Overcoming Forage Challenges in Mesophytic Grasslands—The Advantages of Lotus tenuis
Abstract
1. Introduction
2. Materials and Methods
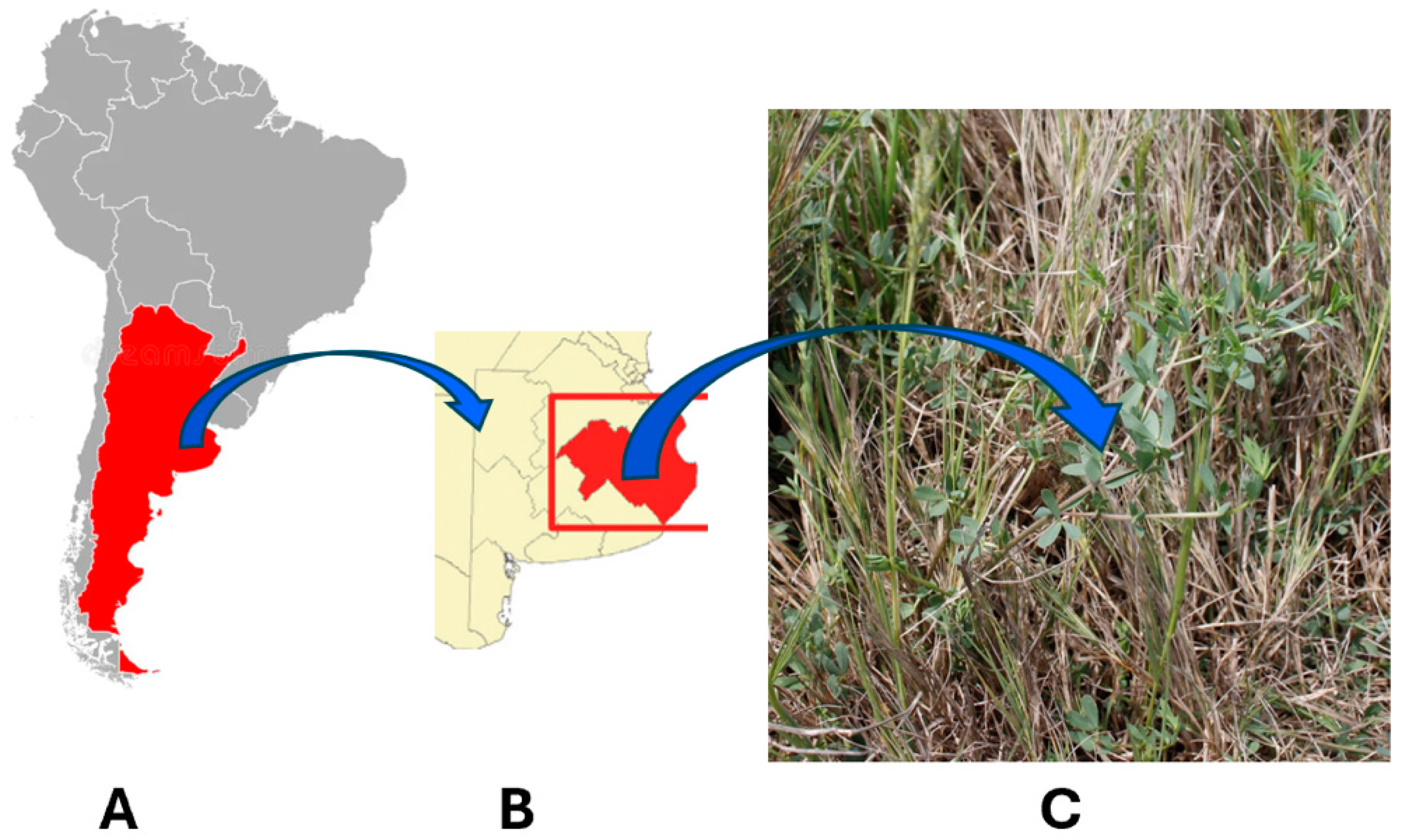
2.1. Plant Sampling and Experimental Farm Description
2.2. Soil Analysis
2.3. Analysis of Sodium and Potassium Contents
2.4. Photosynthetic Activity Analysis
2.5. Nutritional Parameters and Digestibility Using the In Vitro Gas Production Technique
2.6. Alcohol-Insoluble and Hot-Water-Insoluble Residue Determination
2.7. Analysis of Cell Wall Chemical Composition
2.8. Spectroscopy ATR-FTIR Analysis
2.9. Statistical Analysis
3. Results
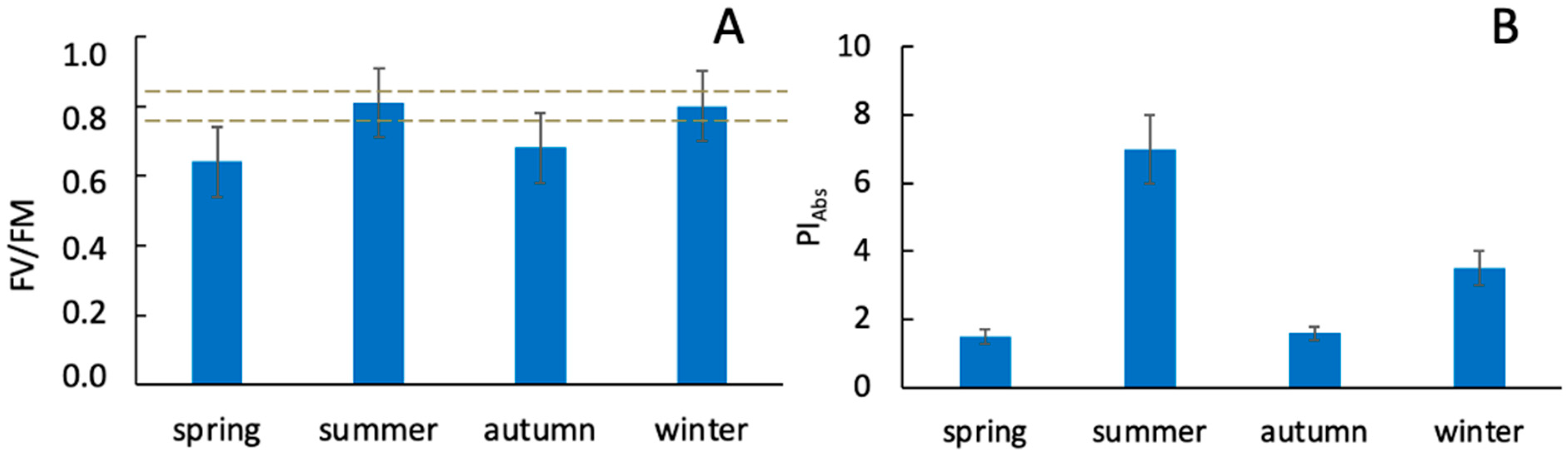
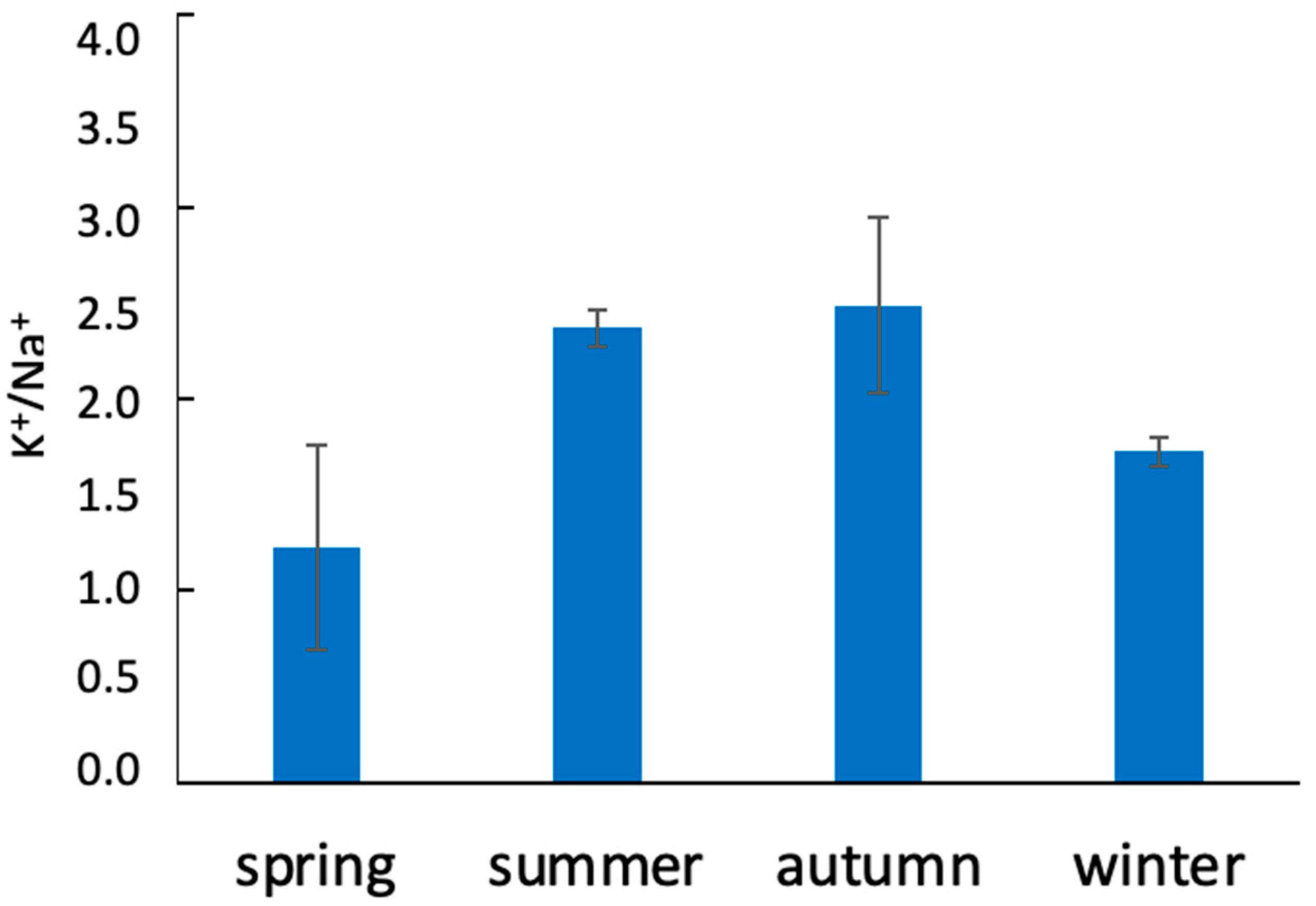
3.1. Characterization of Soil and Plants in the Areas of This Study Using Physicochemical and Physiological Parameters
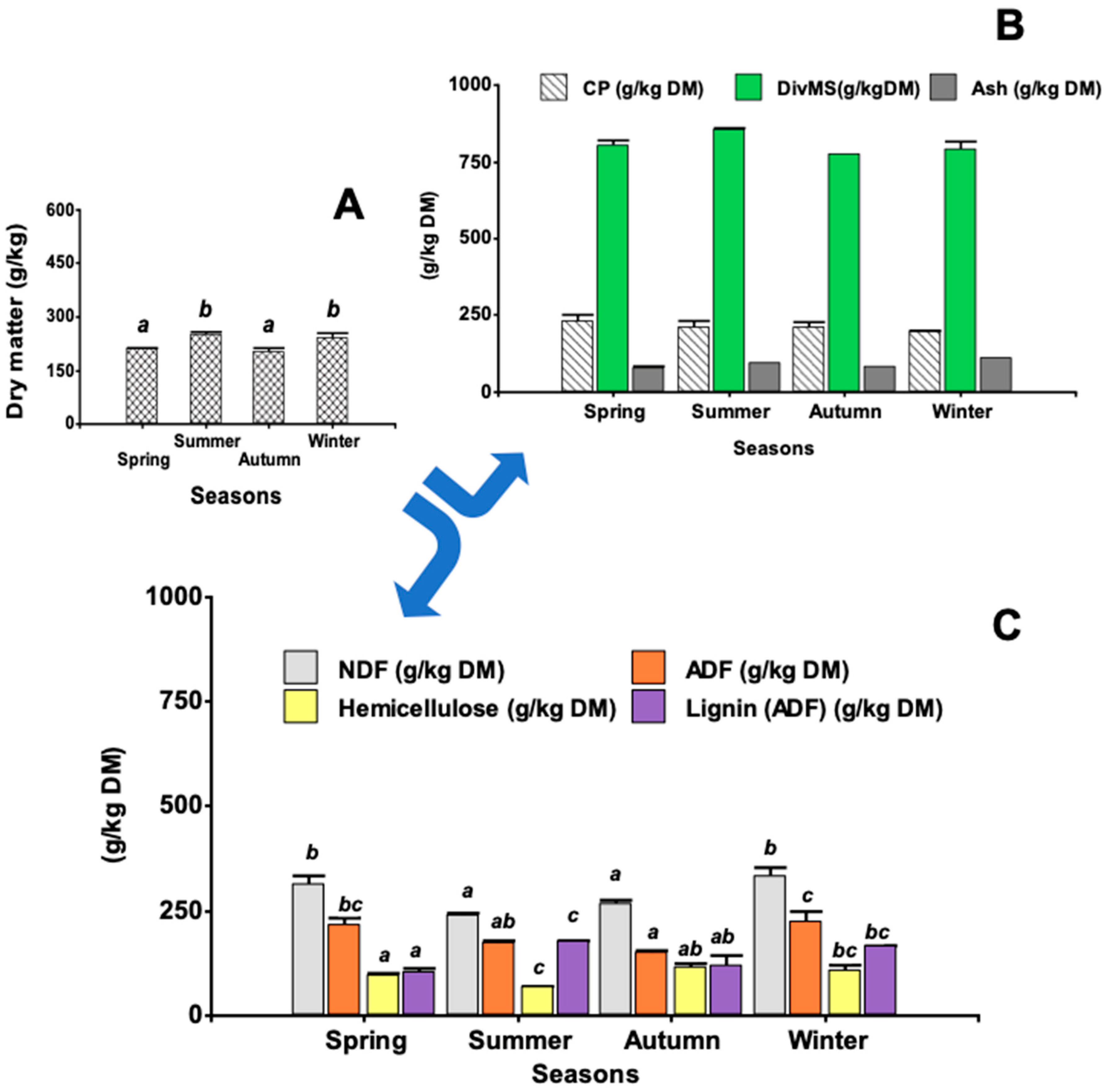
3.2. Nutritional Parameters and Characterization of Cell Wall Material
3.2.1. Nutritional Characterization
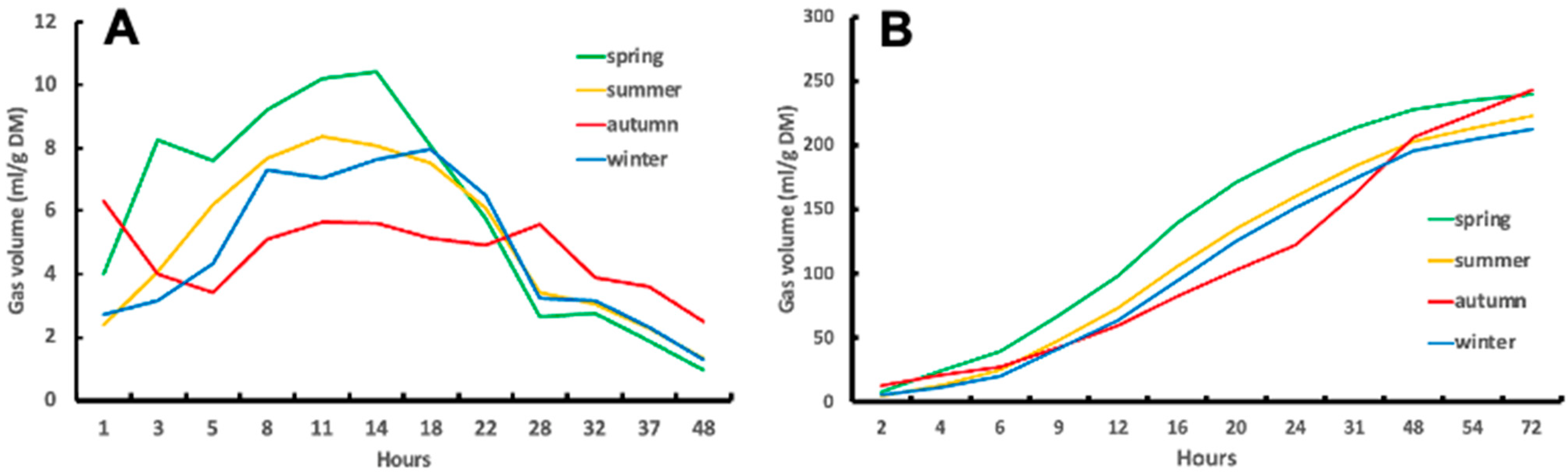
3.2.2. Gas Production Kinetics
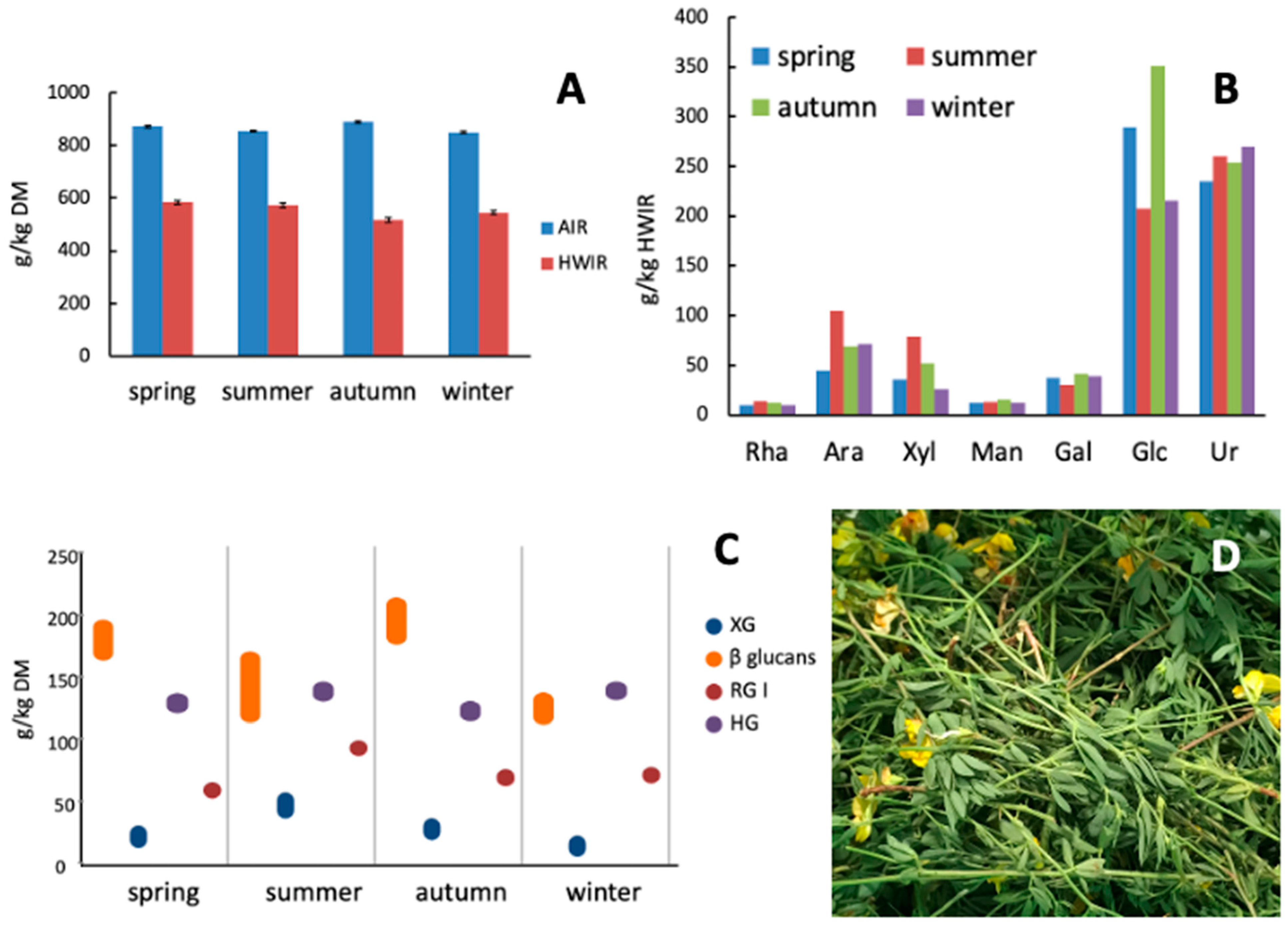
3.2.3. Characterization of Cell Wall Materials
3.2.4. ATR-FTIR Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, A.; Jacobo, E.; Preliasco, P.; Roitman, G.; Miñarro, F. Pastoreo controlado: Una herramienta para el manejo de los pastizales naturales en sistemas ganaderos extensivos. In Buenas prácticas para una ganadería sustentable de pastizal: Kit de extensión para las pampas y campos; Fundación Vida Silvestre Argentina, Aves argentinas/AOP; FAUBA: Buenos Aires, Argentina, 2012. [Google Scholar]
