Phosphorus Use Efficiency: Morphogenetic and Productive Responses of Brachiaria decumbens Genotypes (Syn: Urochloa decumbens)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site, Soil, and Fertilization
2.2. Experimental Design
2.3. Morphogenic and Productive Characteristics
2.4. Phosphorus Use Efficiency
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Duarte, C.F.D.; Paiva, L.M.; Fernandes, H.J.; Prochera, D.L.; Cassaro, L.H.; Breure, M.F.; Flores, L.S.; Fernandes, R.L.; Souza, E.R.C.; Fleitas, A.C.; et al. Piata palisadegrass fertilized with different sources of phosphorus for solubility in water. Arq. Bras. Med. Veterinária Zootec. 2016, 15, 58–63. [Google Scholar] [CrossRef][Green Version]
- Heinrichs, R.; Monreal, C.M.; Santos, E.T.; Soares Filho, C.V.; Rebonatti, M.D.; Teixeira, N.M.; Moreira, A. Phosphorus Sources and Rates Associated with Nitrogen Fertilization in Mombasa Grass Yield. Commun. Soil Sci. Plant Anal. 2016, 47, 657–669. [Google Scholar] [CrossRef]
- Costa, K.S.d.Q.; de Oliveira, C.F.; Melo, M.P.; Vaz, C.F.; Melo, N.C.; Moraes, F.K.C. Fósforo no sistema solo-planta: Uma revisão. Obs. De La Econ. Latinoam. 2024, 22, e5361. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J. Fósforo em Solo e Planta em Condições Tropicais; Universidade Federal de Viçosa: Viçosa, Brazil, 1999. [Google Scholar]
- Withers, P.J.A.; Rodrigues, M.; Soltangheisi, A.; de Carvalho, T.S.; Guilherme, L.R.G.; Benites, V.d.M.; Gatiboni, L.C.; de Sousa, D.M.G.; Nunes, R.d.S.; Rosolem, C.A.; et al. Transições para o manejo sustentável do fósforo na agricultura brasileira. Sci. Rep. 2018, 8, 2537. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; Neill, C.; Porder, S. The phosphorus footprint of food production: Where should we focus for reducing environmental impacts? J. Ind. Ecol. 2016, 20, 432–444. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Jarvie, H.P.; Powers, S.M.; Sharpley, A.N.; Elser, J.J.; Shen, J.; Peterson, H.M.; Chan, N.; Howden, N.J.K.; Burt, T.; et al. Sustainable phosphorus management and the need for a long-term perspective: The legacy hypothesis. Environ. Sci. Technol. 2014, 48, 8417–8419. [Google Scholar] [CrossRef]
- Costa, K.A.P.; Faquin, V.; Oliveira, I.P.; Severiano, E.C.; Simon, G.A.; Carrijo, M.S. Nutrient extraction by Marandu-grass phytomass under doses and nitrogen sources. Rev. Bras. Saúde Produção Anim. 2009, 10, 801–810. Available online: https://periodicos.ufba.br/index.php/rbspa/article/view/40060 (accessed on 19 March 2025).
