Therapeutic Perspectives on ROCK Inhibition for Cerebral Cavernous Malformations
Abstract
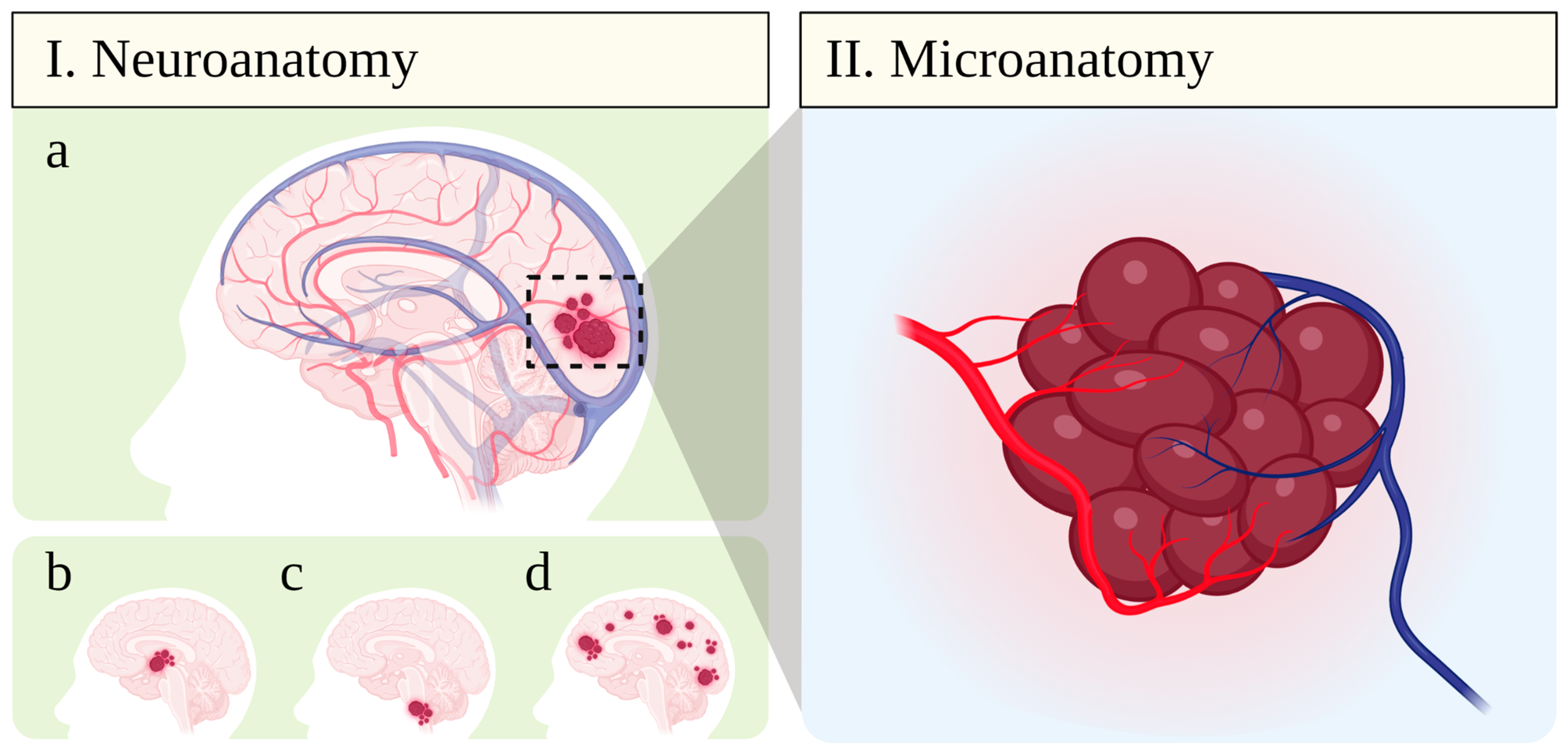
:1. Cerebral Cavernous Malformations (CCM): Epidemiology, Clinical Manifestations, and Current Therapy
2. Pathobiology of the Neurovascular Unit in CCM
3. The Role of Rho-Activated Coiled Coil-Containing Protein Kinases (ROCK) in CCM
3.1. Endothelial Cell and Blood–Brain Barrier Function
3.2. Leukocytes and Inflammation
3.3. Platelets and Thrombosis
3.4. Activated Glia and Epileptogenesis
4. Recent Development of CNS-Targeted ROCK Inhibitors
5. Concluding Remarks
6. Data Collection and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- McCormick, W.F. The Pathology of Vascular (“Arteriovenous”) Malformations. J. Neurosurg. 1966, 24, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, C.J.; Barker, F.G. 2nd Cranial Cavernous Malformations: Natural History and Treatment. Stroke 2018, 49, 1029–1035. [Google Scholar] [CrossRef]
- Fernando, P.M.; Munasinghe, B.M.; Jayamanne, M.D.C.J.P.; Jayasundara, K.A.; Arambepola, W.S.N.W.B.M.A.G.; Pranavan, S.; Ranathunge, N.D. Cerebral cavernous malformation in a child leading to a fatal subarachnoid hemorrhage—“Silent but sinister:” A case report and literature review. Surg. Neurol. Int. 2021, 12, 253. [Google Scholar] [CrossRef]
- Gao, X.; Yue, K.; Sun, J.; Fang, Z.; Cao, Y.; Zhao, B.; Zhang, H.; Dai, S.; Zhang, L.; Luo, P.; et al. A systematic review and meta-analysis of surgeries performed for cerebral cavernous malformation-related epilepsy in pediatric patients. Front. Pediatr. 2022, 10, 892456. [Google Scholar] [CrossRef]
- Flemming, K.D.; Lanzino, G. Cerebral Cavernous Malformation: What a Practicing Clinician Should Know. Mayo Clin. Proc. 2020, 95, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Retta, S.F.; Perrelli, A.; Trabalzini, L.; Finetti, F. From Genes and Mechanisms to Molecular-Targeted Therapies: The Long Climb to the Cure of Cerebral Cavernous Malformation (CCM) Disease. Methods Mol. Biol. 2020, 2152, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Polster, S.P. Cavernous angiomas: Deconstructing a neurosurgical disease. J. Neurosurg. 2019, 131, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paddock, M.; Lanham, S.; Gill, K.; Sinha, S.; Connolly, D.J.A. Pediatric Cerebral Cavernous Malformations. Pediatr. Neurol. 2021, 116, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, I.; Knöpfel, N. Venous Malformations in Childhood: Clinical, Histopathological and Genetics Update. Dermatopathology 2021, 8, 477–493. [Google Scholar] [CrossRef]
- Akers, A.; Al-Shahi Salman, R.; Awad, I.A.; Dahlem, K.; Flemming, K.; Hart, B.; Kim, H.; Jusue-Torres, I.; Kondziolka, D.; Lee, C.; et al. Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery 2017, 80, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, K.D. Incidence, Prevalence, and Clinical Presentation of Cerebral Cavernous Malformations. Methods Mol. Biol. 2020, 2152, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Merlino, L.; Del Prete, F.; Titi, L.; Piccioni, M.G. Cerebral cavernous malformation: Management and outcome during pregnancy and puerperium. A systematic review of literature. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101927. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.K.; Kumar, S.; Brown, R.D.J.; Lanzino, G.; Flemming, K.D. Influence of Pregnancy on Hemorrhage Risk in Women With Cerebral and Spinal Cavernous Malformations. Stroke 2021, 52, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Nelson, J.; Pawlikowska, L.; McCulloch, C.E.; Akers, A.; Baca, B.; Khan, Y.; Hart, B.; Morrison, L.; Kim, H. Association of cardiovascular risk factors with disease severity in cerebral cavernous malformation type 1 subjects with the common Hispanic mutation. Cerebrovasc. Dis. 2014, 37, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Saban, D.; Rauscher, S.; Herten, A.; Rauschenbach, L.; Santos, A.; Li, Y.; Schmidt, B.; Zhu, Y.; Jabbarli, R.; et al. Modifiable Cardiovascular Risk Factors in Patients With Sporadic Cerebral Cavernous Malformations: Obesity Matters. Stroke 2021, 52, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Dammann, P.; Santos, A.N.; Wan, X.-Y.; Zhu, Y.; Sure, U. Cavernous Malformations: Updates in Surgical Management and Biology. Neurosurg. Clin. N. Am. 2022, 33, 449–460. [Google Scholar] [CrossRef]
- Mahajan, U.V.; Patel, M.; Pace, J.; Rothstein, B.D. Presentation and management of nervous system cavernous malformations in children: A systematic review and case report. Brain Circ. 2022, 8, 121–126. [Google Scholar] [CrossRef]
- Kiratli, H.; Koç, I.; Toprak, H.; Yildirim, S.; Söylemezoğlu, F. Recurrence of a Totally Excised Cavernous Venous Malformation 25 Years Later. Ophthal. Plast. Reconstr. Surg. 2021, 37, e59–e60. [Google Scholar] [CrossRef]
- Patet, G.; Bartoli, A.; Meling, T.R. Natural history and treatment options of radiation-induced brain cavernomas: A systematic review. Neurosurg. Rev. 2022, 45, 243–251. [Google Scholar] [CrossRef]
- Hart, B.L.; Mabray, M.C.; Morrison, L.; Whitehead, K.J.; Kim, H. Systemic and CNS manifestations of inherited cerebrovascular malformations. Clin. Imaging 2021, 75, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Fotakopoulos, G.; Kivelev, J.; Andrade-Barazarte, H.; Tjahjadi, M.; Goehre, F.; Hernesniemi, J. Outcome in Patients with Spinal Cavernomas Presenting with Symptoms Due to Mass Effect and/or Hemorrhage: Conservative versus Surgical Management: Meta-analysis of Direct Comparison of Approach-Related Complications. World Neurosurg. 2021, 152, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, G.G.; Cofano, F.; Santonio, F.V.; Vincitorio, F.; Zenga, F.; Garbossa, D. Natural History, Clinical, and Surgical Management of Cavernous Malformations. Methods Mol. Biol. 2020, 2152, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-J.; Liu, P.-P.; Wang, L.; Zhang, L.-W.; Zhang, J.-T.; Li, D.; Wu, Z.; Wu, Y.-M. Natural history of incidentally diagnosed brainstem cavernous malformations in a prospective observational cohort. Neurosurg. Rev. 2021, 44, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Alonso-Vanegas, M.A.; Baumgartner, C.; Blümcke, I.; Carreño, M.; Gizewski, E.R.; Hamer, H.M.; Knake, S.; Kahane, P.; Lüders, H.O.; et al. Cavernoma-related epilepsy: Review and recommendations for management--report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2013, 54, 2025–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.M.; Karahalios, K.; Rumalla, K.; Shlobin, N.A.; Rahmani, R.; Scherschinski, L.; Benner, D.; Catapano, J.S.; Labib, M.A.; Graffeo, C.S.; et al. Giant cerebral cavernous malformations: Redefinition based on surgical outcomes and systematic review of the literature. J. Neurosurg. 2022, 137, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Quadri, S.A.; Farooqui, M.; Ikram, A.; Robinson, M.; Hart, B.L.; Mabray, M.C.; Vigil, C.; Tang, A.T.; Kahn, M.L.; et al. Familial Cerebral Cavernous Malformations. Stroke 2019, 50, 1294–1301. [Google Scholar] [CrossRef]
