SMURF1/2 Are Novel Regulators of WNK1 Stability
Abstract
1. Introduction
2. Results
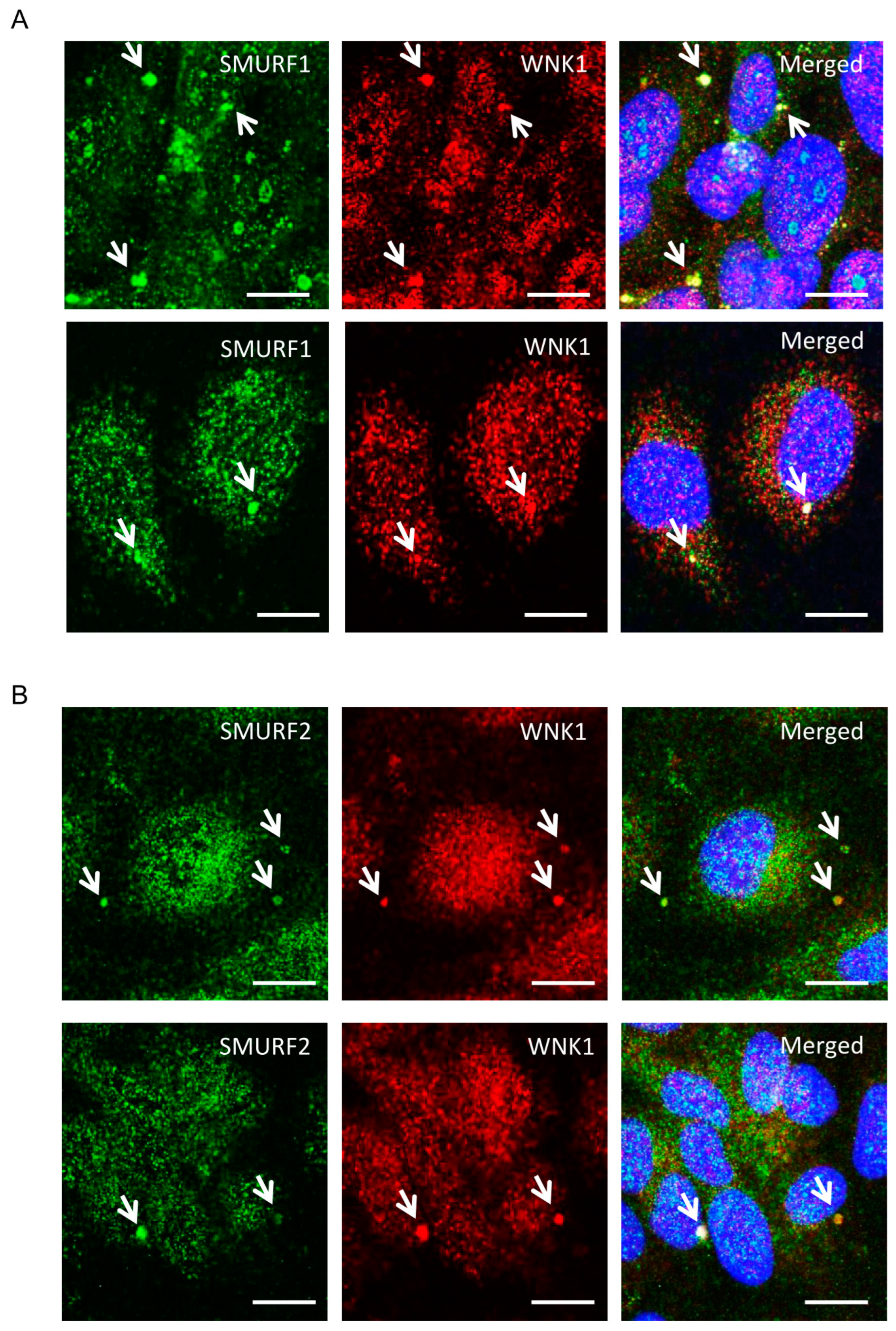
2.1. WNK1 Colocalizes with SMURF1/2
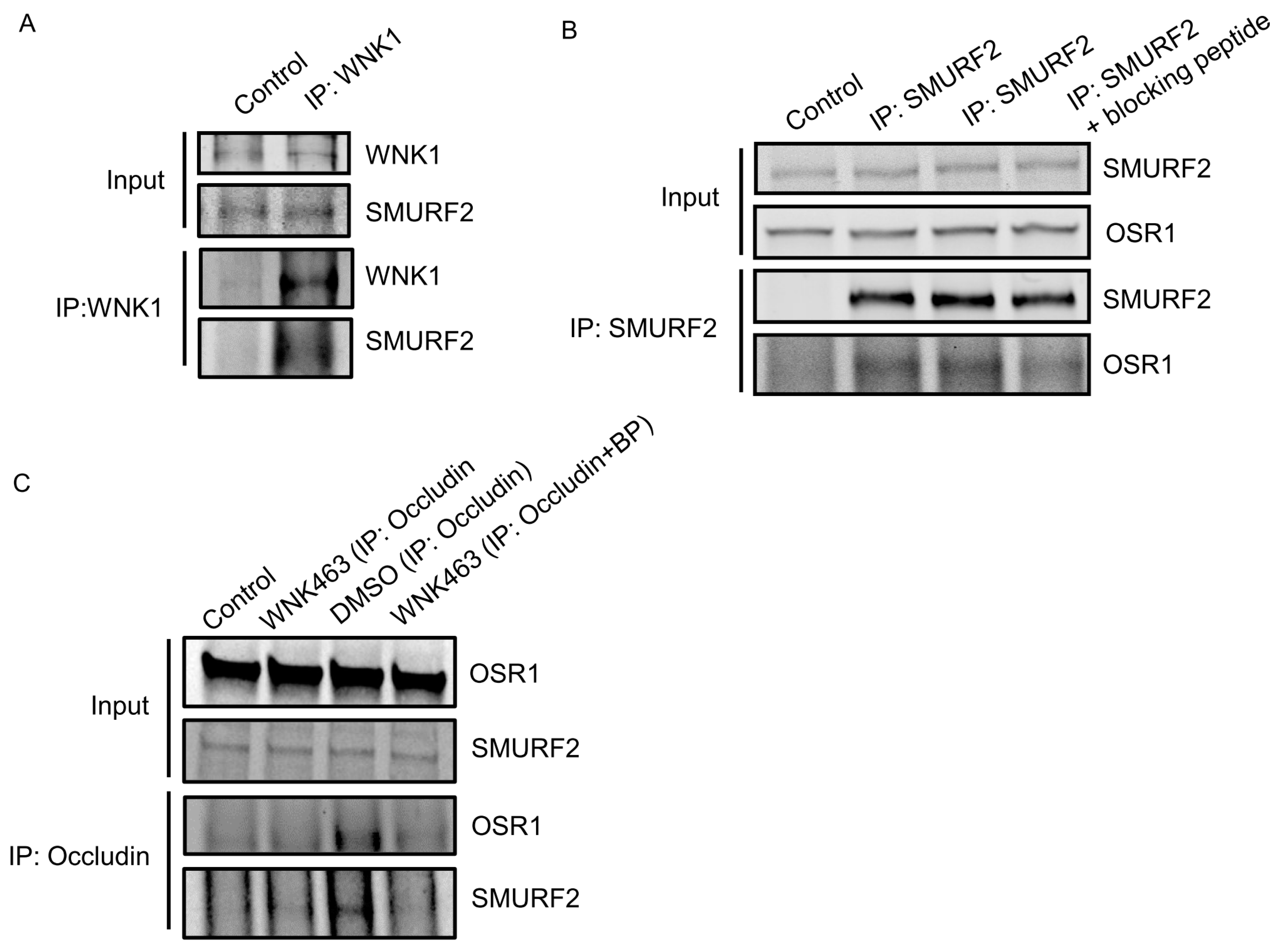
2.2. WNK1 Kinase Activity Regulates Association of WNK1/OSR1 with SMURF2
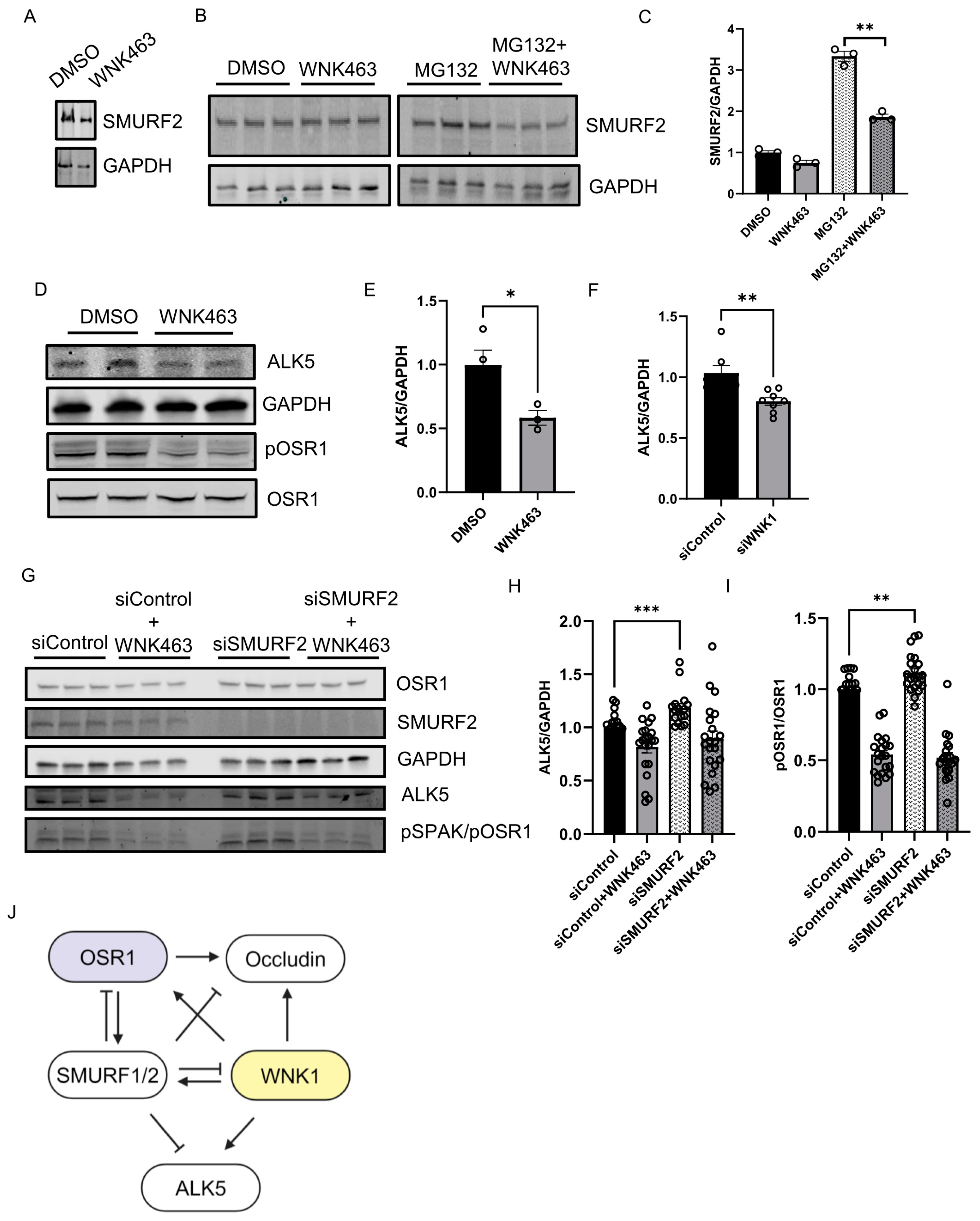
2.3. WNK1/OSR1 Regulates SMURF1/2 and Vice Versa
2.4. WNK1 Kinase Activity Mediates SMURF2-Dependent Regulation of ALK5
3. Discussion
4. Methods
4.1. Cell Lines
4.2. Co-Immunoprecipitation
4.3. Immunofluorescence
4.4. siRNA Knockdown
4.5. Immunoblotting
4.6. Reagents
4.7. Statistics and Reproducibility
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, C.J.; Massagué, J. Contextual Determinants of TGFβ Action in Development, Immunity and Cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, T.; Xu, K.; Huang, I.K.; Cleaver, O.; Huang, C.-L. Endothelial-Specific Expression of WNK1 Kinase Is Essential for Angiogenesis and Heart Development in Mice. Am. J. Pathol. 2009, 175, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yoon, J.; Yang, S.-S.; Lin, S.-H.; Huang, C.-L. WNK1 Protein Kinase Regulates Embryonic Cardiovascular Development through the OSR1 Signaling Cascade. J. Biol. Chem. 2013, 288, 8566–8574. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-E.; English, J.M.; Wilsbacher, J.L.; Stippec, S.; Goldsmith, E.J.; Cobb, M.H. WNK1, a Novel Mammalian Serine/Threonine Protein Kinase Lacking the Catalytic Lysine in Subdomain II. J. Biol. Chem. 2000, 275, 16795–16801. [Google Scholar] [CrossRef]
- Vitari, A.C.; Deak, M.; Morrice, N.A.; Alessi, D.R. The WNK1 and WNK4 Action in Development Protein Kinases That Are Mutated in Gordon’s Hypertension Syndrome Phosphorylate and Activate SPAK and OSR1 Protein Kinases. Biochem. J. 2005, 391, 17–24. [Google Scholar] [CrossRef]
