Electronic Health Record-Based Calcium Pyrophosphate Deposition Disease Registry: A Feasibility Study
Nils Bürgisser 1,2,*, Denis Mongin 2,3, Samia Mehouachi 2, Delphine Courvoisier 2,3 and Kim Lauper 2
- 1
- Division of General Internal Medicine, Geneva University Hospitals, 1205 Geneva, Switzerland
- 2
- Division of Rheumatology, Geneva University Hospitals, 1205 Geneva, Switzerland
- 3
- Quality of Care Division, Geneva University Hospitals, 1205 Geneva, Switzerland
- *
- Correspondence: nils.burgisser@hug.ch
- Abstract: Background: Despite its prevalence, calcium pyrophosphate deposition disease (CPPD) remains insufficiently studied. Leveraging electronic health record (EHR) data, a rich source of patient information, could facilitate the automatic enrollment of CPPD patients in registries without the need for manual recruitment [1], enhancing research opportunities. However, registry reliability is often compromised due to the poor accuracy of diagnostic coding for CPPD. We describe the process and validation of creating a CPPD registry out of EHR data from a tertiary university hospital in Geneva, Switzerland, integrating various sources of information to improve its accuracy. Methods: We screened EHR data from patients over 18 years old with any out- or inpatient visit at the Geneva University Hospital—Switzerland’s largest tertiary hospital—between 1 January 2013, and 31 December 2023. Screening criteria consisted of the presence of at least one of the following: ICD-10-GM codes for CPPD (M11) or positive joint aspiration for CPP crystal or CPPD-related terms (“pseudogout”, “chondrocalcinosis”) in the list of diagnosis or documents or imaging reports. A random sample of 200 medical charts was fully reviewed by a trained nurse and physician. The positive predictive value (PPV) to detect CPPD was established if any form of it was mentioned by any doctor in any part of the EHR. Results: The four criteria identified 6709 patients presumably suffering from CPPD (Figure 1A). Most patients were identified by screening medical reports (15.0%), followed by the list of diagnosis (5.1%). A proportion of 3% of the patients had a positive joint aspiration for CPPD, without any documented diagnosis in the EHR. In the randomly selected 200 charts manually reviewed (Table 3), the overall (any criterion) positive predictive value was 85.0% (CI 95% 79.3 to 89.6%). When taken alone (i.e., without any other criteria), both the ICD-10 codes and the list of diagnosis showed the best PPV (100% and 92.7%, respectively). The sole use of these two criteria decreased the number of included patients to 1953. By using a combination of criteria (ICD-10 codes or list of diagnosis or ≥ 1 other criteria), the PPV reached 96.1% (CI 91.2% to 98.7%), leading to a registry of 5320 patients (Figure 1B). Conclusions: The creation of a CPPD register with automatic enrollment is feasible and valid. Incorporating various elements of the EHR, beyond the diagnostic codes alone, improves patient detection accuracy and increases registry numbers. Notably, a subset of patients exhibited positive joint aspirations without a corresponding documented diagnosis, indicating a documentation gap. The next step is to estimate the negative predictive value in a sample of patients without any criteria but with risk factors for CPPD, and develop a method to classify CPPD patients by clinical presentation. The final register will help assess pertinent clinical outcomes using clinical indicators to evaluate current quality of CPPD care and trajectories within the disease.

Table 3.
Results of the chart review looking for any CPPD-related terms (CPPD, pseudogout, chondrocalcinosis) among patients detected by the queries.
Table 3.
Results of the chart review looking for any CPPD-related terms (CPPD, pseudogout, chondrocalcinosis) among patients detected by the queries.
| Query | Total | CPPD | No CPPD | Positive Predictive Value (CI 95%) |
|---|---|---|---|---|
| Present irrespective of other criteria | ||||
| ICD-10-GM codes | 75 | 72 | 3 | 96.0 (88.8 to 99.2) |
| List of diagnoses | 66 | 64 | 2 | 97.0 (89.5 to 99.6) |
| Joint aspiration | 69 | 62 | 7 | 89.7 (80.2 to 95.8) |
| Documents | 140 | 119 | 21 | 85.0 (78.0 to 90.5) |
| Radiology reports | 29 | 26 | 3 | 89.7 (72.6 to 97.8) |
| Present alone | ||||
| ICD-10-GM codes | 10 | 10 | 0 | 100 (69.1 to 100) |
| List of diagnoses | 14 | 13 | 1 | 92.7 (66.1 to 99.8) |
| Joint aspiration | 9 | 3 | 6 | 33.3 (7.5 to 70.1) |
| Documents | 54 | 37 | 17 | 68.5 (54.5 to 80.5) |
| Radiology reports | 7 | 5 | 2 | 71.4 (29.0 to 96.3) |
| Overall result | ||||
| Any criteria | 200 | 170 | 30 | 85.0 (79.3 to 89.6) |
| Combination query | ||||
| ICD-10 or list of diagnosis or ≥1 other criteria | 130 | 125 | 5 | 96.1 (91.2 to 98.7) |
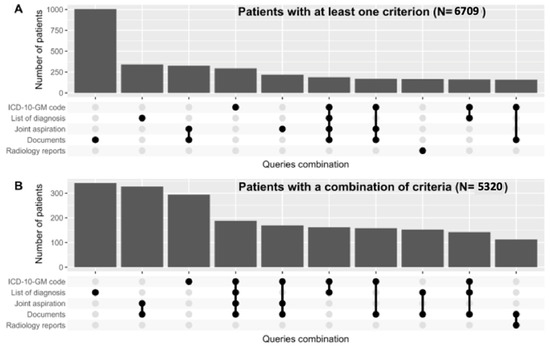
Figure 1.
(A) Upset plot showing patients identified with calcium pyrophosphate deposition disease (CPPD) using any criterion. (B) Upset plot showing patients identified using a combination of criteria (ICD-10-GM codes or diagnosis list or ≥ 1 other criteria), resulting in higher positive predictive value but fewer patients.
Reference
- Bürgisser, N.; Mongin, D.; Mehouachi, S.; Buclin, C.P.; Guemara, R.; Darbellay Farhoumand, P.; Braillard, O.; Kauper, K.; Courvoisier, D.S. Development and validation of a self–updating gout register from electronic health records data. RMD Open. 2024, 10, e004120.
3. Urate-Induced Immunometabolic Adaptations of Myeloid Cells
Georgiana Cabău 1, Viola Kluck 2, Valentin Nica 1, Orsolya Gaal 1,2, Medeea Badii 1,2, HINT–Consortium, Tania O. Crișan 1 and Leo A. B. Joosten 1,2
- 1
- Department of Medical Genetics, “Iuliu Haţieganu” University of Medicine and Pharmacy, 400012 Cluj–Napoca, Romania
- 2
- Department of Internal Medicine, Radboud University Medical Center, Nijmegen 6525 GA, The Netherlands
- *
- Correspondence: georgiana.cabau@gmail.com
- Abstract: Background: Hyperuricemia is the main risk factor for gout and is associated with a higher incidence of cardiometabolic disorders. Urate exposure in vivo alters the serum proteome towards higher inflammation, while in vitro studies reveal that soluble urate primes myeloid cells for higher induction of inflammatory cytokines, by increasing IL-1beta production and reducing the anti-inflammatory cytokine, IL-1Ra. Moreover, the inflammatory memory induced by urate modifies the chromatin landscape, suggesting that epigenetic modifications could mediate the long-term detrimental effects of urate exposure. In the present study, we aim to unravel the underlying metabolic adaptations of urate-exposed myeloid cells. This will not only expand our understanding of the multifaceted nature of urate-induced inflammation but can potentially reveal new therapeutic targets aimed at preventing cardiometabolic complications associated with hyperuricemia. Methods: In a urate priming design, Percoll-enriched monocytes from healthy volunteers were treated with increasing concentrations of soluble urate in the absence or presence of pharmacological modulators 3PO, an inhibitor of the rate-limiting glycolysis enzyme PFKFB3, acetyl-CoA, and the fatty acid synthase (FASN) inhibitor C75 for 24 h. After incubation, cells were washed and stimulated for another 24 h with LPS and monosodium urate (MSU) crystals. Cytokine production was determined by ELISA. Transcriptomic data from in vitro soluble urate stimulated PBMCs were assessed for the differential expression of genes involved in the glycolytic and fatty acid synthesis pathways. Results: In vitro soluble urate exposure enhances pro-inflammatory cytokine production. Inhibition of glycolysis lowers the urate-induced inflammatory response. Addition of acetyl-CoA, the substrate for fatty acid synthesis on 3PO pre-treated myeloid cells, rescues the urate-induced pro-inflammatory phenotype. Inhibition of FASN, the rate-limiting enzyme in the FA synthesis pathway, blocks urate priming, lowers pro-inflammatory cytokine production, and restores the anti-inflammatory IL-1Ra. Transcriptomic analysis corroborates these findings and reveals upregulation of the PFKFB3 and FASN in urate-exposed PBMCs. Conclusions: Our preliminary data reveal that soluble urate rewires the metabolism of myeloid cells towards enhanced glycolysis and fatty acid synthesis. Cooperation of these two pathways converge to sustain the long-term inflammatory memory of urate exposed cells. Our data support the idea of a role for urate-induced metabolic adaptations and reveal new targetable pathways as a strategy to prevent hyperuricemia-induced inflammation.
4. Associations of Physical Volume and Intensity with Incident Gout: A Prospective Cohort Study in the UK Biobank
Qi Chen * and Haibing Chen
- School of Medicine, Tongji University, 200070 Shanghai, China
- * Correspondence: chenqi911229@163.com
- Abstract: Background: The association between physical volume and the intensity and incident of gout has not been examined in a large prospective cohort study. We aimed to evaluate the association of physical volume and intensity with the risk of incident gout in the general population. Methods: A total of 436,594 participants (55.4% female) at baseline who completed physical activity screening in the UK Biobank were enrolled. Physical activity was assessed using the self-reported short-form international physical activity questionnaire (IPAQ). From IPAQ questions, the total physical activity volume can be computed in metabolic equivalent minutes per week (MET-min/wk). Additionally, the MET-min/wk within each type of activity (i.e., walking, moderate intensity, and vigorous intensity) was calculated. We used Cox proportional hazard models to estimate the associations between physical activity and incident gout, with restricted cubic spline added to the Cox models to evaluate the dose–response relationships. Subgroup analysis was performed in subjects with gout history and in asymptomatic patients with hyperuricemia. Incident gout was determined based on information from hospital admission records. Results: During a median follow-up period of 12.6 years, we recorded 6919 gout cases. U-shaped relationships were discovered between summed MET scores with incident gout. After multivariable adjustment for major risk factors, we found a nonlinear association between summed MET scores and gout (p for non-linearity <0.001), rapidly reaching the lowest risk at 1455 MET-min/wk and rising gradually. For vigorous activity, J-shaped association between Met scores with gout risk were observed. In patients with gout history in particular, the risk of incident gout increased significantly as vigorous Met scores increased (p for trend <0.001). For moderate activity and walking, an L-shaped association was observed between Met scores with gout risk, and higher moderate or walking MET scores showed a strong dose–response association with a lower risk of gout (p for trend = 0.012). Conclusions: Physical volume had a strong nonlinear association in gout risk reduction. Vigorous activity was related to higher risk of gout, while moderate activity and walking were associated with reduced risk of gout.
5. Prevalence and Severity of Osteoarthritis in the Spines of Patients with Gout Versus Non-Gout Controls
Allyson Covello 1,2,*,†, Salim Zenkhri 3,†, Cheongeun Oh 4, Michael H. Pillinger 1,2, Fabio Becce 3,‡ and Michael Toprover 1,2,‡
- 1
- Division of Rheumatology, Department of Medicine, New York University Grossman School of Medicine, New York, NY 10016, USA
- 2
- Rheumatology Section, NY Harbor Health Care System New York Campus, United States Department of Veterans Affairs, New York, NY 10010-5011, USA
- 3
- Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, University of Lausanne, 1015 Lausanne, Switzerland
- 4
- Division of Biostatistics, Department of Population Health, New York University Grossman School of Medicine, New York, NY 10016, USA
- *
- Correspondence: allyson.covello@nyulangone.org
- †
- Co–first authors.
