Abstract
Erratic rainfall and extended dry periods challenge forage production and livestock feed sustainability in dryland agriculture regions. This study investigated the effects of planting dates and genotype selection on the nutritive value and in-vitro dry matter degradability (IVDMD) of fodder radish genotypes in Midlands of KwaZulu-Natal, South Africa. The experiment followed a completely randomised design with three fodder radish genotypes (Endurance, Line 2, and Nooitgedacht) and five planting dates (December, January, February, March and May). After three months of growth in each planting date, crops were harvested, prepared and analysed for various nutritional parameters including crude protein, fibre content, and IVDMD. Results revealed that December had the highest crude protein (28–31%) across genotypes, while March plantings optimised total non-structural carbohydrates (13.31%) and metabolisable energy (6.64 MJ/kg). The Nooitgedacht genotype demonstrated improved performance, achieving higher IVDMD of 85.54% for leaves in December plantings and 77.51% for tubers in February plantings. Significant interactions between planting dates and genotypes were observed for ash, crude protein, and cellulose in leaves. In conclusion, these findings highlight the crucial role of planting date selection and genotype choice in optimising fodder radish production under dryland conditions, offering valuable insights for enhancing livestock productivity and supporting sustainable rural livelihoods.
1. Introduction
Livestock production, especially under dryland agriculture, faces growing challenges in maintaining sustainable feed production as climate variability increasingly affects forage availability and quality [1,2]. In South Africa (SA), where the livestock sector drives rural economic development, smallholder farmers struggle to maintain productive herds due to feed scarcity, particularly during dry seasons [3,4]. The country’s semi-arid climate and reliance on rainfed agriculture compound these difficulties, as farmers depend heavily on natural pastures and crop residues that provide poor nutritional value during dry periods [5,6]. These constraints significantly impact food security and rural livelihoods, where smallholder farmers make substantial contributions to local food production [7,8]. Moreover, poor feed quality and resource inefficiency lead to environmental degradation and elevated greenhouse gas emissions [9,10]. Climate change further threatens the sustainability of livestock production systems through increased rainfall variability [1,2]. Thus, there is an urgent need to identify climate-resilient forage species that combine rapid growth characteristics with high nutritional value, enabling smallholder farmers to maintain productive livestock systems while adapting to increasingly variable rainfall patterns and extended dry periods.
Raphanus sativus L. (fodder radish) is a resilient annual or biennial crop within the Brassicaceae family and has emerged as a valuable forage resource for livestock production systems [11,12]. This forage crop demonstrates significant potential through its rapid growth cycle, climate adaptability, and high nutritive value [13,14,15]. Its nutritional profile, mainly crude protein (CP), ranges from 15% to 25% in leaves and 10% to 18% in tubers [16]. The fodder radish’s ability to thrive under dryland conditions [17] aligns with smallholder farmers’ resource constraints [18]. Recent breeding advances in SA have produced two promising dual-purpose genotypes, Endurance and Line 2 [19], which significantly improve upon the Nooitgedacht cultivar’s rough leaf structure and early flowering characteristics. These novel genotypes were developed explicitly for delayed flowering and enhanced forage production during drought periods [16]. Their improved traits include softer leaf texture for increased palatability, biomass production, and elevated total non-structural carbohydrate (TNC) levels. Despite the aforementioned advantages, these novel genotypes, similar to other crops, can be affected by agronomic factors, including planting date, soil fertility, plant density, and water availability, which significantly affect their growth and forage quality [20,21,22,23].
From an agronomic viewpoint, planting date emerges as the most critical factor governing both productivity and quality of fodder radish [24], especially in water-limited environments. Supporting this notion, planting timing is vital in dryland agriculture, where water availability serves as the primary limiting factor for crop productivity and can vary depending on geographical location [25]. For instance, in New Zealand, farmers plant fodder radish in summer for late summer/early autumn use [26]. In contrast, northwestern Canadian farmers opt for late May to early June planting to enable late summer/fall grazing [27]. Australian temperate regions adhere to a February-March planting schedule for winter feed provision [28]. In SA, optimal planting occurs from February to mid-March, capitalising on late summer rainfall patterns for establishment whilst ensuring adequate bulb development before winter [13]. Despite this extensive global cultivation experience and regional planting recommendations, a significant knowledge gap persists regarding the nutritive value and in-vitro dry matter degradability (IVDMD) of fodder radish genotypes across different planting dates. This gap is particularly pronounced for newly developed genotypes adapted to water-limited environments in the Midlands of KwaZulu-Natal, SA, where local farmers are already utilising these fodder radish genotypes as winter feed without a comprehensive understanding of their quality and response to the agronomical viewpoint, including planting date responses.
While March planting is traditionally recommended in SA for fodder radish, this timing recommendation has primarily focused on optimising biomass production and energy-related nutritional parameters such as total non-structural carbohydrates and metabolisable energy [13,19,28]. However, different planting dates may optimise various nutritional aspects differently—for instance, early season plantings (December) might favour protein accumulation due to more favourable soil moisture and temperature conditions during initial growth stages. This nutritional optimisation paradox creates a situation where farmers must prioritise specific nutritional outcomes based on their livestock requirements and management systems. Moreover, the anticipated superior performance of March plantings must be considered alongside the challenges that later plantings face, particularly for May establishment. During these later plantings, crops may experience advanced maturity under suboptimal environmental conditions, leading to accelerated lignification, reduced leaf-to-stem ratios, and overall quality decline [29].
Furthermore, each fodder radish genotype responds differently to environmental stresses associated with various planting dates. The newer genotypes (Endurance and Line 2) were specifically bred for improved stress tolerance, particularly to heat and moisture limitations [16,19], but their performance across diverse planting windows remains inadequately characterised. Meanwhile, the traditional Nooitgedacht cultivar, despite its early flowering tendency and rougher leaf texture, may exhibit different tissue-specific responses to seasonal variations in temperature, photoperiod, and moisture availability. These genotype-specific responses are likely to manifest differently between leaves and tubers, as these plant structures serve distinct biological functions and follow independent developmental patterns [30,31,32]. The advanced maturity and environmental stress factors associated with later plantings (particularly May) are expected to have pronounced effects on tissue composition, accelerating lignification processes and potentially compromising digestibility and overall nutritive value for ruminants.
Therefore, this research aimed to address this knowledge gap by evaluating the effect of plating dates and fodder radish genotypes on nutritive and IVDMD. Thus, understanding the impact of planting date is crucial as it affects nutritional quality, mainly in changing climatic conditions [33]. Furthermore, the IVDMD analysis provides a cost-effective and rapid method to assess feed digestibility compared to in-vivo trials [34]. Therefore, optimal planting dates can significantly enhance fodder radish while minimising climate-related risks, making it essential for sustainable livestock production [35]. The study focused on genotypes developed or adapted in the Midlands of KwaZulu-Natal, SA recognising the importance of locally relevant solutions by conducting research that mimics smallholder farming conditions without irrigation or fertiliser application. This research contributes to improving livestock productivity and sustainability in smallholder farming systems, with findings that can inform agricultural extension services, policymakers, and farmers, ultimately supporting food security, rural livelihoods, and the resilience of agricultural systems in the face of climate variability [36]. Hence, the study hypothesised that nutritive value and IVDMD would vary among fodder radish genotypes depending on the planting date. Subsequently, March planting optimised performance across genotypes, with a slight decline in Nooitgedacht during May planting.
