Abstract
The European Food Safety Authority (EFSA) has raised concerns regarding the use of alkaline phosphatase (ALP) as a pasteurization marker in non-cow milk due to compositional differences. This study investigates the thermal inactivation kinetics of ALP in six milk species (cow, sheep, goat, donkey, buffalo and camel) to assess its reliability as an indicator. The thermal inactivation of ALP in different milk types was evaluated by heating samples at 63–75 °C at various times, then measuring residual enzyme activity using a spectrophotometric method. The results revealed a sharp increase in ALP inactivation with rising temperatures, consistent with previous findings on the enzyme’s thermal sensitivity. Notably, donkey milk exhibited the highest ALP inactivation at 72 °C, probably due to lower fat content compared to the rest of milk types studied, while camel milk showed the lowest inactivation rate constant (kT) at 75 °C, highlighting its higher heat resistance compared to bovine milk. These findings highlight potential limitations of using the ALP test to verify pasteurization in non-bovine milk, which is directly linked to microbial safety, as well as the preservation of nutritional and sensory characteristics. This study reinforces the importance of considering milk composition, particularly fat and protein structures, in optimizing pasteurization conditions for diverse milk varieties.
1. Introduction
According to the Food and Agriculture Organization (FAO), cows produce 82.4% of the world’s milk, which is a key raw material for processing products in the food industry, followed by buffalo (13.6%), goats (2.3%), sheep (1.3%) and camels (0.4%) [1]. Horse and donkey milk have the lowest contribution to global milk production (<0.1%) [2]. Although the majority of dairy products in the markets are of bovine origin, there has been a growing demand for dairy products derived from other milks such as sheep and goat [3].
The need to sanitize raw milk is evident, as it is well established that it serves as a vehicle for the transmission of pathogenic microorganisms. These include Listeria monocytogenes, Yersinia enterocolitica, Campylobacter jejuni, Salmonella spp., Escherichia coli (including EHEC), Staphylococcus aureus and others [4]. As a result, the vast majority of dairy products undergo heat treatment. Depending on the intensity, heat treatments of milk include thermization, pasteurization, ultra-high temperature treatment and commercial sterilization [3].
Considering industrial requirements, the alkaline phosphatase (ALP) test has been widely adopted by many countries as the standard assay for verifying the adequacy of milk pasteurization. This test is obligatory in the dairy industry, and Regulation (EC) No. 853/2004 mandates its application to all types of milk. It is recognized as a validation indicator for confirming the effectiveness of pasteurization, particularly in cow milk.
ALP is one of the numerous (>60) endogenous enzymes found in raw milk [5]. It is a membrane-bound glycoprotein that catalyzes the hydrolysis of phosphoric acid monoesters (at alkaline pH), producing phosphate and the corresponding alcohol [6]. The ALP test is used to evaluate milk pasteurization based on the principle that ALP is naturally present in raw milk and is more heat-resistant than most pathogens of concern [7]. For cows’ milk, a negative result is defined as an ALP activity not higher than 350 mU/L, indicating effective pasteurization [3,8].
However, concerns have been raised about the suitability of ALP activity as an indicator to verify heating treatment of raw milk from non-bovine species. The recent report by European Food Safety Authority (EFSA) [3] highlighted the concerns regarding the sufficiency of ALP testing due to differences in heat resistance and inactivation kinetics among different types of milk. Studies have investigated the thermal inactivation of ALP in milk originating from various species, indicating that it occurs more slowly in goat and sheep milk, and faster in horse milk, compared to in cow’s milk. In general, due to variations in ALP activities and differences in thermal inactivation rates, a negative test does not seem to be sufficient to determine the effectiveness of pasteurization for all types of milk [3,9,10,11]. Most studies on ALP detection on non-cow milk so far have been conducted on goat and sheep milk, while few have focused on milk originating from other species [12].
In accordance with Regulation (EC) No. 852/2004 of the European Parliament, milk quality is to be ensured through pasteurization at 72 °C for 15 s, a standard process aiming to inactivate ALP and thereby validating the safety of the milk. However, the regulation does not specify whether this standard applies to all types of milk. Given that the ALP test is accepted as an indicator of pasteurization only for cow’s milk, an in-depth study of thermal inactivation kinetics of milk from different animal species is recommended to obtain reliable data. Therefore, thermal inactivation kinetic studies on different types of milk are needed to provide data for determining D- and z-values of ALP inactivation in milk. The D-value (decimal reduction time) represents the time required at a specific temperature to reduce enzyme activity by 90%, indicating heat resistance. The z-value expresses how much the temperature must increase to cause a tenfold reduction in the D-value, showing the temperature sensitivity of the inactivation rate constant, kT, of ALP to temperature changes. These are commonly used parameters in food processing to assess the thermal stability and resistance of enzymes and microorganisms, which largely determine the time required for efficient food thermal treatment. According to Claeys et al. [13], ALP thermal inactivation in milk follows first-order kinetics, therefore, the remaining enzyme activity during thermal treatment can be mathematically modeled as an exponential function of treatment time.
