Metal–Organic Frameworks for Enzyme Modulation in Protein Kinase and Phosphatase Regulation—Mechanisms and Biomedical Applications
Abstract
1. Introduction
2. Metal–Organic Frameworks (MOFs) as Platforms for Protein Kinase and Phosphatase Inhibition
2.1. Fundamentals of MOFs Relevant to Enzyme Modulation
2.2. Synthesis Methods of MOFs for Enzyme Inhibition Applications
2.3. Synthesis Influences MOF Properties (Porosity, Stability, Functional Groups)
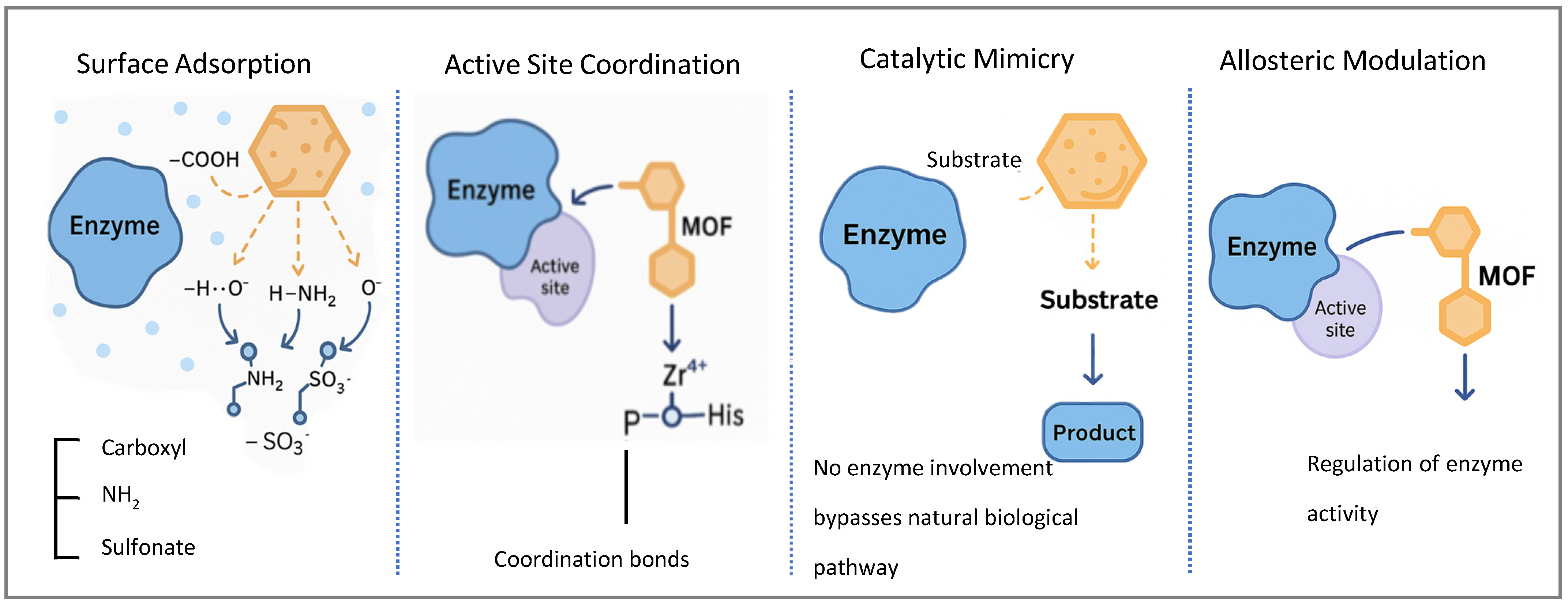
3. MOFs as Enzyme Inhibitors: Mechanisms, Efficacy, and Translational Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balbaied, T.; Moore, E. Overview of Capillary Electrophoresis Analysis of Alkaline Phosphatase (ALP) with Emphasis on Post-Translational Modifications (PTMs). Kinases Phosphatases 2023, 1, 206–219. [Google Scholar] [CrossRef]
- Hunter, T. Signaling—2000 and beyond. Cell 2000, 100, 113–127. [Google Scholar] [CrossRef]
- Bononi, A.; Agnoletto, C.; De Marchi, E.; Marchi, S.; Patergnani, S.; Bonora, M.; Giorgi, C.; Missiroli, S.; Poletti, F.; Rimessi, A.; et al. Protein kinases and phosphatases in the control of cell fate. Enzym. Res. 2011, 2011, 329098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, P. The role of protein phosphorylation in human health and disease. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef]
- Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Baldi, S.; Long, N.; Ma, S.; Liu, L.; Al-Danakh, A.; Yang, Q.; Deng, X.; Xie, J.; Tang, H. Advancements in Protein Kinase Inhibitors: From Discovery to Clinical Applications. Research 2025, 8, 747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pawson, T.; Scott, J.D. Protein phosphorylation in signaling–50 years and counting. Trends Biochem. Sci. 2005, 30, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.C.; Royer, F.; Grimsey, N.J. Spatiotemporal control of kinases and the biomolecular tools to trace activity. J. Biol. Chem. 2024, 300, 107846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, W.; Scott, J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Wu, H.; Hu, C.; Wang, H.; Liu, J.; Wang, W.; Liu, O. An overview of kinase downregulators and recent advances in discovery approaches. Signal Transduct. Target. Ther. 2021, 6, 423. [Google Scholar] [CrossRef]
- Klebe, G. Transferase Inhibitors. In Drug Design; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Hefayathullah, M.; Singh, S.; Ganesan, V.; Maduraiveeran, G. Metal-organic frameworks for biomedical applications: A review. Adv. Colloid Interface Sci. 2024, 331, 103210. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, P.; Gulcay-Ozcan, E.; Vučkovski, M.; Bondžić, A.M.; Erucar, I.; Keskin, S. Biomedical Applications of Metal-Organic Frameworks Revisited. Ind. Eng. Chem. Res. 2025, 64, 1907–1932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alamro, A.; Balbaied, T. Boron Nitride Nanostructures (BNNs) Within Metal–Organic Frameworks (MOFs): Electrochemical Platform for Hydrogen Sensing and Storage. Analytica 2024, 5, 599–618. [Google Scholar] [CrossRef]