2. Materials and Methods
2.1. Study Site
The experimental trial was carried out at the Agricultural Research Council (ARC)-API Range and Forage Institute, located at the Cedara Research Station in the Midlands of KwaZulu-Natal, SA (29°32′15.33″ S, 30°16′09.19″ E) during the 2021 and 2022 growing seasons. The site generally experienced an average annual rainfall of 885 mm, with temperatures ranging from a minimum of 9.4 °C to a maximum of 22 °C, and a long-term mean temperature of 16 °C. During winter months, a characteristic 75-day frost period occurred from June to August. According to the Soil Classification Working Group [37], Cedara’s soil was classified as Hutton soil, characterised by its deep, red, kaolinitic nature and heavy clay loam texture. Three fodder radish genotypes, namely, Endurance, Line 2, and Nooitgedacht) were developed at ARC, except Nooitgedacht. According to [16,19], both Line 2 and Endurance were specifically bred to provide high-quality forage for livestock grazing during late winter. Moreover, these genotypes resulted from crossing a late-flowering fodder radish line “PG 1” (sourced from Pyne Gould Wrightson Seeds, New Zealand) with ARC-AP Cedara fodder radish varieties Geisha and Sterling. Both Endurance and Line 2 were selectively bred for late flowering, high biomass production, and higher TNC, a characteristic that contributes to improved milk production in dairy cattle.
2.2. Experimental Design
The experiment was established as a split-plot design within a completely randomised block arrangement, replicated three times. The main plots were formed by the three fodder radish genotypes (Endurance, Line 2 and Nooitgedacht), while the five planting dates (15 December 2021, 15 January, 15 February, 15 March and 15 May 2022) constituted the subplots. All genotypes were grown under dryland agriculture conditions with no fertiliser application. The trial encompassed a total area of 144 m2, with each individual plot measuring 6 × 6 m (36 m2). Plots were separated by a 1 m inter-plot spacing to minimise edge effects. Each subplot contained six rows spaced 1 metre apart, with 12 plants per row spaced at 0.5 metres, resulting in 72 plants per subplot. The three innermost rows were designated for data collection to minimise border effects. Before planting, soil samples were collected at a 0–15 cm depth using a soil auger and analysed for their physical and chemical properties [38,39]. The soil at the experimental site was classified as a heavy clay loam with acidic properties (pH 4.91), which is typical of the Hutton soil form in the region. The soil organic matter content was low (<2%), indicating potentially limited natural nutrient cycling capacity. The meteorological data was collected during the fodder radish genotypes’ growth period from December 2021 to August 2022, as recorded by the ARC’s Cedara weather station and presented in Table 1. Fodder radish genotypes were harvested after three months of growing, where the leaves (upper part) and tuber (underground part) were separated. They were separated for the following reasons:

Table 1.
Weather conditions at Cedara research station during the fodder radish growing season (December 2021–August 2022).
- ▪
- Different tissue functions: leaves and tubers serve distinct biological functions—leaves are photosynthetic organs while tubers are storage organs [31,32]; hence, these essential differences likely respond differently to planting dates and genotypes.
- ▪
- Resource allocation: plants balance resource distribution between leaves and tubers based on environmental conditions and developmental stage [30,40]. Thus, a specific planting date might favour leaf development while potentially reducing tuber growth, or vice versa.
- ▪
- Lastly, independent development patterns: leaves and tubers have different growth patterns and formation timing. Leaf development is more immediate and directly influenced by environmental conditions, while tuber development occurs later and depends on accumulated resources [30,31,32,40].
The freshly chopped plant samples were then dried in a forced-air oven at 60 °C for 48 h. After drying, the samples were milled to pass through a 1 mm sieve and placed in air-sealed plastic bags. They were then stored in a cold room until they were ready for further analysis.
2.3. Determination of Chemical Composition
The samples were analysed for dry matter (DM), ash, CP, ether extract (EE), neutral detergent fibre (NDF), acid detergent fibre (ADF), acid detergent lignin (ADL), cellulose (Cel), hemicellulose (Hem), and TNC. All analyses were performed in triplicate to ensure accuracy and reproducibility. Dry matter and ash content were determined following the procedures described by [41] (methods 934.01 and 942.05, respectively). Approximately 1 g of each sample was weighed into pre-weighed crucibles and oven-dried at 105 °C for 12 h to determine DM content. The dried samples were ashed in a muffle furnace at 550 °C for 12 h to determine ash content. Crude protein content was determined using the macro-method [41] (method 984.13) by multiplying nitrogen content by 6.25. The ether extract was determined using the Soxhlet method with petroleum ether as the solvent, as described by [41] (method 920.39). Fibre fractions (NDF, ADF, and ADL) were determined sequentially using the procedure described by [42], with modifications by Ankom Technology (Macedon, NY, USA). Heat-stable α-amylase and sodium sulphite were used in the NDF determination. Cellulose was calculated as the difference between ADF and ADL, while hemicellulose was calculated as the difference between NDF and ADF, as described by [43]. The TNC was determined using the standard wet chemistry method, as detailed by [44].
2.4. Estimated Feeding Value
Estimated feeding value were calculated using chemical composition values according to the following formulas. The relative feed value (RFV%) was calculated using Equation (1), where DMD is dry matter digestibility and DMI is dry matter intake, as described by Moore and Undersander [45]. Following this, digestible energy (DE, Mcal/kg) was determined using Equation (2), which establishes the relationship with DMD% as detailed by Fonnesbeck et al. [46]. Subsequently, metabolisable energy (ME%) was calculated using Equation (3) [47].
RFV% = (%DMD × %DMI)/1.29
DE (Mcal/kg) = 0.27 + 0.0428 (DMD%)
ME% = 0.821 × DE
2.5. Detection of Soluble Sugars
The procedure described by [48] was followed to determine soluble sugars. Briefly, a sample of 0.1 grams (g) dry weight was mixed with 10 mL of 80% v/v ethanol/H2O and homogenised for 1 min. The mixture was then incubated at a temperature of 80°C for 1 h in a water bath. After incubation, the samples were refrigerated at 4 °C for 24 h and then centrifuged at 10,000 rpm for 15 min in a refrigerated centrifuge at 4 °C. The samples were filtered through glass wool and dried overnight in a GenVac personal evaporator (EZ-2.3, SP scientific, Genevac Ltd., Ipswich, UK). The dried samples were resuspended in 2 mL of ultrapure water and filtered through a 0.4-micron nylon syringe filter before being examined using an HPLC (LC 20 AT, Shimadzu Corporation, Kyoto, Japan) equipped with a refractive index detector (RID-10 A, Shimadzu Corporation, Kyoto, Japan) and a Raze RCMmonosaccharide column (300 mm 7.8 mm) (8 mm pore size; Phenomenex, Torrance, CA, USA). The content of each detected sugar was determined by comparing it with authentic standards, and the units were presented in mg/g.
2.6. In-Vitro Dry Matter Degradability
The in-vitro study was conducted at the North-West University, Mahikeng, Northwest province, SA (25°49′22″ S and 25°36′54″ E), situated 1290 m above sea level. This region experiences temperatures fluctuating between 11 °C and 38 °C, with annual precipitation averaging 450 mm. In-vitro dry matter degradability (IVDMD) of forage samples was determined using the ANKOM DaisyII Incubator (ANKOM Technology Corp., Fairport, NY, USA), which consists of a thermostatic chamber (39 °C) with four circling jars, following the [49] protocol. Samples weighing 0.45–0.5 g were placed in ANKOM F57 filter bags, heat-sealed, and distributed among the digestion jars. Two buffer solutions were prepared and combined at a 1:5 ratio. Each jar received 1600 mL of the combined buffer, prewarmed to 39 °C. Rumen fluid was obtained from two fistulated Bonsmara cows (approximately 550 kg) fed a diet of Lucerne and Cenchrus ciliaris grass hay. The study adhered to protocols approved by the Animal Ethics Committee of the University of KwaZulu-Natal (reference number AREC/00004427/2022). The rumen fluid was collected in pre-warmed thermos flasks and promptly transported to the laboratory. It was mixed and strained through two layers of warm muslin cloth there. Each jar received 400 mL of the prepared rumen inoculum, the ANKOM buffer, and F57 bags. The jars were continuously purged with CO2 gas during the 39 °C incubation period to maintain anaerobic conditions. The F57 filter bags were removed at 48 hours (h) after incubation and sample bags were rinsed with cold running water for 20 min and dried for 12 h at 105 °C. The bags were weighed before and after this process to calculate IVDMD. The formula used for calculating IVDMD was as follows:
where: W1 = bag tare weight, W2 = sample weight, W3 = final bag weight after in-vitro treatment, C1 = blank bag correction factor (final oven-dried weight ÷ original blank bag weight).