- Kapadia, M.; Walwema, M.; Smith, T.R.; Bellinski, I.; Batjer, H.; Getch, C.; Rosenow, J.M.; Bendok, B.R.; Schuele, S.U. Seizure outcome in patients with cavernous malformation after early surgery. Epilepsy Behav. 2021, 115, 107662. [Google Scholar] [CrossRef]
- Gao, X.; Yue, K.; Sun, J.; Cao, Y.; Zhao, B.; Zhang, H.; Dai, S.; Zhang, L.; Luo, P.; Jiang, X. Treatment of Cerebral Cavernous Malformations Presenting With Seizures: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 590589. [Google Scholar] [CrossRef]
- Hoffman, J.E.; Wittenberg, B.; Morel, B.; Folzenlogen, Z.; Case, D.; Roark, C.; Youssef, S.; Seinfeld, J. Tailored Treatment Options for Cerebral Cavernous Malformations. J. Pers. Med. 2022, 12, 831. [Google Scholar] [CrossRef]
- Chohan, M.O.; Marchiò, S.; Morrison, L.A.; Sidman, R.L.; Cavenee, W.K.; Dejana, E.; Yonas, H.; Pasqualini, R.; Arap, W. Emerging Pharmacologic Targets in Cerebral Cavernous Malformation and Potential Strategies to Alter the Natural History of a Difficult Disease: A Review. JAMA Neurol. 2019, 76, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Shanker, M.D.; Webber, R.; Pinkham, M.B.; Huo, M.; Olson, S.; Hall, B.; Jayalath, R.; Watkins, T.; Foote, M.C. Gamma Knife® stereotactic radiosurgery for intracranial cavernous malformations. J. Clin. Neurosci. 2022, 106, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, O.; Sabahi, M.; Malcolm, J.; Adada, B.; Borghei-Razavi, H. Laser Interstitial Thermal Therapy for Cavernous Malformations: A Systematic Review. Front. Surg. 2022, 9, 887329. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yue, K.; Sun, J.; Cao, Y.; Zhao, B.; Zhang, H.; Dai, S.; Zhang, L.; Luo, P.; Jiang, X. Microsurgery vs. Gamma Knife Radiosurgery for the Treatment of Brainstem Cavernous Malformations: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 600461. [Google Scholar] [CrossRef]
- Cox, E.M.; Bambakidis, N.C.; Cohen, M.L. Pathology of cavernous malformations. Handb. Clin. Neurol. 2017, 143, 267–277. [Google Scholar] [CrossRef]
- Phillips, C.M.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Cerebral Cavernous Malformation Pathogenesis: Investigating Lesion Formation and Progression with Animal Models. Int. J. Mol. Sci. 2022, 23, 5000. [Google Scholar] [CrossRef]
- Marotta, D.; Hendricks, B.K.; Zaher, M.; Watanabe, G.; Grasso, G.; Cohen-Gadol, A. Resection of Brainstem Cavernous Malformations: Pearls and Pitfalls for Minimizing Complications. World Neurosurg. 2022, 159, 390–401. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Sure, U.; Freman, S.; Bozinov, O.; Benes, L.; Siegel, A.M.; Bertalanffy, H. Biological activity of adult cavernous malformations: A study of 56 patients. J. Neurosurg. 2005, 102, 342–347. [Google Scholar] [CrossRef]
- DiStefano, P.V.; Glading, A.J. VEGF signalling enhances lesion burden in KRIT1 deficient mice. J. Cell. Mol. Med. 2020, 24, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Finetti, F.; Trabalzini, L. Bidimentional In Vitro Angiogenic Assays to Study CCM Pathogenesis: Endothelial Cell Proliferation and Migration. Methods Mol. Biol. 2020, 2152, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Hojo, M.; Tanigaki, K.; Miyamoto, S. Contribution of Endothelial-to-Mesenchymal Transition to the Pathogenesis of Human Cerebral and Orbital Cavernous Malformations. Neurosurgery 2017, 81, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravi, L.; Malinverno, M.; Pisati, F.; Rudini, N.; Cuttano, R.; Pallini, R.; Martini, M.; Larocca, L.M.; Locatelli, M.; Levi, V.; et al. Endothelial Cells Lining Sporadic Cerebral Cavernous Malformation Cavernomas Undergo Endothelial-to-Mesenchymal Transition. Stroke 2016, 47, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Riolo, G.; Ricci, C.; Battistini, S. Molecular Genetic Features of Cerebral Cavernous Malformations (CCM) Patients: An Overall View from Genes to Endothelial Cells. Cells 2021, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Snellings, D.A.; Hong, C.C.; Ren, A.A.; Lopez-Ramirez, M.A.; Girard, R.; Srinath, A.; Marchuk, D.A.; Ginsberg, M.H.; Awad, I.A.; Kahn, M.L. Cerebral Cavernous Malformation: From Mechanism to Therapy. Circ. Res. 2021, 129, 195–215. [Google Scholar] [CrossRef]
- Abdelilah-Seyfried, S.; Tournier-Lasserve, E.; Derry, W.B. Blocking Signalopathic Events to Treat Cerebral Cavernous Malformations. Trends Mol. Med. 2020, 26, 874–887. [Google Scholar] [CrossRef]
- Tu, T.; Peng, Z.; Ren, J.; Zhang, H. Cerebral Cavernous Malformation: Immune and Inflammatory Perspectives. Front. Immunol. 2022, 13, 922281. [Google Scholar] [CrossRef]
- Florian, I.A.; Buruiana, A.; Timis, T.L.; Susman, S.; Florian, I.S.; Balasa, A.; Berindan-Neagoe, I. An Insight into the microRNAs Associated with Arteriovenous and Cavernous Malformations of the Brain. Cells 2021, 10, 1373. [Google Scholar] [CrossRef]
- Subhash, S.; Kalmbach, N.; Wegner, F.; Petri, S.; Glomb, T.; Dittrich-Breiholz, O.; Huang, C.; Bali, K.K.; Kunz, W.S.; Samii, A.; et al. Transcriptome-wide Profiling of Cerebral Cavernous Malformations Patients Reveal Important Long noncoding RNA molecular signatures. Sci. Rep. 2019, 9, 18203. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Bali, K.K.; Baisantry, A.; Geffers, R.; Hartmann, C.; Samii, A.; Bertalanffy, H. Genome-Wide Sequencing Reveals Small Nucleolar RNAs Downregulated in Cerebral Cavernous Malformations. Cell. Mol. Neurobiol. 2018, 38, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Ferraris, C.; Berni, E.; Glading, A.J.; Retta, S.F. KRIT1: A Traffic Warden at the Busy Crossroads Between Redox Signaling and the Pathogenesis of Cerebral Cavernous Malformation Disease. Antioxid. Redox Signal. 2022. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Goitre, L.; Salzano, A.M.; Moglia, A.; Scaloni, A.; Retta, S.F. Biological Activities, Health Benefits, and Therapeutic Properties of Avenanthramides: From Skin Protection to Prevention and Treatment of Cerebrovascular Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 6015351. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Perrelli, A.; Armeni, T.; Nicola Talesa, V.; Retta, S.F. Dicarbonyl Stress and S-Glutathionylation in Cerebrovascular Diseases: A Focus on Cerebral Cavernous Malformations. Antioxidants 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Globisch, M.A.; Onyeogaziri, F.C.; Smith, R.O.; Arce, M.; Magnusson, P.U. Dysregulated Hemostasis and Immunothrombosis in Cerebral Cavernous Malformations. Int. J. Mol. Sci. 2022, 23, 12575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X. The Roles of TGF-β Signaling in Cerebrovascular Diseases. Front. Cell Dev. Biol. 2020, 8, 567682. [Google Scholar] [CrossRef] [PubMed]
- Previch, L.; Lanzino, G.; Brown, R.D.J.; Flemming, K.D. The Influence of Select Medications on Prospective Hemorrhage Risk in Patients with Spinal or Cerebral Cavernous Malformations. World Neurosurg. 2022, 163, e678–e683. [Google Scholar] [CrossRef]
- Snellings, D.A.; Girard, R.; Lightle, R.; Srinath, A.; Romanos, S.; Li, Y.; Chen, C.; Ren, A.A.; Kahn, M.L.; Awad, I.A.; et al. Developmental venous anomalies are a genetic primer for cerebral cavernous malformations. Nat. Cardiovasc. Res. 2022, 1, 246–252. [Google Scholar] [CrossRef]