- Khafaga, D.S.; El-Morsy, M.T.; Faried, H.; Diab, A.H.; Shehab, S.; Saleh, A.M.; Ali, G.A. Metal–organic frameworks in drug delivery: Engineering versatile platforms for therapeutic applications. RSC Adv. 2024, 14, 30201–30229. [Google Scholar] [CrossRef]
- van Veldhuisen, T.W.; Dijkstra, R.M.J.; Koops, A.A.; Cossar, P.J.; van Hest, J.C.M.; Brunsveld, B. Modulation of Protein–Protein Interactions with Molecular Glues in a Synthetic Condensate Platform. J. Am. Chem. Soc. 2025, 147, 5386–5397. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mandal, M.; Maiti, D.K. A review on zirconium-based metal–organic frameworks: Synthetic approaches and biomedical applications. Mater. Adv. 2024, 5, 51–67. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chakraborty, T.; Bhaumik, A. A Ce-MOF as an alkaline phosphatase mimic: Ce-OH2 sites in catalytic dephosphorylation. Inorg. Chem. Front. 2022, 9, 5735–5744. [Google Scholar] [CrossRef]
- Cun, J.E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-based metal–organic frameworks for biomedical applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef]
- Zhu, R.; Cai, M.; Fu, T.; Yin, D.; Peng, H.; Liao, S.; Du, Y.; Kong, J.; Ni, J.; Yin, X. Fe-Based Metal Organic Frameworks (Fe-MOFs) for Bio-Related Applications. Pharmaceutics 2023, 15, 1599. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Wang, Y.; Liang, Z.; Hu, B.; Xie, J.; Wong, W.-L.; Wong, K.-Y.; Qiu, B.; Peng, W. A new fluorescent biosensor based on inner filter effect and competitive coordination with the europium ion of non-luminescent Eu-MOF nanosheets for the determination of alkaline phosphatase activity in human serum. Sens. Actuators B Chem. 2023, 380, 133379. [Google Scholar] [CrossRef]
- Yu, L.; Feng, L.; Li, X.; Li, S.; Xu, Q.; Pan, X.; Xiao, Y. Rational Design of Dual-Emission Lanthanide Metal–Organic Framework for Visual Alkaline Phosphatase Activity Assay. ACS Appl. Mater. Interfaces 2021, 13, 11646–11656. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.T.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef]
- Pramanik, B.; Sahoo, R.; Das, M.C. pH-stable MOFs: Design principles and applications. Coord. Chem. Rev. 2023, 493, 215301. [Google Scholar] [CrossRef]
- Han, W.; Shi, M.; Jiang, H.-L. Scalable and Low-energy Synthesis of Metal-organic Frameworks by a Seed-mediated Approach. Angew. Chem. Int. Ed. 2025, 64, e202421942. [Google Scholar] [CrossRef]
- Nazari, M.; Zadehahmadi, F.; Sadiq, M.M.; Sutton, A.L.; Mahdavi, H.; Hill, M.R. Challenges and solutions to the scale-up of porous materials. Commun. Mater. 2024, 5, 170. [Google Scholar] [CrossRef]
- Karami, Z.; Khodaei, M.M. Post-synthetic modification of IR-MOF-3 as acidic-basic heterogeneous catalyst for one-pot synthesis of pyrimido [4,5-b]quinolones. Res. Chem. Intermed. 2022, 48, 1773–1792. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, W.; Pan, Y.; Wang, W.; Wang, D.; Ding, J. Progress in nanocarriers-based approaches for the delivery of tyrosine kinase inhibitors in bone cancer: Trends and prospects. IUBMB Life 2025, 77, e70052. [Google Scholar] [CrossRef]