2.7. Statistical Description
The nutritive value and IVDMD were evaluated using a two-way analysis of variance (ANOVA) in GenStat 23rd edition software [50]. The analysis examined two fixed factors: five planting dates (December, January, February, March, and May) and three genotypes (Endurance, Line 2, and Nooitgedacht). Given the distinct biological functions and independent developmental patterns of leaves and tubers [31,32], separate analyses were conducted to evaluate planting date and genotype interactions for each tissue type, as these organs might respond uniquely to environmental conditions. This analytical approach enabled precise interpretation of treatment effects while preventing complex interfering interactions from masking tissue-specific responses. This approach aligns with the study by [16,46], who also separated radish roots/tubers from leaves when performing chemical characterisation and antioxidant capacity, as well as IVDMD analyses. Subsequently, in cases where no significant interaction was detected between planting dates and genotypes, one-way ANOVA was performed for each factor. Shapiro-Wilk and Bartlett’s tests were used to validate the normality and homogeneity of variances for each parameter. For parameters that failed to meet these assumptions, double log transformation successfully achieved both normality and homogeneity of variances. Mean comparisons were conducted using Tukey’s post-hoc test, with statistical significance determined at p < 0.05.
3. Results
3.1. Chemical Composition, Estimated Feeding Value and Soluble Sugars
The results revealed significant planting date × genotype interactions for several nutritional parameters (Table 2 and Table 3). In leaf (Table 2), Nooitgedacht achieved the highest ash (19.46%) and CP (31.23%) during December plantings when moderate temperatures (25.49 °C/14.72 °C) and adequate rainfall (209.8 mm) prevailed, whilst ADL reached maximum values (12.49%) in May during cooler conditions (8.27 °C minimum). Line 2 demonstrated optimal TNC (13.31%) in March under moderate temperatures with declining humidity (48.83%), and highest fructose (13.50 mg/g) in December when temperature and moisture conditions favoured carbohydrate accumulation. In tubers (Table 3), January plantings, characterised by peak rainfall (241.55 mm) and temperatures (27.83 °C/16.49 °C), yielded the highest ash content across genotypes, with Endurance recording maximum values (27.18%) compared to the lowest values in May for Nooitgedacht (7.37%). Line 2 exhibited the highest glucose (10.07 mg/g) in December, whilst ADL peaked in May plantings across all genotypes (13.03–13.36%) under cooler, drier conditions, representing a 152% increase compared to the lowest values observed in March for Line 2 (5.30%).

Table 2.
Mean ± standard error (SE) for effects of genotype and planting date on Ash content, crude protein, cellulose, total non-structural carbohydrates and fructose of fodder radish leaves.

Table 3.
Mean ± standard error (SE) for effects of genotype and planting date on Ash content, acid detergent lignin and glucose of fodder radish tubers.
Relative feed value in leaves was significantly (p < 0.030) higher in Endurance (43.77) compared to other genotypes (Figure 1), demonstrating its superior digestibility and potential intake characteristics. In contrast, DM, EE, NDF, ADF, ADL, Hem, TDN, ME, DE, glucose, and sucrose showed no significant genotypic differences (p > 0.05), and were therefore omitted from the presentation. Most nutritional parameters were significantly affected by the planting date (Table 4). Specifically, May plantings, characterised by lower temperatures (8.27 °C minimum) and reduced rainfall (117.35 mm), produced the highest DM (92.03%) and fibre levels, with NDF reaching 40.28% and ADL 12.49%. March plantings, under moderate temperatures (27.17 °C/15.44 °C) and declining humidity (48.83%), had maximum DE (23.63 Mcal/kg) and ME (6.64 MJ/kg), showing 12.4% improvements over the lowest January values. With optimal rainfall (209.80 mm) and moderate temperatures, December plantings consistently accumulated the highest concentrations of soluble sugars, with glucose and sucrose reaching 6.74 mg/g and 6.16 mg/g, respectively.
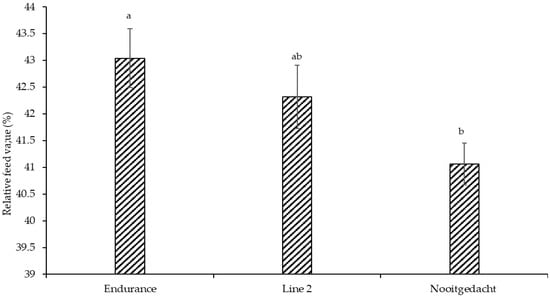
Figure 1.
Mean (±SE) for the effect of genotype relative feed value in leaves. Different superscripts indicate significant differences.

Table 4.
Mean (±SE) for the effect of planting date on chemical composition, estimated feeding value and soluble sugars of fodder radish leaves.
Dry matter varied significantly (p = 0.028) among genotypes, with Nooitgedacht showing highest values (91.83%) compared to Line 2 (90.22%) and Endurance (89.68%), highlighting genotypic differences in resource allocation to tuber development (Figure 2). In contrast, EE, CP, NDF, ADF, Hem, TDN, RFV, ME, DE, fructose, and sucrose showed no significant genotypic differences (p > 0.05) and were therefore omitted from presentation. With respect to planting date, January, characterised by peak rainfall (241.55 mm) and optimal temperatures (27.83 °C/16.49 °C), produced the highest CP content in tubers (21.03%), whilst March exhibited the lowest CP values (14.16%) (Table 5). Furthermore, NDF and ADF were markedly higher in May plantings under cooler, drier conditions, reaching 40.05% and 30.42%, respectively, compared to their minimum March values of 27.78% and 18.67%. Moreover, March plantings, with moderate temperatures (27.17 °C/15.44 °C) and declining humidity (48.83%), exhibited superior TDN (72.18%), DE (23.26 Mcal/kg), and ME (6.54 MJ/kg) values, whilst December plantings, under optimal early-season conditions, demonstrated the highest fructose (7.30 mg/g) and sucrose (5.69 mg/g) concentrations, compared to their lowest values of 0.58 mg/g and 1.08 mg/g observed in May and March, respectively.
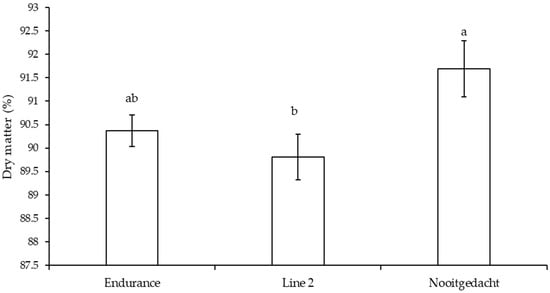
Figure 2.
Mean (±SE) for the effect of genotype dry matter in tubers. Different superscripts indicate significant differences.

Table 5.
Mean (±SE) for the effect of planting date on chemical composition, estimated feeding value and soluble sugars of fodder radish tubers.