- Peyre, M.; Miyagishima, D.; Bielle, F.; Chapon, F.; Sierant, M.; Venot, Q.; Lerond, J.; Marijon, P.; Abi-Jaoude, S.; Le Van, T.; et al. Somatic PIK3CA Mutations in Sporadic Cerebral Cavernous Malformations. N. Engl. J. Med. 2021, 385, 996–1004. [Google Scholar] [CrossRef]
- Ren, A.A.; Snellings, D.A.; Su, Y.S.; Hong, C.C.; Castro, M.; Tang, A.T.; Detter, M.R.; Hobson, N.; Girard, R.; Romanos, S.; et al. PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature 2021, 594, 271–276. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, A.T.; Wong, W.-Y.; Bamezai, S.; Goddard, L.M.; Shenkar, R.; Zhou, S.; Yang, J.; Wright, A.C.; Foley, M.; et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature 2016, 532, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, V.L.; Calderwood, D.A. Signalling through cerebral cavernous malformation protein networks. Open Biol. 2020, 10, 200263. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.A.; Shi, C.; Shenkar, R.; Gallione, C.J.; Akers, A.L.; Li, S.; De Castro, N.; Berg, M.J.; Corcoran, D.L.; Awad, I.A.; et al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: Evidence for a common biochemical pathway for CCM pathogenesis. Hum. Mol. Genet. 2014, 23, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, H.A.; Girard, R.; Saadat, L.; Shen, L.; Lightle, R.; Moore, T.; Cao, Y.; Hobson, N.; Shenkar, R.; Avner, K.; et al. Phenotypic characterization of murine models of cerebral cavernous malformations. Lab. Investig. 2019, 99, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Polster, S.P.; Stadnik, A.; Akers, A.L.; Cao, Y.; Christoforidis, G.A.; Fam, M.D.; Flemming, K.D.; Girard, R.; Hobson, N.; Koenig, J.I.; et al. Atorvastatin Treatment of Cavernous Angiomas with Symptomatic Hemorrhage Exploratory Proof of Concept (AT CASH EPOC) Trial. Neurosurgery 2019, 85, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Eisa-Beygi, S.; Hatch, G.; Noble, S.; Ekker, M.; Moon, T.W. The 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) pathway regulates developmental cerebral-vascular stability via prenylation-dependent signalling pathway. Dev. Biol. 2013, 373, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Westover, M.B.; Bianchi, M.T.; Eckman, M.H.; Greenberg, S.M. Statin Use Following Intracerebral Hemorrhage. Arch. Neurol. 2011, 68, 573–579. [Google Scholar] [CrossRef]
- Mabray, M.C.; Caprihan, A.; Nelson, J.; McCulloch, C.E.; Zafar, A.; Kim, H.; Hart, B.L.; Morrison, L. Effect of Simvastatin on Permeability in Cerebral Cavernous Malformation Type 1 Patients: Results from a Pilot Small Randomized Controlled Clinical Trial. Transl. Stroke Res. 2020, 11, 319–321. [Google Scholar] [CrossRef]
- Eisa-Beygi, S.; Wen, X.-Y.; Macdonald, R.L. A call for rigorous study of statins in resolution of cerebral cavernous malformation pathology. Stroke 2014, 45, 1859–1861. [Google Scholar] [CrossRef] [Green Version]
- Barlow, H.R.; Cleaver, O. Building Blood Vessels-One Rho GTPase at a Time. Cells 2019, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- Lampugnani, M.G.; Dejana, E.; Giampietro, C. Vascular Endothelial (VE)-Cadherin, Endothelial Adherens Junctions, and Vascular Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029322. [Google Scholar] [CrossRef] [PubMed]
- Shenkar, R.; Peiper, A.; Pardo, H.; Moore, T.; Lightle, R.; Girard, R.; Hobson, N.; Polster, S.P.; Koskimaki, J.; Zhang, D.; et al. Rho Kinase Inhibition Blunts Lesion Development and Hemorrhage in Murine Models of Aggressive Pdcd10/Ccm3 Disease. Stroke 2019, 50, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Shenkar, R.; Shi, C.; Austin, C.; Moore, T.; Lightle, R.; Cao, Y.; Zhang, L.; Wu, M.; Zeineddine, H.A.; Girard, R.; et al. RhoA Kinase Inhibition With Fasudil Versus Simvastatin in Murine Models of Cerebral Cavernous Malformations. Stroke 2017, 48, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef]
- Leung, T.; Manser, E.; Tan, L.; Lim, L. A Novel Serine/Threonine Kinase Binding the Ras-related RhoA GTPase Which Translocates the Kinase to Peripheral Membranes. J. Biol. Chem. 1995, 270, 29051–29054. [Google Scholar] [CrossRef] [Green Version]
- Hajdú, I.; Szilágyi, A.; Végh, B.M.; Wacha, A.; Györffy, D.; Gráczer, É.; Somogyi, M.; Gál, P.; Závodszky, P. Ligand-induced conformational rearrangements regulate the switch between membrane-proximal and distal functions of Rho kinase 2. Commun. Biol. 2020, 3, 721. [Google Scholar] [CrossRef]
- Feng, J.; Ito, M.; Kureishi, Y.; Ichikawa, K.; Amano, M.; Isaka, N.; Okawa, K.; Iwamatsu, A.; Kaibuchi, K.; Hartshorne, D.J.; et al. Rho-associated Kinase of Chicken Gizzard Smooth Muscle. J. Biol. Chem. 1999, 274, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.; Hayakawa, K.; Swenson, L.; Bellon, S.; Fleming, M.; Taslimi, P.; Doran, J. The Structure of Dimeric ROCK I Reveals the Mechanism for Ligand Selectivity. J. Biol. Chem. 2006, 281, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Doran, J.D.; Liu, X.; Taslimi, P.; Saadat, A.; Fox, T. New insights into the structure–function relationships of Rho-associated kinase: A thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem. J. 2004, 384, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Sladojevic, N.; Blair, J.E.; Liao, J.K. Targeting Rho-associated coiled-coil forming protein kinase (ROCK) in cardiovascular fibrosis and stiffening. Expert Opin. Ther. Targets 2020, 24, 47–62. [Google Scholar] [CrossRef]
- Amin, F.; Ahmed, A.; Feroz, A.; Khaki, P.S.S.; Khan, M.S.; Tabrez, S.; Zaidi, S.K.; Abdulaal, W.H.; Shamsi, A.; Khan, W.; et al. An Update on the Association of Protein Kinases with Cardiovascular Diseases. Curr. Pharm. Des. 2019, 25, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, L.; Wei, L. Rho-Kinase in Development and Heart Failure: Insights From Genetic Models. Pediatr. Cardiol. 2011, 32, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisowska, J.; Rodel, C.J.; Manet, S.; Miroshnikova, Y.A.; Boyault, C.; Planus, E.; De Mets, R.; Lee, H.-H.; Destaing, O.; Mertani, H.; et al. The CCM1-CCM2 complex controls complementary functions of ROCK1 and ROCK2 that are required for endothelial integrity. J. Cell Sci. 2018, 131, jcs216093. [Google Scholar] [CrossRef] [Green Version]
- Vannier, D.R.; Shapeti, A.; Chuffart, F.; Planus, E.; Manet, S.; Rivier, P.; Destaing, O.; Albiges-Rizo, C.; Van Oosterwyck, H.; Faurobert, E. CCM2-deficient endothelial cells undergo a ROCK-dependent reprogramming into senescence-associated secretory phenotype. Angiogenesis 2021, 24, 843–860. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.A.; Shi, C.; Shenkar, R.; Stockton, R.A.; Liu, F.; Ginsberg, M.H.; Marchuk, D.A.; Awad, I.A. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke 2012, 43, 571–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.-A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef] [Green Version]
- McKerracher, L.; Shenkar, R.; Abbinanti, M.; Cao, Y.; Peiper, A.; Liao, J.K.; Lightle, R.; Moore, T.; Hobson, N.; Gallione, C.; et al. A Brain-Targeted Orally Available ROCK2 Inhibitor Benefits Mild and Aggressive Cavernous Angioma Disease. Transl. Stroke Res. 2020, 11, 365–376. [Google Scholar] [CrossRef]