- Akhuli, A.; Chakraborty, D.; Preeyanka, N.; Simanchal, A.; Sarkar, D.M. Copper nanoclusters as an effective enzyme inhibitor on the activity modulation of α-chymotrypsin. ACS Appl. Nano Mater. 2023, 6, 4910–4924. [Google Scholar] [CrossRef]
- Pan, X.; Yao, Y.; Zhang, M.; Yuan, X.; Yao, Q.; Hua, W. Enzyme-mimic catalytic activities and biomedical applications of noble metal nanoclusters. Nanoscale 2024, 16, 8196–8215. [Google Scholar] [CrossRef] [PubMed]
- Gomari, M.M.; Abkhiz, S.; Pour, T.G.; Lotfi, E.; Rostami, N.; Monfared, F.N.; Ghobari, B.; Mosavi, M.; Alipour, B.; Dokholyan, N.V. Peptidomimetics in cancer targeting. Mol. Med. 2022, 28, 146. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.-J.; Melrose, A.R.; Pang, J.; Schofield, K.; Song, S.D.; Parra-Izquierdo, I.; Zheng, T.J.; Lyssikatos, J.P.; Gross, S.D.; et al. Pharmacological effects of small molecule BCR-ABL tyrosine kinase inhibitors on platelet function. J. Pharmacol. Exp. Ther. 2025, 392, 100020. [Google Scholar] [CrossRef]
- Yao, S.-X.; Huang, Y.-J.; Zhang, Y.-X.; Cui, Z.-X.; Lu, H.-Y.; Wang, R.; Shi, L. Revisiting VEGF/VEGFR-2 signaling as an anticancer target and its inhibitor discovery: Where are we and where should we go? J. Drug Target. 2025, 33, 1471–1494. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, M.S. Deubiquitination of epidermal growth factor receptor by ubiquitin-specific peptidase 54 enhances drug sensitivity to gefitinib in gefitinib-resistant non-small cell lung cancer cells. PLoS ONE 2025, 20, e0320668. [Google Scholar] [CrossRef]
- Ramos-Alvarez, I.; Jensen, R.T. Elucidation of roles of serine/threonine phosphatases PP1 and PP2A in mediating CCK-stimulated growth and enzyme secretion in pancreatic acinar cells. AJP Gastrointest. Liver Physiol. 2025, 329, G102–G121. [Google Scholar] [CrossRef]
- Guo, H.; Ren, W.; Guo, M.; Wu, X.; Guo, Q. A Comprehensive Review on Ethnopharmacology, Phytochemistry of Mylabris, and Pharmacology of Cantharidin. Chem. Biodivers. 2025, 22, e202500266. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal organic frameworks mimicking natural enzymes: A structural and functional analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Huang, X.; Min, Q.; Zhu, J.-J. Multiplexed Quantitative MALDI MS Approach for Assessing Activity and Inhibition of Protein Kinases Based on Postenrichment Dephosphorylation of Phosphopeptides by Metal–Organic Framework-Templated Porous CeO2. Anal. Chem. 2018, 90, 9859–9867. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Hsu, Y.-C.; Lin, H.; Han, Z.; Pang, J.; Yang, Z.; Liang, R.-R.; Shi, W.; Zhou, H.-C. Bioinspired Framework Catalysts: From Enzyme Immobilization to Biomimetic Catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Xu, Y.; Wei, G. Recent Advances in Biomolecule-Engineered Metal-Organic Frameworks (Bio-MOFs): From Design, Bioengineering, and Structural/functional Regulation to Biocatalytic Applications. Chem. Rec. 2025, 25, e202500001. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Yusran, Y.; Li, H.; Qiu, S.; Fang, Q. Exploring high-connectivity three-dimensional covalent organic frameworks: Topologies, structures, and emerging applications. Chem. Soc. Rev. 2024, 54, 484–514. [Google Scholar] [CrossRef]