- ‡
- Co–senior authors.
- Abstract: Background: Prior research suggests a connection between osteoarthritis (OA) and gout at sites commonly affected by gouty attacks, such as the knee, foot, and ankle. Whether a relationship exists between gout and radiological OA at sites with known monosodium urate (MSU) crystal deposition [1] but which are less commonly affected by gouty attacks, such as the lumbosacral (LS) spine, has not been previously investigated. Additionally, the potential role of MSU crystal deposition in mediating a relationship between OA and gout remains unclear. We assessed whether LS OA is more prevalent and more severe in gout patients vs. controls, and whether LS OA is associated with higher levels of spinal MSU crystal deposition. Methods: A total of 50 gout subjects and 25 controls, ages 45–80, underwent DECT imaging of the lumbosacral spine. We assessed LS OA using a modification of a previously validated computed tomography scoring system, incorporating grade of intervertebral disc narrowing and facet joint OA, and the presence or absence of spondylolysis and spondylolisthesis [2]. We quantified spinal MSU crystal deposition using the manufacturer’s default post-processing algorithm, as well as a maximally specific algorithm to exclude potential artifact [3]. Results: 71 subjects (46 gout and 25 controls) were included in the final analysis. The average age was 62 years. Gout subjects had higher BMI, more kidney disease and hypertension, lower exercise frequency, and higher mean serum urate and creatinine vs. controls. Both gout and control subjects exhibited high rates of facet joint OA and degenerative disc disease, with no difference in prevalence or severity between the groups (Table 4, Figure 2). Gout subjects also did not have differing prevalence of spondylolysis and spondylolisthesis versus controls (Table 4). Subjects with LS OA did not have higher levels of spinal MSU crystal deposition versus subjects without LS OA (Figure 3). Conclusions: LS OA was not more prevalent or more severe in gout patients vs. controls, and spinal MSU crystal deposition did not differ between patients with and without LS OA. These findings suggest that gout and MSU crystal deposition may not play a role in the development of radiological OA in joints not commonly affected by gouty attacks.

Table 4.
Prevalence of lumbosacral facet joint osteoarthritis, degenerative disc disease, spondylolysis, and spondylolisthesis in gout patients versus controls.
Table 4.
Prevalence of lumbosacral facet joint osteoarthritis, degenerative disc disease, spondylolysis, and spondylolisthesis in gout patients versus controls.
| Gout n = 46 | Control n = 25 | p-Value | |
|---|---|---|---|
| Facet joint OA | 42 (91%) | 24 (96%) | 0.650 |
| Degenerative disc disease | 41 (89%) | 24 (96%) | 0.414 |
| Spondylolysis | 3 (7%) | 2 (8%) | >0.999 |
| Spondylolisthesis | 16 (35%) | 10 (40%) | 0.663 |
Abbreviation: OA = osteoarthritis. Note: Values are n (%), and p-values were generated using Pearson’s chi-squared test and Fisher’s exact test.
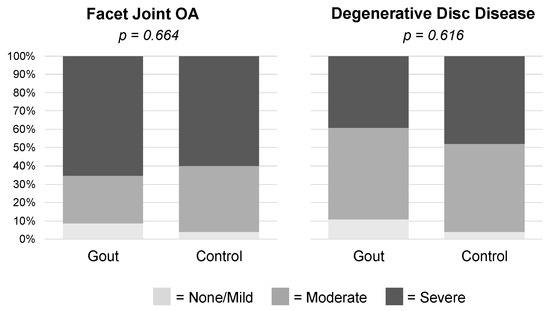
Figure 2.
Severity of lumbosacral facet joint osteoarthritis and degenerative disc disease in gout patients versus controls.
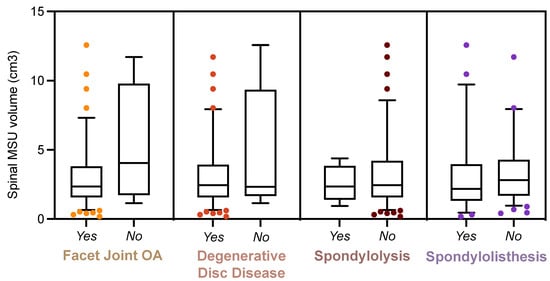
Figure 3.
Monosodium urate crystal deposition in the lumbosacral spines of patients with and without spinal osteoarthritis.
References
- Toprover, M.; Mechlin, M.; Fields, T.; Oh, C.; Becce, F.; Pillinger, M.H. Monosodium urate deposition in the lumbosacral spine of patients with gout compared with non–gout controls: A dual–energy CT study. Semin. Arthritis Rheum. 2022, 56, 152064.
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Hunter, D.J. Computed tomography–evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self–reported low back pain. Spine J. 2010, 10, 200–208.
- Døssing, A.; Müller, F.C.; Becce, F.; Stamp, L.; Bliddal, H.; Boesen, M. Dual–energy computed tomography for detection and characterization of monosodium urate, calcium pyrophosphate, and hydroxyapatite: A phantom study on diagnostic performance. Invest. Radiol. 2021, 56, 417–424.
6. The Intersection of CD38, NAD Metabolism, and NLRP3 Inflammasome Activation in Synovial Fibroblasts in Gouty Arthritis
Carlisle DeJulius, John Varga, Tristan Maerz and Puja Khanna
- University of Michigan, 500 S State St, Ann Arbor, MI 48109, USA
- * Correspondence: cdejuliu@med.umich.edu
- Abstract: Background: Gout is a common, age-related, hyperuricemia-induced autoinflammatory condition associated with painful flares and which represents a growing public health crisis, with enormous socioeconomic impact. Flares lead to joint destruction and progressive functional impairment [1], and current treatments are associated with serious side effects. There is a critical need to better understand the mechanism of gout flares to develop effective, disease-modifying treatments. An emerging target in understanding the acute cytokine cascade is inflammasome activation, the assembly of NLRP3 and ASC for the activation of CASP-1, leading to increased secretion of inflammatory cytokines [2]. However, upstream mediators of inflammasome activation remain unclear. The transmembrane ectoenzyme CD38, which cleaves extracellular NAD [3], has now been implicated in inflammasome activation in inflammatory conditions, including gout [4]. NAD consumption is linked to many diseases of aging, and emerging studies demonstrate that NAD rescue through CD38 inhibition can be protective [5]. Here, we test the hypothesis that NAD depletion in synovial fibroblasts through upregulated CD38 activity is a key checkpoint in the inflammasome-mediated pathomechanism of gout (Figure 4). Methods: Fibroblast-like synoviocytes (FLS) were harvested from the hindpaws of C57/Bl6 wild-type (WT) and CD38 knockout (KO) mice. FLS were treated with 0–200 µg/mL MSU crystals for 6 or 24 h, and expression of key genes was evaluated by qPCR. Statistical significance was determined via two-way ANOVA and Tukey’s post-hoc test. Results: After MSU treatment, we measured the expression of four key disease-relevant genes: Nlrp3, for inflammasome activation (Figure 5A,B); Mmp13, as a representative protease (Figure 5C,D); Il6, as a representative cytokine (Figure 5E,F); and Cd38 (in WT cells), to determine the effect of MSU treatment on Cd38 expression (Figure 5G,H). In WT FLS, 200 µg/mL of MSU strongly and rapidly induced the expression of Nlrp3, Mmp13, and Il6. Nlrp3 and Il6 expression was increased 6 h post-treatment (10- and 40-fold, respectively) and began to taper at 24 h. The effect on Mmp13 was more sustained, increasing from 6 to 24 h (12- and 80-fold, respectively). In all cases, the absence of CD38 significantly blunted the effect of the MSU crystal, indicating a strong tie between CD38 expression and MSU crystal response in FLS. Notably, MSU crystal treatment of WT FLS caused a ~50% reduction in Cd38 expression. Comprehensive characterization of transcriptional changes induced by MSU in WT and CD38 KO cells is ongoing. Additionally, the effect of a selective CD38 inhibitor, MK-0159, on MSU crystal-induced inflammation and cellular NAD metabolism is being tested. Conclusions: Genetic ablation of CD38 in FLS blunts MSU crystal-induced inflammasome, protease, and cytokine expression. Ongoing experiments are evaluating the role of NAD metabolism in vitro and in vivo to further characterize the links between CD38 and MSU crystal-induced inflammation, and its therapeutic potential in treatment-resistant gout.
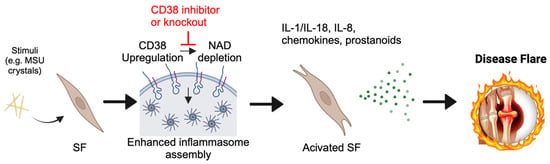
Figure 4.
Proposed mechanism for fibroblast inflammasome response to monosodium urate (MSU) crystals. MSU crystals may cause CD38 upregulation, resulting in nicotinamide adenine dinucleotide (NAD) depletion and the triggering of enhanced inflammasome assembly. SF = synovial fibroblast.
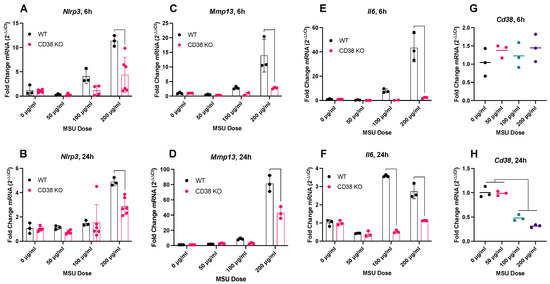
Figure 5.
In vitro transcriptional response of fibroblast-like synoviocytes (FLS) to monosodium urate (MSU) crystals measured by qPCR 6 and 24 h after treatment. (A,B), Nlrp3; (C,D), Mmp13; (E,F), Il6 6 and 24 h after treatment; (G,H), Cd38 expression in WT cells.
References
- Punjwani, S.; Jani, C.; Liu, W.; Kakoullis, L.; Salciccioli, I.; Al Omari, O.; Merchant, A.; Singh, H.; Marshall, D.; Shalhoub, J.; et al. Burden of gout among different WHO regions, 1990–2019: estimates from the global burden of disease study. Sci. Rep. 2024, 14, 15953.
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Tr. Biochem. Sci. 2016, 41, 1012–1021.
- Camacho–Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M., Galina, A.; et al. CD38 dictates age–related NAD decline and mitochondrial dysfunction through and SIRT3–dependent mechanism. Cell. Metab. 2016, 23, 1127–1139.
- Wen, S.; Arakawa, H.; Tamai, I. CD38 activation by monosodium, urate crystals contributes to inflammatory responses in human and murine macrophages. Biochem. Biophys. Res. Commun. 2021, 581, 6–11.
- Zeidler, J.D.; Hogan, K.A.; Agorrody, G.A.; Peclat, T.R.; Kashyap, S.; Kanamori, K.S.; Sales Gomez, L.; Mazdeh, D.Z.; Warner, G.M.; Thompson, K.L.; et al. The CD38 glycohydrolase and the NAD sink: implications for pathological conditions. Biochem. Biophys. Res. Commun. 2021, 581, 6–11.