- Lambers, H.; Hayes, P.E.; Laliberté, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.-R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, M.; Vázquez, E.; Villegas, D.M.; Rao, I.M.; Gutierrez, J.F.; Vivas-Quila, N.J.; Amado, M.; Berdugo, C.; Arango, J. Optimizing nitrogen use efficiency of six forage grasses to reduce nitrogen loss from intensification of tropical pastures. Agric. Ecosys. Environ. 2024; 367, 108970. [Google Scholar] [CrossRef]
- Santos, H.P.; Gatiboni, L.C.; Kaminski, J.; Siva, L.S.; Leites, L.; Tessier, D. Manejo da fertilidade do solo e adubação em pastagens. In Manejo Sustentável de Pastagens; Cecato, U., Santos, G.T., Branco, A.F., Soares Filho, C.V., Eds.; EDUEM: Maringá, Brazil, 2014; pp. 195–242. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification of Making and Interpreting Soil Surveys, 2nd ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 1999; 869p. [Google Scholar]
- Emizael, M.A.; Araújp, A.R.; Difante, G.S.; Macedo, M.C.M.; Montagner, D.B.; Gurgel, A.L.C.; Zimmer, A.H.; Ferreira, A.D. Do different soil use and management systems change root eight? New Zealand J. Agric. Res. 2023, 67, 479–497. [Google Scholar] [CrossRef]
- Fageria, N.K.; Stalon, N.A.; Baligar, V.C. Nutrient Management for Improving Lowland Rice Productivity and Sustainability. Adv. Agron. 2003, 80, 63–152. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. Defoliation, Shoot Plasticity, Sward Structure and Herbage Utilization in Pasture: Review of the Underlying Ecophysiological Processes. Agriculture 2015, 5, 1146–1171. [Google Scholar] [CrossRef]