- Tang, H.; Fan, D.; Chen, Y.; Han, S.-Y. Exploring Enzyme-MOF (Metal-Organic Framework) Catalytic Systems: Trade-offs Between Enzyme Activity and MOF Stability. Green Chem. 2024, 27, 2605–2628. [Google Scholar] [CrossRef]
- Ellis, G.A.; Klein, W.P.; Lasarte-Aragonés, G.; Thakur, M.; Walper, S.A.; Medintz, I.L. Artificial Multienzyme Scaffolds: Pursuing in Vitro Substrate Channeling with an Overview of Current Progress. ACS Catal. 2019, 9, 10812–10869. [Google Scholar] [CrossRef]
- Jia, C.; Bai, J.; Liu, Z.; Gao, S.; Han, Y.; Yan, H. Application of a titanium-based metal-organic framework to protein kinase activity detection and inhibitor screening. Anal. Chim. Acta 2020, 1128, 99–106. [Google Scholar] [CrossRef]
- Bai, J.; Liu, L.; Jia, C.; Liu, Z.; Gao, S.; Han, Y.; Yan, H. Fluorescence Method for the Detection of Protein Kinase Activity by Using a Zirconium-Based Metal–Organic Framework as an Affinity Probe. ACS Appl. Bio Mater. 2019, 2, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Radfar, S.; Sheikh, M.; Akhavantabib, A.; Heidari, A.; Ghasemi, M.; Naghavi, M.; Ghanbari, R.; Zibadi, F.; Jamshidi, B.; Alizadeh, A. Application of a porous zirconium-based MOF nanoplate as an affinity ECL platform for the detection of protein kinase activity and inhibitor screening. Talanta 2025, 287, 127675. [Google Scholar] [CrossRef]
- Alizadeh, A.; Sheikh, M.; Akhavantabib, A.; Heidari, A.; Ghasemi, M.; Naghavi, M.; Ghanbari, R.; Radfar, S. A Porous Zirconium-Based MOF Nanoplate as an Affinity ECL Platform for the Detection of Protein Kinase Activity and Inhibitor Screening. Preprint 2024. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, F.; Deng, P.; Wang, Y.; Cai, K.; Chen, Y.; Wang, Z.; Liu, Y. Sensitive electrogenerated chemiluminescence biosensors for protein kinase activity analysis based on bimetallic catalysis signal amplification and recognition of Au and Pt loaded metal-organic frameworks nanocomposites. Biosens. Bioelectron. 2018, 109, 132–138. [Google Scholar] [CrossRef]
- Chang, Y.; Gao, F.; Wu, T.; Pan, Q.; Liu, L.; Song, Q. Electrochemical detection of protein kinases with methylene blue-functionalized Zr-based metal-organic frameworks as signal labels. Int. J. Electrochem. Sci. 2023, 18, 100338. [Google Scholar] [CrossRef]
- Yu, L.; Shen, Y.; Xu, Q.; Gan, Z.; Feng, Y.; Yang, C.; Xiao, Y. Enhancing Kinase Activity Detection with a Programmable Lanthanide Metal–Organic Framework via ATP-to-ADP Conversion. Anal. Chem. 2024, 96, 12139–12146. [Google Scholar] [CrossRef]
- Jodaeeasl, N.; Wang, S.; Hu, A.; Peslherbe, G.H. Comprehensive DFT investigation of small-molecule adsorption on the paradigm M-mof-74 family of metal–organic frameworks. Phys. Chem. Chem. Phys. 2025, 27, 3068–3082. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ostovar, S.; Eshaghi, M.M.; Rajabzadeh-Khosroshahi, M.; Safakhah, S.; Ghotekar, S.; Rahdar, A.; Díez-Pascual, A.M. Nanoscale metallic-organic frameworks as an advanced tool for medical applications: Challenges and recent progress. Appl. Organomet. Chem. 2023, 37, e6982. [Google Scholar] [CrossRef]