The objective of this study was to investigate the thermal inactivation of ALP in milk originating from six different species (cow, sheep, goat, donkey, buffalo and camel) by conducting a kinetic study in the temperature domain of milk pasteurization.
2. Results and Discussion
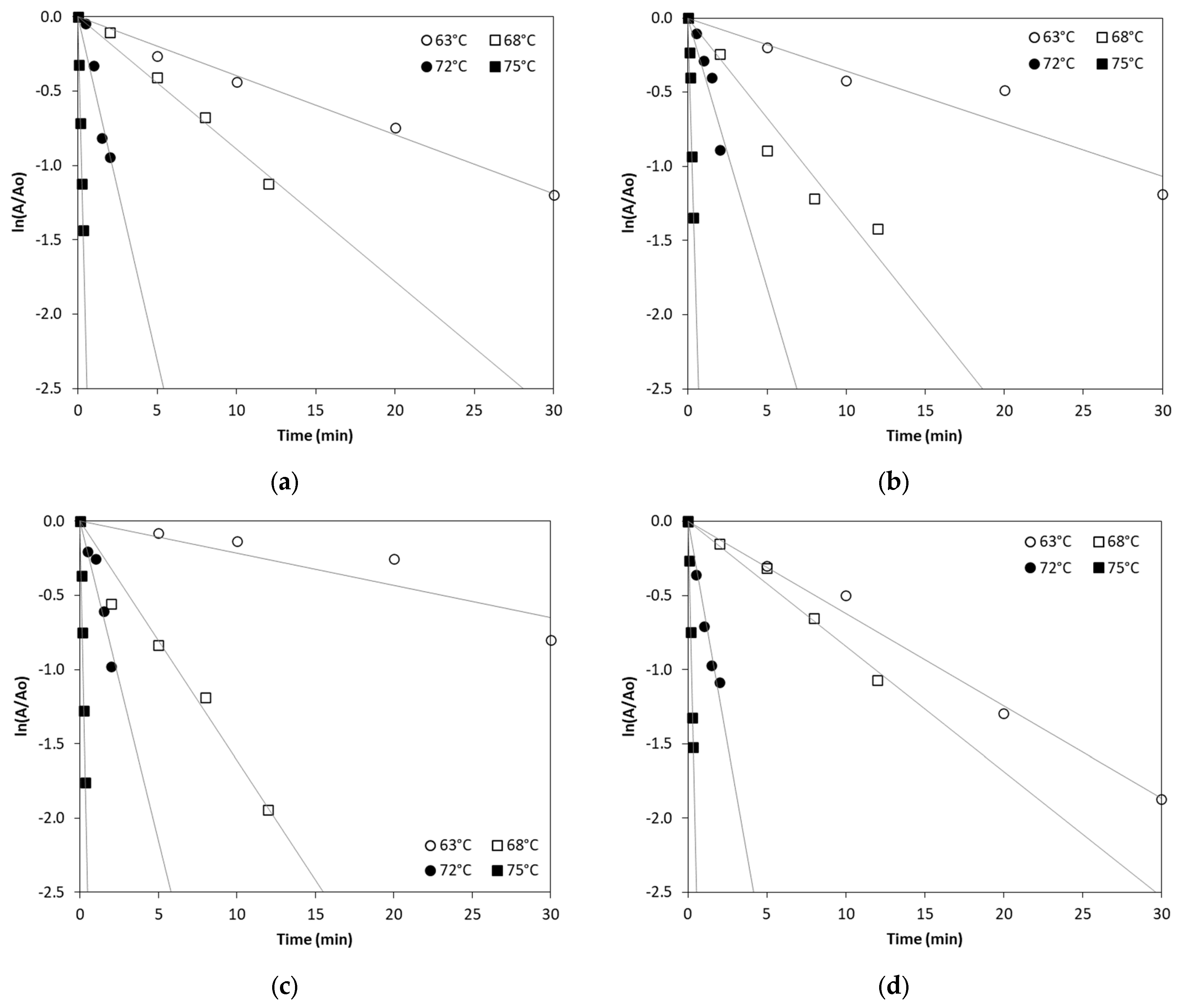
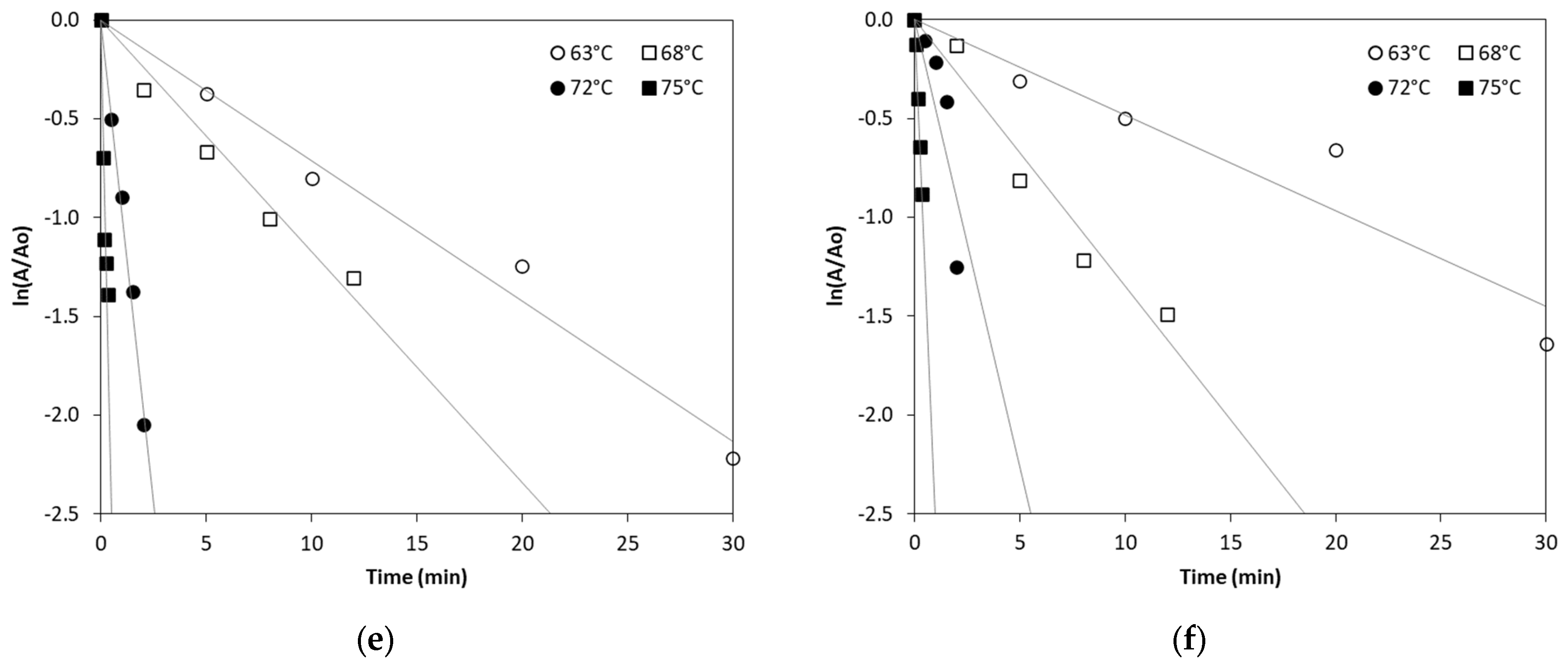
In order to kinetically study ALP thermal inactivation across different milk types, enzyme inactivation was monitored under isothermal treatment conditions of 63, 68, 72 and 75 °C. The remaining ALP activity was plotted against the time of treatment and fitted by exponential curves confirming first order inactivation kinetics. The linearized Equation (2) (see Section 3.3) was used to estimate ALP inactivation rate constant (kT) in all types of milk at the studied temperatures by linear regression, as depicted in Figure 1.
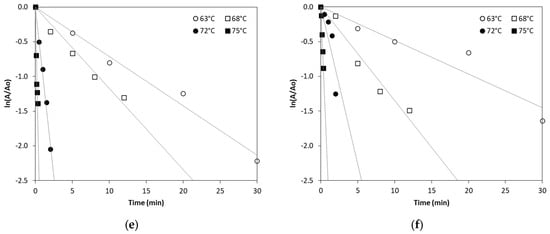
Figure 1.
Thermal inactivation of ALP in (a) cow milk, (b) sheep milk, (c) goat milk, (d) buffalo milk, (e) donkey milk and (f) camel milk at temperatures of 63, 68, 72 and 75 °C. (Each data point is the average of three samples).
According to Table 1, the inactivation of ALP in different milk types is temperature-dependent, with higher temperatures leading to significantly increased inactivation rates. Our findings show a sharp increase in ALP inactivation as the temperature rises, consistent with previously published studies that report the enzyme’s thermal sensitivity [14]. The inactivation rate constant values at 75 °C suggest near-complete inactivation in all milk types, which confirms that pasteurization at the temperature of 75 °C for 30–60 s effectively inactivates ALP in bovine and other milk types, although there is a clear variability of inactivation rate constant values that depends on the milk type. Donkey milk shows a particularly high ALP inactivation at 72 °C compared to all other milk types studied. However, camel milk exhibits the lowest ALP inactivation rate constant values at 75 °C when compared to the other studied milk types, suggesting that camel milk ALP is more heat-resistant, requiring higher temperatures for complete inactivation.