- Imbellone, P.A.; Taboada, M.A.; Damiano, F.; Lavado, R.S. Genesis, Properties and Management of Salt-Affected Soils in the Flooding Pampas, Argentina. In Saline and Alkaline Soils in Latin America; Taleisnik, E., Lavado, R.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 191–208. ISBN 978-3-030-52591-0. [Google Scholar]
- Campestre, M.P.; Antonelli, C.J.; Bailleres, M.A.; Gortari, M.; Maguire, V.G.; Ezquiaga, J.P.; Taboada, M.A.; Ruiz, O.A. An Efficiently Biological Nitrogen Fixation of Non-Native Lotus Tenuis Justifies Its Key Role in the Flooding Pampas (Argentina). Farming Syst. 2025, 3, 100122. [Google Scholar] [CrossRef]
- Cid, M.S.; Grecco, R.C.F.; Oesterheld, M.; Paruelo, J.M.; Cibils, A.F.; Brizuela, M.A. Grass-Fed Beef Production Systems of Argentina’s Flooding Pampas: Understanding Ecosystem Heterogeneity to Improve Livestock Production. Outlook Agric. 2011, 40, 181–189. [Google Scholar] [CrossRef]
- Nieva, A.S.; Ruiz, O.A. Lotus spp.: A Foreigner That Came to Stay Forever: Economic and Environmental Changes Caused by Its Naturalization in the Salado River Basin (Argentina). In Saline and Alkaline Soils in Latin America; Taleisnik, E., Lavado, R.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 431–446. ISBN 978-3-030-52591-0. [Google Scholar]
- Teakle, N.L.; Snell, A.; Real, D.; Barrett-Lennard, E.G.; Colmer, T.D. Variation in Salinity Tolerance, Early Shoot Mass and Shoot Ion Concentrations within Lotus Tenuis: Towards a Perennial Pasture Legume for Saline Land. Crop Pasture Sci. 2010, 61, 379. [Google Scholar] [CrossRef]