- Dalapati, S.; Jana, R.; Banerjee, R. Solvothermal synthesis of metal-organic frameworks for catalytic applications. Coord. Chem. Rev. 2022, 471, 214729. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, C.; Xu, R.; Wang, J.; Chen, Q.; Zhu, Z.; Hu, Q.; Shen, Q.; Shen, J.-W. Copper-based metal–organic frameworks for antitumor application. J. Nanobiotechnol. 2025, 23, 135. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, S.-S.; Wu, J.-X.; Wang, H.; Yu, X.; Shang, W.; Chen, G.-Q.; Gu, Z.-Y. Highly Selective Capture of Monophosphopeptides by Two-Dimensional Metal–Organic Framework Nanosheets. Anal. Chem. 2019, 91, 9093–9101. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Zhao, X. Microwave-assisted synthesis of MOFs: Methods and applications. Microporous Mesoporous Mater. 2021, 318, 111036. [Google Scholar] [CrossRef]
- Altharawi, A.; Alqahtani, S.M.; Aldakhil, T.; Ahmad, I. Microwave-assisted synthesis of novel Ti/BTB-MOFs as porous anticancer and antibacterial agents. Front. Chem. 2024, 12, 1386311. [Google Scholar] [CrossRef] [PubMed]
- Iorkula, T.H.; Osayawe, O.J.K.; Odogwu, D.A.; Ganiyu, L.O.; Faderin, E.; Awoyemi, R.F.; Akodu, B.O.; Ifijen, I.H.; Aworinde, O.R.; Agyemang, P.; et al. Advances in pyrazolo [1,5-a]pyrimidines: Synthesis and their role as protein kinase inhibitors in cancer treatment. RSC Adv. 2025, 15, 3756–3828. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Zhang, G. Recent advances in sonochemical synthesis of MOFs. Ultrason. Sonochem. 2023, 94, 106352. [Google Scholar] [CrossRef]
- Wang, J.; Hu, T.; Han, Q.; Luo, W.; Zhong, J.; Ding, M. The synthesis and functionalization of metal organic frameworks and their applications for the selective separation of proteins/peptides. Anal. Bioanal. Chem. 2023, 415, 5859–5874. [Google Scholar] [CrossRef]
- Scattolin, T.; Tonon, G.; Botter, E.; Canale, V.C.; Hasanzadeh, M.; Cuscela, D.M.; Buschini, A.; Zarepour, A.; Khosravi, A.; Cordani, M.; et al. Synergistic applications of cyclodextrin-based systems and metal–organic frameworks in transdermal drug delivery for skin cancer therapy. J. Mater. Chem. B 2024, 12, 3807–3839. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Mahjoub, A.R.; Molaie, S.; Ghaffarifar, F.; Ghasemi, E.; Slawin, A.M.; Carpenter-Warren, C.L. The effect of different parameters under ultrasound irradiation for synthesis of new nanostructured Fe3O4@bio-MOF as an efficient anti-leishmanial in vitro and in vivo conditions. Ultrason. Sonochem. 2018, 43, 248–261. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Hu, Y. Mechanochemical synthesis of bio-compatible MOFs: Towards green and scalable production. Adv. Mater. Interfaces 2024, 11, 2400190. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Tu, M.; Wang, S.; Zhao, J.; Zhang, H.; Cao, Y. Recent Advances on the Metal-Organic Frameworks-Based Biosensing Methods for Cancer Biomarkers Detection. Crit. Rev. Anal. Chem. 2024, 54, 1273–1289. [Google Scholar] [CrossRef]