- Stockton, R.A.; Shenkar, R.; Awad, I.A.; Ginsberg, M.H. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J. Exp. Med. 2010, 207, 881–896. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Li, Y.; Polster, S.P.; Weber, C.R.; Awad, I.A.; Shen, L. Cerebral Cavernous Malformation Proteins in Barrier Maintenance and Regulation. Int. J. Mol. Sci. 2020, 21, 675. [Google Scholar] [CrossRef] [Green Version]
- Padarti, A.; Zhang, J. Recent advances in cerebral cavernous malformation research. Vessel Plus 2018, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, S.; Lin, J.; Shenkar, R.; Moore, T.; Lightle, R.; Faurobert, E.; Lopez-Ramirez, M.-A.; Awad, I.; Ginsberg, M.H. β1 integrin monoclonal antibody treatment ameliorates cerebral cavernous malformations. FASEB J. 2022, 36, e22629. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, N.; Furmston, J.; Ridley, A.J. The STRIPAK complex components FAM40A and FAM40B regulate endothelial cell contractility via ROCKs. BMC Cell Biol. 2018, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, H.J.; Wang, M. CCM3 and cerebral cavernous malformation disease. Stroke Vasc. Neurol. 2019, 4, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Swamy, H.; Glading, A.J. Is Location Everything? Regulation of the Endothelial CCM Signaling Complex. Front. Cardiovasc. Med. 2022, 9, 954780. [Google Scholar] [CrossRef]
- Kryczka, J.; Przygodzka, P.; Bogusz, H.; Boncela, J. HMEC-1 adopt the mixed amoeboid-mesenchymal migration type during EndMT. Eur. J. Cell Biol. 2017, 96, 289–300. [Google Scholar] [CrossRef]
- Detter, M.R.; Snellings, D.A.; Marchuk, D.A. Cerebral Cavernous Malformations Develop Through Clonal Expansion of Mutant Endothelial Cells. Circ. Res. 2018, 123, 1143–1151. [Google Scholar] [CrossRef]
- Malinverno, M.; Maderna, C.; Abu Taha, A.; Corada, M.; Orsenigo, F.; Valentino, M.; Pisati, F.; Fusco, C.; Graziano, P.; Giannotta, M.; et al. Endothelial cell clonal expansion in the development of cerebral cavernous malformations. Nat. Commun. 2019, 10, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detter, M.R.; Shenkar, R.; Benavides, C.R.; Neilson, C.A.; Moore, T.; Lightle, R.; Hobson, N.; Shen, L.; Cao, Y.; Girard, R.; et al. Novel Murine Models of Cerebral Cavernous Malformations. Angiogenesis 2020, 23, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Tanumihardja, C.; Kipnis, J. Cerebrovascular Anomalies: Perspectives From Immunology and Cerebrospinal Fluid Flow. Circ. Res. 2021, 129, 174–194. [Google Scholar] [CrossRef]
- Castro, C.; Oyamada, H.A.A.; Cafasso, M.O.S.D.; Lopes, L.M.; Monteiro, C.; Sacramento, P.M.; Alves-Leon, S.V.; da Fontoura Galvão, G.; Hygino, J.; de Souza, J.P.B.M.; et al. Elevated proportion of TLR2- and TLR4-expressing Th17-like cells and activated memory B cells was associated with clinical activity of cerebral cavernous malformations. J. Neuroinflammation 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zou, L.; Wang, H.; He, R.; Liu, K.; Zhu, H. RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability. Molecules 2018, 23, 2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhang, W.; Kang, P.; Zheng, X.; He, K.; Bai, H.; Yu, X. Midazolam Ameliorates Impairment of the Blood–Brain Barrier (BBB) Against LPS. Neurotox. Res. 2022, 40, 751–762. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Coleman, P.; Chen, J.; Ting, K.K.; Choi, J.P.; Zheng, X.; Vadas, M.A.; Gamble, J.R. Low fluid shear stress conditions contribute to activation of cerebral cavernous malformation signalling pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165519. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, L.; Ding, G.; Chen, L.; Jiang, L.; Wang, J.; Wu, J. Oxygen-Glucose Deprivation/Reoxygenation Induces Human Brain Microvascular Endothelial Cell Hyperpermeability Via VE-Cadherin Internalization: Roles of RhoA/ROCK2. J. Mol. Neurosci. 2019, 69, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.L.; Srivastava, K.; Sprigg, N.; Bath, P.M.W.; Bayraktutan, U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J. Neurochem. 2014, 129, 816–826. [Google Scholar] [CrossRef] [PubMed]
- QIAO, F.; ZOU, Z.; LIU, C.; ZHU, X.; WANG, X.; YANG, C.; JIANG, T.; CHEN, Y. ROCK2 mediates the proliferation of pulmonary arterial endothelial cells induced by hypoxia in the development of pulmonary arterial hypertension. Exp. Ther. Med. 2016, 11, 2567–2572. [Google Scholar] [CrossRef] [Green Version]
- Heemskerk, N.; Schimmel, L.; Oort, C.; van Rijssel, J.; Yin, T.; Ma, B.; van Unen, J.; Pitter, B.; Huveneers, S.; Goedhart, J.; et al. F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling. Nat. Commun. 2016, 7, 10493. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, H.; Shu, R.; Zhang, X.; Yu, Y.; Liu, X.; Xu, K. Hydrogen treatment prevents lipopolysaccharide-induced pulmonary endothelial cell dysfunction through RhoA inhibition. Biochem. Biophys. Res. Commun. 2020, 522, 499–505. [Google Scholar] [CrossRef]
- García Ponce, A.; Citalán Madrid, A.F.; Vargas Robles, H.; Chánez Paredes, S.; Nava, P.; Betanzos, A.; Zarbock, A.; Rottner, K.; Vestweber, D.; Schnoor, M. Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Sci. Rep. 2016, 6, 29003. [Google Scholar] [CrossRef] [Green Version]
- Girard, R.; Zeineddine, H.A.; Koskimäki, J.; Fam, M.D.; Cao, Y.; Shi, C.; Moore, T.; Lightle, R.; Stadnik, A.; Chaudagar, K.; et al. Plasma Biomarkers of Inflammation and Angiogenesis Predict Cerebral Cavernous Malformation Symptomatic Hemorrhage or Lesional Growth. Circ. Res. 2018, 122, 1716–1721. [Google Scholar] [CrossRef]
- Girard, R.; Zeineddine, H.A.; Fam, M.D.; Mayampurath, A.; Cao, Y.; Shi, C.; Shenkar, R.; Polster, S.P.; Jesselson, M.; Duggan, R.; et al. Plasma Biomarkers of Inflammation Reflect Seizures and Hemorrhagic Activity of Cerebral Cavernous Malformations. Transl. Stroke Res. 2018, 9, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Chehuen Bicalho, V.; da Fontoura Galvão, G.; Lima Fontes-Dantas, F.; Paulo da Costa Gonçalves, J.; Dutra de Araujo, A.; Carolina França, L.; Emílio Corrêa Leite, P.; Campolina Vidal, D.; Castro Filho, R.; Vieira Alves-Leon, S.; et al. Asymptomatic cerebral cavernous angiomas associated with plasma marker signature. J. Clin. Neurosci. 2021, 89, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Nelsen, B.; Frias-Anaya, E.; Gallego-Gutierrez, H.; Orecchioni, M.; Herrera, V.; Ortiz, E.; Sun, H.; Mesarwi, O.A.; Ley, K.; et al. Neuroinflammation Plays a Critical Role in Cerebral Cavernous Malformation Disease. Circ. Res. 2022, 131, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Shenkar, R.; Zeineddine, H.A.; Girard, R.; Fam, M.D.; Austin, C.; Moore, T.; Lightle, R.; Zhang, L.; Wu, M.; et al. B-Cell Depletion Reduces the Maturation of Cerebral Cavernous Malformations in Murine Models. J. Neuroimmune Pharmacol. 2016, 11, 369–377. [Google Scholar] [CrossRef]