- García, I.V.; Covacevich, F.; Fernández-López, C.; Cabello, M.N. Lotus Tenuis Maintains High Arbuscular Mycorrhizal Diversity in Grasslands Regardless of Soil Properties or Management. Rhizosphere 2023, 27, 100754. [Google Scholar] [CrossRef]
- Vignolio, O.R.; Fernandez, O.N. Bioecología de Lotus glaber Mill. (Fabaceae) en la Pampa Deprimida (provincia de Buenos Aires, Argentina). In Revista Argentina de Producción Animal; Asociación Argentina de Producción Animal: Balcarce, Argentina, 2006; pp. 113–130. [Google Scholar]
- Veblen, T.; Young, K.; Orme, A. The Physical Geography of South America; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-531341-3. [Google Scholar]
- Fernández, P.V.; Vago, M.E.; Ezquiaga, J.P.; Maiale, S.; Rodriguez, A.; Acosta, J.M.; Gortari, M.; Ruiz, O.A.; Ciancia, M. Cell Wall Composition and Nutritional Quality through Seasons of the Saltgrass Distichlis Laxiflora Growing in Halophytic and Mesophytic Meadows. Plant Stress 2024, 13, 100519. [Google Scholar] [CrossRef]
- García, I.; Mendoza, R.; Pomar, M.C. Deficit and Excess of Soil Water Impact on Plant Growth of Lotus Tenuis by Affecting Nutrient Uptake and Arbuscular Mycorrhizal Symbiosis. Plant Soil 2008, 304, 117–131. [Google Scholar] [CrossRef]
- Mendoza, R.; Escudero, V.; García, I. Plant Growth, Nutrient Acquisition and Mycorrhizal Symbioses of a Waterlogging Tolerant Legume (Lotus Glaber Mill.) in a Saline-Sodic Soil. Plant Soil 2005, 275, 305–315. [Google Scholar] [CrossRef]
