Assessing the Repeatability and Reliability of NIRS to Predict Nutritional Values and to Evaluate Two Lignin Methods in Urochloa spp. Grasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Urochloa spp. Samples
2.2. Laboratory Analysis
2.2.1. Standard Reference Methods (SRM)
2.2.2. In Vitro Digestibility
2.3. NIRS Procedures
Multivariate Calibration
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analyses
3.2. NIRS Model Calibrations and Internal Validation
3.3. External Validation of NIRS Calibration Models
3.3.1. Statistical Performance of External Validation Assessed by NIRS
3.3.2. Correlation between SRM and NIRS Methods
3.3.3. Association between SRM and NIRS Methods
3.4. Lignin NIRS-Prediction through ABL or ADL Methods
4. Discussion
4.1. Descriptive Analyses
4.2. NIRS Model Calibrations and Internal Validation
4.3. External Validation of NIRS Calibration Models
4.4. Lignin NIRS-Prediction through ABL or ADL Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutai, C.; Njuguna, J.; Ghimire, S. Brachiaria Grasses (Brachiaria spp.) harbor a diverse bacterial community with multiple attributes beneficial to plant growth and development. MicrobiologyOpen 2017, 6, e00497. [Google Scholar]
- Umami, N.; Widodo, S.; Suhartanto, B.; Suwignyo, B.; Suseno, N.; Noviandi, C.T. The effect of planting material on nutrient quality and production of brachiaria spp. In Yogyakarta, Indonesia. Pak. J. Nutr. 2018, 17, 671–676. [Google Scholar]
- Euclides, V.P.B.; Nantes, N.N.; Montagner, D.B.; De Araújo, A.R.; Barbosa, R.A.; Zimmer, A.H.; Valle, C.B.D. Beef cattle performance in response to Ipyporã and Marandu brachiariagrass cultivars under rotational stocking management. Rev. Bras. Zootec. 2018, 47. [Google Scholar] [CrossRef]
- Wassie, W.A.; Tsegay, B.A.; Wolde, A.T.; Limeneh, B.A. Evaluation of morphological characteristics, yield and nutritive value of Brachiaria grass ecotypes in northwestern Ethiopia. Agric. Food Secur. 2018, 7, 89. [Google Scholar]
- Lascano, C.E.; Schmidt, A.; Barahona, R. Forage Quality and the Environment. In Proceedings of the International Grassland Congress, São Paulo, Brazil, 11–21 February 2001; pp. 1–19. [Google Scholar]
- Deinum, B.; Sulastri, R.D.; Zeinab, M.H.J.; Maassen, A. Effects of light intensity on growth, anatomy and forage quality of two tropical grasses (Brachiaria brizantha and Panicum maximum var. trichoglume). Neth. J. Agric. Sci. 1996, 44, 111–124. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. The Application of near Infrared Reflectance Spectroscopy (NIRS) to Forage Analysis. In Forage Quality, Evaluation, and Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 406–449. [Google Scholar]
- Bell, M.J.; Mereu, L.; Davis, J. The Use of Mobile Near-Infrared Spectroscopy for Real-Time Pasture Management. Front. Sustain. Food. Syst. 2018, 2, 76. [Google Scholar]