3.2. In-Vitro Dry Matter Degradability
The results detected that the 48-h IVDMD of leaves demonstrated significant interactions between planting date and genotype (p < 0.001), with both factors showing significance (p < 0.001) (Figure 3). The Nooitgedacht genotype planted in December exhibited the highest leaf IVDMD (85.54%), whilst Line 2 in May showed the lowest (69.94%) under cooler, drier conditions. Likewise, 48-h IVDMD of tubers revealed significant interactions between planting date and genotype (p = 0.025), where planting date showed significant differences (p < 0.001), whereas genotype showed no significance (p = 0.289) (Figure 4). The Nooitgedacht genotype planted in February, under moderate temperatures (27.84 °C/17.17 °C) and declining rainfall (79.51 mm), produced tubers with maximum IVDMD values (77.51%), whilst Line 2 in May, under cooler temperatures (8.27 °C minimum), yielded the lowest tuber IVDMD (64.61%). Overall, the Nooitgedacht genotype consistently outperformed others in both leaves and tubers, with optimal digestibility achieved through December plantings for leaves and February plantings for tubers, demonstrating this genotype’s superior response to the early and mid-season establishment under favourable climatic conditions.
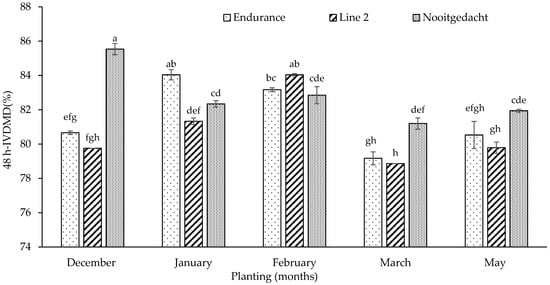
Figure 3.
Effect of planting dates and genotypes on 48 h-IVDMD of fodder radish leaves. Different superscripts indicate significant differences.
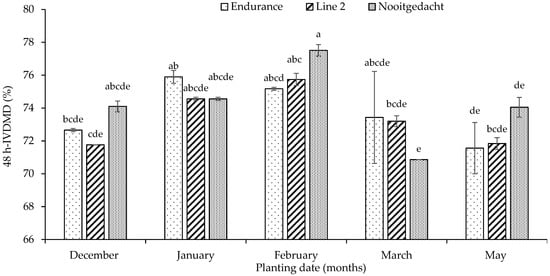
Figure 4.
Effect of planting dates and genotypes on 48 h-IVDMD of fodder radish tubers. Different superscripts indicate significant differences.
4. Discussion
4.1. Chemical Composition
The results supported the hypothesis that planting dates would affect fodder radish genotypes’ nutritive value and IVDMD. However, the hypothesis regarding March plantings optimising performance across genotypes was only partially supported. Whilst March plantings did optimise TNC (13.31%) and ME (6.64 MJ/kg), December plantings produced higher CP levels (28–31%) across genotypes. This nutritional optimisation paradox highlights that no single planting date optimises all quality parameters simultaneously—while March plantings favour energy-related metrics (TNC, ME), December plantings better support protein accumulation. This finding is significant since it demonstrates the need for farmers to prioritise specific nutritional targets based on their livestock requirements rather than adhering to a single “optimal” planting window. The hypothesis regarding Nooitgedacht’s decline in May was supported, as evidenced by increased NDF (40.28%) and ADF (30.42%) contents during this period, likely due to advanced maturity and environmental stress factors [51,52]. This quality decline in May plantings can be attributed to two primary factors: first, the accelerated maturation process triggered by declining temperatures and photoperiod changes, and second, the physiological stress responses induced by suboptimal growing conditions. Plants encountering environmental stress typically divert resources toward protective mechanisms, including increased lignification and structural carbohydrate production, at the expense of protein synthesis and nutrient uptake [53]. This decline aligns with previous studies showing that late-season plantings often reduce forage quality due to accelerated lignification under declining temperatures [54,55].
The high CP observed in December plantings (28–31%) across genotypes represents a significant improvement over conventional dryland forages available during dry seasons. Therefore, this improvement is vital since local feed resources during prolonged dry seasons fail to meet ruminant nutritional needs, with CP falling below 7% [55]. Moreover, all the CP levels across genotypes exceed the minimum requirement of 7–8% needed for effective rumen function in cattle [42] and approach levels found in legume forages like alfalfa (18–22%) [56], which is considered as a king of forage. These findings align with comparative studies of radish leaf and tuber content conducted in different ecological contexts. For instance, Goyeneche et al. [57] found that red radish cultivars grown in temperate Mediterranean conditions exhibited CP values of 15–22% in leaves, substantially lower than our December plantings but comparable to our May results. Similarly, Keogh et al. [58] observed CP ranges of 18–24% in Brassica varieties grown in Irish conditions, highlighting our South African genotypes’ superior protein accumulation capacity under local conditions. Furthermore, previous research by Barry [59] demonstrated that Brassica species grown in New Zealand exhibited variable CP content (16–24%) depending on environmental conditions, suggesting that the unique combination of temperature, photoperiod, and moisture conditions in our December plantings created an optimal environment for protein synthesis. Although studies in forage Brassica, including fodder radish in some regions, have reported CP ranges of 18.6–25.5% [60], our findings suggest that these dryland genotypes perform exceptionally well across different planting dates. The difference in CP between the aforementioned studies and our findings may be attributed to differences in management practices, environmental conditions, and other biological factors, as supported by [61]. The CP quality of most brassica crops, including fodder radish, is highly digestible, with effective rumen degradability rates exceeding 75% [59,60], making them excellent protein sources for ruminant nutrition. Moreover, studies have demonstrated that brassica forages can maintain protein content better than traditional forages under water-limited conditions [62,63].
Higher NDF and ADF contents were detected in May planting, reflecting typical maturity-related plant cell wall composition changes and the physiological stress responses as discussed earlier. Although these values remain more favourable than mature grass hay (65–75% NDF), they approach the threshold where fibre content begins limiting voluntary ruminant intake [64]. This limitation is significant since NDF levels above 45% can reduce ruminant DM intake [64]. The relationship between NDF and DM intake has been well established, with studies showing a decrease of 0.17% in intake for each percentage unit increase in NDF above 45% [65,66]. Moreover, the observed ADF values align with findings from studies on other brassica forages, where environmental stress increased lignification [67,68]. Studies into the cell wall composition of brassica forages have revealed that their fibre structure maintains higher digestibility than grass species at similar maturity stages [69,70]. This unique characteristic of the Brassicaceae family is attributed to differences in lignin composition and cross-linking patterns with structural carbohydrates [71], resulting in more readily degradable fibre despite comparable quantitative measurements. Furthermore, the relatively lower content of indigestible components in brassica cell walls than in grasses contributes to their superior digestibility even under stress conditions. Despite increased fibre content, these findings explain the relatively favourable IVDMD values observed in May plantings.
Beyond fibre content, the variation in TNC levels presents another promising aspect, with March plantings achieving peak levels (13.31%) in Line 2. This improvement is because environmental conditions promote higher photosynthetic efficiency during cool season periods like March while reducing respiratory losses [72], creating an optimal environment for TNC accumulation in forage plants. Interestingly, this pattern differs from the findings by Francisco et al. [69], who reported peak TNC accumulation in Brassica rapa during warmer periods in temperate European conditions, highlighting the unique response of our dryland-adapted genotypes to the specific environmental conditions of KwaZulu-Natal. The nutritional importance of these findings is emphasised by research demonstrating that forages with higher TNC levels can improve microbial protein synthesis [73]. Studies have shown that increasing TNC can enhance rumen microbial efficiency by up to 15% [74] and improve nitrogen utilisation by reducing urinary nitrogen losses [75]. The concurrent availability of protein degradation and energy has been identified as a key factor in optimising rumen fermentation patterns [76,77].