- Bezerra, J.D.D.V.; Neto, J.V.E.; Alves, D.J.d.S.; Neta, I.E.B.; Neto, L.C.G.; Santos, R.d.S.; Difante, G.d.S. Productive, morfhogenic and structural characteristics of Brachiaria brizantha cultivars grown in two types of soil. Res. Soc. Dev. 2020, 9, e129972947. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ulah, A.; Sabia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Nunes, F.N.; Cantarutti, R.B.; Novais, R.F.; Silva, I.R.; Tótola, M.R.; Ribeiro, B.N. Atividades de fosfatases em gramíneas forrageiras em resposta à disponibilidade de fósforo no solo e à altura de corte das plantas. Rev. Bras. Ciência Solo 2008, 32, 1899–1909. [Google Scholar] [CrossRef]
- Garcez Neto, A.F.; Nascimento Junior, D.; Regazzi, A.J.; Fonseca, D.M.; Mosquim, P.R.; Gobbi, K.F. Respostas morofgênicas e estruturais de Panicum maximum cv. Mombaça sob diferentes níveis de adubação nitrogenada e alturas de corte. Braz. J. Anim. Sci. 2002; 31, 1890–1900. [Google Scholar] [CrossRef]
- Lemaire, G.; Da Silva, M.; Agnusdei, M.; Wade, J.; Hodgson, J. Interactions between leaf lifespan and defoliation frequency in temperate and tropical pastures: A review. Grass Forage Sci. 2009, 64, 341–353. [Google Scholar] [CrossRef]
- Francisquini Junior, A.; Calonego, J.C.; Rosolem, C.A.; Santos, C.H.; Tiritan, C. Increase of nitrogen-use efficiency by phosphorus fertilization in grass–legume pastures. Nutr. Cycl. Agroecosyst. 2020, 118, 165–175. [Google Scholar] [CrossRef]
- Nunes, J.O.; Pompeu, R.C.F.F.; Bueno, L.G.; Tavares, R.K.O.; Clark, M.V.G.; Sagrilo, E.; Oliveira Júnior, J.O.L.; Souza, H.A. Response of Urochloa mosambicensis genotypes to phosphorus fertilization in soil with low phosphorus levels. Rev. Bras. Saúde Produção Anim. 2023, 24, e20230028. [Google Scholar] [CrossRef]