- Moriguchi, T.; Urushiyama, S.; Hisamoto, N.; Iemura, S.; Uchida, S.; Natsume, T.; Matsumoto, K.; Shibuya, H. WNK1 regulates phos-phorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 2005, 280, 42685–42693. [Google Scholar] [CrossRef]
- Xu, B.-E.; Stippec, S.; Chu, P.-Y.; Lazrak, A.; Li, X.-J.; Lee, B.-H.; English, J.M.; Ortega, B.; Huang, C.-L.; Cobb, M.H. WNK1 Activates SGK1 to Regulate the Epithelial Sodium Channel. Proc. Natl. Acad. Sci. USA 2005, 102, 10315–10320. [Google Scholar] [CrossRef]
- Anselmo, A.N.; Earnest, S.; Chen, W.; Juang, Y.C.; Kim, S.C.; Zhao, Y.; Cobb, M.H. WNK1 and OSR1 Regulate the Na+, K+, 2Cl− Co-Transporter in HeLa cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10883–10888. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A., 4th; An, S.W.; Kankanamalage, S.G.; Stippec, S.; Earnest, S.; Trivedi, A.T.; Yang, J.Z.; Mirzaei, H.; Huang, C.L.; Cobb, M.H. OSR1 Regulates a Subset of Inward Rectifier Potassium Channels via a Binding Motif Variant. Proc. Natl. Acad. Sci. USA 2018, 115, 3840–3845. [Google Scholar] [CrossRef]
- Jaykumar, A.B.; Plumber, S.; Barry, D.M.; Binns, D.; Wichaidit, C.; Grzemska, M.; Earnest, S.; Goldsmith, E.J.; Cleaver, O.; Cobb, M.H. WNK1 Collaborates with TGF-β in Endothelial Cell Junction Turnover and Angiogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2203743119. [Google Scholar] [CrossRef]
- Lee, B.H.; Chen, W.; Stippec, S.; Cobb, M.H. Biological Cross-Talk between WNK1 and the Transforming Growth Factor Beta-Smad Signaling Pathway. J. Biol. Chem. 2007, 282, 17985–17996. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.; Rasmussen, R.K.; Causing, C.G.; Bonni, S.; Zhu, H.; Thomsen, G.H.; Wrana, J.L. SMAD7 Binds to SMURF2 to Form an E3 Ubiquitin Ligase That Targets the TGF Beta Receptor for Degradation. Mol. Cell. 2000, 6, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, T.; Fukuchi, M.; Murakami, G.; Chiba, T.; Tanaka, K.; Imamura, T.; Miyazono, K. SMURF1 Interacts with Transforming Growth Factor-Beta Type I Receptor through SMAD7 and Induces Receptor Degradation. J. Biol. Chem. 2001, 276, 12477–12480. [Google Scholar] [CrossRef] [PubMed]
- Koefoed, K.; Skat-Rørdam, J.; Andersen, P.; Warzecha, C.B.; Pye, M.; Andersen, T.A.; Ajbro, K.D.; Bendsen, E.; Narimatsu, M.; Vilhardt, F.; et al. The E3 Ubiquitin Ligase SMURF1 Regulates Cell-Fate Specification and Outflow Tract Septation during Mammalian Heart Devel-Opment. Sci. Rep. 2018, 8, 9542. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, M.; Feng, X.H. SMURF2 Is a Ubiquitin E3 Ligase Mediating Proteasome-Dependent Degradation of SMAD2 in Trans-Forming Growth Factor-Beta Signaling. J. Biol. Chem. 2000, 275, 36818–36822. [Google Scholar] [CrossRef]
- Koganti, P.; Levy-Cohen, G.; Blank, M. Smurfs in Protein Homeostasis, Signaling, and Cancer. Front. Oncol. 2018, 8, 295. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C.; Gehling, D.J.; Hemmati-Brivanlou, A.; Derynck, R. Regulation of Smad Degradation and Activity by SMURF2, an E3 Ubiquitin Ligase. Proc. Natl. Acad. Sci. USA 2001, 98, 974–979. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Yamashita, M.; Coussens, N.P.; Tang, Y.; Wang, X.; Li, C.; Deng, C.-X.; Cheng, S.Y.; Zhang, Y.E. Ablation of SMURF2 Reveals an Inhibition in TGF-β Signalling through Multiple Mono-Ubiquitination of SMAD3. EMBO J. 2011, 30, 4777–4789. [Google Scholar] [CrossRef]