4.2. Estimated Feeding Value
Endurance’s improved RFV performance further demonstrates the potential for genetic improvement to enhance forage quality and ruminant nutrition outcomes. Even Nooitgedacht, despite its lower RFV, consistently exceeded the nutritional quality of natural pastures during dry seasons [78]. Research has shown that genetic selection for improved RFV can produce substantial gains in animal performance [79], particularly in optimising rumen fermentation patterns and microbial protein synthesis [80]. Progressive breeding programs targeting enhanced fibre digestibility have improved dairy cattle’s DM intake and milk production [81]. Modern genomic selection techniques have accelerated the development of superior forage varieties with enhanced RFV characteristics that directly benefit rumen function and nutrient utilisation [82]. The ME content showed significant variation, reaching its peak in March plantings (6.64 MJ/kg). This energy level proves particularly valuable as it exceeds maintenance requirements for small ruminants and approaches levels needed for moderate growth [83]. Some studies have confirmed that ME values above 6.5 MJ/kg can support daily gains of 100–150 g in small ruminants under dryland conditions [84], with enhanced rumen microbial protein synthesis and improved nitrogen utilisation efficiency [85]. These nutritional characteristics and optimal rumen-degradable protein levels have improved volatile fatty acid production and overall rumen fermentation efficiency [86], leading to better feed conversion ratios and reduced methane emissions in ruminants [87].
4.3. Soluble Sugars
The differential accumulation of soluble sugars across planting dates demonstrates the crucial effect of environmental conditions on carbohydrate metabolism in fodder radish. The higher sugar concentrations observed in December plantings align with findings by Hajihashemi et al. [88], who reported that optimal temperature and moisture conditions enhance photosynthetic efficiency and subsequent carbohydrate synthesis. Some studies highlighted that variations in sugar accumulation among Brassica genotypes reflect differences in carbohydrate partitioning strategies, with some cultivars prioritising fructose accumulation while others favour glucose or sucrose synthesis [89,90]. The glucose-dominated sugar profile observed in earlier plantings is particularly beneficial. Saidi et al. [91] identified glucose as promoting more efficient volatile fatty acid production patterns than other sugar types. The decline in sugar concentrations during later planting dates likely results from metabolic adaptations to suboptimal growing conditions. Fletcher et al. [92] stated that Brassica crops subjected to temperature stress redirect carbohydrates toward protective compounds rather than storage sugars. According to Shao et al. [93], this metabolic shift is a fundamental stress response mechanism that diverts energy from growth and storage processes toward defence pathways. Under environmental stress conditions, plants allocate more carbon to secondary metabolites and structural components, resulting in reduced accumulation of soluble sugars as part of a comprehensive physiological adaptation strategy [94].
Comparative studies from other ecological zones confirm these findings. Research by Bhandari et al. [89] examining cabbage cultivars grown in temperate zones found that soluble sugar concentrations were 30–45% higher in optimal growing seasons than in stress periods, closely mirroring our observed decline from December to May plantings. Likewise, Ghafoor et al. [90], studying Brassica napus in semi-arid conditions, reported that early-season plantings accumulated 20–35% more soluble sugars than late-season plantings, further validating our findings. These cross-ecological comparisons highlight that Brassica species exhibit similar carbohydrate metabolism responses to environmental cues regardless of geographical region, with optimal growing conditions consistently favouring higher sugar accumulation.
4.4. In-Vitro Dry Matter Degradability
The improved performance of Nooitgedacht was evident through its highest IVDMD of 85.54% when planted in December for leaves, which synergistically aligned with its high CP (Table 2). Notably, the strong correlation between high IVDMD and CP indicates enhanced microbial protein synthesis in the rumen, whereby higher protein availability coupled with improved degradability leads to more efficient fermentation and nutrient utilisation by rumen microorganisms [87,95]. The observed IVDMD values in this study (70–85%) were comparatively lower than those reported by [60], who found IVDMD ranges of 88.7–91.9% in forage brassicas during fall grazing. Nevertheless, our findings demonstrated improved degradability compared to [16], who reported IVDMD values ranging from 60–80% for fodder radish under various water regimes, thus suggesting that optimal planting dates may enhance forage digestibility beyond water management alone. Consequently, IVDMD variations across these studies reflect differences in environmental conditions, genotypes, and management practices, with values ranging from 60–91.9% depending on growing conditions and cultivar selection.
The contrasting IVDMD patterns observed between leaves and tubers across genotypes and planting dates reveal intriguing tissue-specific responses that merit further examination. Particularly notable is Nooitgedacht’s superior leaf IVDMD in December (85.54%) compared to its relatively lower tuber IVDMD performance during the same period (73.47%), while it exhibited peak tuber IVDMD in February (77.51%). This tissue-specific response is likely attributable to several factors. First, the differential allocation of nutrients between tissues during different growth phases plays a crucial role. Favourable conditions in early plantings (December) support rapid leaf development with minimal lignification, resulting in highly digestible foliar tissue [96]. This aligns with findings by Goyeneche et al. [57], who observed that red radish leaves contained significantly lower structural carbohydrate content than tubers during early growth stages, contributing to their superior digestibility. Second, the physiological maturity of tubers in February plantings creates an optimal balance between starch accumulation and structural development. By mid-season, tubers have accumulated sufficient non-structural carbohydrates while maintaining relatively low lignin content, creating an ideal composition for rumen degradability [97,98].
Comparative studies by Barry [59] on various Brassica species found that root tissues reached peak digestibility during mid-season growth, with significant declines in later maturities, supporting our February optimisation findings for tubers. Finally, the differential response of tissue types to environmental factors explains the contrasting seasonal patterns. Leaf tissues respond more immediately to ambient temperature and moisture conditions, while tuber development follows a more programmed developmental pattern with delayed environmental responses [30,40]. This explains why December conditions optimised leaf IVDMD, while February conditions were more conducive to tuber digestibility. These findings align with those reported by Villalobos and Brummer [60], who found that Brassica tissue types exhibited independent digestibility responses to environmental conditions across growing seasons.
4.5. Practical Implications for Farmers
The findings from this study have significant practical implications for farmers, particularly regarding the implementation of optimal planting schedules for fodder radish. While our results demonstrate the nutritional benefits of specific planting windows (December for CP, March for energy parameters), converting these findings into practical farm management strategies requires careful consideration of farmers’ logistical challenges and resource constraints. One primary challenge is the resource allocation dilemma created by staggered planting recommendations. Smallholder farmers in SA typically face labour constraints during peak agricultural seasons [4], making it difficult to implement sequential planting schedules across December, February, and March, as would be optimal for balancing protein and energy objectives. Additionally, the limited availability of tillage equipment during optimal planting windows can force compromises, as farmers must prioritise either protein-optimised (December) or energy-optimised (March) planting dates based on their specific livestock needs.
Water resource management presents another practical barrier, particularly for dryland farmers. Despite the superior CP content achieved with December plantings, this timing coincides with uncertain early-season rainfall in many parts of KwaZulu-Natal, creating establishment risks for resource-limited farmers without irrigation capacity [7]. While offering superior energy characteristics, March plantings have risks related to potentially declining soil moisture as the dry season approaches. Seed cost and availability also influence practical implementation decisions. The newer genotypes (Endurance and Line 2) often command premium prices and may have limited availability compared to traditional varieties like Nooitgedacht [19]. This economic consideration must be balanced against the demonstrated performance benefits, particularly for resource-constrained smallholder operations. To address these practical barriers, a pragmatic approach for smallholder farmers might involve dividing available land into two planting windows (December and March) when possible, prioritising Nooitgedacht for December plantings to optimise protein production, and utilising either Endurance or Line 2 for March plantings to maximise energy parameters. Alternatively, for farmers unable to implement split planting schedules, February plantings offer a reasonable compromise, delivering balanced nutritional profiles with acceptable, though not optimal, levels of both protein and energy parameters. These practical recommendations acknowledge the real-world constraints faced by farmers while still enabling them to capitalise on the key nutritional findings from this research.