- Pereira, E.A.; Martuscello, J.A.; Fonseca, D.M.; Santos, M.V.; Cecon, P.R. Características morfogênicas e estruturais de gramíneas do gênero Brachiaria sob doses de nitrogênio. Ciência Anim. Bras. 2015, 16, 68–79. [Google Scholar]
- Camacho, M.A.; Silveira, L.P.O.; Silveira, M.V. Efficiency of genotypes of Brachiaria brizantha Stapf. (Syn: Urochloa brizantha) in biomass production under phosporus application. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1133–1140. [Google Scholar] [CrossRef][Green Version]
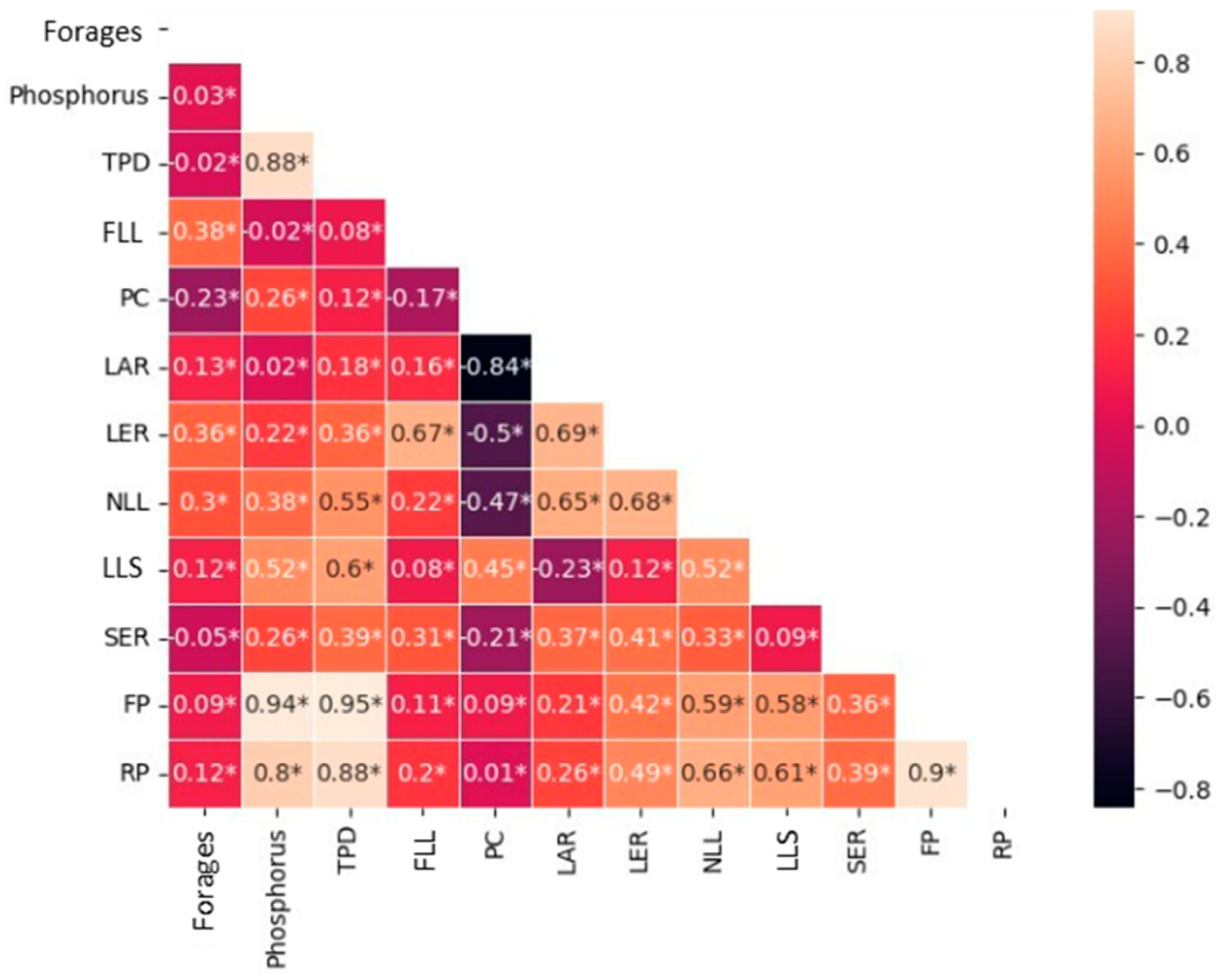
| Soil | pH | Ca2+ | Mg2+ | K+ | Al3+ | H + Al | S | T | t | V | m | MO | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | cmolc dm−3 | % | mg dm−3 | ||||||||||
| Ox | 5.04 | 1.33 | 1.36 | 0.19 | 0.08 | 5.49 | 2.87 | 8.37 | 2.96 | 34.38 | 2.92 | 3.72 | 3.31 |
| Doses of P (mg dm−3) | Equation | R2 | p-Value | MSE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 0 | 13 | 26 | 52 | 104 | ||||
| LAR | 0.12 | 0.13 | 0.13 | 0.13 | 0.12 | y = 0.12 + 0.005x − 0.0004x2 | 0.78 | <0.0001 | 0.04 |
| PC | 9.18 | 8.27 | 8.57 | 8.81 | 9.75 | y = 8.88 − 0.01x + 0.002x2 | 0.75 | 0.0349 | 0.31 |
| LLS | 41.87 | 42.01 | 46.22 | 50.23 | 49.85 | y = 40.78 +0.24x − 0.001x2 | 0.93 | 0.0356 | 1.30 |
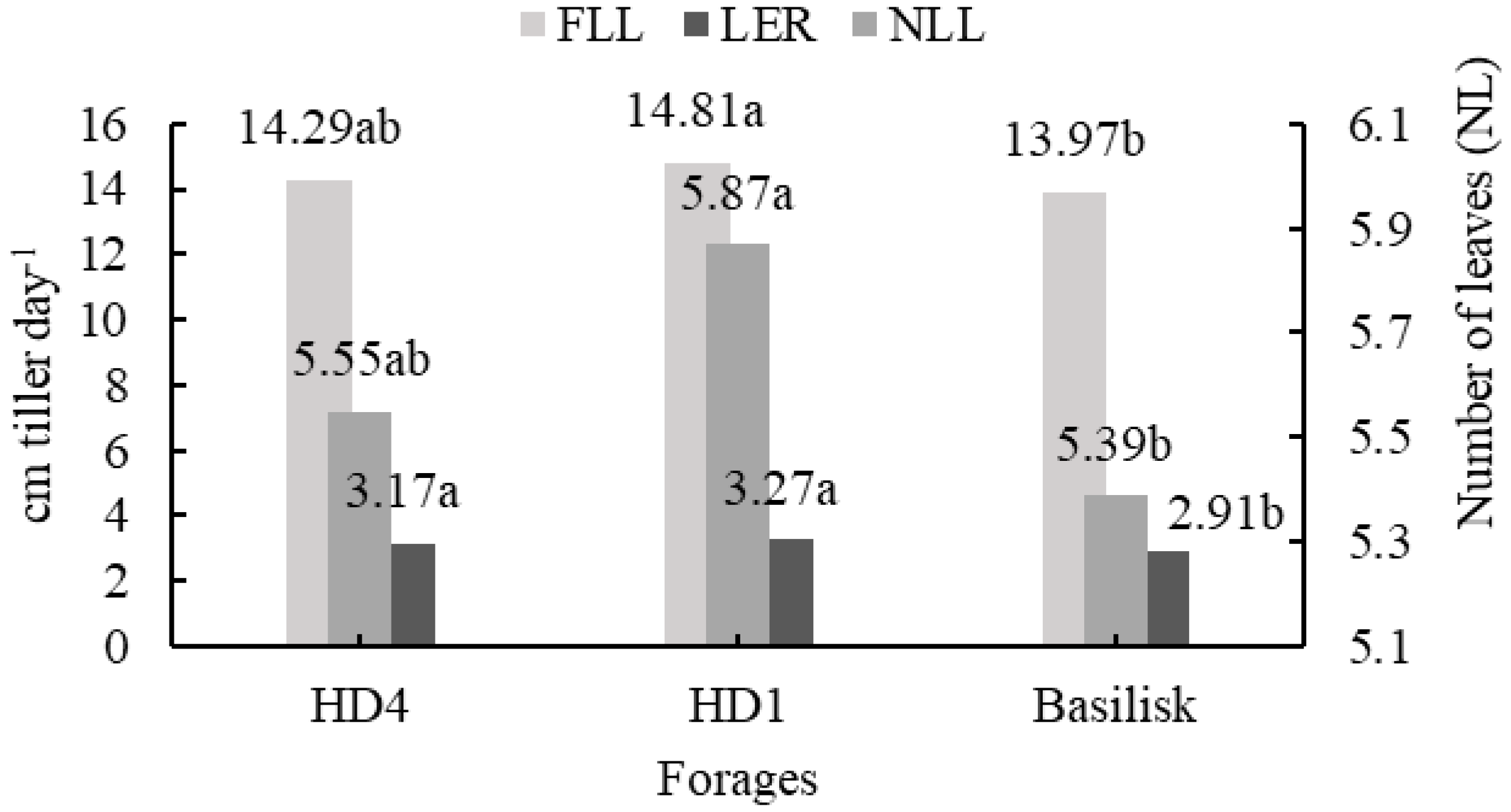
| LER | 2.78 | 3.07 | 3.23 | 3.43 | 3.09 | y = 2.16 + 0.02x − 0.001x2 | 0.99 | 0.0345 | 0.08 |
| SER | 0.81 | 0.90 | 0.90 | 0.99 | 0.93 | y = 0.71 + 0.11x − 0.14x2 | 0.81 | 0.0174 | 0.02 |