- Mund, T.; Pelham, H.R. Substrate Clustering Potently Regulates the Activity of WW-HECT Domain–Containing Ubiquitin Ligases. J. Biol. Chem. 2018, 293, 5200–5209. [Google Scholar] [CrossRef]
- Wang, H.-R.; Ogunjimi, A.A.; Zhang, Y.; Ozdamar, B.; Bose, R.; Wrana, J.L. Degradation of RhoA by Smurf1 Ubiquitin Ligase. Methods Enzymol. 2006, 406, 437–447. [Google Scholar] [CrossRef]
- Ozdamar, B.; Bose, R.; Barrios-Rodiles, M.; Wang, H.R.; Zhang, Y.; Wrana, J.L. Regulation of the Polarity Protein Par6 by TGFbeta Receptors Controls Epithelial Cell Plasticity. Science 2005, 307, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodiles, M.; Brown, K.R.; Ozdamar, B.; Bose, R.; Liu, Z.; Donovan, R.S.; Shinjo, F.; Liu, Y.; Dembowy, J.; Taylor, I.W.; et al. High-Throughput Mapping of a Dynamic Signaling Network in Mammalian Cells. Science 2005, 307, 1621–1625. [Google Scholar] [CrossRef]
- Wong, V. Phosphorylation of Occludin Correlates with Occludin Localization and Function at the Tight Junction. Am. J. Physiol. Physiol. 1997, 273, C1859–C1867. [Google Scholar] [CrossRef]
- Du, D.; Xu, F.; Yu, L.; Zhang, C.; Lu, X.; Yuan, H.; Huang, Q.; Zhang, F.; Bao, H.; Jia, L.; et al. The Tight Junction Protein, Occludin, Regulates the Directional Migration of Epithelial Cells. Dev. Cell 2010, 18, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Roy, A.; Namboodiri, H.N.; Nkashama, L.J.; Xie, J.; McClain, K.L.; Marciszyn, A.; Kleyman, T.R.; Tan, R.J.; et al. Potassium-Regulated Distal Tubule Wnk Bodies Are Kidney-Specific WNK1 Dependent. Mol. Biol. Cell 2018, 29, 499–509. [Google Scholar] [CrossRef]