- Mannias, G. Development of New Synthesis Methods of Proteins/Enzymes-Metal Organic Frameworks (MOFs) Hybrid Composite Materials for Biomedical Applications. Depositolegale.it; Università degli Studi di Sassari. Available online: https://tesidottorato.depositolegale.it/handle/20.500.14242/117581 (accessed on 21 February 2025).
- Griffin, S.L.; Briuglia, M.L.; ter Horst, J.H.; Forgan, R.S. Assessing crystallisation kinetics of zr metal–organic frameworks through turbidity measurements to inform rapid microwave-assisted synthesis. Chem.-A Eur. J. 2020, 26, 6910–6918. [Google Scholar] [CrossRef]
- Abaszadeh, N.; Afzali, D.; Sargazi, G.; Golpayegani, A. Sonochemical-assisted method for efficient synthesis of Cu-MOF and evaluating its antibacterial properties. Heliyon 2024, 10, e31024. [Google Scholar] [CrossRef] [PubMed]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of MOF-303 and its CO2 adsorption at ambient conditions. Molecules 2024, 29, 2698. [Google Scholar] [CrossRef]
- Phan, P.T.; Hong, J.; Tran, N.; Le, T.H. The Properties of Microwave-Assisted Synthesis of Metal–Organic Frameworks and Their Applications. Nanomaterials 2023, 13, 352. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, X.; Yang, X.; Ma, M.; Ji, S.; Jiang, F. Microwave- and ultrasonic-assisted synthesis of 2D La-based MOF nanosheets by coordinative unsaturation degree to boost phosphate adsorption. RSC Adv. 2022, 12, 35517–35530. [Google Scholar] [CrossRef] [PubMed]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Recent Developments in Sonochemical Synthesis of Nanoporous Materials. Molecules 2023, 28, 2639. [Google Scholar] [CrossRef] [PubMed]
- Ismail, K.M.; Hassan, S.S.; Medany, S.S.; Hefnawy, M.A. A facile sonochemical synthesis of the Zn-based metal–organic framework for electrochemical sensing of paracetamol. Mater. Adv. 2024, 5, 5870–5884. [Google Scholar] [CrossRef]
- Steenhaut, T.; Grégoire, N.; Barozzino-Consiglio, G.; Filinchuk, Y.; Hermans, S. Mechanochemical defect engineering of HKUST-1 and impact of the resulting defects on carbon dioxide sorption and catalytic cyclopropanation. RSC Adv. 2020, 10, 19822–19831. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Liu, R.; Chi, L.; Wang, X.; Wang, Y.; Sui, Y.; Xie, T.; Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168. [Google Scholar] [CrossRef]
- Alexander, C.; Guo, Z.; Glover, P.B.; Faulkner, S.; Pikramenou, Z. Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics. Chem. Rev. 2025, 125, 2269–2370. [Google Scholar] [CrossRef]
- Shteinman, A.A. Metallocavitins as Advanced Enzyme Mimics and Promising Chemical Catalysts. Catalysts 2023, 13, 415. [Google Scholar] [CrossRef]
- Changeux, J.-P.; Christopoulos, A. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell 2016, 166, 1084–1102. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Pandit, S.; Lee, S.; Ebrahimi, S.B.; Samanta, D. Modulating Enzyme Activity using Engineered Nanomaterials. ChemBioChem 2024, 26, e202400520. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Wang, T.; Wu, Y.; He, Y.; Yang, R.; Zheng, S.-R. Regulation of the surface area and surface charge property of MOFs by multivariate strategy: Synthesis, characterization, selective dye adsorption and separation. Microporous Mesoporous Mater. 2018, 272, 101–108. [Google Scholar] [CrossRef]