- Lavado, R.S.; Taboada, M.A. Water, Salt and Sodium Dynamics in a Natraquoll in Argentina. CATENA 1988, 15, 577–594. [Google Scholar] [CrossRef]
- Perelman, S.B.; León, R.J.C.; Oesterheld, M. Cross-scale Vegetation Patterns of Flooding Pampa Grasslands. J. Ecol. 2001, 89, 562–577. [Google Scholar] [CrossRef]
- Bondi, A. Animal Nutrition; John Wiley and Sons: West Sussex, UK, 1987. [Google Scholar]
- Vago, M.E.; Jaurena, G.; Estevez, J.M.; Castro, M.A.; Zavala, J.A.; Ciancia, M. Salt Stress on Lotus Tenuis Triggers Cell Wall Polysaccharide Changes Affecting Their Digestibility by Ruminants. Plant Physiol. Biochem. 2021, 166, 405–415. [Google Scholar] [CrossRef]
- Buxton, D.R.; Casler, M.D. Environmental and Genetic Effects on Cell Wall Composition and Digestibility. In ASA, CSSA, and SSSA Books; Jung, H.G., Buxton, D.R., Hatfield, R.D., Ralph, J., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 685–714. ISBN 978-0-89118-238-2. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 8th ed.; Pearson Education Limited: Essex, UK, 2011; ISBN 978-1-292-25166-0. [Google Scholar]
- Cosgrove, D.J. Growth of the Plant Cell Wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and Growth of Plant Cell Walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a Systems Approach to Understanding Plant Cell Walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, F.; Fry, S.C. Plant Cell Walls. From Chemistry to Biology. Ann. Bot. 2011, 108, viii–ix. [Google Scholar] [CrossRef]
- Begenisic, F. Recopilación de las técnicas de Laboratorio Vigentes y Reconocidas Por El SAMLA, En Proceso de Revisión y Actualización En El Marco Del Convenio IRAM-SAGPyA. Resolución Nro. 478/98 y 238/03. 2004. [Cd- Rom].