Table 1.
ALP thermal inactivation rate constant k (min−1) in Cow, sheep, goat, buffalo, donkey and camel milk at the temperature range of 63–75 °C.
In the current study, the activation energy (Ea) values ranged from 320 to 410 kJ/mol, depending on the type of milk corresponding to a z-value ranging from 6.99 °C to 5.45 °C, as summarized in Table 2. The results align with previous kinetic models of ALP inactivation, which often follow first-order kinetics. Previous studies on ALP thermal inactivation kinetics report Ea values ranging between 200 and 460 kJ/mol, which are compared with the present experimental results. Dumitraşcu et al. [14] performed a kinetic study on ALP thermal inactivation in the temperature range of 60–72.5 °C in different milk types, reporting that ALP in bovine milk was less thermoresistant compared to goat and sheep milk, supporting the findings of varying inactivation rates among different milk types. In the same study, Ea values for heat-labile and heat-resistant isoenzymes were determined, reporting values of 280.97± 44.71 and 434.44 ± 46.45 kJ/mol for goat milk, 462.54 ± 47.31 and 227.76 ± 29.59 kJ/mol for sheep milk, and 249.62 ± 26.89 and 336.46 ± 49.05 kJ/mol for cow milk for labile and stable fraction, respectively [14]. Claeys et al. [15] studied ALP thermal inactivation kinetics in raw bovine milk under isothermal conditions, reporting first-order kinetics with a D-value of approximately 24.6 min at 60 °C, a z-value of 5.3 °C, and a relatively high activation energy (Ea) of 432.1 ± 6.3 kJ/mol. In contrast, Stănciuc et al. [16] reported a significantly lower Ea value of 222.02 ± 5.6 kJ/mol in raw bovine milk compared to our results. Moreover, Wilińska et al. [17] reported Ea values of 421 ± 15.1 kJ/mol and 340 ± 6.76 kJ/mol for bovine milk and its corresponding buffer (0.1 M potassium phosphate buffer, pH 6.6, which matches the pH of raw milk), respectively, and 406 ± 12.4 kJ/mol and 366 ± 4.65 kJ/mol for caprine milk and the same buffer, respectively.

Table 2.
Thermal inactivation kinetic parameters of ALP in cow, sheep, goat, buffalo, donkey and camel milk at the temperature range of 63–75 °C (reference temperature (Tref): 70 °C).
As reported in Table 2, the donkey milk inactivation rate constant at reference temperature (70 °C) was the highest among all milk types studied. The main difference between donkey milk and the rest of the studied milk types with regards composition is the lower fat content of 1.2% when compared to the fat content of 3.9% for cow milk, 6.9% for sheep milk, 4.5% for goat milk, 8.5% for buffalo milk and 3.1% for camel milk. Although a further clear correlation between the milk constituent’s variation as summarized in Table 3 (see Section 3.1) and the variations of reported ALP thermal inactivation kinetic parameters (Table 2) could not be established, our study confirms previous studies indicating that ALP thermal inactivation kinetics could vary among different milk types. In particular, the composition of milk, including factors such as fat content, protein concentration and the presence of other components, may influence the thermal stability of ALP. The differences observed in the ALP thermal inactivation kinetics among the various milk types studied in the present study could be attributed to variations in milk composition among the milk types studied. It is generally recognized that thermal tolerance of ALP in milk can vary among different types of milk, and this can be attributed mainly to fat content variation. Fat globules in milk have been recognized as thermal insulators due to their lower thermal conductivity compared to the aqueous phase, thereby reducing heat transfer efficiency [18]. The thermal denaturation of heat-sensitive enzymes found in milk such as ALP can be reduced by the protective microenvironment created by the fat globules and the surrounding membrane structure [19]. In this context, experimental studies have demonstrated that higher fat levels in milk slow down the rate of ALP inactivation compared to low-fat or skimmed milk [20]. Thus, the milk matrix, particularly its fat content, plays a critical role in modulating enzyme stability during thermal treatment [21]. In addition to fat content, milk proteins can also influence the thermal stability of enzymes. The heat stable structure of casein micelles can interact with enzymes such as ALP, creating a protective microenvironment against thermal denaturation [22]. Studies have demonstrated that the association of ALP with casein micelles can increase heat resistance [23]. Moreover, whey proteins, and particularly β-lactoglobulin, can either stabilize or destabilize ALP, depending on their state. The increased thermal resistance of ALP has been linked with the native state of whey proteins. On the other hand, whey proteins can promote the aggregation of ALP and lead to enhanced thermal inactivation once denatured by heat [24,25]. Therefore, the protein matrix of milk is considered to play a significant role in modulating the thermal inactivation kinetics of ALP. In this context, the presence of fat and other milk constituents can affect the enzyme’s resistance to heat, thereby altering the Ea values determined during inactivation studies [13,16]. Therefore, when comparing Ea values across studies, it is crucial to consider these variables to ensure accurate interpretations.