- Harris, P.A.; Nelson, S.; Carslake, H.B.; Argo, C.M.; Wolf, R.; Fabri, F.B.; Brolsma, K.M.; van Oostrum, M.J.; Ellis, A.D. Comparison of NIRS and Wet Chemistry Methods for the Nutritional Analysis of Haylages for Horses. J. Equine Vet. Sci. 2018, 71, 13–20. [Google Scholar]
- Conrad, H.; Weiss, W.; Odwongo, W.; Shockey, W. Estimating Net Energy Lactation from Components of Cell Solubles and Cell Walls. J. Dairy Sci. 1984, 67, 427–436. [Google Scholar]
- Weiss, W.; Conrad, H.; Pierre, N.S. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed. Sci. Technol. 1992, 39, 95–110. [Google Scholar]
- Moreira-Vilar, F.C.; Siqueira-Soares, R.D.C.; Finger-Teixeira, A.; de Oliveira, D.M.; Ferro, A.P.; da Rocha, G.J.; Ferrarese, M.D.L.L.; dos Santos, W.D.; Ferrarese-Filho, O. The Acetyl Bromide Method Is Faster, Simpler and Presents Best Recovery of Lignin in Different Herbaceous Tissues than Klason and Thioglycolic Acid Methods. PLoS ONE 2014, 9, e110000. [Google Scholar]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed. Sci. Technol. 2015, 201, 25–37. [Google Scholar]
- Lowry, J.B.; Conlan, L.L.; Schlink, A.C.; McSweeney, C.S. Acid detergent dispersible lignin in tropical grasses. J. Sci. Food Agric. 1994, 65, 41–49. [Google Scholar]
- Kondo, T.; Mizuno, K.; Zuee, S. Short Report, Variation in Solubilities of Lignin in Acid Detergent and in Alkali. Jpn. J. Grassl. Sci. 1987, 33, 296–299. [Google Scholar]
- Monrroy, M.; Gutiérrez, D.; Miranda, M.; Hernández, K.; García, J.R. Determination of Brachiaria spp. forage quality by near-infrareD spectroscopy anD partial least squares regression. J. Chil. Chem. Soc. 2017, 62, 3472–3477. [Google Scholar]
- Norris, K.H.; Barnes, R.F.; Moore, J.E.; Shenk, J.S. Predicting Forage Quality by Infrared Replectance Spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar]
- Givens, D.I.; De Boever, J.L.; Deaville, E.R. The principles, practices and some future applications of near infrared spectroscopy for predicting the nutritive value of foods for animals and humans. Nutr. Res. Rev. 1997, 10, 83–114. [Google Scholar]
- Mazabel, J.; Worthington, M.; Castiblanco, V.; Peters, M.; Arango, J. Using near infrared reflectance spectroscopy for estimating nutritional quality of Brachiaria humidicola in breeding selections. Agrosyst. Geosci. Environ. 2020, 3, e20070. [Google Scholar]
- Simeone, M.; Gontijo Neto, M.M.; Guimaraes CD, C.; Medeiros, E.; Barrocas, G.; Pasquini, C. Use of NIR and PLS to Predict Chemical Composition of Brachiaria. In Proceedings of the 17th International Conference on Near Infrared Spectroscopy, Foz do Iguaçú, Brazil, 18–23 October 2015. [Google Scholar]