- Shi, C.; Shenkar, R.; Du, H.; Duckworth, E.; Raja, H.; Batjer, H.H.; Awad, I.A. Immune response in human cerebral cavernous malformations. Stroke 2009, 40, 1659–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernis, A.B.; Ricker, E.; Weng, C.-H.; Rozo, C.; Yi, W. Rho Kinases in Autoimmune Diseases. Annu. Rev. Med. 2016, 67, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Bros; Haas; Moll; Grabbe RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [CrossRef] [Green Version]
- Niermann, C.; Gorressen, S.; Klier, M.; Gowert, N.S.; Billuart, P.; Kelm, M.; Merx, M.W.; Elvers, M. Oligophrenin1 protects mice against myocardial ischemia and reperfusion injury by modulating inflammation and myocardial apoptosis. Cell. Signal. 2016, 28, 967–978. [Google Scholar] [CrossRef]
- Azreq, M.-A.; El Kadiri, M.; Boisvert, M.; Pagé, N.; Tessier, P.A.; Aoudjit, F. Discoidin domain receptor 1 promotes Th17 cell migration by activating the RhoA/ROCK/MAPK/ERK signaling pathway. Oncotarget 2016, 7, 44975–44990. [Google Scholar] [CrossRef] [Green Version]
- Biro, M.; Munoz, M.A.; Weninger, W. Targeting Rho-GTP ases in immune cell migration and inflammation. Br. J. Pharmacol. 2014, 171, 5491–5506. [Google Scholar] [CrossRef] [PubMed]
- Zanin-Zhorov, A.; Flynn, R.; Waksal, S.D.; Blazar, B.R. Isoform-specific targeting of ROCK proteins in immune cells. Small GTPases 2016, 7, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, Y.; Matoba, K.; Kawanami, D.; Nagai, Y.; Akamine, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Utsunomiya, K. ROCK2 Regulates Monocyte Migration and Cell to Cell Adhesion in Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Dai, W.; Lin, Y.; Zhang, Z.; Pan, Y.; Han, H.; Jia, H.; Peng, J.; Zhao, J.; Xu, L. Leukocyte Rho kinase activity and serum cystatin C affect cardiovascular events in acute coronary syndrome. Medicine 2020, 99, e20060. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Moya, J.; Jalil, J.E.; Lavandero, S.; Kalergis, A.M.; Molina, C.; Gabrielli, L.; Godoy, I.; Córdova, S.; Castro, P.; et al. Rho-kinase pathway activation and apoptosis in circulating leucocytes in patients with heart failure with reduced ejection fraction. J. Cell. Mol. Med. 2020, 24, 1413–1427. [Google Scholar] [CrossRef]
- Ricker, E.; Chowdhury, L.; Yi, W.; Pernis, A.B. The RhoA-ROCK pathway in the regulation of T and B cell responses. F1000Research 2016, 5, 2295. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Nagpal, K.; Suárez-Fueyo, A.; Ferretti, A.; Yoshida, N.; Tsokos, M.G.; Tsokos, G.C. The Regulatory Subunit PPP2R2A of PP2A Enhances Th1 and Th17 Differentiation through Activation of the GEF-H1/RhoA/ROCK Signaling Pathway. J. Immunol. 2021, 206, 1719–1728. [Google Scholar] [CrossRef]
- Chen, W.; Nyuydzefe, M.S.; Weiss, J.M.; Zhang, J.; Waksal, S.D.; Zanin-Zhorov, A. ROCK2, but not ROCK1 interacts with phosphorylated STAT3 and co-occupies TH17/TFH gene promoters in TH17-activated human T cells. Sci. Rep. 2018, 8, 16636. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, P.; Tang, D.; Song, R.; Zhang, Y.; Lei, S.; Wu, S. Targeting the RhoA-ROCK pathway to regulate T-cell homeostasis in hypoxia-induced pulmonary arterial hypertension. Pulm. Pharmacol. Ther. 2018, 50, 111–122. [Google Scholar] [CrossRef]
- Dai, K.; Wang, Y.; Tai, S.; Ni, H.; Lian, H.; Yu, Y.; Liao, W.; Zheng, C.; Chen, Q.; Kuver, A.; et al. Fasudil exerts a cardio-protective effect on mice with coxsackievirus B3-induced acute viral myocarditis. Cardiovasc. Ther. 2018, 36, e12477. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Dai, F.; Tang, L.; Le, Y.; Yao, W. Macrophage differentiation induced by PMA is mediated by activation of RhoA/ROCK signaling. J. Toxicol. Sci. 2017, 42, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saclier, M.; Lapi, M.; Bonfanti, C.; Rossi, G.; Antonini, S.; Messina, G. The Transcription Factor Nfix Requires RhoA-ROCK1 Dependent Phagocytosis to Mediate Macrophage Skewing during Skeletal Muscle Regeneration. Cells 2020, 9, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Zhou, H.; Wang, Q.; Zhou, S.; Li, C.; Liu, R.; Qiu, J.; Shi, C.; Lu, L. RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1–TLR4–NF-κB pathway in macrophages. FASEB J. 2019, 33, 11180–11193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.; Harris, G.J.; Pinder, E.M.; Macfarlane, J.G.; Hellyer, T.P.; Rostron, A.J.; Conway Morris, A.; Thickett, D.R.; Perkins, G.D.; McAuley, D.F.; et al. Exchange protein directly activated by cyclic AMP (EPAC) activation reverses neutrophil dysfunction induced by β2-agonists, corticosteroids, and critical illness. J. Allergy Clin. Immunol. 2016, 137, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filina, J.V.; Gabdoulkhakova, A.G.; Safronova, V.G. RhoA/ROCK downregulates FPR2-mediated NADPH oxidase activation in mouse bone marrow granulocytes. Cell. Signal. 2014, 26, 2138–2146. [Google Scholar] [CrossRef]
- Nam, G.-H.; Lee, E.J.; Kim, Y.K.; Hong, Y.; Choi, Y.; Ryu, M.-J.; Woo, J.; Cho, Y.; Ahn, D.J.; Yang, Y.; et al. Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer. Nat. Commun. 2018, 9, 2165. [Google Scholar] [CrossRef] [Green Version]
- Silveira, A.A.A.; Dominical, V.M.; Lazarini, M.; Costa, F.F.; Conran, N. Simvastatin abrogates inflamed neutrophil adhesive properties, in association with the inhibition of Mac-1 integrin expression and modulation of Rho kinase activity. Inflamm. Res. 2013, 62, 127–132. [Google Scholar] [CrossRef]
- Li, M.; Lyu, X.; Liao, J.; Werth, V.P.; Liu, M.-L. Rho Kinase regulates neutrophil NET formation that is involved in UVB-induced skin inflammation. Theranostics 2022, 12, 2133–2149. [Google Scholar] [CrossRef]
- Schneble, H.-M.; Soumare, A.; Hervé, D.; Bresson, D.; Guichard, J.-P.; Riant, F.; Tournier-Lasserve, E.; Tzourio, C.; Chabriat, H.; Stapf, C. Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations. Stroke 2012, 43, 3196–3199. [Google Scholar] [CrossRef] [Green Version]
- Zuurbier, S.M.; Hickman, C.R.; Tolias, C.S.; Rinkel, L.A.; Leyrer, R.; Flemming, K.D.; Bervini, D.; Lanzino, G.; Wityk, R.J.; Schneble, H.-M.H.-M.; et al. Long-term antithrombotic therapy and risk of intracranial haemorrhage from cerebral cavernous malformations: A population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2019, 18, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Akbar, H.; Duan, X.; Saleem, S.; Davis, A.K.; Zheng, Y. RhoA and Rac1 GTPases Differentially Regulate Agonist-Receptor Mediated Reactive Oxygen Species Generation in Platelets. PLoS ONE 2016, 11, e0163227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, P.K.; Han, J.-S.; Jee, Y.; Lee, S.-H.; Kim, S. Pyk2 downstream of G12/13 pathways regulates platelet shape change through RhoA/p160ROCK. Biochem. Biophys. Res. Commun. 2020, 526, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Feghhi, S.; Tooley, W.W.; Sniadecki, N.J. Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase. J. Biomech. Eng. 2016, 138, 104506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sladojevic, N.; Oh, G.T.; Kim, H.-H.; Beaulieu, L.M.; Falet, H.; Kamiński, K.; Freedman, J.E.; Liao, J.K. Decreased thromboembolic stroke but not atherosclerosis or vascular remodelling in mice with ROCK2-deficient platelets. Cardiovasc. Res. 2017, 113, 1307–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, H.; Hiraishi, T.; Itoh, Y.; Oishi, M.; Fujii, Y.; Fukuda, M.; Kakita, A. Reactive astrocytes contribute to epileptogenesis in patients with cavernous angioma. Epilepsy Res. 2021, 176, 106732. [Google Scholar] [CrossRef]