Table 3.
Milk samples’ compositions.
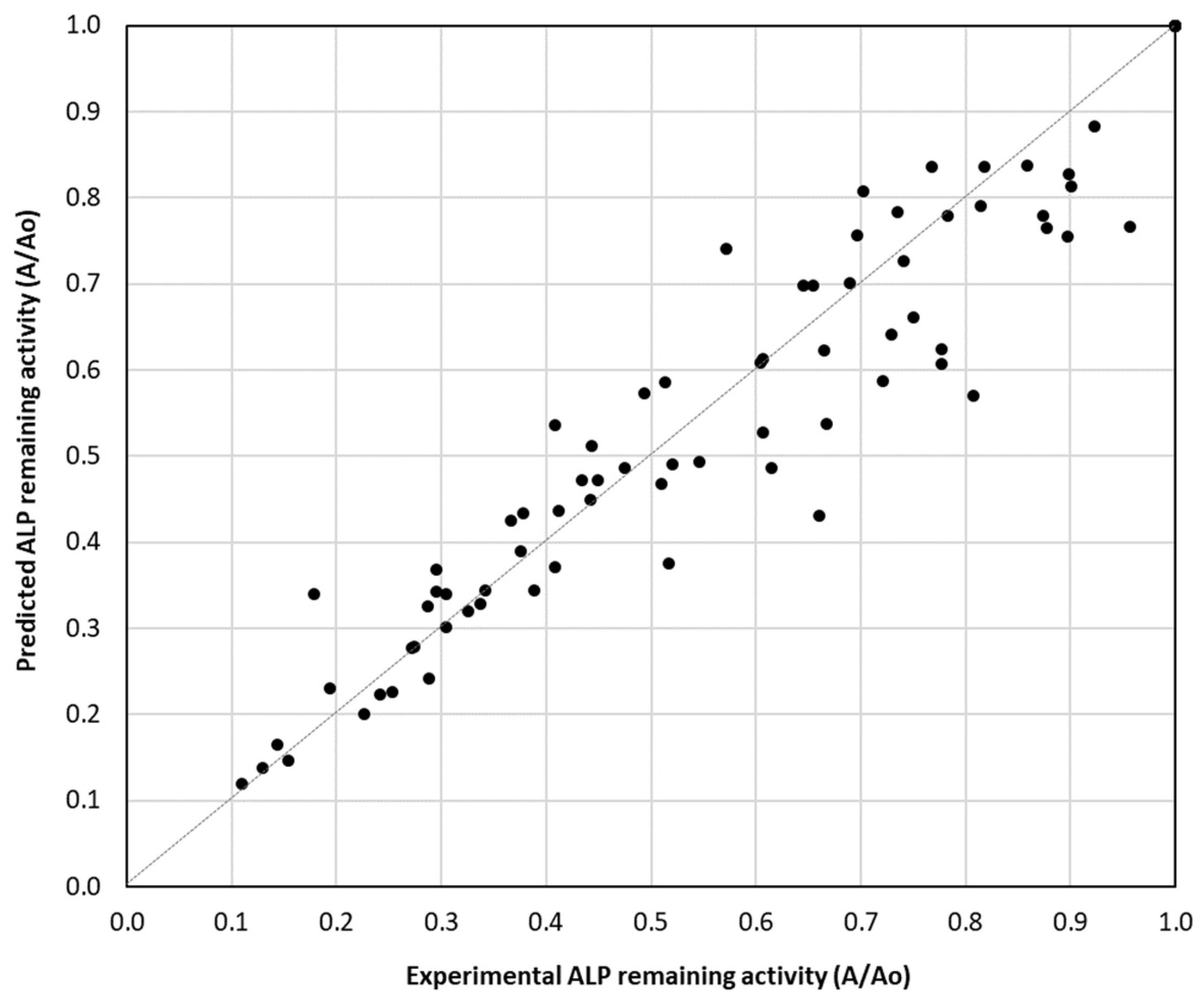
The scatter plot depicted in Figure 2 compares the experimental and predicted ALP remaining activity values (A/A0), providing insights into the accuracy of the kinetic models developed in the present study. The data points closely follow the diagonal reference line (y = x), indicating that the predicted values align well with the experimental values of ALP remaining activity. This suggests that the estimated inactivation kinetic parameters accurately describe the thermal inactivation kinetics of ALP within the studied temperature range.