- AOAC. Official Methods of Analysis—Animal Feed. In Official Methods of Analysis, 17th ed.; Association of Officiating Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- van Soest, P.J.; Mason, V.C. The influence of the Maillard reaction upon the nutritive value of fibrous feeds. Anim. Feed. Sci. Technol. 1991, 32, 45–53. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Fukushima, R.S.; Hatfield, R.D. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food. Chem. 2001, 49, 3133–3139. [Google Scholar]
- Van Soest, P.J. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Fukushima, R.S.; Kerley, M.S. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin method. J. Agric. Food Chem. 2011, 59, 3505–3509. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression—A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar]
- Chen, J.; Zhu, R.; Xu, R.; Zhang, W.; Shen, Y.; Zhang, Y. Evaluation of Leymus chinensis quality using near-infrared reflectance spectroscopy with three different statistical analyses. PeerJ 2015, 2015, e1416. [Google Scholar]
- Rushing, J.B.; Saha, U.K.; Lemus, R.; Sonon, L.; Baldwin, B.S. Analysis of Some Important Forage Quality Attributes of Southeastern Wildrye (Elymus glabriflorus) Using Near-Infrared Reflectance Spectroscopy. Am. J. Analyt. Chem. 2016, 7, 642–662. [Google Scholar]
- Balehegn, M.; Varijakshapanicker, P.; Zampaligre, N.; Blummel, M.; Ayantunde, A.; Jones, C.; Prasad, K.V.S.V.; Duncan, A.; Dejene, M.; Adegbola, T. Near-infrared reflectance spectroscopy for forage nutritive value analysis in sub-Saharan African countries. Agron. J. 2022, 114, 1001–1014. [Google Scholar]
- Berauer, B.J.; Wilfahrt, P.A.; Reu, B.; Schuchardt, M.A.; Garcia-Franco, N.; Zistl-Schlingmann, M.; Dannenmann, M.; Kiese, R.; Kühnel, A.; Jentsch, A. Predicting forage quality of species-rich pasture grasslands using vis-NIRS to reveal effects of man-agement intensity and climate change. Agric. Ecosyst. Environ. 2020, 296, 106929. [Google Scholar]
- Yang, Z.; Nie, G.; Pan, L.; Zhang, Y.; Huang, L.; Ma, X.; Zhang, X. Development and validation of near-infrared spectroscopy for the prediction of forage quality parameters in Lolium multiflorum. PeerJ 2017, 2017, e3869. [Google Scholar]
- NASEM. National Academy of Sciences, Engineering, and Medicine, Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- NASEM. National Academies of Sciences, Engineering, and Medicine, Nutrient Requirements of Dairy Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Filho, C.V.S.; de Andrade Rodrigues, L.R.; Perri, S.H. Produção e valor nutritivo de dez gramíneas forrageiras na região Noroeste do Estado de São Paulo. Acta Sci. Agron. 2008, 24, 1377–1384. (In Portuguese) [Google Scholar]
- Balsalobre, M.A.A.; Corsi, M.; Santos, P.M.; Penati, M.A.; Demetrio, C.G.B. Cinética da degradação ruminal do capim Tanzânia irrigado sob três níveis de resíduo pós-pastejo. Rev. Bras. Zootec. 2003, 32 (Suppl. 1), 1747–1762. (In Portuguese) [Google Scholar]