7. Mapping Monosodium Urate Crystal Deposition Within Joints in Tophaceous Gout: A Dual Energy CT Study
Chamaya de Silva 1,*, Cèsar Díaz–Torné 2, Greg Gamble 3, Anne Horne 3, Anthony Doyle 3, Lisa K. Stamp 4 and Nicola Dalbeth 3
- 1
- Health New Zealand, 1023 Auckland, New Zealand
- 2
- Hospital de la Santa Creu I Sant Pau, 08025 Barcelona, Spain
- 3
- Department of Medicine, University of Auckland, 1023 Auckland, New Zealand
- 4
- Christchurch School of Medicine, University of Otago, 8011 Christchurch, New Zealand
- *
- Correspondence: chamaya.desilva@gmail.com
- Abstract: Background: In gout, monosodium urate (MSU) crystal deposition occurs preferentially at certain joints, most frequently the first metatarsophalangeal (MTP) joint. Dual energy CT (DECT) allows for the visualization of MSU crystals. The aim of this DECT study was to map the distribution of MSU crystal deposition within the MTP joints in people with tophaceous gout. The study hypothesis was that MSU crystal deposition is evenly distributed within the joint in tophaceous gout. Methods: Bilateral feet DECT scans of 124 people with tophaceous gout (57% male, mean disease duration 21 years, 98% on urate-lowering therapy, mean serum urate 6.9 mg/dL) were analyzed. All participants met the ACR/EULAR 2015 classification criteria for gout. The metatarsal head and phalangeal base of each MTP joint was analyzed by dividing each bone into four quadrants (dorsal, plantar, medial, and lateral) as described by Pecherstorfer et al. [1]. Each bone quadrant was scored for the presence or absence of MSU crystal deposition (8 sites per joint, 80 sites per participant). MSU crystal deposition was considered present if in contact with or directly adjacent (within 1 mm) to bone or cartilage in at least two planes. All images were analyzed independently by two trained readers (inter-reader kappa 0.78 and percentage agreement 98.9%), and differences between readers was resolved by image review. Data were analyzed using general estimating equations undertaken to adjust for the likely closer agreement between bones in the same individual. Results: There was marked variation in MSU crystal deposition across the analyzed sites (Figure 6). The medial quadrant of the first metatarsal head was most affected (57%) and significantly more affected than any other analyzed site. The next most common affected sites were the plantar quadrants of the first and second metatarsal heads and medial quadrant of the first phalangeal base (all >30%). MSU crystal deposition was very rare in the phalangeal base of the third and fourth MTP joints, particularly the lateral quadrants (1%). Across all joints, there was more MSU crystal deposition at the first MTP joint compared with other joints, at the metatarsal head compared with the phalangeal base, and at the plantar and medial quadrants compared with other quadrants (p < 0.001 for all comparisons). Conclusions: In people with tophaceous gout, MSU crystal deposition is not evenly distributed. There is a marked lack of uniformity with preferential sites of deposition not only between different joints, but also within joints. Several biological mechanisms may explain these findings, including the influence of localized tissue planes, temperature or microtrauma.
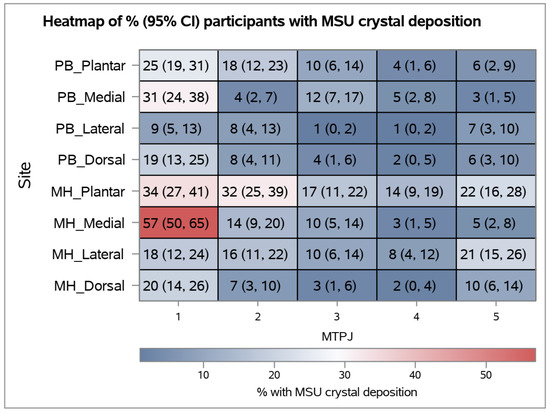
Figure 6.
Heatmap showing the percentage (95% CI) of participants with MSU crystal deposition. PB: phalangeal base, MH: metatarsal head.
Reference
- Percherstorfer, C.; Simon, D.; Unbehend, S.; Ellmann, H.; Enlbrecht, M.; Hartmann, F.; Figueiredo, C.; Hueber, A.; Haschka, J.; Kocijan, R. et al. A detailed analysis of the association between urate deposition and erosions and osteophytes in gout. ACR Open Rheumatol. 2020, 2, 565–572.
8. Should the Hyperuricemia Threshold for Women Be Lowered? Examining Gender-Specific Differences in Prevalence and Associated Risk Factors
Florina–Iulia Ionete 1,2,*, Simona Chirica 1,2, Oana Vutcanu 1,2 and Mihai Bojinca 1,2
- 1
- University of Medicine and Pharmacy “Carol Davila” Bucharest, Bucharest 050474, Romania
- 2
- Dr. Ion Cantacuzino Clinical Hospital Bucharest, Bucharest 030167, Romania
- *
- Correspondence: florina.ionete@yahoo.com
- Abstract: Background: Hyperuricemia, even when asymptomatic, is a known risk factor for renal, cardiovascular, and metabolic disorders. Traditionally, hyperuricemia is more prevalent in men than women, suggesting gender-specific differences in its impact, although the commonly used diagnostic threshold of >7 mg/dL for hyperuricemia may not fully capture the associated risks, particularly in women. This study explores gender-specific differences in hyperuricemia prevalence and evaluates whether a lower threshold for women would better reflect their risk of complications. The primary objective of this study was to assess gender-specific differences in the prevalence of asymptomatic hyperuricemia and its association with cardiovascular and metabolic risk factors. Additionally, the study aimed to evaluate whether lowering the diagnostic threshold for women from >7 mg/dL to >6 mg/dL would more accurately capture risk and prevalence. Methods: A total of 1602 unselected adults from rural Romania participated in this study. Blood tests measured serum urate levels and other biomarkers, and hyperuricemia was defined as serum urate >7 mg/dL for both genders, with a secondary analysis considering >6 mg/dL for women. The population was divided into hyperuricemic and non-hyperuricemic groups. Exclusion criteria included individuals under 18 or over 85 years of age and those on urate-lowering medication. Results: The study comprised 29.6% males and 70.4% females, with a mean age of 54.25 ± 15.64 years. Hyperuricemia prevalence was significantly higher in men (26.05%) compared with women (4.52%) using the >7 mg/dL threshold. However, using a >6 mg/dl threshold for women increased their hyperuricemia prevalence to 14.9%. Men with hyperuricemia had higher values for cardiovascular risk factors, including BMI, fasting glucose, cholesterol, triglycerides, and serum creatinine. Furthermore, when the female population was categorized into three groups—normouricemic (SUA < 6 mg/dL), “impaired” (SUA 6–7 mg/dL), and hyperuricemic (SUA >7 mg/dL)—both the “impaired” and hyperuricemic groups exhibited significantly higher average values for BMI, waist and hip circumference, systolic blood pressure, cholesterol, triglycerides, GGT, serum creatinine, fasting blood glucose, and CRP levels compared with the normouricemic women. The graphic representations also show different distributions for serum uric acid in males and females (Figure 7 and Figure 8). Conclusions: The current >7 mg/dL threshold for diagnosing hyperuricemia in women may not adequately capture the risk associated with elevated uric acid levels. Women with uric acid levels between 6–7 mg/dL showed significant increases in cardiovascular and metabolic risk markers. This suggests that a lower threshold could improve risk assessment and early intervention. This study highlights significant gender-specific differences in hyperuricemia and its associated risks. Lowering the diagnostic threshold for women may enable earlier identification and better management of hyperuricemia-related health risks.
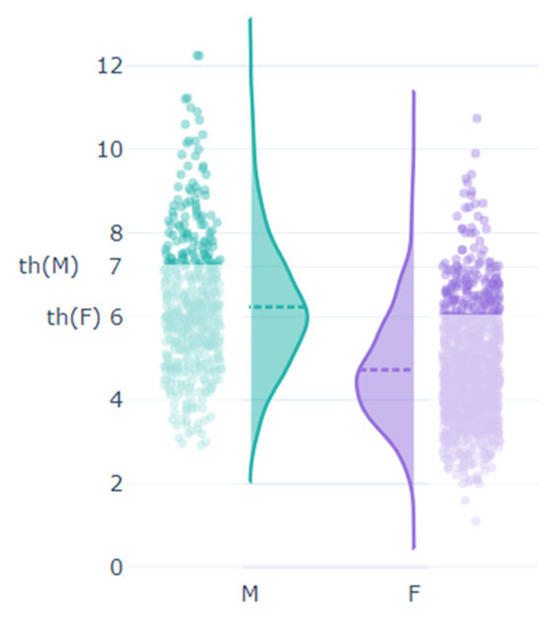 Figure 7. Serum urate levels by gender.
Figure 7. Serum urate levels by gender.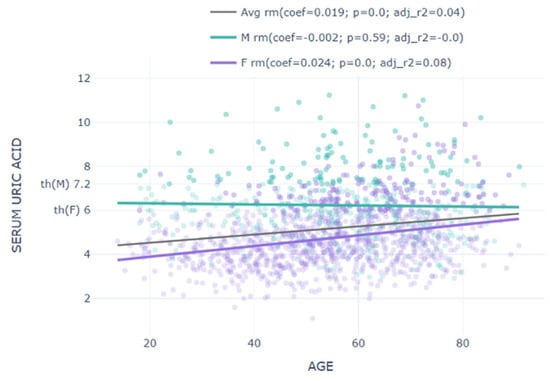 Figure 8. Correlation of serum urate with age by gender.
Figure 8. Correlation of serum urate with age by gender.
9. A Gout GWAS in African-Ancestry Population (N = 204,060) Identifies 22 Gout-Associated Loci
Sydney Grooms 1,2,*, Riku Takei 1,2, Vinodh Srinivasasainagendra 1,2, Nick Sumpter 1,3, Louis Dell’Italia 1,2, Betty Pat 1,2 and Tony R. Merriman 1,2
- 1
- Birmingham Veterans Affairs Health Care System, Birmingham, AL 35233, USA
- 2
- University of Alabama at Birmingham, Birmingham, AL 35294, USA
- 3
- Radboud University Medical Center, Nijmegen 6525 GA, The Netherlands
- *
- Correspondence: segrooms@uabmc.edu
- Abstract: Background: Gout is an innate immune response to monosodium urate crystals deposited in the joints. The prevalence of gout in the United States is 5.1%, however the prevalence is higher in the Black population, at 5.7% [1]. Despite this, previous gout genome-wide association studies (GWAS) have had a notable under-representation of participants of African descent, with only two loci (ABGC2 and SLC2A9) previously detected [2]. We therefore conducted a larger GWAS specifically on an African population. Methods: We meta-analyzed the summary statistics of two separate gout GWAS of African ancestry using the program METAL [3]. The separate GWAS included age, sex, and PC as covariates. The first GWAS comprised 3053 cases and 77,944 controls [2], and the second GWAS comprised 18,345 cases and 104,718 controls using the Million Veterans Project cohort, resulting in 21,398 cases and 182,662 controls in total. Loci were identified by Manhattan plot and visualized by Locus Zoom plots. Results: We detected 22 loci associated with gout (p-value threshold = 5 × 10−8) of which seven have not been previously reported in other populations. We detected signals at established gout loci such as SLC2A9, ABCG2, PDZK1, GCKR, SLC22A12, and SLC16A9. We also detected signals at XDH, FADS2, DGAT2, and NRG4. Notably 14 of the 22 loci were on chromosome 11 including all of the 7 newly reported loci. Of these seven the locus at 5.2 MB was notable. It was detected by SNP rs334, an African-specific missense variant (A-allele frequency 10%) in the hemoglobin subunit delta (HBD) gene and is causal of sickle cell anemia. Additionally, variant rs2217366 at the XDH (codes for urate-producing enzyme xanthine dehydrogenase) locus showed a stronger effect in African (OR = 1.16, SE = 0.03) compared with European (OR = 1.05, SE = 0.01) populations [2]. Furthermore, the signal was more focused in African than European populations, which will facilitate efforts towards the fine-mapping of the genetic effect at XDH. Conclusions: By substantially increasing the sample size we provide new insights into the genetic basis of gout in people of African ancestry. Key findings were the concentration of loci on chromosome 11 and the associations at the XDH and HBD loci. Several other loci have been previously detected in Europeans and are strongly implicated in the innate inflammatory response (THBS3, FADS2, DGAT2). We plan to increase power to detect gout-associated loci by including additional cohorts such as the All of Us cohort. Not only will this work increase our understanding of the molecular pathogenesis of gout in the Black population, but will assist in the fine-mapping of gout-associated loci shared between populations.
References
- Yokose, C.; McCormick, N.; Lu, N.; Tanikella, S.; Lin, K.; Joshi, A.D.; Raffield L.M.; Merriman T.; Hsu, J.; Saag, K. Trends in prevalence of gout among US Asian adults, 2011–2018. JAMA Netw. Open 2023, 6, e239501.