- Saint-Laurent, C.; Mazeyrie, L.; Tajan, M.; Paccoud, R.; Castan-Laurell, I.; Valet, P.; Edouard, T.; Pradère, J.P.; Dray, C.; Yart, A. The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance? Biomedicines 2022, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, J. Toward Scalable and Sustainable Synthesis of Metal–Organic Frameworks. ACS Mater. Lett. 2024, 6, 2400–2408. [Google Scholar] [CrossRef]
- Shrivastav, V.; Mansi; Gupta, B.; Dubey, P.; Deep, A.; Nogala, W.; Shrivastav, V.; Sundriyal, S. Recent advances on surface mounted metal-organic frameworks for energy storage and conversion applications: Trends, challenges, and opportunities. Adv. Colloid Interface Sci. 2023, 318, 102967. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.P.L.; Gapan, A.B.; Angeles, R.A.; Lopez, E.C.R. Stability of Metal–Organic Frameworks: Recent Advances and Future Trends. Eng. Proc. 2023, 56, 146. [Google Scholar] [CrossRef]
- Chen, G.; Dai, B.; Hao, J.-N.; Li, Y. A dual-excitation-driven full-component-responsive lanthanide-based metal-organic framework for switchable profiling of multi-disease markers. Sci. China Mater. 2025, 68, 666–676. [Google Scholar] [CrossRef]
- Baa, E.; Watkins, G.M.; Krause, R.W.; Tantoh, D.N. Current Trend in Synthesis, Post-Synthetic Modifications and Biological Applications of Nanometal-Organic Frameworks (NMOFs). Chin. J. Chem. 2019, 37, 378–404. [Google Scholar] [CrossRef]
- Bagheri, A.; Hoseinzadeh, H.; Hayati, B.; Mahmoodi, N.M.; Mehraeen, E. Post-synthetic functionalization of the metal-organic framework: Clean synthesis, pollutant removal, and antibacterial activity. J. Environ. Chem. Eng. 2020, 9, 104590. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.; Sharma, A.L.; Deep, A. Green Synthesis and Applications of Metal-Organic Frameworks; Springer: Cham, Switzerland, 2022; pp. 1–20. [Google Scholar] [CrossRef]


| Platform | Key Features | Advantages | Limitations | Examples in Enzyme Modulation |
|---|---|---|---|---|
| MOFs | Crystalline porous frameworks with metal nodes and organic linkers | High porosity, tunable pore size, modular functionalization, catalytic metal sites, multifunctionality | Potential toxicity, stability issues in biological fluids, scalability challenges | Zr-MOFs for kinase inhibition; Ce-MOFs for phosphatase inhibition |
| Polymeric Nanocarriers | Synthetic polymers (micelles, dendrimers, block copolymers, PEGylated systems) | High drug-loading capacity, biocompatibility, ability to prolong circulation time | Premature drug release, limited structural rigidity, less precise control of enzyme interactions | PEGylated micelles delivering kinase inhibitors in cancer therapy |
| Nanoclusters | Ultra-small (<2 nm) metal or metal oxide aggregates (e.g., Au, Cu, Fe) | Intrinsic catalytic activity, strong optical/electronic properties for biosensing | Potential long-term toxicity, rapid clearance, aggregation under physiological conditions | Au nanoclusters mimicking oxidase or phosphatase activity |