- Chen, S.; Li, J.; Wang, S.; Hüttermann, A.; Altman, A. Salt, Nutrient Uptake and Transport, and ABA of Populus Euphratica; a Hybrid in Response to Increasing Soil NaCl. Trees 2001, 15, 186–194. [Google Scholar] [CrossRef]
- Campestre, M.P.; Antonelli, C.J.; Castagno, N.L.; Maguire, V.G.; Ruiz, O.A. Interspecific Hybridization and Inoculation with Pantoea Eucalypti Improve Forage Performance of Lotus Crop Species under Alkaline Stress. Plant Biol. 2024, 26, 245–256. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.P.; Baker, N.R.; Oquist, G.; Schreiber, U.; Lechner, E.G. Chlorophyll Fluorescence as a Probe of the Photosynthetic Competence of Leaves in the Field: A Review of Current Instrumentation. Funct. Ecol. 1989, 3, 497. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Danelón, J.L. Mejoramiento de la técnica de producción de gas in vitro para evaluar alimentos para rumiantes. In Revista Argentina de Producción Animal; Asociación Argentina de Producción Animal: Balcarce, Argentina, 2004; pp. 187–197. [Google Scholar]
- Fry, S.C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis; Longman Scientific & Technical: Harlow, UK, 1988. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A Simplified Method for Accurate Determinationof Cell Wall Uronide Content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Shui, G.; Leong, L.P. Residue from Star Fruit as Valuable Source for Functional Food Ingredients and Antioxidant Nutraceuticals. Food Chem. 2006, 97, 277–284. [Google Scholar] [CrossRef]