- Major, T.J.; Takei, R.; Leask, M.P.; Sumpter, N.A.; Topless, R.K.; Shirai, Y.; Wang, W.; Cadzow, M.J.; Phipps–Green, A.J.; Li, Z. A genome wide association analysis reveals new pathogenic pathways in gout. Nat. Genet. 2024, 56, 2392–2406.
- Willer, C.J.; Li, Y.; Abecasis, G.R. METAL: fast and efficient meta–analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191.
10. Unravelling Systemic Inflammation in Gout and CPPD Disease: New Insights into Biomarker Discovery
Brenda Kischkel 1,*, Nicholas Sumpter 1, Charles Leroy 2, Twinu Wilson Chirayath 2, Mihai G Netea 1, Frédéric Lioté 2, Pascal Richette 2, Tristan Pascart 3, Hang Korng Ea 2 and Leo A. B. Joosten 1,4
- 1
- Department of Internal Medicine, Radboud University Medical Center, 6500HB, Geert Grooteplein Zuid 10, Nijmegen 6525 GA, The Netherlands
- 2
- Université de Paris, INSERM UMR 1132, Hôpital Lariboisière, AP–HP, 75010 Paris, France
- 3
- Department of Rheumatology, Hôpital Saint–Philibert, Université Catholique de Lille, 59800 Lille, France
- 4
- Department of Medical Genetics, Iuliu Hatieganu University of Medicine and Pharmacy, 400012 Cluj–Napoca, Romania
- *
- Correspondence: brenda.kischkel@radboudmc.nl
- Abstract: Objective: Gout and CPPD disease are prevalent types of inflammatory arthritis particularly affecting elderly individuals and those with multiple medical comorbidities. The primary distinction between these conditions lies in their etiology: gout results from the deposition of monosodium urate (MSU) crystals, whereas CPPD disease is caused by the deposition of calcium pyrophosphate (CPP) crystals. Clinical differentiation between gout and CPPD disease is challenging, as, during acute flares, patients with either condition typically exhibit symptoms such as pain, swelling, and reduced range of motion in the affected joint. A thorough understanding of the unique characteristics of each condition is essential for accurate diagnosis, effective treatment, and the prevention of flare-ups. This study aims to enhance our comprehension of these conditions by accessing systemic inflammation in patients experiencing gout and CPPD flares. Methods: Proximity extension assay technology (Olink) was used to measure 92 inflammation-related proteins in 71 patients with gout (GOUTROS), 86 patients with CPPD disease (COLCHICORT), and 96 healthy controls (BCG elderly). Differently expressed proteins were obtained by linear regression models using age and sex as covariates (Limma R package). Results: Comparison between gout and CPPD disease revealed 13 downregulated and 2 upregulated proteins, including, CASP-8 (FDR 3.30 × 10−18), FGF-5 (4.25 × 10−5), TGF-α (1.0 × 10−4), CCL28 (0.001), AXIN1 (0.005) and 4E-BP1 (0.04). Notably, CASP-8, TGF-α and 4E-BP1 were also differently expressed compared with healthy controls. To explore the potential of these proteins in distinguishing between gout, CPPD disease, and healthy controls, a multinomial logistic regression model adjusted for age and sex (nnet R package) was performed. As a result, TGF-α and CASP-8 presented an area under the curve (AUC) above 0.97 for all three cohorts. Of importance, between CASP-8, TGF-α and 4E-BP1, only TGF-α was strongly correlated with CRP in gout and CPPD disease patients (p ≤ 0.001). Additionally, human peripheral blood mononuclear cells (PBMCs) from 195 healthy donors (200FG, 2023) were exposed to MSU and mCPP crystals, with and without LPS. We observed that PBMCs stimulated with both crystals alone did not produce pro-inflammatory cytokines. However, the combination of MSU crystal + LPS increased the production of IL-1β, IL-6, IL-1Ra and IL-10 compared with LPS alone. Interestingly, PBMCs stimulated with mCPP + LPS showed increased production of IL-1β and IL-1Ra and decreased production of IL-6 and IL-10 compared with LPS alone. Conclusions: Our findings collectively indicate significant differences in systemic inflammation between patients with gout and CPPD disease. Of the 76 proteins tested, 19.7% exhibited differential expression during a gout or CPPD flare. Notably, TGF-α and CASP-8 have potential as biomarkers for differentiating between these two conditions. However, further studies are required to validate these results in an independent cohort of patients. Moreover, our experiments in PBMCs demonstrate that mCPP crystals induce higher levels of pro-inflammatory cytokines compared with MSU crystals, suggesting an IL-1β-dependent inflammatory response.
11. Intracellular Fate of Monosodium Urate and Calcium Pyrophosphate Crystals in Macrophages and Their Dissolution
Charles Leroy *, Nghia Pham, François Brial, Brenda Kischkel, Gwénaëlle Jayat, Elena Ishow, Christèle Combes, Leo Joosten, Augustin Latourte, Pascale Richette and Hang–Korng Ea
- Bioscar, Lariboisiere Hospital, Université Paris Cité, Inserm U1132, 75010 Paris, France
- * Correspondence: charles.leroy@inserm.fr
- Abstract: Background: Gout, due to the presence of monosodium urate crystals (MSU) and calcium pyrophosphate (CPP) crystal deposition disease are both responsible for recurrent inflammation flares. Both crystals stimulate interleukin-1β production by macrophages. Macrophages can interact and phagocytose both MSU and CPP crystals. In vivo, giant multinucleated cells (GMCs) are observed surrounding the MSU crystal deposition in tophi from gout patients. The objective was to describe the intracellular fates of MSU and CPP crystals in macrophages. Methods: Synthetic pyrogen-free MSU and CPP crystals were used to stimulate different type of mouse (RAW 264.7 cell line, bone marrow-derived macrophages (BMDMs)) and human (THP-1 cell line and peripheral blood mononuclear cells (PBMCs)) macrophages. To assess crystal phagocytosis in vitro, macrophages were stimulated by MSU and CPP crystals engrafted with fluorescent organic nanoparticles (FONs) and recorded for 5 days using the Incucyte instrument (Sartorius). Intracellular outcomes were assessed by 18-h recording using apotome Zeiss microscopy. Image analysis was performed by ImageJ software. To observe crystal phagocytosis by GMCs ex vivo, tophi from gouty patients were fixed and stained by Von Kossa and HE staining. In vitro, GMC were obtained after stimulation of RAW 264.7 cells by MSU crystal, after complete dissolution of crystals and macrophage fusion to form GMC at day 10, GMC were re-stimulated by MSU crystal and recorded. Results: All types of macrophages, including RAW 264.7, BMDM, THP-1 and PBMC, phagocytosed MSU and CPP crystals within one hour (Figure 9). Interestingly, in a few RAW 264.7 macrophages and PBMCs, phagocytosed MSU crystals were expulsed into the cytosol of other macrophages. MSU crystals were dissolved after phagocytosis while CPP crystals remained undissolved even after 7 days of stimulation. MSU crystal dissolution occurred only 48 h after phagocytosis. No dissolution was observed in the first 48 h while all MSU crystals were dissolved after 7 days of stimulation. Macrophages displayed different capacities to dissolve MSU crystals: after 48 h of stimulation, 17.5% of MSU crystals (in one recording field) were dissolved in RAW 264.7 vs. 24.3% and 56.0% for BMDM and THP-1 cells, respectively (Figure 10A,B). Interestingly, we observed that dissolution of MSU crystals was followed by cell death and burst in RAW 264.7 and PBMCs. THP-1 cells also dissolved MSU crystals but without burst nor death. Treatment with the inhibitor of the V-ATPase pump, bafilomycin A1, completely blocked the dissolution of MSU crystals without affecting crystal phagocytosis (Figure 10C). Ex vivo, tophi analysis showed that the GMC surrounding MSU crystal deposition had numerous intracellular MSU crystals, suggesting that they had been phagocytosed. Under MSU crystal stimulation these macrophages differentiated into GMC, which phagocytosed and dissolved numerous MSU crystals (Figure 11). Conclusions: Our results suggest that dissolution of MSU crystals is a late cellular response involving cell lysosomes and acidification. GMC seem to present a higher capacity to phagocyte and dissolve MSU crystals in vitro. Further studies are ongoing to decipher the underlying mechanisms.
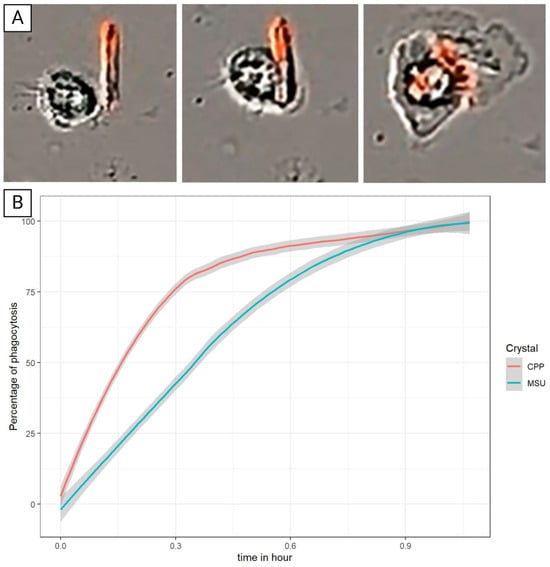 Figure 9. (A) Phagocytosis of FON-coupled CPP by PBMC during the first hour. (B) Percentage of phagocytosis of MSU and CPP crystals by PBMC.
Figure 9. (A) Phagocytosis of FON-coupled CPP by PBMC during the first hour. (B) Percentage of phagocytosis of MSU and CPP crystals by PBMC.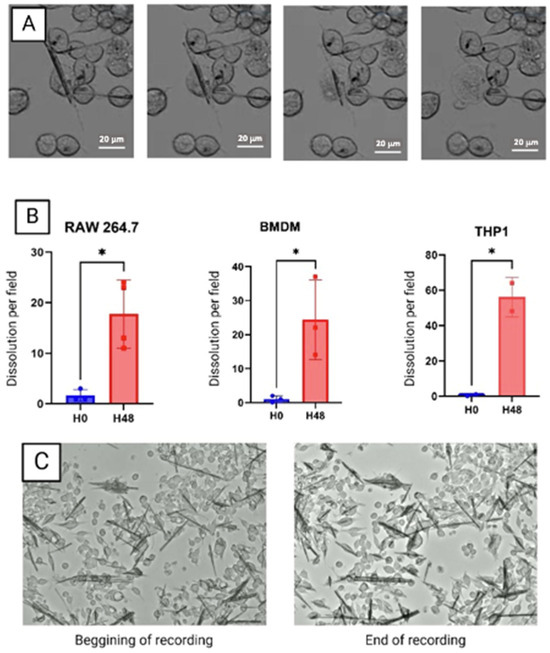 Figure 10. (A) Dissolution of MSU crystal by RAW 264.7 cells accompanied by the bursting and death of the cell after 48 h of stimulation. (B) Quantification of the number of dissolutions of MSU crystals per field of three types of cells. * Significantly different comparisons. (C) Absence of MSU crystal dissolution by RAW 264.7 after 48 h of stimulation by MSU and Bafilomycin A1.
Figure 10. (A) Dissolution of MSU crystal by RAW 264.7 cells accompanied by the bursting and death of the cell after 48 h of stimulation. (B) Quantification of the number of dissolutions of MSU crystals per field of three types of cells. * Significantly different comparisons. (C) Absence of MSU crystal dissolution by RAW 264.7 after 48 h of stimulation by MSU and Bafilomycin A1.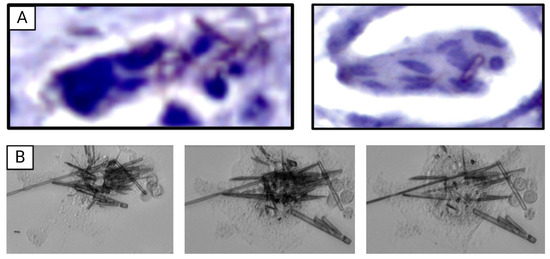 Figure 11. (A) Giant multinucleated cells containing MSU crystals (red arrow) and CPP crystals (green arrow). (B) Dissolution of MSU crystals by giant multinucleated cells accompanied by bursting of the cell after 48 h of stimulation.