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin Phosphorylation and Ubiquitination Regulate Tight Junction Trafficking and Vascular Endothelial Growth Factor-induced Permeability. J. Biol. Chem. 2009, 284, 21036–21046. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dreffs, A.; Díaz-Coránguez, M.; Runkle, E.A.; Gardner, T.W.; Chiodo, V.A.; Hauswirth, W.W.; Antonetti, D.A. Occludin S490 Phosphorylation Regulates Vascular Endothelial Growth Factor–Induced Retinal Neovascularization. Am. J. Pathol. 2016, 186, 2486–2499. [Google Scholar] [CrossRef]
- McCormick, J.A.; Yang, C.L.; Zhang, C.; Davidge, B.; Blankenstein, K.I.; Terker, A.S.; Yarbrough, B.; Meermeier, N.P.; Park, H.J.; McCully, B.; et al. Hyper-Kalemic Hypertension-Associated Cullin 3 Promotes WNK Signaling by Degrading KLHL3. J. Clin. Investig. 2014, 124, 4723–4736. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.U.; B. Ghosh, A.; Earnest, S.; Deaton, S.L.; Cobb, M.H. UBR5 Is a Novel Regulator of WNK1 Stability. Am. J. Physiol. Cell Physiol. 2022, 322, C1176–C1186. [Google Scholar] [CrossRef]
- Roy, A.; Al-Qusairi, L.; Donnelly, B.F.; Ronzaud, C.; Marciszyn, A.L.; Gong, F.; Chang, Y.C.; Butterworth, M.B.; Pastor-Soler, N.M.; Hallows, K.R.; et al. Alternatively Spliced Proline-Rich Cassettes Link WNK1 to Aldosterone Action. J. Clin. Investig. 2015, 125, 3433–3448. [Google Scholar] [CrossRef]
- Heise, C.J.; Xu, B.E.; Deaton, S.L.; Cha, S.K.; Cheng, C.J.; Earnest, S.; Sengupta, S.; Juang, Y.C.; Stippec, S.; Xu, Y.; et al. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010, 285, 25161–25167. [Google Scholar] [CrossRef] [PubMed]
- Al-Qusairi, L.; Basquin, D.; Roy, A.; Stifanelli, M.; Rajaram, R.D.; Debonneville, A.; Nita, I.; Maillard, M.; Loffing, J.; Subramanya, A.R.; et al. Renal Tubular SGK1 Deficiency Causes Impaired K+ Excretion via Loss of Regulationof NEDD4–2/WNK1 and ENaC. Am. J. Physiol. Renal Physiol. 2016, 311, F330–F342. [Google Scholar] [CrossRef] [PubMed]