- Balsalobre, A.M.; Corsi, M.; Santos, P.M.; Vieira, I.; Cárdenas, R.R. Composição Química e Fracionamento do Nitrogênio e dos Carboidratos do Capim—Nutriotinal Quality of Irrigated Tanzaniagrass under Three Post Grazed Stubbles Intensities. Rev. Bras. Zootec. 2003, 32, 519–528. (In Portuguese) [Google Scholar]
- ASTM E1655; Standard Practices For Infrared Multivariate Quantitative Analysis. ASTM: West Conshohocken, PA, USA, 2012.
- Kohn, R.A.; Kalscheur, K.F.; Hanigan, M. Evaluation of Models for Balancing the Protein Requirements of Dairy Cows. J. Dairy Sci. 1998, 81, 3402–3414. [Google Scholar]
- Nie, Z.; Han, J.; Liu, T.; Liu, X. Hot Topic: Application of support vector machine method in prediction of alfalfa protein fractions by near infrared reflectance spectroscopy. J. Dairy Sci. 2008, 91, 2361–2369. [Google Scholar]
- Rabotnikof, C.M.; Planas, G.M.; Colomer, J.S.; Stritzler, N.P. Near infrared reflectance spectroscopy (NIRS) for predicting forage quality of perennial warm-season grasses in La Pampa, Argentina. Ann. Zootech. 1995, 44, 97–100. [Google Scholar]
- Pires, F.F.; Prates, Ê.R. Uso da técnica da espectrofotometria de refletância no infravermelho proximal (NIRS) na predição da composição química da alfafa (medicago sativa, L.). Rev. Bras. Zootec. 1998, 27, 1076–1081. (In Portuguese) [Google Scholar]
- Freitas, J.C.; Santos, S.A.; Tomich, T.R.; Franco, G.L. Predição do valor nutritivo de gramínea nativa e exótica no pantanal por meio do método de reflectância no infravermelho próximo. Veterinária Zootec. 2016, 23, 251–259. (In Portuguese) [Google Scholar]
- Clark, D.H.; Lamb, R.C. Near Infrared Reflectance Spectroscopy, A Survey of Wavelength Selection to Determine Dry Matter Digestibility. J. Dairy Sci. 1991, 74, 2200–2205. [Google Scholar]
- Euclides, V.P.B.; Macedo, M.C.M.; Valle, C.B.D.; Difante, G.D.S.; Barbosa, R.A.; Cacere, E.R. Valor nutritivo da forragem e produção animal em pastagens de Brachiaria brizantha. Pesqui. Agropecu. Bras. 2009, 44, 98–106. (In Portuguese) [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Barry, M.C. Soluble Lignin and Its Relation To Klason Lignin, Acid-Detergent Lignin and Digestibility of NDF; Cornell University: Itheca, NY, USA, 2016; pp. 1–12. [Google Scholar]
- Jung, H.G.; Allen, M.S. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar]
- Jung, H.G.; Mertens, D.R.; Payne, A.J. Correlation of Acid Detergent Lignin and Klason Lignin with Digestibility of Forage Dry Matter and Neutral Detergent Fiber. J. Dairy Sci. 1997, 80, 1622–1628. [Google Scholar]
- Jung, H.G.; Vogel, K.P. Influence of Lignin on Digestibility of Forage Cell Wall Material. J. Anim. Sci. 1986, 62, 1703–1712. [Google Scholar]
- Fukushima, R.S.; Hatfield, R.D. Comparison of the Acetyl Bromide Spectrophotometric Method with Other Analytical Lignin Methods for Determining Lignin Concentration in Forage Samples. J. Agric. Food Chem. 2004, 52, 3713–3720. [Google Scholar]