| NLL | 4.2 | 5.4 | 5.9 | 6.1 | 5.8 | y = 4.92 + 0.04x − 0.003x2 | 0.97 | <0.0001 | 0.14 |
| Doses of P (mg dm−3) | Equation | R2 | p-Value | MSE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Forage Plant | 0 | 13 | 26 | 52 | 104 | ||||
| TPD (Tillers Pot−1) | |||||||||
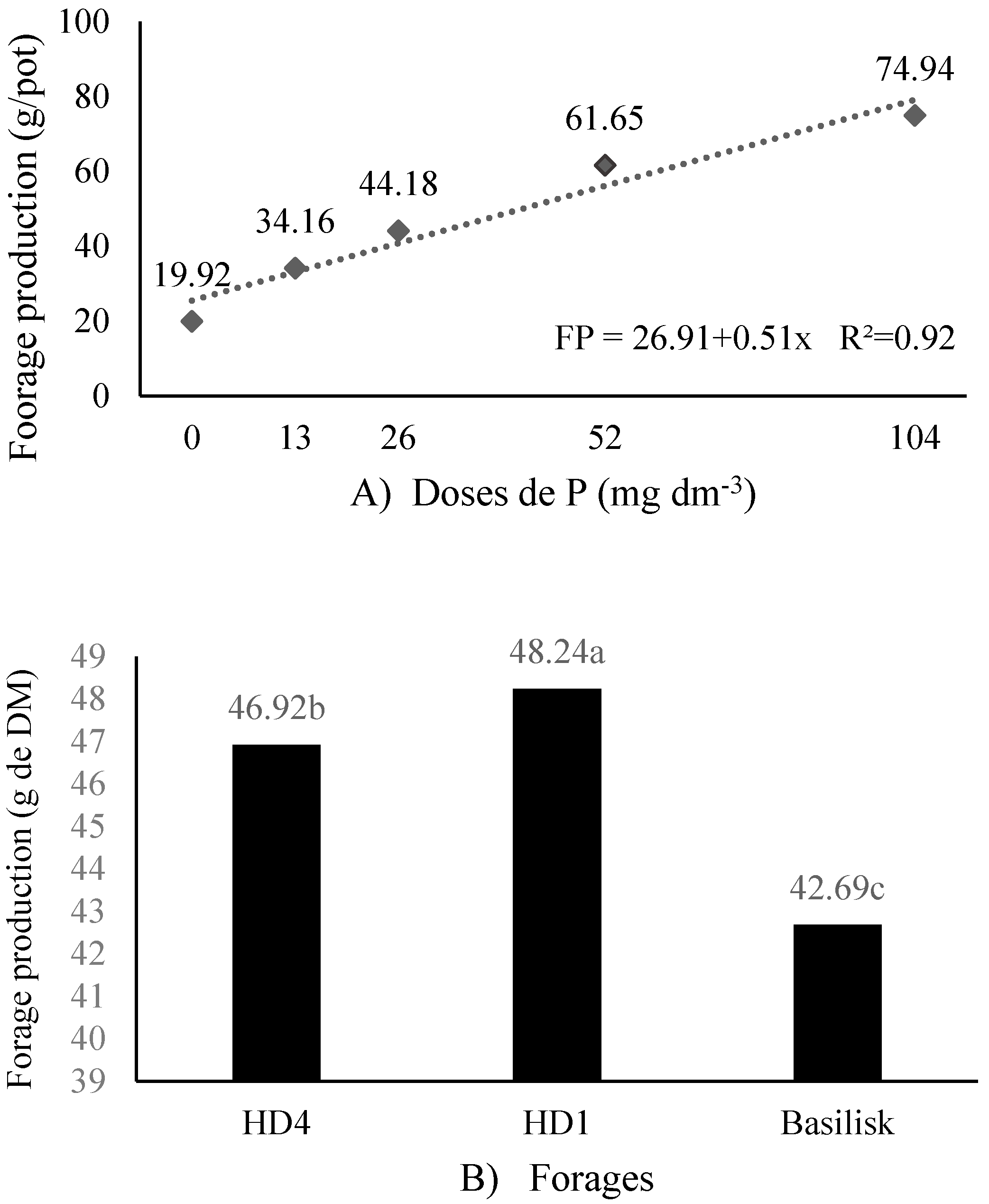
| HD4 | 11.75 b | 20.55 a | 28.00 a | 36.40 ab | 40.70 b | y = 17.17 + 0.26x | 0.84 | <0.0001 | 2.14 |
| HD1 | 15.20 ab | 23.05 a | 26.30 a | 34.50 b | 45.40 a | y = 18.01 + 0.28x | 0.97 | <0.0001 | 2.14 |
| Basilisk | 16.50 a | 22.90 a | 30.00 a | 40.05 a | 41.80 ab | y = 20.83 + 0.24x | 0.90 | <0.0001 | 2.14 |
| Doses of P (mg dm−3) | Equation | R2 | p-Value | MSE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Forage Plant | 0 | 13 | 26 | 52 | 104 | ||||
| Root Production (g of DM) | |||||||||
| HD4 | 4.67 a | 8.06 a | 10.43 a | 13.53 a | 14.92 ab | y = 6.83 + 0.09x | 0.81 | <0.0001 | 0.87 |
| HD1 | 6.83 a | 7.67 a | 9.05 a | 11.77 a | 15.70 a | y = 6.77 + 0.08x | 0.99 | <0.0001 | 0.87 |
| Basilisk | 6.91 a | 5.68 a | 10.90 a | 11.12 a | 12.16 b | y = 7.10 + 0.05x | 0.66 | <0.0001 | 0.87 |
| p-value | 0.1355 | 0.1323 | 0.3119 | 0.2729 | 0.0170 | ||||
| Forage Plant | Doses of P (mg dm−3) | p-Value | MSE | |||
|---|---|---|---|---|---|---|
| 13 | 26 | 52 | 102 | |||
| Phosphorus Use Efficiency (%) | ||||||
| HD4 | 61.49 abC | 69.35 aC | 61.79 abC | 48.07 bB | 0.0311 | 6.54 |
| HD1 | 141.69 aA | 125.12 abA | 101.73 bA | 64.02 cA | 0.0155 | 6.54 |
| Basilisk | 97.62 aB | 85.54 abB | 76.69 bB | 50.99 cB | 0.0199 | 6.54 |