- Morrison, I.M.; Stewart, D. Plant Cell Wall Fragments Released on Solubilisation in Trifluoroacetic Acid. Phytochemistry 1998, 49, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops–What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the Contribution of ATR-FTIR Spectroscopy to Characterize Plant Cell Wall Polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Di Bella, C.E.; Kotula, L.; Striker, G.G.; Colmer, T.D. Submergence Tolerance and Recovery in Lotus: Variation among Fifteen Accessions in Response to Partial and Complete Submergence. J. Plant Physiol. 2020, 249, 153180. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Buxton, D.R.; Redfearn, D.D. Plant Limitations to Fiber Digestion and Utilization. J. Nutr. 1997, 127, 814S–818S. [Google Scholar] [CrossRef]
- González, F.A.; Noemí Cosentino, V.R.; Loza, C.; Cerón-Cucchi, M.E.; Williams, K.E.; Bualó, R.; Costantini, A.; Gere, J.I. Inclusion of Lotus Tenuis in Beef Cattle Systems in the Argentinian Flooding Pampa as an Enteric Methane Mitigation Strategy. N. Z. J. Agric. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Bailey, R.W.; Ulyatt, M.J. Pasture Quality and Ruminant Nutrition: II. Carbohydrate and Lignin Composition of Detergent-Extracted Residues from Pasture Grasses and Legumes. N. Z. J. Agric. Res. 1970, 13, 591–604. [Google Scholar] [CrossRef]
- Vignolio, O.R.; Cambareri, G.; Maceira, N. Lotus tenuis (Fabaceae). Productividad y manejo agronómico. Rev. Argent. De Prod. Animal 2013, 30, 97–116. [Google Scholar]