5. Conclusions
In conclusion, this study underlines the critical role of planting date selection and genotype choice in fodder radish cultivation for enhancing feed quality and availability in smallholder farming systems. The findings reveal that early planting in December maximises CP levels, while March plantings optimise TNC and ME content. Nooitgedacht demonstrated superior performance among the genotypes evaluated, exhibiting the highest IVDMD when planted in December for leaves and February for tubers. These results have far-reaching implications for revolutionising livestock production systems in dryland regions. Fodder radish’s high nutritive value and digestibility can significantly reduce reliance on expensive protein supplements, improve rumen function, and foster more resilient and sustainable farming practices. To further advance this research, it is recommended that future studies investigate the impact of season and environment on fodder radish growth and nutritional composition. Conducting grazing trials to evaluate dietary selection and preference by ruminants would provide valuable insights into the practical application of fodder radish in livestock feeding systems. Moreover, exploring the potential of using fodder radish as a protein supplement to low-quality grasses, such as Eragrostis curvula, during extended dry periods could offer a promising strategy for mitigating feed scarcity and maintaining animal productivity.
Author Contributions
Conceptualization, L.M. and N.R.M.; methodology, L.M., N.R.M., L.N., S.M., L.S., M.S.N. and P.N.R.; software, L.M. and S.M.; validation, N.R.M., T.D.E.M., Z.T.R.-K. and TJ.T; formal analysis, L.M.; investigation, L.M. and N.R.M.; resources, L.S., S.M. and L.M.; data curation, L.M., S.M. and N.R.M.; writing—original draft preparation, L.M.; writing—review and editing, L.M., N.R.M., T.D.E.M., Z.T.R.-K., L.N., M.S.N., P.N.R., S.M., L.S. and T.J.T.; visualization, L.M.; supervision, N.R.M., T.D.E.M. and Z.T.R.-K.; project administration, L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from the Range and Forage Unit of the Agricultural Research Council. The authors thank the National Research Foundation (Grant number: 118141 and 139101) and FoodBev SETA Bursary for awarding a doctoral scholarship to Lwando Mbambalala at the University of KwaZulu-Natal.
Institutional Review Board Statement
The study adhered to protocols approved by the Animal Ethics Committee of the University of KwaZulu-Natal (reference number AREC/00004427/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from this study are available from the corresponding author upon request.
Acknowledgments
We extend our gratitude to the technical staff and general workers of the Agricultural Research Council at Cedara Research Station for their invaluable assistance in land preparation, planting, and management of fodder radish. Our appreciation also goes to the laboratory technicians of the KwaZulu-Natal Department of Agriculture and Rural Development’s Soil Fertility and Analytical Service for their laboratory support. We are particularly grateful to Dave Goodenough and Alan Stewart for their significant contributions to the breeding of Endurance and Line 2 genotypes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Archer, E.; Landman, W.; Malherbe, J.; Tadross, M.; Pretorius, S. South Africa’s winter rainfall region drought: A region in transition? Clim. Risk Manag. 2019, 25, 100188. [Google Scholar] [CrossRef]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.J.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C.; et al. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2022, 17, 024009. [Google Scholar] [CrossRef]
- Mapiye, C.; Chikwanha, O.C.; Chimonyo, M.; Dzama, K. Strategies for sustainable use of indigenous cattle genetic resources in Southern Africa. Diversity 2020, 11, 214. [Google Scholar] [CrossRef]
- Aliber, M.; Hall, R. Support for smallholder farmers in South Africa: Challenges of scale and strategy. Dev. S. Afr. 2012, 29, 548–563. [Google Scholar] [CrossRef]
- Müller, C.; Cramer, W.; Hare, W.L.; Lotze-Campen, H. Climate change risks for African agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 4313–4315. [Google Scholar] [CrossRef] [PubMed]
- Amole, T.A.; Zijlstra, M.; Descheemaeker, K.; Ayantunde, A.A.; Duncan, A.J. Assessment of lifetime performance of small ruminants under different feeding systems. Animal 2022, 11, 1024. [Google Scholar] [CrossRef]
- Pienaar, L.; Traub, L.N. Understanding the smallholder farmer in South Africa: Towards a sustainable livelihood’s classification. In Proceedings of the International Conference of Agricultural Economists, Milan, Italy, 9–14 August 2015. [Google Scholar] [CrossRef]
- Sinyolo, S.; Mudhara, M.; Wale, E. The impact of social grant dependency on smallholder maize producers’ market participation in South Africa: Application of the double-hurdle model. S. Afr. J. Econ. Manag. Sci. 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Scholtz, M.M.; Van Ryssen, J.B.J.; Meissner, H.H.; Laker, M.C. A South African perspective on livestock production in relation to greenhouse gases and water usage. S. Afr. J. Anim. Sci. 2013, 43, 247–254. [Google Scholar] [CrossRef]
- Panchasara, H.; Samrat, N.H.; Islam, N. Greenhouse gas emissions trends and mitigation measures in Australian agriculture sector—A review. Agriculture 2021, 11, 85. [Google Scholar] [CrossRef]
- Rethman, N.F.G.; Heyns, G. Grazing of Raphanus sativus L. (Japanese radish). J. Grassl. Soc. S. Afr. 1987, 4, 154. [Google Scholar] [CrossRef]
- Altman, B.; Hayes, M.; Janes, S.; Forbes, R. Wildlife of westside grassland and chaparral habitats. In Wildlife-Habitat Relationships in Oregon and Washington; Johnson, D.H., O’Neil, T.A., Eds.; Oregon State University Press: Corvallis, OR, USA, 2001; pp. 261–291. [Google Scholar]
- Ammann, S.; Nash, D.; Goodenough, D. Fodder radish for autumn and winter: Technology. Dairy Mail 2009, 16, 70–71. [Google Scholar]
- Tsytsiura, Y.H. Evaluation of the efficiency of oil radish agrofitocenosis construction by the factor of reproductive effort. Bulg. J. Agric. Sci 2019, 25, 1161–1174. [Google Scholar]
- Mbambalala, L.; Rani, Z.T.; Mpanza, T.D.E.; Mthana, M.S.; Ncisana, L.; Mkhize, N.R. Fodder radish as a potential alternative feed source for livestock in South Africa. Agriculture 2023, 13, 1625. [Google Scholar] [CrossRef]
- Ncisana, L.; Mabhaudhi, T.; Mkhize, N.R.; Ravhuhali, K.; Tjelele, T.J.; Nyathi, M.K.; Mbambalala, L.; Msiza, N.H.; Nzeru, M.S.; Modi, A.T. Water regimes in selected fodder radish (Raphanus sativus) genotypes: Effects on nutritional value and in vitro ruminal dry matter degradability. Heliyon 2024, 10, e29203. [Google Scholar] [CrossRef]
- Tsytsiura, Y.H. Modular-vitality and ideotypical approach in evaluating the efficiency of construction of oilseed radish agrophytocenosises (Raphanus sativus var. oleifera Pers.). Agraarteadus 2020, 31, 219–243. [Google Scholar]
- Mutengwa, C.S.; Mnkeni, P.; Kondwakwenda, A. Climate-smart agriculture and food security in Southern Africa: A review of the vulnerability of smallholder agriculture and food security to climate change. Sustainability 2023, 15, 2882. [Google Scholar] [CrossRef]
- Rakau, P. New fodder radish varieties can boost dairy production. In Food for Thought; Johns Hopkins University Press: Baltimore, MD, USA, 2021. [Google Scholar]
- Jahanzad, E.; Jorat, M.; Moghadam, H.; Sadeghpour, A.; Chaichi, M.R.; Dashtaki, M. Response of a new and a commonly grown forage sorghum cultivar to limited irrigation and planting density. Agric. Water Manag. 2013, 117, 62–69. [Google Scholar] [CrossRef]