- Martín-Cámara, O.; Cores, Á.; López-Alvarado, P.; Menéndez, J.C. Emerging targets in drug discovery against neurodegenerative diseases: Control of synapsis disfunction by the RhoA/ROCK pathway. Eur. J. Med. Chem. 2021, 225, 113742. [Google Scholar] [CrossRef]
- Song, X.; He, R.; Han, W.; Li, T.; Xie, L.; Cheng, L.; Chen, H.; Xie, M.; Jiang, L. Protective effects of the ROCK inhibitor fasudil against cognitive dysfunction following status epilepticus in male rats. J. Neurosci. Res. 2019, 97, 506–519. [Google Scholar] [CrossRef]
- Çarçak, N.; Yavuz, M.; Eryiğit Karamahmutoğlu, T.; Kurt, A.H.; Urhan Küçük, M.; Onat, F.Y.; Büyükafsar, K. Suppressive effect of Rho-kinase inhibitors Y-27632 and fasudil on spike-and-wave discharges in genetic absence epilepsy rats from Strasbourg (GAERS). Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 1275–1283. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Qu, X.-P.; Jin, J.; Wang, C.; Jiang, X.; Gao, L.; Li, G.; Wang, D.; Shen, L.; et al. Increased expression of Rho-associated protein kinase 2 confers astroglial Stat3 pathway activation during epileptogenesis. Neurosci. Res. 2022, 177, 25–37. [Google Scholar] [CrossRef]
- Piao, C.; Ranaivo, H.R.; Rusie, A.; Wadhwani, N.; Koh, S.; Wainwright, M.S. Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway. Exp. Neurol. 2015, 273, 288–300. [Google Scholar] [CrossRef]
- Xiang, Y.; Niu, Y.; Xie, Y.; Chen, S.; Zhu, F.; Shen, W.; Zeng, L. Inhibition of RhoA/Rho kinase signaling pathway by fasudil protects against kainic acid-induced neurite injury. Brain Behav. 2021, 11, e2266. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liao, X.; Wu, L.; Huang, J.; Li, Z.; Li, Y.; Guo, F. FOXO4 alleviates hippocampal neuronal damage in epileptic mice via the miR-138-5p/ROCK2 axis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2022, 189, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Thumkeo, D. Rho signaling research: History, current status and future directions. FEBS Lett. 2018, 592, 1763–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Song, L.-J.; Ding, Z.-B.; Chai, Z.; Yu, J.-Z.; Xiao, B.-G.; Ma, C.-G. Advantages of Rho-associated kinases and their inhibitor fasudil for the treatment of neurodegenerative diseases. Neural Regen. Res. 2022, 17, 2623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The Blood–Brain Barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. [Google Scholar] [CrossRef] [PubMed]
- Rosen, K.M.; Abbinanti, M.D.; Ruschel, J.; Mckerracher, L.; Moritz, L.B. Rho Kinase Inhibitor BA-1049 (R) and Active Metabolites Thereof. U.S. Patent Application US2018/0297982, 18 October 2018. [Google Scholar]
- Lampe, J.W.; Navratil, T.; Peterson, W.M.; Boyer, J.L.; Fulcher, E.H.; Sorensen, S.D. Method for Treating Cardiovascular Diseases Using Rho Kinase Inhibitor Compounds. U.S. Patent 8,968, 14 January 2010. [Google Scholar]
- Takase, S.; Liao, J.; Liu, Y.; Tanaka, R.; Miyagawa, Y.; Sawahata, M.; Sobue, A.; Mizoguchi, H.; Nagai, T.; Kaibuchi, K.; et al. Antipsychotic-like effects of fasudil, a Rho-kinase inhibitor, in a pharmacologic animal model of schizophrenia. Eur. J. Pharmacol. 2022, 931, 175207. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, N. Fasudil hydrochloride ameliorates memory deficits in rat model of streptozotocin-induced Alzheimer’s disease: Involvement of PI3-kinase, eNOS and NFκB. Behav. Brain Res. 2018, 351, 4–16. [Google Scholar] [CrossRef]
- Song, Y.; Chen, X.; Wang, L.-Y.; Gao, W.; Zhu, M.-J. Rho Kinase Inhibitor Fasudil Protects against β -Amyloid-Induced Hippocampal Neurodegeneration in Rats. CNS Neurosci. Ther. 2013, 19, 603–610. [Google Scholar] [CrossRef]
- Takata, M.; Tanaka, H.; Kimura, M.; Nagahara, Y.; Tanaka, K.; Kawasaki, K.; Seto, M.; Tsuruma, K.; Shimazawa, M.; Hara, H. Fasudil, a rho kinase inhibitor, limits motor neuron loss in experimental models of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2013, 170, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sun, H.; Ding, J.; Niu, C.; Su, M.; Zhang, L.; Li, Y.; Wang, C.; Gamper, N.; Du, X.; et al. Selective targeting of M-type potassium K v 7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour. Br. J. Pharmacol. 2017, 174, 4277–4294. [Google Scholar] [CrossRef]
- Satoh, S.; Toshima, Y.; Ikegaki, I.; Iwasaki, M.; Asano, T. Wide therapeutic time window for fasudil neuroprotection against ischemia-induced delayed neuronal death in gerbils. Brain Res. 2007, 1128, 175–180. [Google Scholar] [CrossRef]
- Ohbuchi, M.; Kimura, T.; Nishikawa, T.; Horiguchi, T.; Fukuda, M.; Masaki, Y. Neuroprotective Effects of Fasudil, a Rho-Kinase Inhibitor, After Spinal Cord Ischemia and Reperfusion in Rats. Anesth. Analg. 2018, 126, 815–823. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Wang, X.; Ding, Y.; Ren, Z.; Fang, J.; Sun, T.; Guo, Y.; Chen, Z.; Wen, J. CSE-Derived H 2 S Inhibits Reactive Astrocytes Proliferation and Promotes Neural Functional Recovery after Cerebral Ischemia/Reperfusion Injury in Mice Via Inhibition of RhoA/ROCK 2 Pathway. ACS Chem. Neurosci. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Tatenhorst, L.; Tönges, L.; Saal, K.-A.; Koch, J.C.; Szegő, É.M.; Bähr, M.; Lingor, P. Rho Kinase Inhibition by Fasudil in the Striatal 6-Hydroxydopamine Lesion Mouse Model of Parkinson Disease. J. Neuropathol. Exp. Neurol. 2014, 73, 770–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Lopez, A.; Labandeira, C.M.; Labandeira-Garcia, J.L.; Muñoz, A. Rho kinase inhibitor fasudil reduces l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Br. J. Pharmacol. 2020, 177, 5622–5641. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Guo, D.; Zhou, X.; Jiang, W.; Wang, J.; Tang, J.; Zou, Y.; Bi, M.; Li, Q. Fasudil alleviates brain damage in rats after carbon monoxide poisoning through regulating neurite outgrowth inhibitor/oligodendrocytemyelin glycoprotein signalling pathway. Basic Clin. Pharmacol. Toxicol. 2019, 125, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.; Zhang, Q.; Yu, J.; Zhao, Y.; Ma, C.; Xiao, B. Fasudil regulates T cell responses through polarization of BV-2 cells in mice experimental autoimmune encephalomyelitis. Acta Pharmacol. Sin. 2014, 35, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Minohara, M.; Kikuchi, H.; Ishizu, T.; Tanaka, M.; Piao, H.; Osoegawa, M.; Ohyagi, Y.; Shimokawa, H.; Kira, J.-I. The selective Rho-kinase inhibitor Fasudil is protective and therapeutic in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006, 180, 126–134. [Google Scholar] [CrossRef]
- Hara, M.; Takayasu, M.; Watanabe, K.; Noda, A.; Takagi, T.; Suzuki, Y.; Yoshida, J. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J. Neurosurg. Spine 2000, 93, 94–101. [Google Scholar] [CrossRef]