| p-value | 0.0029 | 0.0074 | 0.0012 | 0.0430 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frontado, N.E.V.; Difante, G.d.S.; Araújo, A.R.d.; Montagner, D.B.; Rodrigues, J.G.; Monteiro, G.O.d.A.; Macedo, M.C.M.; Pereira, M.d.G.; Moura, A.E.S.; Arze, E.W. Phosphorus Use Efficiency: Morphogenetic and Productive Responses of Brachiaria decumbens Genotypes (Syn: Urochloa decumbens). Grasses 2025, 4, 20. https://doi.org/10.3390/grasses4020020
Frontado NEV, Difante GdS, Araújo ARd, Montagner DB, Rodrigues JG, Monteiro GOdA, Macedo MCM, Pereira MdG, Moura AES, Arze EW. Phosphorus Use Efficiency: Morphogenetic and Productive Responses of Brachiaria decumbens Genotypes (Syn: Urochloa decumbens). Grasses. 2025; 4(2):20. https://doi.org/10.3390/grasses4020020
Chicago/Turabian StyleFrontado, Néstor Eduardo Villamizar, Gelson dos Santos Difante, Alexandre Romeiro de Araújo, Denise Baptaglin Montagner, Jéssica Gomes Rodrigues, Gabriela Oliveira de Aquino Monteiro, Manuel Cláudio Motta Macedo, Marislayne de Gusmão Pereira, Amanda Eunice Silva Moura, and Eduardo Weisz Arze. 2025. "Phosphorus Use Efficiency: Morphogenetic and Productive Responses of Brachiaria decumbens Genotypes (Syn: Urochloa decumbens)" Grasses 4, no. 2: 20. https://doi.org/10.3390/grasses4020020
APA StyleFrontado, N. E. V., Difante, G. d. S., Araújo, A. R. d., Montagner, D. B., Rodrigues, J. G., Monteiro, G. O. d. A., Macedo, M. C. M., Pereira, M. d. G., Moura, A. E. S., & Arze, E. W. (2025). Phosphorus Use Efficiency: Morphogenetic and Productive Responses of Brachiaria decumbens Genotypes (Syn: Urochloa decumbens). Grasses, 4(2), 20. https://doi.org/10.3390/grasses4020020