Figure 11. (A) Giant multinucleated cells containing MSU crystals (red arrow) and CPP crystals (green arrow). (B) Dissolution of MSU crystals by giant multinucleated cells accompanied by bursting of the cell after 48 h of stimulation.
12. Rapid Access Microscopy and Real Time Case Discussion via a Secure Messaging App Improves Diagnostic Accuracy and Management of Acute Hot Swollen Joints
Anouchka Lewis 1,2,*, John Stack 1,2, Tomás Breslin 1,2, Frank Lyons 1,2, Eavan Muldoon 1,2, Cian McDermott 1,2, Sharon Cowley 1,2, Helina Alemayehu 1,2, Oliver Boughton 1,2, Oisin Corish 1,2, Callum Swift 1,2, Khaled Taha 1,2 and Geraldine McCarthy 1,2
- 1
- Rheumatology Department, Mater Misericordiae University Hospital, University College Dublin, Dublin, Ireland
- 2
- Academic Track Internship Programme, Dublin South–East Network, Trinity College Dublin, The University of Dublin, Ireland
- *
- Correspondence: anlewis@tcd.ie
- Abstract: Background: Patients with acute swollen joints are often presumed to have septic arthritis, leading to treatment with intravenous antibiotics and arthroscopic washout. Previously at our center, joint fluid aspirates often lacked crystal analysis, resulting in excess culture-negative septic arthritis diagnoses. To address this, stakeholders from the emergency department (ED), infectious disease, orthopedic, and rheumatology services developed a ‘Hot Joint Pathway.’ We hypothesized that, as acute crystal arthropathy can be misdiagnosed as ‘culture-negative septic arthritis,’ introducing the pathway would increase diagnostic accuracy and decrease unnecessary admissions, intravenous antibiotics and joint washouts. Methods: A ‘Hot Joint Pathway’ was developed to provide a structured approach for investigating acutely swollen joints, outlining necessary blood tests, sepsis assessment, indications for joint aspiration and guidance on distinguishing septic arthritis from other conditions such as crystal arthritis. Key features of the pathway include a secure messaging app for multidisciplinary discussion and rheumatology-led rapid access polarized light microscopy (PLM) within 24 h. A service evaluation, approved by our clinical audit committee, was completed following the pathway’s introduction. Hospital inpatient data from the Mater Misericordiae University Hospital, Dublin, identified patients labelled with septic arthritis (including culture-negative cases) discharged between two periods: before the pathway (1 January 2019 to 30 November 2020) and after its introduction (22 September 2022 to 21 February 2024). Data were also recorded for patients presenting with an acute swollen joint at the ED who were subsequently discussed on the secure messaging app between 27 September 2022 and 25 September 2023. Patient medical records, laboratory results, and radiology reports were reviewed. Results: Of the patients discussed on the secure messaging app who presented to the ED, 92% received rheumatology input, and 100% underwent joint aspiration with crystal analysis by a rheumatologist in less than 24 h. A proportion of 68% of patients avoided hospital admission, receiving same-day discharge. Of these, 53% received same-day diagnoses of crystal arthropathy and were discharged with a plan for rheumatology outpatient follow-up. Diagnostic accuracy increased substantially following introduction of the pathway (Table 5). Joint aspirates increased from 50% to 76%. Culture-negative cases of septic arthritis reduced from 34% to 17%. Culture-positive cases of septic arthritis increased from 41% to 76%. Crystal analysis increased from 19% to 28%. Positive blood cultures increased from 28% to 41%. The average length of stay reduced by 6 days. Conclusions: A structured care pathway combining rheumatology-led rapid access PLM and multidisciplinary case discussion via a secure messaging app increases diagnostic accuracy for patients presenting with acute swollen joints. This pathway facilitates admission avoidance, reduces length of stay, and prevents unnecessary surgical intervention, whilst positively impacting antimicrobial stewardship. Access to rheumatology-led point-of-care PLM is essential to the success of this pathway.
 Table 5. Inpatient data showing positive blood cultures, joint aspirations, crystal analyses and culture-positive and negative septic arthritis cases pre- and post-introduction of the ‘Hot Joint Pathway.’
Table 5. Inpatient data showing positive blood cultures, joint aspirations, crystal analyses and culture-positive and negative septic arthritis cases pre- and post-introduction of the ‘Hot Joint Pathway.’
13. Treat to Target in Gout Yields Superior Outcomes Compared with the Treat to Avoid Symptoms Approach (Results from the Gout TrEatment Strategy (GO TEST) Overture Trial)
Anusha Moses 1, Martijn Oude Voshaar 1,2, Tim Jansen 3 and Mart Van de Laar 4
- 1
- Department of Personalized Diagnostics and Therapeutics Department of Personalized Diagnostics and Therapeutics/Medical Cell BioPhysics &TechMed Center, University of Twente, 7522 Enschede, The Netherlands
- 2
- Department of Public Health, Erasmus Medical Center, 3015 Rotterdam, The Netherlands
- 3
- Department of Rheumatology, Viecuri Medical Center, 5912 Venlo, The Netherlands
- 4
- Department of Psychology, University of Twente, 7522 Enschede, The Netherlands
- *
- Correspondence: a.moses@utwente.nl
- Abstract: Background: Both the European League Against Rheumatism (EULAR) and the American College for Rheumatology (ACR) state that gout can be effectively managed by reducing serum urate (sU) levels below the physiological threshold of saturation using urate-lowering therapy (ULT), a treat-to-target approach (T2T). An alternative, more reactive management strategy, advocated by the American College of Physicians (ACP) is to treat symptoms and to base the start and dosing of ULT on avoiding (recurrent) symptoms, without monitoring sU levels. This treat-to-avoid symptom (T2S) approach has been criticized by rheumatologists for potentially ignoring ongoing urate deposition until potentially severe disease manifestations become apparent. Although the solid underlying mechanism of gout is ignored, the lack of a head-to-head comparison of both strategies led the ACP to advise the T2S strategy for the treatment of gout. The objective of the Gout TrEatment Strategy (GO TEST) Overture trial is to compare a T2T strategy versus a T2S strategy for the management of gout. Methods: The GO TEST Overture study is a multicenter randomized controlled open-label pragmatic trial. Patients with a clinical diagnosis of gout, fulfilling the 2015 ACR–EULAR criteria, currently not using ULT but with an indication for the use of ULT were included. Patients with a contraindication for allopurinol, benzbromarone, and febuxostat, including those with an eGFR < 30 mL/min, were excluded from the study. Patients in the T2T group started ULT at the discretion of the attending physician. Their sU levels were regularly measured and ULT dosages were titrated to achieve an sU target of <0.36 mmol/L. In the T2S group, patients were instructed to self-monitor their gout symptoms and to contact the clinic in case of recurrent symptoms, with a potential ULT dosage increase in case of flare recurrence. The incidence of patient-reported gout flares was assessed every three months using the Gaffo gout flare criteria. The major outcomes of the study were the proportions of patients meeting the sU target and the number of Gaffo flares during 1-year follow-up. The proportions of patients achieving the sU target level were compared using z-tests for proportions. The number of flares over 1 year was compared using Poisson regression analysis. Results: A total of 308 patients were eligible and included in the trial (T2T n = 145; T2S n =163) with a mean sU of 0.5 and 0.4 mmol/L and mean age of 62.5 at baseline in each group, respectively. After 1 year, the proportion of patients achieving the target sU level <0.36 mmol/L s was significantly higher in the T2T group (77%) than in the T2S group (29%) (p < 0.001). In addition, the incidence rate ratio for gout flares was 1.541 for T2S versus T2T (p < 0.001), indicating that patients in the T2S group experienced a 54% increase in the rate of gout flares. Conclusions: Our study is the first randomized trial to directly compare T2T with T2S as a control group in gout. We were able to show the superiority of the T2T management approach in gout.
14. A Genotype-Guided Approach to Hypouricemia Diagnosis
Junhyoung Park *, Yunjin Kim, Seong–ho Cho and Sung Kweon Cho
- Department of Pharmacology, Ajou University School of Medicine, 164, Worldcup–ro, Yeongtong–gu, Suwon 16499, Republic of Korea
- * Correspondence: wontan2000@gmail.com
- Abstract: Background: Hypouricemia, defined as a serum urate level below 2 mg/dL, is generally asymptomatic but can occasionally result in conditions such as exercise-induced acute kidney injury (E–AKI), kidney stones, and neurodegenerative diseases. Hypouricemic patients are often unrecognized or ignored and the diagnostic study is not standardized. In the Korean population, a known cause is the SLC22A12 gene mutation. This study aims to assess the genetic influence on serum urate levels (sU) and to propose a diagnostic guideline. Methods: This study utilized data from the Korean Genome and Epidemiology Study (KoGES), a cohort study by the National Institutes of Health of the Korea Disease Control and Prevention Agency (114,780 subjects). We focused on two specific mutations in the SLC22A12 gene (W258X, R90H). The impact of these two SLC22A12 mutations on sU level was analyzed using linear regression. Additionally, we assessed the feasibility of the genetic testing of SLC22A12 gene mutations for hypouricemia diagnosis. Evaluations were conducted under two definitions of hypouricemia: sU < 1.3 mg/dL and sU < 2.0 mg/dL. Furthermore, we also evaluate the usefulness of predicting sU based on the statistical results in 92 samples. Results: We identified that SLC22A12 mutations significantly lower sU level (β = −0.1, p < 0.01). The homozygous mutation exhibits a more pronounced sU-lowering effect than the heterozygous mutation. The compound heterozygous and homozygous mutations of the W258X variant have the most substantial sU-lowering impact (β = −0.4, p < 0.01). Furthermore, we demonstrated that a genetic test for SLC22A12 mutations would have high specificity (>0.99) under both hypouricemia criteria. In addition, with regard to the association between predicted sU and observed sU in 92 samples, the QQplot shows that a right-skewed distribution and a 0.417 correlation coefficient is deduced. Conclusions: The genetic diagnosis for hypouricemia is expected to be accurate and reliable. Further research is required to validate the use of genetic testing for screening purposes.
15. Three Novel Metabolomic Signatures of Inflammation for Female Gout Risk: A Prospective Cohort Study over 26 Years
Sharan K. Rai *, Hyon K. Choi, Chio Yokose and Natalie McCormick
- Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Boston, MA 02114, USA
- * Correspondence: srai1@mgh.harvard.edu
- Abstract: Background: Only 20% of people with hyperuricemia develop clinically evident gout, suggesting that other, likely inflammatory, factors influence NLRP3 inflammasome activation and progression to gout. Unpublished work has found that a proinflammatory diet is associated with an approximate doubling of female gout risk (between extreme quintiles); however, specific mechanisms are unknown. Metabolomics may provide novel insights into the role of metabolites in mediating the associations between a proinflammatory diet and gout. We prospectively examined the relation between three novel plasma metabolomic signatures of inflammation and female gout risk in the Nurses’ Health Study over 26 years. Methods: We analyzed data from 5936 women with complete metabolite data who were free of gout at baseline. Diet, covariates, and new physician-diagnosed gout cases were ascertained by validated questionnaires every 2–4 years. We calculated an empirical dietary inflammatory pattern (EDIP) score [1] for all participants, with higher scores reflecting a habitual proinflammatory diet. To evaluate the interplay between the EDIP, plasma metabolome, and gout, we examined three novel metabolomic signatures constructed using elastic net regression [2]. These three signatures included metabolites that were predominantly associated with (1) the EDIP dietary score, regardless of the metabolites’ association with any inflammatory biomarkers (i.e., EDIP-only signature); (2) a panel of four inflammatory biomarkers, regardless of the metabolites’ association with diet (i.e., CRP, IL-6, TNF-α-R2, and adiponectin; biomarker-only signature); and (3) a combination of both the EDIP and four biomarkers (i.e., combined signature). We used multivariable Cox models to evaluate the associations between each metabolomic signature and gout. Results: We ascertained 228 cases of incident gout over 26 years. In our multivariable models, women in the highest EDIP quartile (most proinflammatory diet) had 2.04-times (95% CI 1.32, 3.14) greater gout risk compared with the lowest quartile (Table 6). We observed strong positive associations with gout for the EDIP-only (HR for extreme quartiles 2.20 (1.39, 3.47)) and biomarker-only (2.52 (1.61, 3.92)) metabolomic signatures. Greater scores for the combined signature were associated with the highest gout risk among all three signatures (HR for extreme quartiles 2.94 (1.81, 4.80)). The association between the EDIP (dietary intake) and gout was attenuated with further adjustment for each metabolomic signature, although it remained significant (HR for extreme quartiles 1.70 (1.09, 2.67), 1.81 (1.17, 2.80), and 1.83 (1.18, 2.83) after adjusting for the EDIP-only, biomarker-only, and combined signatures, respectively), suggesting that diet’s effect on gout may be partly mediated by these metabolomic profiles. Conclusions: Three novel metabolomic signatures of inflammation were each strongly associated with gout, suggesting that both adherence and intrinsic metabolic response to a proinflammatory dietary pattern may modulate gout risk.