- Bustan, A.; Pasternak, D.; Pirogova, I.; Durikov, M.; Devries, T.T.; El-Meccawi, S.; Degen, A.A. Evaluation of Saltgrass as a Fodder Crop for Livestock. J. Sci. Food Agric. 2005, 85, 2077–2084. [Google Scholar] [CrossRef]
- Wangui, J.C.; Millner, J.P.; Kenyon, P.R.; Tozer, P.R.; Morel, P.C.H.; Pain, S.J. In Vitro Fermentation of Browsable Native Shrubs in New Zealand. Plants 2022, 11, 2085. [Google Scholar] [CrossRef]
- Boga, M.; Yurtseven, S.; Kilic, U.; Aydemir, S.; Polat, T. Determination of Nutrient Contents and In Vitro Gas Production Values of Some Legume Forages Grown in the Harran Plain Saline Soils. Asian Australas J. Anim. Sci. 2014, 27, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Rymer, C.; Huntington, J.A.; Williams, B.A.; Givens, D.I. In Vitro Cumulative Gas Production Techniques: History, Methodological Considerations and Challenges. Anim. Feed. Sci. Technol. 2005, 123–124, 9–30. [Google Scholar] [CrossRef]
- Jung, H.G.; Allen, M.S. Characteristics of Plant Cell Walls Affecting Intake and Digestibility of Forages by Ruminants. J. Anim. Sci. 1995, 73, 2774. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural Models of Primary Cell Walls in Flowering Plants: Consistency of Molecular Structure with the Physical Properties of the Walls during Growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- CSIRO. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Melbourne, Australia, 2007. [Google Scholar]