- Akama, T.; Dong, C.; Virtucio, C.; Sullivan, D.; Zhou, Y.; Zhang, Y.-K.; Rock, F.; Freund, Y.; Liu, L.; Bu, W.; et al. Linking Phenotype to Kinase: Identification of a Novel Benzoxaborole Hinge-Binding Motif for Kinase Inhibition and Development of High-Potency Rho Kinase Inhibitors. J. Pharmacol. Exp. Ther. 2013, 347, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Huentelman, M.J.; Stephan, D.A.; Talboom, J.; Corneveaux, J.J.; Reiman, D.M.; Gerber, J.D.; Barnes, C.A.; Alexander, G.E.; Reiman, E.M.; Bimonte-Nelson, H.A. Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav. Neurosci. 2009, 123, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Satoh, S.; Utsunomiya, T.; Tsurui, K.; Kobayashi, T.; Ikegaki, I.; Sasaki, Y.; Asano, T. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001, 69, 1441–1453. [Google Scholar] [CrossRef]
- Satoh, S.; Hitomi, A.; Ikegaki, I.; Kawasaki, K.; Nakazono, O.; Iwasaki, M.; Mohri, M.; Asano, T. Amelioration of endothelial damage/dysfunction is a possible mechanism for the neuroprotective effects of Rho-kinase inhibitors against ischemic brain damage. Brain Res. Bull. 2010, 81, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Q.; Wen, R.; Yu, Z.; Li, N.; Ma, L.; Feng, W. Rho-kinase inhibitor prevents acute injury against transient focal cerebral ischemia by enhancing the expression and function of GABA receptors in rats. Eur. J. Pharmacol. 2017, 797, 134–142. [Google Scholar] [CrossRef]
- Fujii, M.; Duris, K.; Altay, O.; Soejima, Y.; Sherchan, P.; Zhang, J.H. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem. Int. 2012, 60, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.-L.; Yu, J.-Z.; Yang, X.-W.; Liu, C.-Y.; Li, Y.-H.; Feng, L.; Chai, Z.; Yang, W.-F.; Wang, Q.; Jiang, W.-J.; et al. FSD-C10: A more promising novel ROCK inhibitor than Fasudil for treatment of CNS autoimmunity. Biosci. Rep. 2015, 35, e00247. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Yu, J.; Wu, H.; Li, Y.; Liu, C.-Y.; Feng, L.; Zhang, G.; Xiao, B.; Ma, C. Therapeutic effect of Rho kinase inhibitor FSD-C10 in a mouse model of Alzheimer’s disease. Exp. Ther. Med. 2018, 16, 3929–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-H.; Yu, J.-Z.; Liu, C.-Y.; Zhang, H.; Zhang, H.-F.; Yang, W.-F.; Li, J.-L.; Feng, Q.-J.; Feng, L.; Zhang, G.-X.; et al. Intranasal delivery of FSD-C10, a novel Rho kinase inhibitor, exhibits therapeutic potential in experimental autoimmune encephalomyelitis. Immunology 2014, 143, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, K.M.; Riesinger, S.W.; McKerracher, L.; Mortiz, L.B. Treatment of Cerebral Cavernous Malformations and Cerebral Aneurysms with Rho Kinase Inhibitors. U.S. Patent 246,181, 31 August 2017. [Google Scholar]
- Chen, M.; Liu, Q.; Liu, A.; Tan, M.; Xie, Z.; Uri, A.; Chen, Z.; Huang, G.; Sun, Y.; Ge, H.; et al. Simply combining fasudil and lipoic acid in a novel multitargeted chemical entity potentially useful in central nervous system disorders. RSC Adv. 2014, 4, 37266–37269. [Google Scholar] [CrossRef]
- Chen, J.; Yin, W.; Tu, Y.; Wang, S.; Yang, X.; Chen, Q.; Zhang, X.; Han, Y.; Pi, R. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: Involvement of NF-κB inhibition and Nrf2 pathway activation. Eur. J. Pharmacol. 2017, 806, 1–9. [Google Scholar] [CrossRef]
- Luo, L.; Chen, J.; Su, D.; Chen, M.; Luo, B.; Pi, R.; Wang, L.; Shen, W.; Wang, R. L-F001, a Multifunction ROCK Inhibitor Prevents 6-OHDA Induced Cell Death Through Activating Akt/GSK-3beta and Nrf2/HO-1 Signaling Pathway in PC12 Cells and Attenuates MPTP-Induced Dopamine Neuron Toxicity in Mice. Neurochem. Res. 2017, 42, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bye, N.; Christie, K.J.; Turbic, A.; Basrai, H.S.; Turnley, A.M. Rho kinase inhibition following traumatic brain injury in mice promotes functional improvement and acute neuron survival but has little effect on neurogenesis, glial responses or neuroinflammation. Exp. Neurol. 2016, 279, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Quan, Y.; Cai, Q.; Le, Y.; Ma, T.; Liu, Z.; Wu, G.; Wang, F.; Bao, C.; et al. Inhibition of RhoA/ROCK Pathway in the Early Stage of Hypoxia Ameliorates Depression in Mice via Protecting Myelin Sheath. ACS Chem. Neurosci. 2020, 11, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Jianjun, Z.; Baochun, Z.; Limei, M.; Lijun, L. Exploring the beneficial role of ROCK inhibitors in sepsis-induced cerebral and cognitive injury in rats. Fundam. Clin. Pharmacol. 2021, 35, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Cao, Y. The ROCK inhibitor Y-27632 ameliorates blood-spinal cord barrier disruption by reducing tight junction protein degradation via the MYPT1-MLC2 pathway after spinal cord injury in rats. Brain Res. 2021, 1773, 147684. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Rechberger, E.; Banczerowska-Górska, M.; Poleszak, E.; Dudka, J.; Skorupska, K.; Miotła, P.; Semczuk, A.; Kulik-Rechberger, B.; et al. Inhibition of Rho kinase by GSK 269962 reverses both corticosterone-induced detrusor overactivity and depression-like behaviour in rats. Eur. J. Pharmacol. 2018, 837, 127–136. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, C.; Sitkoff, D.; Cheadle, N.L.; Xu, S.; Muckelbauer, J.K.; Adam, L.P.; Wexler, R.R.; Quan, M.L. Identification of 5H-chromeno[3,4-c]pyridine and 6H-isochromeno[3,4-c]pyridine derivatives as potent and selective dual ROCK inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127474. [Google Scholar] [CrossRef]
- Hyun Lee, J.; Zheng, Y.; Bornstadt, D.; Wei, Y.; Balcioglu, A.; Daneshmand, A.; Yalcin, N.; Yu, E.; Herisson, F.; Atalay, Y.B.; et al. Selective ROCK 2 inhibition in focal cerebral ischemia. Ann. Clin. Transl. Neurol. 2014, 1, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, L.P.; Kietzman, H.W.; Guo, J.; Rainnie, D.G.; Gourley, S.L. Rho-kinase inhibition has antidepressant-like efficacy and expedites dendritic spine pruning in adolescent mice. Neurobiol. Dis. 2019, 124, 520–530. [Google Scholar] [CrossRef]
- Findlay, J.M.; Nisar, J.; Darsaut, T. Cerebral Vasospasm: A Review. Can. J. Neurol. Sci. 2016, 43, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Hamano, T.; Shirafuji, N.; Yen, S.-H.; Yoshida, H.; Kanaan, N.M.; Hayashi, K.; Ikawa, M.; Yamamura, O.; Fujita, Y.; Kuriyama, M.; et al. Rho-kinase ROCK inhibitors reduce oligomeric tau protein. Neurobiol. Aging 2020, 89, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, X.-J.; He, W.; Ding, J.; Jia, J.-T.; Fu, G.; Wang, H.-X.; Guo, L.-J. Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell. Mol. Neurobiol. 2009, 29, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, Y.Q.; Lu, Y.F.; Wu, Y.; Liu, R.; Zheng, Y.R.; Yin, M. Exploring the potential of RhoA inhibitors to improve exercise-recoverable spinal cord injury: A systematic review and meta-analysis. J. Chem. Neuroanat. 2021, 111, 101879. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Wu, Y.; Li, Q.; Wang, X.; Liu, Y.; Di, X. Aldehyde oxidase-dependent species difference in hepatic metabolism of fasudil to hydroxyfasudil. Xenobiotica 2018, 48, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Nakao, H.; Yoshizaki, H.; Shiratsuchi, M.; Shigyo, H.; Yamada, H.; Ozawa, T.; Totsuka, J.; Hidaka, H. Development of specific Rho-kinase inhibitors and their clinical application. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1754, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, J.; Yi, X.; Li, Y.; Zeng, Y. Absorption, tissue disposition, and excretion of fasudil hydrochloride, a RHO kinase inhibitor, in rats and dogs. Biopharm. Drug Dispos. 2020, 41, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Wright, J.; Adam, J.; Bennett, J.; Boucharens, S.; Black, D.; Cook, A.; Brown, A.R.; Epemolu, O.; Fletcher, D.; et al. Fragment-based discovery of 6-substituted isoquinolin-1-amine based ROCK-I inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 97–101. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Surma, M.; Vemula, S.; Zhang, L.; Yang, Y.; Kapur, R.; Wei, L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013, 4, e483. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Wen, J.; Chen, Z. Distinct Roles of ROCK1 and ROCK2 on the Cerebral Ischemia Injury and Subsequently Neurodegenerative Changes. Pharmacology 2020, 105, 3–8. [Google Scholar] [CrossRef]