Table 6.
Associations of an inflammatory dietary pattern and three novel metabolomic signatures of inflammation with gout risk.
Table 6.
Associations of an inflammatory dietary pattern and three novel metabolomic signatures of inflammation with gout risk.
| Model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per 1–Standard Deviation Increase | p Value of Continuous Score |
|---|---|---|---|---|---|---|
| Empirical dietary inflammatory pattern (EDIP) score | ||||||
| Cases | 34 | 51 | 60 | 83 | –– | –– |
| Person-years | 32,324 | 32,260 | 32,346 | 32,300 | –– | –– |
| Age-adjusted model | 1.0 | 1.51 (0.97, 2.35) | 1.81 (1.18, 2.77) | 2.55 (1.70, 3.83) | 1.40 (1.22, 1.62) | <0.01 |
| Multivariable model | 1.0 | 1.51 (0.96, 2.37) | 1.72 (1.10, 2.68) | 2.04 (1.32, 3.14) | 1.25 (1.08, 1.45) | <0.01 |
| Multivariable model + EDIP-only signature | 1.0 | 1.43 (0.91, 2.25) | 1.54 (0.98, 2.41) | 1.70 (1.09, 2.67) | 1.14 (0.98, 1.33) | 0.10 |
| Multivariable model + biomarker-only signature | 1.0 | 1.45 (0.92, 2.27) | 1.67 (1.07, 2.61) | 1.81 (1.17, 2.80) | 1.17 (1.01, 1.36) | 0.03 |
| Multivariable model + combined signature | 1.0 | 1.45 (0.92, 2.27) | 1.64 (1.05, 2.56) | 1.83 (1.18, 2.83) | 1.19 (1.02, 1.38) | 0.02 |
| EDIP-only signature (comprising metabolites associated with an inflammatory dietary pattern, not considering their associations with biomarkers) | ||||||
| Cases | 28 | 45 | 67 | 88 | –– | –– |
| Person years | 32,325 | 32,365 | 32,293 | 32,248 | –– | –– |
| Age-adjusted model | 1.0 | 1.63 (1.01, 2.62) | 2.40 (1.53, 3.75) | 3.23 (2.09, 4.98) | 1.58 (1.39, 1.80) | <0.01 |
| Multivariable model | 1.0 | 1.51 (0.94, 2.45) | 2.02 (1.28, 3.19) | 2.20 (1.39, 3.47) | 1.36 (1.18, 1.56) | <0.01 |
| Multivariable model + EDIP (diet) score | 1.0 | 1.40 (0.86, 2.27) | 1.82 (1.14, 2.89) | 1.89 (1.18, 3.05) | 1.29 (1.11, 1.51) | <0.01 |
| Biomarker-only signature (metabolites associated with circulating inflammatory biomarkers, not considering their associations with EDIP) | ||||||
| Cases | 29 | 29 | 59 | 111 | –– | –– |
| Person years | 32,401 | 32,395 | 32,283 | 32,152 | –– | –– |
| Age-adjusted model | 1.0 | 1.03 (0.61, 1.74) | 2.04 (1.30, 3.20) | 3.79 (2.50, 5.76) | 1.74 (1.53, 1.97) | <0.01 |
| Multivariable model | 1.0 | 0.92 (0.55, 1.56) | 1.68 (1.07, 2.66) | 2.52 (1.61, 3.92) | 1.46 (1.27, 1.67) | <0.01 |
| Multivariable model + EDIP (diet) score | 1.0 | 0.89 (0.53, 1.51) | 1.61 (1.02, 2.55) | 2.35 (1.50, 3.68) | 1.42 (1.23, 1.63) | <0.01 |
| Combined signature (reflecting both an inflammatory dietary pattern and circulating inflammatory biomarkers) | ||||||
| Cases | 23 | 40 | 61 | 104 | –– | –– |
| Person years | 32,465 | 32,371 | 32,285 | 32,109 | –– | –– |
| Age-adjusted model | 1.0 | 1.74 (1.03, 2.93) | 2.52 (1.54, 4.12) | 4.39 (2.76, 6.99) | 1.68 (1.48, 1.91) | <0.01 |
| Multivariable model | 1.0 | 1.56 (0.92, 2.63) | 2.11 (1.28, 3.46) | 2.94 (1.81, 4.80) | 1.41 (1.22, 1.63) | <0.01 |
| Multivariable model + EDIP (diet) score | 1.0 | 1.50 (0.89, 2.54) | 2.01 (1.22, 3.31) | 2.75 (1.69, 4.49) | 1.37 (1.19, 1.58) | <0.01 |
All multivariable models were adjusted for age, original case-control status, total energy intake, alcohol intake, smoking status, body mass index, history of hypertension, diuretic use, physical activity, race, menopausal status, and postmenopausal hormone use.
References
- Hoon Lee, D.; Li, J.; Li, Y.; Liu, G.; Wu, K.; Bhupathiraju, S.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C. et al. Dietary inflammatory and insulinemic potential and risk of type 2 diabetes: results from three prospective U.S. cohort studies. Diabetes Care. 2020 43, 2675–2683.
- Hoon Lee, D.; Jin, Q.; Shi, N.; Wang, F.; Bever, A.M.; Liang, L.; Hu, F.B.; Song, M.; Zeleznik, O.A.; Zhang, X. et al. The metabolic potential of inflammatory and insulinaemic dietary patterns and risk of type 2 diabetes. Diabetologia 2024, 67, 88–101.
16. Effects of Long-Term Exposure to Air Pollution on Hyperuricemia and Gout: A Nationwide Cohort Study in Korea
Seong–ho Cho, Yunjin Kim, Junhyoung Park and Sung kweon Cho *
- Department of Pharmacology, Ajou University of School of Medicine, 164, Kyeonggi–do, Republic of Korea
- * Correspondence: wontan2000@gmail.com
- Abstract: Background: The impact of environmental factors on gout remains largely unexplored. Previous research suggests that ambient air pollution may be a significant risk factor for various inflammation-related health problems. This study aims to examine the association between air pollutants and hyperuricemia. Methods: The study analyzed data from 552,064 participants in the Korean Genome and Epidemiology Study (KoGES) who had serum urate measurements. Air pollution data, including measurements for PM10, PM2.5, CO, NOx, SOx, TSP, VOCs, and NH3, were provided by the Ministry of Environment, covering the years 1999 to 2023. PM2.5 data were available from 2011 to 2023. The study assessed exposure to air pollutants by calculating the yearly average levels in participants’ regions. Logistic regression analysis was used to evaluate the impact of each pollutant, divided into quartiles. Results: The study revealed that the risk of hyperuricemia was highest in the second quartile (Q2) for most air pollutants, except for sulfur dioxide (SOx), where the highest risk was observed in the third quartile (Q3). Odds ratio at Q2 compared with Q1 of PM10, PM2.5, CO, NOx, SOx, TSP, VOCs, NH3 are 1.34, 1.14, 1.38, 1.16, 1.31, 1.36, 1.42, 1.22, respectively. Conversely, the risk of gout was highest in the fourth quartile (Q4) for all pollutants. The odds ratios for gout in the second quartile (Q2) compared with the first quartile (Q1) were 1.13 for PM10, 2.11 for PM2.5, 1.22 for CO, 1.14 for NOx, 1.35 for SOx, 1.20 for TSP, 1.30 for VOCs, and 1.13 for NH3, respectively. Conclusions: Long-term exposure to ambient air pollutants shows an inverted U-shaped association with hyperuricemia and a U-shaped association with gout. This is the first study to explore the different association between air pollution and two health outcomes.
17. The Association Between Anemia and Hyperuricemia: A Serendipitous Finding from Machine Learning to Confirmation Through Epidemiological Methods
Junhyoung Park, Yunjin Kim, Seong–ho Cho and Sung kweon Cho *
- Department of Pharmacology, Ajou University of School of Medicine, 164, Kyeonggi–do, Republic of Korea
- * Correspondence: wontan2000@gmail.com
- Abstract: Background: Although factors associated with hyperuricemia have been identified, the complete picture remains unclear. Using machine learning techniques, we analyzed data from the Korean Genome and Epidemiology Study (KoGES) and the Korea National Health and Nutrition Examination Survey (KNHANES) to uncover novel factors associated with hyperuricemia. A serendipitous finding prompted further investigation. Methods: Using random forest classification and light gradient boosting machine algorithms, we identified hemoglobin and hematocrit as significant factors associated with urate levels. We also discovered an inverted U-shaped association between hemoglobin and urate. This finding was validated using logistic regression. The dataset was stratified by sex and chronic kidney disease status, and the association with anemia was examined across eight quantile groups of urate levels. The analysis was adjusted by age, education status and regions. Results: Our study found that, as serum urate levels rise, hemoglobin, hematocrit, and red blood cell counts decrease in both men and women. This trend was consistent across regional cohort groups. Additionally, the risk of anemia significantly increases in individuals with reduced kidney function, with odds ratios of 1.37 and 2.66 for men and women, respectively. Conclusions: Machine learning proved to be an effective tool for analyzing large-scale population datasets. In our study, we uncovered a previously unrecognized association between urate levels and hemoglobin. This novel finding further corroborates the link between hyperuricemia and an increased risk of anemia, providing new insights into the interplay between these conditions.
18. Analysis of Gout Remission Definitions in a Randomized Controlled Trial of Colchicine Prophylaxis for People with Gout-Initiating Allopurinol
Adwoa Dansoa Tabi–Amponsah 1,*, Lisa K Stamp 2, Anne Horne 1, Jill Drake 2, Sarah Stewart 3, Greg Gamble 1, Keith J Petrie 3 and Nicola Dalbeth 1
- 1
- Department of Medicine, Faculty of Medical and Health Sciences, University of Auckland, Auckland 1010, New Zealand
- 2
- Christchurch School of Medicine, University of Otago, Christchurch 8011, New Zealand
- 3
- School of Clinical Sciences, Auckland University of Technology, Auckland 1010, New Zealand
- *
- Correspondence: dansoa.tabi-amponsah@auckland.ac.nz
- Abstract: Background: To investigate the effect of colchicine prophylaxis on gout remission when commencing urate lowering therapy (ULT), and illness perceptions of people in remission, using two definitions of gout remission. Methods: Data from a 12–month double-blind placebo-controlled trial of 200 people with gout commencing allopurinol were analyzed [1]. Participants were randomly assigned to prophylaxis with 0.5 mg daily colchicine or placebo for six months, followed by six months of additional follow-up. Gout remission was assessed using the 2016 preliminary definition [2] or simplified definition without patient reported outcomes. Binary logistic regression was used to compare intervention groups. Illness perceptions were assessed using a gout-specific brief illness perception questionnaire (BIPQ). Results: In the first six months, few participants were in remission according to either the 2016 preliminary definition (3% for colchicine and 4% for placebo) or the simplified definition (7% for colchicine and 12% for placebo) (Table 7). In the second six months, after study drug (colchicine or placebo) discontinuation, fewer participants in the colchicine group than in the placebo group were in remission according to the 2016 preliminary definition (4% vs. 14%, p = 0.03), and the simplified definition (14% vs. 28%, p = 0.02) (Table 7). Participants fulfilling remission using either definition had more favorable perceptions about their gout symptoms and illness concerns, as well as consequences, when using the simplified definition. Conclusions: Using either definition, six months of colchicine prophylaxis when initiating ULT does not provide an advantage in the fulfilment of gout remission. People fulfilling either definition report fewer symptoms, lower concern about their gout, and when using the simplified definition, are less affected by gout.