| Peptidomimetics | Synthetic molecules mimicking natural peptide motifs or regulatory domains | High selectivity, potential to target protein–protein interactions inaccessible to small molecules | Poor stability in vivo, susceptibility to proteolysis, limited oral bioavailability | Peptidomimetic inhibitors of EGFR and SHP2 |
| Synthesis Method | Key Features | Pros for Enzyme Studies | Limitations |
|---|---|---|---|
| Solvothermal | Metal salts and organic linkers reacted in solvents (e.g., DMF) under heat and pressure (100–220 °C, 6–72 h) | High crystallinity, tunable porosity, yields 40–60%. | Long reaction time, toxic solvents, particle size > 200 nm. |
| Microwave-assisted | Rapid uniform heating (120–200 °C, 10–20 min). | Short reaction time, smaller particles (50–120 nm), high phase purity. | Equipment cost, possible non-uniform heating at scale |
| Sonochemical | Ultrasonic irradiation enhances nucleation | Uniform nanoparticles (50–150 nm), mild conditions. | Limited scalability, moderate crystallinity. |
| Mechanochemical | Solid-state grinding with minimal solvent. | Green synthesis, fast (5–30 min), yields >80%. | Lower crystallinity, less control over morphology |
| MOF Type | Metal Node | Target Enzyme | Reported Mechanism | Notes | References |
|---|---|---|---|---|---|
| UiO-66 | Zr(IV) | Protein Kinase CK2 | Surface blocking, ATP-pocket disruption. | High stability in aqueous media. | [48,52] |
| MIL-101 | Fe(III) | Protein Tyrosine Phosphatase (PTP1B) | Surface anchoring, redox modulation. | Potential oxidative stress. | [78] |
| Ce-MOF | Ce(IV) | SHP2 Phosphatase | Catalytic inhibition via Lewis acid sites. | pH-sensitive structure. | [19] |
| Ln-MOFs (Gd, Eu) | Lanthanides | Dual Kinase/Phosphatase | Electrostatic trapping, enzyme conformation disruption. | Fluorescent properties useful. | [53,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, A.; Balbaied, T. Metal–Organic Frameworks for Enzyme Modulation in Protein Kinase and Phosphatase Regulation—Mechanisms and Biomedical Applications. Kinases Phosphatases 2025, 3, 21. https://doi.org/10.3390/kinasesphosphatases3040021
Alamro A, Balbaied T. Metal–Organic Frameworks for Enzyme Modulation in Protein Kinase and Phosphatase Regulation—Mechanisms and Biomedical Applications. Kinases and Phosphatases. 2025; 3(4):21. https://doi.org/10.3390/kinasesphosphatases3040021
Chicago/Turabian StyleAlamro, Azizah, and Thanih Balbaied. 2025. "Metal–Organic Frameworks for Enzyme Modulation in Protein Kinase and Phosphatase Regulation—Mechanisms and Biomedical Applications" Kinases and Phosphatases 3, no. 4: 21. https://doi.org/10.3390/kinasesphosphatases3040021
APA StyleAlamro, A., & Balbaied, T. (2025). Metal–Organic Frameworks for Enzyme Modulation in Protein Kinase and Phosphatase Regulation—Mechanisms and Biomedical Applications. Kinases and Phosphatases, 3(4), 21. https://doi.org/10.3390/kinasesphosphatases3040021