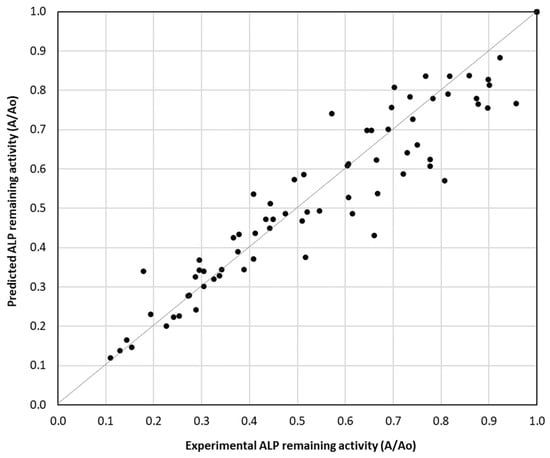
Figure 2.
Correlation between predicted and experimental ALP remaining activity in bovine, sheep, goat, buffalo, donkey and camel milk at the temperature range of 63–75 °C using Equation (1) and estimated ALP inactivation rate constant values in Table 1.
Although most points align well with the diagonal, there is some deviation, especially at higher remaining activity values indicating potential limitations in the model’s predictive ability, especially in the domain of high values of remaining ALP activity. The overall findings suggest a high degree of agreement between experimental and predicted remaining ALP activity, validating the accuracy of the estimated inactivation rate constants. Further refinements may improve the accuracy of the developed kinetic model, particularly in the low ALP inactivation domain.
3. Materials and Methods
3.1. Samples Collection and Treatment
A total of 6 samples of raw milk, 500 mL each, were collected in sterile containers, from cow, sheep and goat farms in the mountainous Pindos region in Greece, buffalo farms near Kerkini Lake in Greece, and colostrum and camel milk farms from Cyprus. The samples were transported frozen (−18 °C) in special refrigerators to the Laboratory of Chemistry, Biochemistry and Food Technology of the Department of Dietetics and Nutrition and the Laboratory of Animal Food Origin of the Animal Production Science Department of the University Thessaly, where they remained frozen until analysis. After controlled thawing, the pH was measured and the composition was determined as summarized in Table 3.
3.2. Evaluation of Alkaline Phosphatase Enzyme Activity
Milk samples were subjected to different isothermal treatments at constant temperature of 63, 68, 72 and 75 °C. ALP thermal inactivation was determined by measuring the residual ALP activity in milk samples after thermal treatment as a function of processing time. Isothermal treatments were performed in temperature-controlled water baths (Μemmert Type: WB 14, Schwabach, Germany) where 2 mL of milk samples were placed in glass centrifuge tubes to ensure fast temperature equilibrium. Temperature was continuously monitored using a digital thermometer (Brannan, Cumbria, UK) with accuracy −50 to +200 °C/−58 to +392 °F, and an equilibration period of 30 s was allowed to ensure isothermal conditions, and the first sample taken 30 s after immersion in the water bath, was considered to be the zero treatment time sample. After thermal treatment for predetermined times (up to 30 min at 63 °C, up to 12 min at 68 °C, up to 2 min at 72 °C and up to 20 s at 75 °C) milk samples were placed in ice-water baths to stop further inactivation of the enzyme, before the determination of remaining ALP activity. Three replicates were used for all thermal treatments.
The remaining ALP activity in milk samples was determined following the methods described by Levieux et al. [26] and Upadhyay and Verma [27], with slight modifications. To ensure optimal performance in measuring ALP activity, a suitable substrate and buffer solution were selected. Tris HCl 0.5 M (TCI ACS reagent, ≥99.8%, Tokyo, Japan) as a buffer solution at pH 9 ensures the enzymatic conversion of the substrate p-Nitrophenol (pNPP) Disodium 4-Nitrophenyl Phosphate Hexahydrate (PNPP) (TCI Purity: >98.0%) into phenol and phosphate with its highest ALP activity. For sample preparation, 10 mL test tubes were used to dilute sheep, goat, camel and colostrum milk in a 1:10 ratio, and buffalo milk in a 1:100 ratio using the 0.5 M Tris HCl solution. The approach of using diluted milk samples after thermal treatment and prior to spectrophotometrically-based ALP determination ensured that absorbance values (A405nm) were measured within the linear range rather than at the upper limit of absorption measurements (according to Beer–Lambert law). In the case of buffalo milk, a higher dilution factor (1:100) was required due to the highest ALP content found in the specific milk type. This dilution was performed for all temperature-time combinations, followed by mixing with a vortex stirrer. Each tube contained 400 µL of 0.5 M Tris HCl buffer, 300 µL of 5 mM Disodium 4-Nitrophenyl Phosphate Hexahydrate (PNPP) as the substrate and 300 µL of the appropriately diluted milk sample. The mixtures were incubated in a water bath at 37 °C for 30 min. ALP activity was then assessed by measuring the resulting yellow coloration spectrophotometrically at 405 nm using a UV/VIS Spectrophotometer (LLG, Meckenheim, Germany). The linearity of the assay between the absorbance read in the spectrophotometer and the enzyme activity was confirmed and the ratio of the recorded absorbance of the sample under consideration, A, and the recorded absorbance of the sample at zero treatment time, A0, was considered as the remaining enzyme activity (A/A0).