Table 7.
Six–monthly fulfilment of gout remission by intervention group.
Table 7.
Six–monthly fulfilment of gout remission by intervention group.
| Preliminary Gout Remission Definition | |||||
|---|---|---|---|---|---|
| All N = 200 | Colchicine N = 100 | Placebo N = 100 | * OR (95% CI) | p-value | |
| Months 1 to 6 | 7 (3.5%) | 3 (3%) | 4 (4%) | 0.62 (0.12–3.18) | 0.57 |
| Months 7 to 12 | 18 (9%) | 4 (4%) | 14 (14%) | 0.29 (0.09–0.90) | 0.03 |
| Simplified Gout Remission Definition | |||||
| All N = 200 | Colchicine N = 100 | Placebo N = 100 | * OR (95% CI) | p-value | |
| Months 1 to 6 | 19 (9.5%) | 7 (7%) | 12 (12%) | 0.61 (0.22–1.68) | 0.34 |
| Months 7 to 12 | 42 (21%) | 14 (14%) | 28 (28%) | 0.41 (0.20–0.85) | 0.02 |
* Participants in placebo group were used as the reference.
References
- Stamp, L.; Horne, A.; Mihov, B.; Drake, J.; Haslett J.; Chapman, P.T.; Frampton, C.; Dalbeth, N. Is colchicine prophylaxis required with start–low go–slow allopurinol dose escalation in gout? A non–inferiority randomized double–blind placebo– controlled trial. Ann. Rheum. Dis. 2023 82, 1626–1634.
- de Lautour, H.; Taylor, W.J.; Adebajo, A.; Alten, R.; Burgos–Vargas, R.; Chapman, P.; Cimmino, M.A.; da Rocha Castelar Pinheiro, G.; Day, R.; Harrold, L.R.; et al. Development of preliminary remission criteria for gout using Delphi and 1000 Minds consensus exercises. Arthritis Care Res. 2016, 68, 667–672.
19. Performance of Two Gout Remission Definitions in a Two-Year Randomized Controlled Trial of Nurse-Led Care Versus Usual Gout Care
Adwoa Dansoa Tabi–Amponsah 1,*, Michael Doherty 2, Aliya Sarmanova 2, Weiya Zhang 1, Sarah Stewart 3, William J Taylor 4, Lisa K Stamp 5 and Nicola Dalbeth 1
- 1
- Department of Medicine, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
- 2
- Division of Academic Rheumatology, University of Nottingham, Nottingham, UK
- 3
- School of Clinical Sciences, Auckland University of Technology, Auckland, New Zealand
- 4
- Wellington School of Medicine, University of Otago, Wellington, New Zealand
- 5
- Christchurch School of Medicine, University of Otago, Christchurch, New Zealand
- *
- Correspondence: dansoa.tabi-amponsah@auckland.ac.nz
- Abstract: Objective: To compare the performance of the 2016 preliminary gout remission definition and a simplified gout remission definition in a clinical trial of nurse-led gout care. Methods: Data were analyzed from a 2-year parallel arm, non-blinded, randomized controlled trial of 517 people with gout, in which participants were assigned 1:1 to receive nurse-led care or usual care [1]. Remission was defined using the 2016 preliminary gout remission definition [2] and a simplified gout remission definition (without serial measures of patient global assessment and pain scores). Binary logistic regression was used to compare intervention groups. Gout-specific quality of life was assessed using items from the gout impact scale (GIS), measured on a 0–100 scale. General linear models were used to compare GIS scores between those in remission and those not in remission using either definition. Results: During the trial participants in the nurse-led group were more likely to achieve remission using either definition; at year 2 the odds ratio was 7.92 (95% CI 4.86–12.92) using the 2016 preliminary definition and 11.88 (95% CI 7.49–18.84) using the simplified definition (Table 8). For all participants, remission was more common using either definition in year 2 than year 1 (p < 0.001), and at both year 1 and year 2 more participants were defined as being in remission using the simplified definition compared with the 2016 preliminary definition (p < 0.001). People in remission using either definition had better gout outcomes assessed using the GIS, including greater control over their gout, with a mean difference of 9.85 (95% CI 3.95–15.74), p < 0.001 using the preliminary definition, and a mean difference of 9.87 (95% CI 3.72–16.01), p = 0.002 using the simplified definition. Conclusions: The 2016 preliminary gout remission definition and the simplified gout remission definition both discriminated well between nurse-led and usual care groups. The simplified definition identified more people in both groups as being in gout remission and showed high construct validity using a gout-specific health-related quality of life instrument. The simplified definition is a feasible and valid option for defining gout remission in gout clinical trials.

Table 8.
Proportion of participants in remission by intervention group.
Table 8.
Proportion of participants in remission by intervention group.
| All N = 517 | Nurse-Led Group N = 255 | Usual Care Group N = 262 | * OR (95% CI) | p–Value | |
|---|---|---|---|---|---|
| Preliminary gout remission definition | |||||
| Year 1 | 51 (9.9%) | 36 (14.1%) | 15 (5.7%) | 2.54 (1.33–4.87) | 0.005 |
| Year 2 | 147 (28.4%) | 120 (47.1%) | 27 (10.3%) | 7.92 (4.86–12.92) | <0.001 |
| Simplified gout remission definition | |||||
| Year 1 | 91 (17.6%) | 59 (23.1%) | 32 (12.2%) | 2.24 (1.31–3.80) | 0.003 |
| Year 2 | 221 (42.8%) | 178 (69.8%) | 43 (16.3%) | 11.88 (7.49–18.84) | <0.001 |
* Participants in the usual care group were used as reference.
References
- Doherty, M.; Jenkins, W.; Richardson, H.; Sarminova, A.; Abhishek, A.; Ashton, D.; Barclay, C.; Doherty, S.; Duley, L.; Hatton, R.; et al. Efficacy and cost–effectiveness of nurse–led care involving education and engagement of patients and a treat–to–target urate–lowering strategy versus usual care for gout: a randomized controlled trial. Lancet 2018 392, 1403–1412.
- de Lautour, H.; Taylor, W.J.; Adebajo, A.; Alten, R.; Burgos–Vargas, R.; Chapman, P.; Cimmino, M.A.; da Rocha Castelar Pinheiro, G.; Day, R.; Harrold, L.R.; et al. Development of preliminary remission criteria for gout using Delphi and 1000 Minds consensus exercises. Arthritis Care Res. 2016, 68, 667–672.
20. Association of Prostatectomy with Serum Urate Changes
Mariana Urquiaga 1,2,*, Richard Reynolds 1,2, Angelo Gaffo 1,2, Tony Merriman 1,2 and Lisandro D. Colantonio 3
- 1
- Division of Clinical Immunology and Rheumatology, University of Alabama at Birmingham, Birmingham, AL 35294, USA
- 2
- Birmingham VA Medical Center, Birmingham, AL 35233, USA
- 3
- School of Public Health, University of Alabama at Birmingham, Birmingham, AL 35294, USA
- *
- Correspondence: muchanganaqui@uabmc.edu
- Abstract: Objectives: Serum urate (SU) in men is ~1.2 mg/dL higher than in pre-menopausal women [1]. We hypothesized that prostatic production of urate contributes to the observed sex difference. We evaluated whether prostatectomy is associated with a decline in SU in men with prostate cancer (PCa). Methods: The study population consisted of United States (US) veterans who underwent a prostatectomy following a diagnosis of PCa using data from the Veterans Health Administration Clinical Data Warehouse between January 1999 and December 2023. We limited the analysis to men with at least three SU measurements in the 6 months before their prostatectomy (i.e., the baseline period), which were averaged to estimate each individual’s pre-prostatectomy SU level. We further restricted the analysis to men with at least one SU measurement of 0 to 1, 1 to 3, 3 to 6, 6 to 12, 12 to 24, 24 to 36, or 36 to 60 months after the prostatectomy (i.e., the follow-up period). Men who had a prescription for urate-lowering therapy, received hormone therapy, or underwent renal replacement therapy or a kidney transplant were excluded from the analysis. We repeated the stratified analysis based on a baseline SU (hypouricemic (SU < 4.45 mg/dL), normouricemic SU (SU between 4.45 and 7.30 mg/dL), and a hyperuricemic SU (SU > 7.30 mg/dL). Results: We identified 1,246,791 US veterans with PCa. Among them, 76,610 underwent a prostatectomy. Their baseline characteristics are presented in Table 9. Compared with baseline, SU was lower within 1 month (mean SU∆ −0.56 (95% CI −0.66 to −0.45) mg/dL), 1–3 months (−0.11 (−0.17 to −0.04] mg/dL), 3–6 months (−0.07 (−0.12 to −0.02) mg/dL), and 6–12 months (−0.05 (−0.09 to −0.003) mg/dL), but higher 24 to 36 (0.08 (0.03 to 0.12) mg/dL), and 36 to 60 months (0.09 (0.05 to 0.13) mg/dL) after prostatectomy (Figure 12). In the hypouricemic group, SU was higher versus baseline after 1 month of prostatectomy (Figure 12 and Table 10). In the normouricemic group and compared with baseline, SU was lower 0–1 month following prostatectomy, similar from 1 to 24 months, and higher after 24 months following post-prostatectomy. In the hypereuricemic group, SU was lower versus baseline at all timepoints, except 1–3 months. Conclusion: There is a decrease in SU in the first months after prostatectomy. The first month results might be related to hospitalization and surgery conditions, such as intravenous hydration and intra-operative medications. Results from the stratified analysis are consistent with those reported in a previous study [2], in which SU following prostatectomy increased, did not change, and decreased in the hypouricemic, normouricemic, and hyperuricemic groups, respectively. Next steps will include statistical adjustment for relevant covariables, comparing individuals with prostatectomy to matched men who did not undergo prostatectomy, and evaluating differences in gout incidence.

Table 9.
Baseline characteristics.
Table 9.
Baseline characteristics.
| US Veterans with PCa Who Underwent a Prostatectomy (76,610) | |
|---|---|
| Demographics (at the time of PCa diagnosis) | |
| Age, mean (SD) | 62.8 (7.13) |
| Year of diagnosis, mean (SD) | 2011 (9.25) |
| Self-reported race, n (%) | |
| American Indian or Alaska Native | 457 (0.60) |
| Asian | 282 (0.37) |
| Black or African American | 21,415 (27.95) |
| Native Hawaiian or other Pacific Islander | 533 (0.70) |
| White | 49,205 (64.23) |
| Unknown | 4718 (6.16) |
| Comorbidities (by the time of PCa diagnosis) | |
| BMI closest to diagnosis date, mean (SD) | 28.9 (5.02) |
| eGFR closest diagnosis date, mean (SD) | 77.9 (24.3) |
| Hypertension, n (%) | 55,615 (72.60) |
| Type 2 diabetes, n (%) | 20,566 (26.85) |
| Cardiovascular disease 1, n (%) | 19,098 (24.93) |
| PCa related | |
| AJCC stage 2, n (%) | |
| 1 | 3912 (7.68) |
| 2 | 36,057 (70.83) |
| 3 | 8640 (16.97) |
| 4 | 2300 (4.52) |
| Metastatic prostate cancer, n (%) | 8365 (10.92) |
| Gleason score 3, mean (SD) | 7.04 (0.96) |
US: United States, PCa: Prostate cancer, n: number, SD: Standard deviation, BMI: Body mass index, eGFR: Estimated glomerular filtration rate, AJCC: American Joint Committee on Cancer. 1: Cardiovascular disease includes coronary artery disease, myocardial infarction, and congestive heart failure; 2: AJCC stage available for 50,909 veterans; 3: Gleason score available for 68,616 veterans.