- Ozaki, Y.; Christy, A.A.; McClure, W.F. (Eds.) Near-Infrared Spectroscopy in Food Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
| Analyte | Mean | Minimum | Maximum | SD 1 | CV 2 |
|---|---|---|---|---|---|
| Ash | 76.05 | 40.77 | 124.98 | 17.60 | 23.1 |
| Cell wall | 809.82 | 670.12 | 916.44 | 53.13 | 6.56 |
| Neutral detergent fiber | 761.52 | 575.42 | 879.24 | 60.74 | 7.98 |
| Acid detergent fiber | 489.14 | 314.31 | 611.55 | 58.81 | 12.0 |
| Acid detergent lignin | 71.57 | 37.88 | 111.52 | 15.83 | 22.1 |
| Acetyl bromide lignin | 114.77 | 61.12 | 173.9 | 20.54 | 17.9 |
| IVDMD 3 | 602.73 | 331.65 | 791.62 | 93.31 | 15.5 |
| IVNDFD 4 | 543.26 | 298.91 | 740.17 | 90.88 | 16.7 |
| Analyte | Mean | Minimum | Maximum | SD | CV |
|---|---|---|---|---|---|
| Ash | 76.29 | 40.77 | 124.9 | 17.49 | 22.93 |
| Cell wall | 811.6 | 670.1 | 916.4 | 54.05 | 6.66 |
| Neutral detergent fiber | 768.2 | 575.4 | 879.2 | 58.84 | 7.66 |
| Acid detergent fiber | 494.2 | 314.3 | 611.5 | 57.84 | 11.70 |
| Acid detergent lignin | 102.4 | 60.93 | 151.4 | 19.86 | 19.39 |
| Acetyl bromide lignin | 113.8 | 61.12 | 173.9 | 20.32 | 17.86 |
| IVDMD 1 | 598.7 | 331.6 | 791.6 | 93.28 | 15.58 |
| IVNDFD 2 | 542.4 | 298.9 | 740.1 | 90.77 | 16.73 |
| Analyte | SRM | NIRS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | SD | CV | Mean | Minimum | Maximum | SD | CV | |
| Ash | 74.27 | 49.84 | 107.5 | 18.7 | 25.2 | 73.3 | 47.2 | 102.5 | 15.73 | 21.5 |
| Cell wall | 795.9 | 711.9 | 859.4 | 44.35 | 5.57 | 796.1 | 701.1 | 865.4 | 47.53 | 5.97 |
| Neutral detergent fiber | 708.7 | 616.4 | 800.9 | 50.91 | 7.18 | 741.3 | 641.9 | 803.3 | 48.66 | 6.56 |
| Acid detergent fiber | 451.2 | 341.2 | 549.5 | 52.9 | 11.7 | 470.8 | 364.7 | 547.1 | 47.33 | 10.1 |
| Acid detergent lignin | 81.33 | 58.37 | 119.2 | 15.11 | 18.6 | 68.8 | 51.9 | 95.8 | 12.25 | 17.8 |
| Acetyl bromide lignin | 123.9 | 69.88 | 155.4 | 22.47 | 18.1 | 121.9 | 69.9 | 155.3 | 21.29 | 17.5 |
| IVDMD 1 | 632.3 | 445.8 | 774.1 | 90.31 | 14.3 | 639.4 | 491.6 | 784.6 | 76.37 | 11.9 |
| IVNDFD 2 | 560.1 | 372.7 | 708.5 | 99.99 | 17.9 | 567.1 | 398.8 | 774.7 | 92.37 | 16.3 |
| Analyte | Pre-Treatment 1 | PC 2 | n3 (C/V) | Range (C/V) | Q-Value 4 | SEC 5 | SEP 6 | r 7 (C/V) | R2 8(C/V) | Bias 9 (C/V) | SD 10 (C/V) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash | n01 11, dg1 12 | 4 | 180/94 | 40.8–112.8/48.4–107.8 | 0.66 | 0.6 | 0.65 | 0.91/0.90 | 0.83/0.81 | 0/−0.07 | 0.60/0.65 |
| mf | 4 | 180/94 | 40.8–112.8/48.4–107.8 | 0.53 | 0.99 | 0.95 | 0.72/0.79 | 0.52/0.62 | 0/−0.23 | 1.00/0.95 | |
| CW | mf | 3 | 190/102 | 670.1–916.4/672.0–901.2 | 0.65 | 1.88 | 1.86 | 0.93/0.94 | 0.97/0.89 | 0/0.15 | 1.88/1.86 |
| sa3 13 | 3 | 190/102 | 670.1–916.4/672.0–901.2 | 0.65 | 1.85 | 1.86 | 0.93/0.94 | 0.87/0.89 | 0/0.23 | 1.85/1.86 | |
| NDF | sa3 | 4 | 200/94 | 623.6–879.2/631.7–847.2 | 0.64 | 1.72 | 1.51 | 0.95/0.96 | 0.91/0.92 | 0/−0.14 | 1.72/1.51 |