- Kuratomi, G.; Komuro, A.; Goto, K.; Shinozaki, M.; Miyazawa, K.; Miyazono, K.; Imamura, T. NEDD4–2 (Neural Precursor Cell Ex-pressed, Developmentally Down-Regulated 4–2) Negatively Regulates Tgf-Beta (Transforming Growth Factor-Beta) Signalling by Inducing Ubiquitin-Mediated Degradation of SMAD2 and Tgf-Beta Type I Receptor. Biochem. J. 2005, 386 Pt 3, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Gagnon, K.B.E. Genome-Wide Analysis of SPAK/OSR1 Binding Motifs. Physiol. Genom. 2007, 28, 223–231. [Google Scholar] [CrossRef]
- Taylor, C.A., IV; Jung, J.; Kankanamalage, S.G.; Li, J.; Grzemska, M.; Jaykumar, A.B.; Earnest, S.; Stippec, S.; Saha, P.; Sauceda, E.; et al. Predictive and Experimental Motif Interaction Analysis Identifies Functions of the WNK-OSR1/SPAK Pathway. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.; Zhang, X.; Xing, G.; Lu, K.; Gu, Y.; He, F.; Zhang, L. Selective Small Molecule Compounds Increase BMP-2 Respon-Siveness by Inhibiting SMURF1-Mediated SMAD1/5 Degradation. Sci. Rep. 2014, 4, 4965. [Google Scholar] [CrossRef]
- Li, L.; Xie, D.; Yu, S.; Ma, M.; Fan, K.; Chen, J.; Xiu, M.; Xie, K.; Li, Y.; Yong, G. WNK1 Interaction with KEAP1 Promotes NRF2 Stabilization to Enhance the Oxidative Stress Response in Hepatocellular Carcinoma. Cancer Res. 2024, 84, 2776–2791. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Y.; Xu, W.; Wu, C.; Zheng, H.; Liu, L.; Dong, L. Enhanced Liquidity of P62 Droplets Mediated by SMURF1 Links NRF2 Activation and Autophagy. Cell Biosci. 2023, 13, 37. [Google Scholar] [CrossRef]