3.3. Inactivation Kinetics and Data Analysis
Τhermal inactivation in most endogenous food enzymes follows first order kinetics. When the kinetic study of inactivation of an enzyme is carried out at isothermal conditions, the remaining enzyme activity (first order) as a function of heat treatment time described by Equation (1) or its linearized form as given in Equation (2).
where A/A0 is the remaining enzyme activity, kT the thermal inactivation rate constant at temperature T (min−1) and t the thermal treatment time (min) at constant temperature T.
In the current study, the linearized remaining ALP activity values were plotted vs. the time for all temperatures studied for all six milk types. The inactivation rate constant of ALP was determined based on the least square statistical fit via linear regression. The temperature-dependence of the inactivation rate constants, kT, was modelled by the Arrhenius equation (Equation (3)),
where, kT is the ALP inactivation rate constant at a constant temperature T (min−1), kTref is the ALP inactivation rate constant at a reference temperature Tref (min−1), Tref is the reference temperature (in this study 70 °C or 343.15 K), T is the temperature (K), Ea is the activation energy of ALP thermal inactivation (J/mol) and R is the universal gas constant (8.314 J/mol·K). The Ea values were estimated from the slope of Arrhenius plots of lnk vs. (1/T−1/Tref), by linear regression.
In thermal processing of food, it is common to characterize first order reactions in terms of decimal reduction time (DT) at a given temperature (Equation (4)), while the temperature dependence of decimal reduction is expressed with the thermal resistance z (°C), defined as the number of degrees the temperature has to be increased to achieve a tenfold (i.e., 1 log10) reduction in the D-value.
where, DT is the decimal reduction time at a constant temperature T (min) and kT is the inactivation rate constant at the same temperature T (min−1).
3.4. Statistical Analysis
Measurements for all groups were performed in triplicate, and the values were averaged and reported with their standard deviation (S.D.). Data analysis was conducted using One-Way ANOVA followed by Post Hoc tests. p-values less than 0.05 were considered statistically significant. All statistical calculations were performed using SPSS 20.0 software (IBM SPSS Inc., Armonk, NY, USA) for Windows.
4. Conclusions
The ALP thermal inactivation kinetic models developed in this study demonstrated variability in ALP thermal resistance across different types of milk. Although this variability may be attributed in milk composition differences, a clear correlation between milk constituents and the observed variations in ALP thermal inactivation kinetics could not be established. Further research in the field involving a larger number of milk samples is necessary in order to explore potential correlation between ALP thermal inactivation kinetics and intrinsic milk properties, particularly focusing on fat and protein content, as well as on protein structure.
Author Contributions
Conceptualization, E.M., E.G. and A.M.; methodology, A.T., K.P. and E.G.; validation, A.T., G.S. and M.K.; formal analysis, E.G. and A.T.; investigation, M.K., K.P., A.T. and E.G.; resources, E.M. and A.M.; data curation, E.G.; writing—original draft preparation, A.T., G.S. and E.G.; writing—review and editing, M.K., G.S. and E.M.; visualization, E.G.; supervision, A.M. and E.M.; project administration, A.M. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request.
Acknowledgments
We extend thanks to P. Papademas for samples provision and A. Tsiamita and D. Chimona for the preliminary test analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Britt, J.H.; Cushman, R.A.; Dechow, C.D.; Dobson, H.; Humblot, P.; Hutjens, M.F.; Jones, G.A.; Ruegg, P.S.; Sheldon, I.M.; Stevenson, J.S. Invited review: Learning from the future—A vision for dairy farms and cows in 2067. J. Dairy Sci. 2018, 101, 3722–3741. [Google Scholar] [CrossRef] [PubMed]
- Faye, B.; Konuspayeva, G. The sustainability challenge to the dairy sector—The growing importance of non-cattle milk production worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Clawin-Rädecker, I.; De Block, J.; Egger, L.; Willis, C.; Felicio, M.T.D.S.; Messens, W. The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products. EFSA J. 2021, 19, e06576. [Google Scholar] [CrossRef]
- Vasavada, P. Pathogenic Bacteria in Milk—A Review. J. Dairy Sci. 1988, 71, 2809–2816. [Google Scholar] [CrossRef]