- Abbas, G.; Younis, H.; Naz, S.; Fatima, Z.; Hussain, S.; Ahmed, M.; Ahmad, S. Effect of planting dates on agronomic crop production. In Agronomic Crops: Volume 1: Production Technologies; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 131–147. [Google Scholar]
- Chand, S.; Indu; Singhal, R.K.; Govindasamy, P. Agronomical and breeding approaches to improve the nutritional status of forage crops for better livestock productivity. Grass Forage Sci. 2022, 77, 11–32. [Google Scholar] [CrossRef]
- Melo, C.D.; Maduro Dias, C.S.; Wallon, S.; Borba, A.E.; Madruga, J.; Borges, P.A.; Ferreira, M.T.; Elias, R.B. Influence of climate variability and soil fertility on the forage quality and productivity in Azorean pastures. Agriculture 2022, 12, 358. [Google Scholar] [CrossRef]
- Verschoor, A.; Rethman, N.F.G. Forage potential of Japanese radish (Raphanus sativus) as influenced by planting date and cultivar choice. J. Grassl. Soc. S. Afr. 1992, 9, 176–177. [Google Scholar] [CrossRef]
- Bussmann, A.; Elagib, N.A.; Fayyad, M.; Ribbe, L. Sowing date determinants for Sahelian rainfed agriculture in the context of agricultural policies and water management. Land Use Policy 2016, 52, 316–328. [Google Scholar] [CrossRef]
- Stewart, A.V.; Moorhead, A.J. The development of a fodder radish suitable for multiple grazing. Agron. N. Z. 2004, 34, 1–7. [Google Scholar]
- Omokanye, A.; Hernandez, G.; Lardner, H.A.; Al-Maqtari, B.; Gill, K.S.; Lee, A. Alternative forage feeds for beef cattle in Northwestern Alberta, Canada: Forage yield and nutritive value of forage brassicas and forbs. J. Appl. Anim. Res. 2021, 49, 203–210. [Google Scholar] [CrossRef]
- Bell, L.W.; Watt, L.J.; Stutz, R.S. Forage brassicas have the potential for wider use in drier, mixed crop-livestock farming systems across Australia. Crop Pasture Sci. 2020, 71, 924–943. [Google Scholar] [CrossRef]
- Nordheim-Viken, H.; Volden, H.; Jørgensen, M. Effects of maturity stage, temperature and photoperiod on growth and nutritive value of timothy (Phleum pratense L.). Anim. Feed Sci. Technol. 2009, 152, 204–218. [Google Scholar] [CrossRef]
- Stitt, M.; Schulze, E.D. Plant growth, storage, and resource allocation: From flux control in a metabolic chain to the whole plant level. In Flux Control in Biological Systems; Schulze, E.D., Ed.; Academic Press: San Diego, CA, USA, 1994; pp. 57–118. [Google Scholar]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; van Biljon, A.; Ceronio, G.; Labuschagne, M. Effects of planting date, environments and their interaction on grain yield and quality traits of maize hybrids. Heliyon 2023, 9, e21660. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Kumar, P.; Abubakar, A.A.; Verma, A.K.; Umaraw, P.; Ahmed, M.A.; Mehta, N.; Hayat, M.N.; Kaka, U.; Sazili, A.Q. New insights in improving sustainability in meat production: Opportunities and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 11830–11858. [Google Scholar] [CrossRef]
- Tibesigwa, B.; Visser, M. Assessing gender inequality in food security among small-holder farm households in urban and rural South Africa. World Dev. 2016, 88, 33–49. [Google Scholar] [CrossRef]
- Soil Classification Working Group. Soil Classification: A Taxonomic System for South Africa; Department of Agricultural Devel-opment: Pretoria, South Africa, 1991; Volume 15. [Google Scholar]
- Manson, A.D.; Roberts, V.G. Analytical Methods Used by the Soil Fertility and Analytical Services Section; KwaZulu-Natal Agri-Report; Republic of South Africa: Pietermaritzburg, South Africa, 2000; N/A/2001/04; pp. 1–6. [Google Scholar]
- Hunter, A. New techniques and equipment for routine soil/plant analytical procedures. In Soil Management in Tropical America; Borremiza, E., Alvarado, A., Eds.; North Carolina State University: Raleigh, NC, USA, 1975. [Google Scholar]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and tuberous root development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of hemicellulose, cellulose, holocellulose and lignin content using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef]
- Marais, J.P.; De Figueiredo, M.; Goodenough, D.C.W. Dry matter and non-structural carbohydrate content as quality parameters in a Lolium multiflorum breeding programme. Afr. J. Range Forage Sci. 1993, 10, 118–124. [Google Scholar] [CrossRef]
- Jeranyama, P.; Garcia, A.D. Understanding Relative Feed Value (RFV) and Relative Forage Quality (RFQ). Ext. Extra 2004, 352, 1–4. [Google Scholar]
- Fonnesbeck, P.V.; Clark, D.H.; Garret, W.N.; Speth, C.F. Predicting energy utilization from alfalfa hay from the Western Region. In Proceedings of the Cornell Nutrition Conference for Feed Manufacture, Ithaca, NY, USA, 19–20 August 2002; Cornell University: Ithaca, NY, USA, 2002. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, Seventh Revised Edition; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006. [Google Scholar]
- ANKOM Technology. In-Vitro True Digestibility Using the Daisy II Incubator; ANKOM Technology: Macedon, NY, USA, 2005. [Google Scholar]
- Payne, R.W.; Murray, D.A.; Harding, S.A. GenStat for Windows, 23rd ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Nair, J.; Beattie, A.D.; Christensen, D.; Yu, P.; McAllister, T.; Damiran, D.; McKinnon, J.J. Effect of variety and stage of maturity at harvest on nutrient and neutral detergent fiber digestibility of forage barley grown in western Canada. Can. J. Anim. Sci. 2018, 98, 299–310. [Google Scholar] [CrossRef]
- Rakszegi, M.; Darkó, É.; Lovegrove, A.; Molnár, I.; Láng, L.; Bedő, Z.; Molnár-Láng, M.; Shewry, P. Drought stress affects the protein and dietary fiber content of wholemeal wheat flour in wheat/Aegilops addition lines. PLoS ONE 2019, 14, e0211892. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xu, C.; Li, X.; Ferguson, I.; Chen, K. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Moyo, M.; Nsahlai, I. Consequences of increases in ambient temperature and effect of climate type on digestibility of forages by ruminants: A meta-analysis in relation to global warming. Animals 2021, 11, 172. [Google Scholar] [CrossRef]
- Norton, B.W.; Moran, J.B.; Nolan, J.V. Nutrient requirements of domesticated ruminants in tropical regions. Aust. J. Agric. Res. 2009, 60, 214–228. [Google Scholar]
- Yari, M.; Valizadeh, R.; Naserian, A.A.; Jonker, A.; Yu, P. Modeling nutrient availability of alfalfa hay harvested at three stages of maturity and in the afternoon and morning in dairy cows. Anim. Feed Sci. Technol. 2012, 178, 12–19. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Keogh, B.; McGrath, T.; Grant, J. The effect of sowing date and nitrogen on the dry-matter yield and nitrogen content of forage rape (Brassica napus L.) and stubble turnips (Brassica rapa L.) in Ireland. Grass Forage Sci. 2012, 67, 2–12. [Google Scholar] [CrossRef]
- Barry, T.N. The feeding value of forage brassica plants for grazing ruminant livestock: A review. Anim. Feed Sci. Technol. 2013, 181, 15–25. [Google Scholar] [CrossRef]
- Villalobos, L.A.; Brummer, J.E. Forage brassicas stockpiled for fall grazing: Yield and nutritive value. Crop Forage Turfgrass Manag. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Palmer, C.D.; Keller, W.; Singh, J.; Datla, R. Brassica crop species: Improving water use efficiency: Challenges and opportunities. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp. 1301–1314. [Google Scholar] [CrossRef]