Table 10.
Stratified serum urate change from baseline at each timepoint.
Table 10.
Stratified serum urate change from baseline at each timepoint.
| Strata | Time After Baseline | n | Mean SU Change (95% CI) in mg/dL |
|---|---|---|---|
| Hypouricemic | 0–1 month | 32 | 0.38 (−0.38 to 0.46) |
| 1–3 months | 44 | 0.38 (0.09 to 0.66) | |
| 3–6 months | 54 | 0.36 (0.06 to 0.66) | |
| 6–12 months | 99 | 0.57 (0.36 to 0.78) | |
| 12–24 months | 121 | 0.39 (0.13 to 0.64) | |
| 24–36 months | 68 | 0.26 (0.09 to 0.43) | |
| 36–60 months | 84 | 0.91 (0.69 to 1.13) | |
| Normouricemic | 0–1 month | 130 | −0.42 (−0.60 to −0.24) |
| 1–3 months | 241 | −0.09 (−0.21 to 0.03) | |
| 3–6 months | 393 | −0.03 (−0.13 to 0.07) | |
| 6–12 months | 664 | −0.04 (−0.13 to 0.05) | |
| 12–24 months | 908 | 0.06 (−0.02 to 0.14) | |
| 24–36 months | 587 | 0.21 (0.12 to 0.31) | |
| 36 to 60 months | 681 | 0.15 (0.06 to 0.25) | |
| Hyperuricemic | 0–1 month | 40 | −1.61 (−2.20 to −1.01) |
| 1–3 months | 31 | –0.54 (−1.1 to 0.02) | |
| 3–6 months | 62 | −0.40 (−0.66 to −0.15) | |
| 6–12 months | 115 | −0.63 (−0.90 to −0.35) | |
| 12–24 months | 133 | −0.30 (−0.52 to −0.09) | |
| 24–36 months | 84 | −0.43 (−0.74 to −0.11) | |
| 36 to 60 months | 84 | −0.60 (−0.84 to −0.36) |
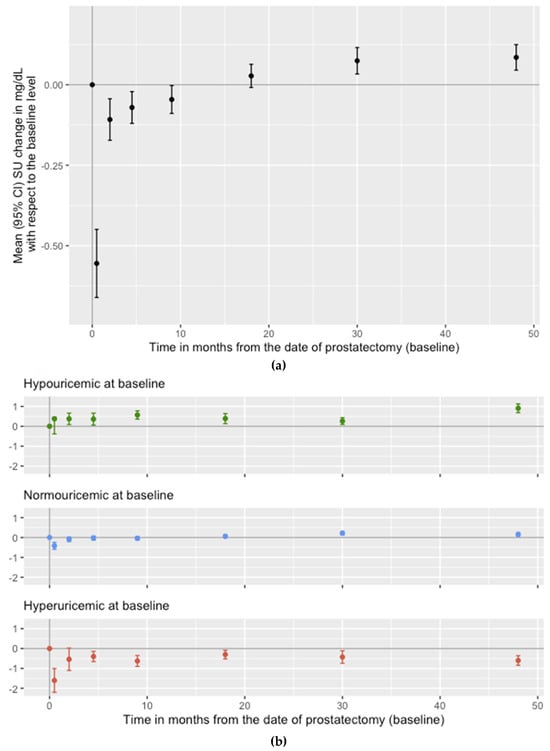
Figure 12.
Serum urate changes ((a) unstratified; (b) stratified) in veterans who underwent prostatectomies.
References
- Zitt, E.; Fischer, A.; Lhotta, K.; Concin, H.; Nagel, G. Sex– and age–specific variations, temporal trends and metabolic determinants of serum uric acid concentrations in a large population–based Austrian cohort. Sci. Rep. 2020, 10, 7578.
- Park, J.W.; Lee, J.H.; Cho, H.J.; Ha, Y.J.; Kang, E.H.; Shin, K.; Byun, S.S.; Lee, E.Y.; Song, Y.W.; Lee, Y.J. Influence of androgen deprivation therapy on serum urate levels in patients with prostate cancer: A retrospective observational study. PLoS ONE 2018, 13, e0209049.
21. Monocytes with Neutrophil-like Transcriptomic Signature Observed in Gout and Asymptomatic Hyperuricemia
Valentin Nica 1,*, Medeea Badii 1,2, Orsolya Gaal 1,2, Georgiana Cabău 1, Andreea–Manuela Mirea 1, Ioana Hotea 3, HINT Consortium, Cristina Pamfil 3, Simona Rednic 3, Radu A. Popp 1, Yang Li 2,4, Tania O. Crișan 1,2 and Leo A.B. Joosten 1,2
- 1
- Department of Medical Genetics, UMF “Iuliu Hatieganu”, Cluj–Napoca 400347, Romania
- 2
- Department of Internal Medicine, Radboud University Nijmegen Medical Center, Nijmegen 6525 GA, The Netherlands
- 3
- Department of Rheumatology, UMF “Iuliu Hatieganu”, Cluj–Napoca 400347, Romania
- 4
- Department of Computational Biology for Individualised Medicine, Centre for Individualised Infection Medicine (CiiM), 30625 Hannover, Germany
- *
- Correspondence: nicavalentin93@gmail.com
- Abstract: Objectives: Gout is one of the most common forms of inflammatory arthritis, characterized by deposits of monosodium urate crystals in the joint, with an infiltration of neutrophils and macrophages. Circulating monocytes play a critical role in rheumatic disease, as local inflammation is often driven not by local macrophages, but by differentiated monocytes. Furthermore, gout is preceded by hyperuricemia, which, despite being an asymptomatic condition is associated with cardiovascular comorbidities. We performed a bulk RNA-seq analysis of circulating blood cells in volunteers with various levels of serum urate and gout patients to identify the signature associated with the two conditions and internally validated the findings in other omics datasets. Methods: Peripheral blood was harvested from volunteers and patients with gout and peripheral blood mononuclear cells (PBMCs) were isolated within the first hour. From a random subset of the same patients, CD14+ monocytes were further isolated by positive selection as an internal validation cohort. Cells were then stored in TRIzol and RNA-seq analysis was performed. After quality control, a total of 197 PBMC (106 normouricemic, 20 hyperuricemic, 71 gout) samples and 30 CD14+ monocyte (15 normouricemic, 15 gout) samples remained. DESeq2 (R) was used to identify differentially expressed genes (DEGs), with sex and age included as covariates. Clustering analysis was performed with STRING; Gene set enrichment analysis with the fgsea package (R), using a pre-ranked list; and transcription factor (TF) enrichment with chea3. Results: The transcriptomic signature observed in hyperuricemia was very similar to the one seen in gout with an overlap of over 60% of DEGs and close to all DEGs being upregulated in both comparisons. The top identified upregulated DEGs were LTF, MMP9, IL1R2, DEFA1B, SLC4A1, CEACAM8 and CYP4F3. Cluster analysis was performed on the common DEGs, which identified two main clusters. Pathway enrichment of said clusters shows activation of Innate immune system, neutrophil activity and activation of matrix metalloproteinases in the main gene cluster and erythrocyte/hemoglobin metabolism in the second. The top observed TFs were LTF, GATA1, CEBPE, KLF1 and TAL1. These findings were largely validated in the CD14+ monocyte group. Out of the identified upregulated genes, three were available in a proteomic analysis previously published by our team—CXCL9, OSM and LIF, and soluble IL1R2 protein level was assessed by ELISA. All of the aforementioned proteins are similarly upregulated in hyperuricemia, gout or both. Conclusion: We identified a neutrophil-like signature in PBMCs and CD14+ monocytes that can be observed in some individuals, the number of which is overrepresented in both gout and asymptomatic hyperuricemia. These findings were internally validated and confirmed in proteomic data. A possible explanation is that hyperuricemia is associated with an increase in specific cell types, such as neutrophil-like monocytes, and the upregulation of CXCR1 and TFAIP6 are suggestive of that. Further investigations are required to assess the specific cell types, cause and function in hyperuricemia and gout.
22. Association Between the Triglyceride–Glucose Index and Incident Gout: A Study from a Nationwide Cohort of Korea and the UK
Yun Jin Kim, Seong Ho Cho, Jun Hyeong Park and Sung Kweon Cho *
- Department of Pharmacology, Ajou University of School of Medicine, 164, Kyeonggi–do, Republic of Korea
- * Correspondence: wontan2000@gmail.com
- Abstract *: Objectives: Several studies have shown associations between gout and insulin resistance. The triglyceride–glucose (TyG) index is a simple but reliable surrogate marker for insulin resistance. We aimed to explore the connection between gout and TyG indices in a multi-ethnic cohort study. Methods: The TyG index is defined as log(triglyceridesxglucose/2), the TyG–waist circumference (TyG–WC) index is defined as TyGxWC. This study included 8167 gout-free adults from the Ansan and Ansung cohorts in the Korean Genome and Epidemiology Study (KoGES), followed for 18 years (2011–2020), and 55,000 gout-free white adults from the UK Biobank (UKBB) with at least two urate follow-up measurements. Incident gout was identified using ICD M10 codes in the UKBB and self-reported cases in KoGES. We employed the Cox proportional hazards model and conducted sensitivity analyses to evaluate the association. Results: Prevalent gout rates were 6.1% in the KoGES cohort and 6.0% in the UKBB. The area under the receiver operating characteristic curve (AUROC) for the TyG index when detecting prevalent gout at baseline was 0.68 in KoGES and 0.65 in the UKBB. For the TyG–waist circumference (TyG–WC) index, AUROC values were 0.72 in both KoGES and the UKBB (Figure 13). The incident gout rate was 0.7 cases per 1000 person years in KoGES and 0.8 in the UKBB (Figure 14). The cut-off for the fourth quartile (Q4) of the TyG index was 9.02 in KoGES and 8.99 in the UKBB. The risk of gout for those in TyG Q4 was 2.29 times higher in KoGES and 2.41 times higher in the UKBB compared with those in Q1 (Table 11). For the TyG–WC Q4, the risk of gout was 2.91 times higher in the UKBB. Conclusion: The TyG index is a significant risk factor for gout and becomes even more predictive when combined with waist circumference. These findings are valuable for both clinicians and epidemiologists, offering a straightforward method for assessing gout risk.
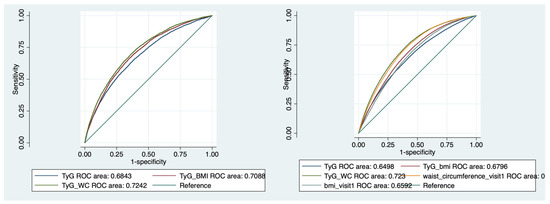 Figure 13. Sensitivity analysis for baseline hyperuricemia by TyG indices in KoGES (left), UKBB (right).
Figure 13. Sensitivity analysis for baseline hyperuricemia by TyG indices in KoGES (left), UKBB (right).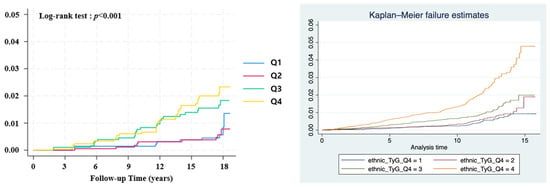 Figure 14. Cumulative incidence of gout according to TyG quartile in KoGES (left), UKBB (right).
Figure 14. Cumulative incidence of gout according to TyG quartile in KoGES (left), UKBB (right). Table 11. Stratified serum urate change from baseline at each timepoint.
Table 11. Stratified serum urate change from baseline at each timepoint.
* This research was supported by a grant of ‘Korea Government Grant Program for Education and Research in Medical AI’ through the Korea Health Industry Development Institute (KHIDI), funded by the Korea government (MOE, MOHW)
Funding
Producing this conference report received no external funding.
Conflicts of Interest
The producers of the conference report declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).