- Wei, Z.; Maxwell, T.M.R.; Robinson, B.; Dickinson, N. Legume Nutrition Is Improved by Neighbouring Grasses. Plant Soil 2022, 475, 443–455. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant Functional Composition Influences Rates of Soil Carbon and Nitrogen Accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Gliessman, S.R. Package Price Agroecology: The Ecology of Sustainable Food Systems; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vago, M.E.; Fernández, P.V.; Ezquiaga, J.P.; Maiale, S.J.; Rodriguez, A.A.; Acosta, J.M.; Gortari, M.; Ruiz, O.A.; Ciancia, M. Overcoming Forage Challenges in Mesophytic Grasslands—The Advantages of Lotus tenuis. Grasses 2025, 4, 19. https://doi.org/10.3390/grasses4020019
Vago ME, Fernández PV, Ezquiaga JP, Maiale SJ, Rodriguez AA, Acosta JM, Gortari M, Ruiz OA, Ciancia M. Overcoming Forage Challenges in Mesophytic Grasslands—The Advantages of Lotus tenuis. Grasses. 2025; 4(2):19. https://doi.org/10.3390/grasses4020019
Chicago/Turabian StyleVago, María Elena, Paula Virginia Fernández, Juan Pedro Ezquiaga, Santiago Javier Maiale, Andrés Alberto Rodriguez, Juan Manuel Acosta, Maximiliano Gortari, Oscar Adolfo Ruiz, and Marina Ciancia. 2025. "Overcoming Forage Challenges in Mesophytic Grasslands—The Advantages of Lotus tenuis" Grasses 4, no. 2: 19. https://doi.org/10.3390/grasses4020019
APA StyleVago, M. E., Fernández, P. V., Ezquiaga, J. P., Maiale, S. J., Rodriguez, A. A., Acosta, J. M., Gortari, M., Ruiz, O. A., & Ciancia, M. (2025). Overcoming Forage Challenges in Mesophytic Grasslands—The Advantages of Lotus tenuis. Grasses, 4(2), 19. https://doi.org/10.3390/grasses4020019