- Deeth, H.C. Heat-induced inactivation of enzymes in milk and dairy products. A review. Int. Dairy J. 2021, 121, 105104. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Rankin, S.; Christiansen, A.; Lee, W.; Banavara, D.; Lopez-Hernandez, A. Invited review: The application of alkaline phosphatase assays for the validation of milk product pasteurization. J. Dairy Sci. 2010, 93, 5538–5551. [Google Scholar] [CrossRef]
- Tsiamita, A.; Valiakos, G.; Natsaridis, N.; Fotiadou, S.; Manouras, A.; Malissiova, E. Preliminary Results on the Comparative Evaluation of Alkaline Phosphatase Commercial Tests Efficiency in Non-Cow Milk Pasteurization. BioTech 2022, 11, 39. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Martin, D.; Clawin-Rädecker, I.; Barth, K.; Knappstein, K. Activities of alkaline phosphatase, γ-glutamyltransferase and lactoperoxidase in cow, sheep and goat’s milk in relation to heat treatment. Small Rumin. Res. 2010, 89, 18–23. [Google Scholar] [CrossRef]
- Marchand, S.; Merchiers, M.; Messens, W.; Coudijzer, K.; De Block, J. Thermal inactivation kinetics of alkaline phosphatase in equine milk. Int. Dairy J. 2009, 19, 763–767. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Park, Y.; Gaucheron, F.; Bouhallab, S. Heat stability and enzymatic modifications of goat and sheep milk. Small Rumin. Res. 2007, 68, 207–220. [Google Scholar] [CrossRef]
- Malissiova, E.; Fotiadou, S.; Tzereme, A.; Cheimona, D.; Soultani, G.; Maisoglou, I.; Manouras, A. Alkaline Phosphatase (ALP) in Non-Cow Milk and Dairy Products: A Review of Current Evidence and Future Trends. Ruminants 2022, 2, 435–447. [Google Scholar] [CrossRef]
- Claeys, W.L.; VAN Loey, A.M.; Hendrickx, M.E. Kinetics of alkaline phosphatase and lactoperoxidase inactivation, and of β-lactoglobulin denaturation in milk with different fat content. J. Dairy Res. 2002, 69, 541–553. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Stănciuc, N.; Stanciu, S.; Râpeanu, G. Inactivation kinetics of alkaline phosphatase from different species of milk using quinolyl phosphate as a substrate. Food Sci. Biotechnol. 2014, 23, 1773–1778. [Google Scholar] [CrossRef]
- Claeys, W.L.; Ludikhuyze, L.R.; VAN Loey, A.M.; Hendrickx, M.E. Inactivation kinetics of alkaline phosphatase and lactoperoxidase, and denaturation kinetics of β-lactoglobulin in raw milk under isothermal and dynamic temperature conditions. J. Dairy Res. 2001, 68, 95–107. [Google Scholar] [CrossRef]
- Stănciuc, N.; Ardelean, A.; Diaconu, V.; Râpeanu, G.; Stanciu, S.; Nicolau, A. Kinetic and thermodynamic parameters of alkaline phosphatase and γ—Glutamyl transferase inactivation in bovine milk. Dairy Sci. Technol. 2011, 91, 701–717. [Google Scholar] [CrossRef]
- Wilińska, A.; Bryjak, J.; Illeová, V.; Polakovič, M. Kinetics of thermal inactivation of alkaline phosphatase in bovine and caprine milk and buffer. Int. Dairy J. 2007, 17, 579–586. [Google Scholar] [CrossRef]
- Lewis, M. Physical and physicochemical properties of milk and milk products. In Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents; Springer: Berlin/Heidelberg, Germany, 2022; pp. 493–551. [Google Scholar]
- Walstra, P.; Wouters, T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Mistry, V.V.; Ma, Y. Effect of homogenization pressure and fat content on the heat stability of milk. J. Dairy Sci. 1985, 68, 1850–1855. [Google Scholar]
- Datta, N.; Deeth, H.C. Heat treatment of milk: Effect on chemical and physical properties. Aust. J. Dairy Technol. 2001, 53, 181–189. [Google Scholar]
- De Kruif, C.; Holt, C. Casein micelle structure, functions and interactions. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Springer: Berlin/Heidelberg, Germany, 2003; pp. 233–276. [Google Scholar]
- Anema, S.G.; McKenna, A.B. Effect of Milk Proteins on the Thermal Denaturation of Milk Enzymes. Int. Dairy J. 1996, 6, 407–422. [Google Scholar]
- Oldfield, D.J.; Singh, H.; Taylor, M.W. Association of β-lactoglobulin and β-lactalbumin with the casein micelles in skim milk heated in an ultra-high temperature plant. Int. Dairy J. 1998, 8, 765–770. [Google Scholar] [CrossRef]
- Havea, P. Protein interactions in milk protein concentrate powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Levieux, D.; Geneix, N.; Levieux, A. Inactivation-denaturation kinetics of bovine milk alkaline phosphatase during mild heating as determined by using a monoclonal antibody-based immunoassay. J. Dairy Res. 2007, 74, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, L.S.B.; Verma, N. A three step approach for the purification of alkaline phosphatase from non-pasteurized milk. J. Food Sci. Technol. 2014, 52, 3140–3146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).