- Watt, L.J.; Bell, L.W.; Pembleton, K.G. A forage brassica simulation model using APSIM: Model calibration and validation across multiple environments. Eur. J. Agron. 2022, 137, 126517. [Google Scholar] [CrossRef]
- Mertens, D.R. Regulation of forage intake. In Forage Quality, Evaluation, and Utilization; Fahey, G.C., Jr., Ed.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 450–493. [Google Scholar]
- Oba, M.; Allen, M.S. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: Effects on dry matter intake and milk yield of dairy cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Vazquez, O.P.; Smith, T.R. Factors affecting pasture intake and total dry matter intake in grazing dairy cows. J. Dairy Sci. 2000, 83, 2301–2309. [Google Scholar] [CrossRef]
- Dong, L.F.; Peng, J.I.A.; Li, B.C.; Wang, B.; Yang, C.L.; Liu, Z.H.; Diao, Q.Y. Quantification and prediction of enteric methane emissions from Chinese lactating Holstein dairy cows fed diets with different dietary neutral detergent fiber/non-fibrous carbohydrate (NDF/NFC) ratios. J. Integr. Agric. 2022, 21, 797–811. [Google Scholar] [CrossRef]
- Pereira, M.C.S.; Yang, W.Z.; Beauchemin, K.A.; McAllister, T.A.; Wood, K.M.; Penner, G.B. Effect of forage types differing in undigested neutral detergent fiber concentration and forage inclusion rate on reticulo-ruminal motility and fermentation, total tract barrier function, and blood metabolites of finishing beef heifers. J. Anim. Sci. 2023, 101, skad043. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Velasco, P.; Lema, M.; Cartea, M.E. Genotypic and environmental effects on agronomic and nutritional value of Brassica rapa. Agron. J. 2011, 103, 735–742. [Google Scholar] [CrossRef]
- Eryilmaz, O. Revalorization of cellulosic fibre extracted from the waste stem of Brassica oleracea var. botrytis L. (cauliflower) by characterizing for potential composite applications. Int. J. Biol. Macromol. 2024, 266, 131086. [Google Scholar] [CrossRef]
- McCartney, D.; Fraser, J.; Ohama, A. Potential of warm-season annual forages and Brassica crops for grazing: A Canadian Review. Can. J. Anim. Sci. 2009, 89, 431–440. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Pelletier, S.; Tremblay, G.F.; Bertrand, A.; Bélanger, G.; Castonguay, Y.; Michaud, R. Drying procedures affect non-structural carbohydrates and other nutritive value attributes in forage samples. Anim. Feed Sci. Technol. 2010, 157, 139–150. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Jones, E.L.; Moorby, J.M.; Humphreys, M.O.; Theodorou, M.K.; Scollan, N.D. Production responses from lambs grazed on Lolium perenne selected for an elevated water-soluble carbohydrate concentration. Anim. Res. 2002, 51, 441–449. [Google Scholar] [CrossRef]
- Miller, L.A.; Moorby, J.M.; Davies, D.R.; Humphreys, M.O.; Scollan, N.D.; MacRae, J.C.; Theodorou, M.K. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne L.): Milk production from late-lactation dairy cows. Grass Forage Sci. 2001, 56, 383–394. [Google Scholar] [CrossRef]
- Edwards, G.R.; Parsons, A.J.; Rasmussen, S.; Bryant, R.H. High sugar ryegrasses for livestock systems in New Zealand. Proc. N. Z. Grassl. Assoc. 2007, 69, 161–171. [Google Scholar] [CrossRef]
- Leng, R.A. Factors affecting the utilization of ‘poor-quality’ forages by ruminants, particularly under tropical conditions. Nutr. Res. Rev. 1990, 3, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Krehbiel, C.R. Invited Review: Applied nutrition of ruminants: Fermentation and digestive physiology. Prof. Anim. Sci. 2014, 30, 129–139. [Google Scholar] [CrossRef]
- Moore, J. Relative forage quality: An alternative to relative feed value and quality index. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 10 January 2002; University of Florida: Gainesville, FL, USA, 2002. [Google Scholar]
- Casler, M.D.; Vogel, K.P. Accomplishments and impact from breeding for increased forage nutritional value. Crop Sci. 1999, 39, 12–20. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane mitigation from ruminants using tannins and saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Combs, D.K. TTNDFD: A new approach to evaluate forages. In Proceedings of the Western States Alfalfa and Forage Symposium, Reno, NV, USA, 2–4 December 2015; University of California: Davis, CA, USA, 2015; pp. 113–118. [Google Scholar]
- Barrett, B.A.; Faville, M.J.; Nichols, S.N.; Simpson, W.R.; Bryan, G.T.; Conner, A.J. Breaking through the feed barrier: Options for improving forage genetics. Anim. Prod. Sci. 2015, 55, 883–892. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson Education Limited: London, UK, 2011. [Google Scholar]
- Ben Salem, H.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rumin. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Karsli, M.; Russell, J.R. Effects of some dietary factors on ruminal microbial protein synthesis. Turk. J. Vet. Anim. Sci. 2001, 25, 681–686. [Google Scholar]
- Chen, P.; Li, Y.; Shen, Y.; Cao, Y.; Li, Q.; Wang, M.; Liu, M.; Wang, Z.; Huo, Z.; Ren, S.; et al. Effect of dietary rumen-degradable starch to rumen-degradable protein ratio on in vitro rumen fermentation characteristics and microbial protein synthesis. Animals 2022, 12, 2633. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.; Djalovic, I.; Siddique, K.H. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Choi, C.S.; Rhee, J.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Lee, J.G. Seasonal variation in agronomic characteristics and sugar content of cabbage genotypes. Chil. J. Agric. Res. 2021, 81, 80–91. [Google Scholar] [CrossRef]
- Ghafoor, A.; Karim, H.; Asghar, M.A.; Raza, A.; Hussain, M.I.; Javed, H.H.; Shafiq, I.; Xiao, P.; Yue, H.; Ahmad, B.; et al. Carbohydrates accumulation, oil quality and yield of rapeseed genotypes at different nitrogen rates. Plant Prod. Sci. 2021, 25, 50–69. [Google Scholar] [CrossRef]
- Saidi, R.; Ziadi, M.; Bouazizi, S.; Bouallagui, H.; Hamdi, M. Biohydrogen and volatile fatty acids production from prickly pear cladodes (Opuntia ficus indica) as renewable feedstock. Euro-Mediterr. J. Environ. Integr. 2025, 10, 1–12. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Sinton, S.M.; Gillespie, R.N.; Maley, S.; Sim, R.E.; de Ruiter, J.M.; Meenken, E.D. Drought response and water use efficiency of forage brassica crops. Agron. N. Z. 2010, 40, 105–117. [Google Scholar]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.A.; Pandey, I.K. Metabolites and Abiotic Stress Tolerance in Plants. In Advancements in Developing Abiotic Stress-Resilient Plants; CRC Press: Boca Raton, FL, USA, 2022; pp. 287–304. [Google Scholar]
- Zain, M.; Tanuwiria, U.H.; Syamsu, J.A.; Yunilas, Y.; Pazla, R.; Putri, E.M.; Makmur, M.; Amanah, U.; Shafura, P.O.; Bagaskara, B. Nutrient digestibility, characteristics of rumen fermentation, and microbial protein synthesis from Pesisir cattle diet containing non-fiber carbohydrate to rumen degradable protein ratio and sulfur supplement. Vet. World 2024, 17, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Forkelová, L.; Hartmann, H. Release of resource constraints allows greater carbon allocation to secondary metabolites and storage in winter wheat. Plant Cell Environ. 2017, 40, 672–685. [Google Scholar] [CrossRef]
- Raffrenato, E.; Fievisohn, R.; Cotanch, K.W.; Grant, R.J.; Chase, L.E.; Van Amburgh, M.E. Effect of lignin linkages with other plant cell wall components on in vitro and in vivo neutral detergent fiber digestibility and rate of digestion of grass forages. J. Dairy Sci. 2017, 100, 8119–8131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).