- Lebrin, F.; Deckers, M.; Bertolino, P.; Ten Dijke, P. TGF-Beta Receptor Function in the Endothelium. Cardiovasc. Res. 2005, 65, 599–608. [Google Scholar] [CrossRef]
- Bellomo, C.; Caja, L.; Moustakas, A. Transforming Growth Factor β as Regulator of Cancer Stemness and Metastasis. Br. J. Cancer 2016, 115, 761–769. [Google Scholar] [CrossRef]
- Nakagawa, T.; Li, J.H.; Garcia, G.; Mu, W.; Piek, E.; Böttinger, E.P.; Chen, Y.; Zhu, H.J.; Kang, D.H.; Schreiner, G.F.; et al. TGF-Beta Induces Proangiogenic and Antiangiogenic Factors via Parallel but Distinct Smad Pathways. Kidney Int. 2004, 66, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Wrana, J.L. Signal Transduction by the TGF-Beta Superfamily. Science 2002, 296, 1646–1647. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaykumar, A.B.; Plumber, S.; Binns, D.; Wichaidit, C.; Luby-Phelps, K.; Cobb, M.H. SMURF1/2 Are Novel Regulators of WNK1 Stability. Kinases Phosphatases 2024, 2, 294-305. https://doi.org/10.3390/kinasesphosphatases2030019
Jaykumar AB, Plumber S, Binns D, Wichaidit C, Luby-Phelps K, Cobb MH. SMURF1/2 Are Novel Regulators of WNK1 Stability. Kinases and Phosphatases. 2024; 2(3):294-305. https://doi.org/10.3390/kinasesphosphatases2030019
Chicago/Turabian StyleJaykumar, Ankita B., Sakina Plumber, Derk Binns, Chonlarat Wichaidit, Katherine Luby-Phelps, and Melanie H. Cobb. 2024. "SMURF1/2 Are Novel Regulators of WNK1 Stability" Kinases and Phosphatases 2, no. 3: 294-305. https://doi.org/10.3390/kinasesphosphatases2030019
APA StyleJaykumar, A. B., Plumber, S., Binns, D., Wichaidit, C., Luby-Phelps, K., & Cobb, M. H. (2024). SMURF1/2 Are Novel Regulators of WNK1 Stability. Kinases and Phosphatases, 2(3), 294-305. https://doi.org/10.3390/kinasesphosphatases2030019