- Yan, J.; Pan, Y.; Zheng, X.; Zhu, C.; Zhang, Y.; Shi, G.; Yao, L.; Chen, Y.; Xu, N. Comparative Study of ROCK1 and ROCK2 in Hippocampal Spine Formation and Synaptic Function. Neurosci. Bull. 2019, 35, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Le, R.Q.; Ionan, A.; Li, R.-J.; Wang, Y.-H.; Gudi, R.; Mitra, S.; Vallejo, J.; Okusanya, O.O.; Ma, L.; et al. FDA Approval Summary: Belumosudil for Adult and Pediatric Patients 12 Years and Older with Chronic GvHD after Two or More Prior Lines of Systemic Therapy. Clin. Cancer Res. 2022, 28, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-J.; Hwang, S.-K.; Kim, N.; Lee, E.-H.; Zhong, W.; Ren, X.-J.; Cho, S.-I.; Bae, J.; Qian, J.; Lim, C.; et al. Abstract WP320: Neuronal ROCK2 Plays Important Role in Ischemic Stroke: GSK-3beta and Tau Signaling Pathways. Stroke 2020, 51, AWP320. [Google Scholar] [CrossRef]
- Sadeghian, H.; Lacoste, B.; Qin, T.; Toussay, X.; Rosa, R.; Oka, F.; Chung, D.Y.; Takizawa, T.; Gu, C.; Ayata, C. Spreading depolarizations trigger caveolin-1-dependent endothelial transcytosis. Ann. Neurol. 2018, 84, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Zabramski, J.M.; Kalani, M.Y.S.; Filippidis, A.S.; Spetzler, R.F. Propranolol Treatment of Cavernous Malformations with Symptomatic Hemorrhage. World Neurosurg. 2016, 88, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Berti, I.; Marchetti, F.; Skabar, A.; Zennaro, F.; Zanon, D.; Ventura, A. Propranolol for cerebral cavernous angiomatosis: A magic bullet. Clin. Pediatr. 2014, 53, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Schuchardt, F.; Meckel, S.; Heinz, J.; Felbor, U.; Sure, U.; Geisen, U. Propranolol stops progressive multiple cerebral cavernoma in an adult patient. J. Neurol. Sci. 2016, 367, 15–17. [Google Scholar] [CrossRef]
- Lanfranconi, S.; Scola, E.; Meessen, J.M.T.A.; Pallini, R.; Bertani, G.A.; Al-Shahi Salman, R.; Dejana, E.; Latini, R. Safety and efficacy of propranolol for treatment of familial cerebral cavernous malformations (Treat_CCM): A randomised, open-label, blinded-endpoint, phase 2 pilot trial. Lancet. Neurol. 2022, 22, 35–44. [Google Scholar] [CrossRef]
- Oliveira, R.G.D.; Guerra, F.S.; Mermelstein, C.D.S.; Fernandes, P.D.; Bastos, I.T.D.S.; Costa, F.N.; Barroso, R.C.R.; Ferreira, F.F.; Fraga, C.A.M. Synthesis and pharmacological evaluation of novel isoquinoline N-sulphonylhydrazones designed as ROCK inhibitors. J. Enzym. Inhib. Med. Chem. 2018, 33, 1181–1193. [Google Scholar] [CrossRef] [Green Version]


| Compound | Affinity (pKD) | Activity (pKI) | BBB Score [155] | Detect. CNS? | CNS Testing | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ROCK1 | ROCK2 | Ref. | ROCK1 | ROCK2 | Ref. | Disease | Animal Model | Ref. | |||
| Non-selective ROCK inhibitors (isoquinolines) | |||||||||||
| Fasudil (1) | 6.04; 7.29 | 6.03; 7.34 | [86,156] | 6.46 | 7.02 | [157] | 4.90 | Yes [158] | fCCM | Ccml+/–Msh2–/– mice | [72,85] |
 | fCCM | Ccm2+/–Trp53–/– mice | [72] | ||||||||
| AD | Wistar rats | [159,160] | |||||||||
| ALS | SOD1G93A mice | [161] | |||||||||
| Depression | Kv7.4−/– mice | [162] | |||||||||
| Epilepsy | GAERS rats | [148] | |||||||||
| Epilepsy | Sprague–Dawley rats | [147] | |||||||||
| I/R | Mongolian gerbils | [163] | |||||||||
| I/R | Sprague–Dawley rats | [164] | |||||||||
| I/R | CSE−/– mice | [165] | |||||||||
| PD | C57BL/6 mice | [166] | |||||||||
| PD | Sprague–Dawley rats | [167] | |||||||||
| Poison CI | Sprague–Dawley rats | [168] | |||||||||
| MS/AE | C57BL/6 mice | [169] | |||||||||
| MS/AE | SJL/J mice | [170] | |||||||||
| Schizophrenia | C57BL/6 mice | [158] | |||||||||
| SCI | Sprague–Dawley rats | [171] | |||||||||
| Hydroxyfasudil (2) | 6.27 | 5.87 | [86] | 7.13 | 7.09 | [172] | 4.43 | Yes [158] | Dementia | Fischer-344 rats | [173] |
 | Ischemia | Rats | [174] | ||||||||
| Ischemia | Sprague–Dawley rats | [175] | |||||||||
| I/R | Sprague–Dawley rats | [176] | |||||||||
| SAH | Sprague–Dawley rats | [177] | |||||||||
| FSD-C10 (3) | n.r. | n.r. | – | 5.94 * | 6.15 * | [178] | 5.28 | n.r. | AD | C57BL/6 mice | [179] |
 | MS/AE | C57BL/6 mice | [180] | ||||||||
| (R)-BA-1049 (4) | 6.96 | 7.23 | [156] | 6.19; 5.47 | 6.49; 6.84 | [156] | 4.70 | Yes [156] | fCCM | Ccml+/–Msh2–/– mice | [87] |
 | fCCM | Ccm3+/–Msh2–/– mice | [87] | ||||||||
| I/R | C57BL/6 mice | [181] | |||||||||
| L-F001 (6) | n.r. | n.r. | – | 6.10 * | 5.98 * | [182] | 4.58 | Yes [182] | AD | C57BL/6 mice | [183] |
 | PD | C57BL/6 mice | [184] | ||||||||
| Y-27632 (5) | n.r. | n.r. | – | 6.71 | 7.22 | [157] | 4.45 | n.r. | Trauma CI | C57BL/6 mice | [185] |
 | Depression | C57BL/6 mice | [186] | ||||||||
| Sespis CI | Wistar rats | [187] | |||||||||
| SAH | Sprague–Dawley rats | [177] | |||||||||
| SCI | Sprague–Dawley rats | [188] | |||||||||
| Epilepsy | GAERS rats | [148] | |||||||||
| GSK269962 (7) | n.r. | n.r. | – | 8.80 ** | 8.40 ** | [146] | 0.59 | n.r. | Depression | Wistar rats | [189] |
 | |||||||||||
| ROCK2-selective inhibitors | |||||||||||
| Belumosudil (8) | 5.13 | 7.19 | [156] | 4.62 | 6.98 | [190] | 2.18 | Yes [191] | I/R | C57BL/6 mice | [191] |
 | Depression | C57BL/6 mice | [192] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagnoli, T.L.; de Oliveira, D.R.; Fraga, C.A.M. Therapeutic Perspectives on ROCK Inhibition for Cerebral Cavernous Malformations. Kinases Phosphatases 2023, 1, 72-96. https://doi.org/10.3390/kinasesphosphatases1010006
Montagnoli TL, de Oliveira DR, Fraga CAM. Therapeutic Perspectives on ROCK Inhibition for Cerebral Cavernous Malformations. Kinases and Phosphatases. 2023; 1(1):72-96. https://doi.org/10.3390/kinasesphosphatases1010006
Chicago/Turabian StyleMontagnoli, Tadeu L., Daniela R. de Oliveira, and Carlos A. Manssour Fraga. 2023. "Therapeutic Perspectives on ROCK Inhibition for Cerebral Cavernous Malformations" Kinases and Phosphatases 1, no. 1: 72-96. https://doi.org/10.3390/kinasesphosphatases1010006
APA StyleMontagnoli, T. L., de Oliveira, D. R., & Fraga, C. A. M. (2023). Therapeutic Perspectives on ROCK Inhibition for Cerebral Cavernous Malformations. Kinases and Phosphatases, 1(1), 72-96. https://doi.org/10.3390/kinasesphosphatases1010006