| mf | 4 | 200/94 | 623.6–879.2/631.7–847.2 | 0.64 | 1.72 | 1.54 | 0.95/0.96 | 0.91/0.92 | 0/−0.23 | 1.72/1.54 | |
| ADF | dg1, n01 | 2 | 190/94 | 379.0–611.5/389.2–588.2 | 0.43 | −0.87 | 3.46 | 0.81/0.79 | 0.65/0.63 | 0/−0.58 | 3.16/3.46 |
| mf | 2 | 190/94 | 379.0–611.5/389.2–588.2 | 0.42 | −0.99 | 3.76 | 0.75/0.75 | 0.56/0.56 | 0/−0.87 | 3.58/3.76 | |
| ADL | sa3, dg2 14, SNV 15 | 4 | 190/90 | 38.8–104.8/45.7–102.0 | 0.53 | 0.88 | 0.97 | 0.80/0.78 | 0.65/0.61 | 0/0.09 | 0.88/0.97 |
| mf | 4 | 190/90 | 38.8–104.8/45.7–102.0 | 0.45 | 1.23 | 1.26 | 0.56/0.59 | 0.31/0.34 | 0/0.21 | 1.23/1.25 | |
| ABL | dg2 | 3 | 200/98 | 61.1–173.9/74.0–164.4 | 0.7 | 0.67 | 0.71 | 0.95/0.92 | 0.90/0.85 | 0/0.03 | 0.67/0.71 |
| mf | 3 | 200/98 | 61.1–173.9/74.0–164.4 | 0.58 | 0.9 | 1.02 | 0.90/0.85 | 0.82/0.72 | 0/0.07 | 0.90/1.02 | |
| IVDMD | sa3, SNV | 5 | 188/96 | 380.6–791.6/393.5–767.7 | 0.59 | 3.34 | 3.28 | 0.93/0.92 | 0.86/0.86 | 0/0.06 | 3.34/3.28 |
| mf | 5 | 188/96 | 380.6–791.6/393.5–767.7 | 0.55 | 3.49 | 3.63 | 0.92/0.91 | 0.85/0.82 | 0/0.12 | 3.49/3.63 | |
| IVNDFD | sa3, dg1 | 4 | 188/92 | 298.9–740.2/334.7–724.9 | 0.57 | 3.62 | 3.6 | 0.92/0.91 | 0.84/0.83 | 0/−0.52 | 3.62/3.59 |
| mf | 4 | 188/92 | 298.9–740.2/334.7–724.9 | 0.5 | 4.29 | 4.44 | 0.88/0.86 | 0.77/0.74 | 0/−0.38 | 4.29/4.44 |
| Propriedade | Mean | Minimum | Maximum | SD 1 | Offset | Slope | RMSEP 2 | SEP 3 | RSD 4 | Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Ash | 73.3 | 47.2 | 102.5 | 15.73 | −0.59 | 1.09 | 1.02 | 1.03 | 1.04 | 0.08 |
| Cell wall | 796.1 | 701.1 | 865.4 | 47.53 | 12.17 | 0.85 | 2.01 | 2.03 | 1.93 | 0.03 |
| Neutral detergent fiber | 741.3 | 641.9 | 803.3 | 48.66 | −3.28 | 1.00 | 3.77 | 2.03 | 2.06 | −3.19 |
| Acid detergent fiber | 470.8 | 364.7 | 547.1 | 47.33 | −5.29 | 1.07 | 3.16 | 2.46 | 2.48 | −2.02 |
| Acid detergent lignin | 68.8 | 51.9 | 95.8 | 12.25 | 0.81 | 0.81 | 1.06 | 0.93 | 0.91 | −0.52 |
| Acetyl bromide lignin | 121.9 | 69.9 | 155.3 | 21.29 | 2.61 | 0.87 | 1.41 | 0.86 | 0.84 | 1.12 |
| IVDMD | 639.4 | 491.6 | 784.6 | 76.37 | −2.87 | 1.04 | 3.67 | 3.71 | 3.75 | −0.34 |
| IVNDFD | 567.1 | 398.8 | 774.7 | 92.37 | −1.94 | 1.01 | 3.64 | 3.46 | 3.51 | −1.30 |
| Item | r 1 | p-Value |
|---|---|---|
| Dry matter | 0.8883 | <0.0001 |
| Ash | 0.8463 | <0.0001 |
| Cell wall | 0.9062 | <0.0001 |
| Neutral detergent fiber | 0.9079 | <0.0001 |
| Acid detergent fiber | 0.8925 | <0.0001 |
| Acid detergent lignin | 0.7518 | <0.0001 |
| Acetyl bromide lignin | 0.9227 | <0.0001 |
| IVDMD 2 | 0.8984 | <0.0001 |
| IVNDFD 3 | 0.9314 | <0.0001 |
| Item | Ash | CW | NDF | ADF | ADL | ABL | IVDMD | IVNDF |
|---|---|---|---|---|---|---|---|---|
| DFresidual 1 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| MSE 2 | 74.18 | 426.4 | 438.9 | 480.9 | 68.90 | 75.25 | 1186.8 | 1192.7 |
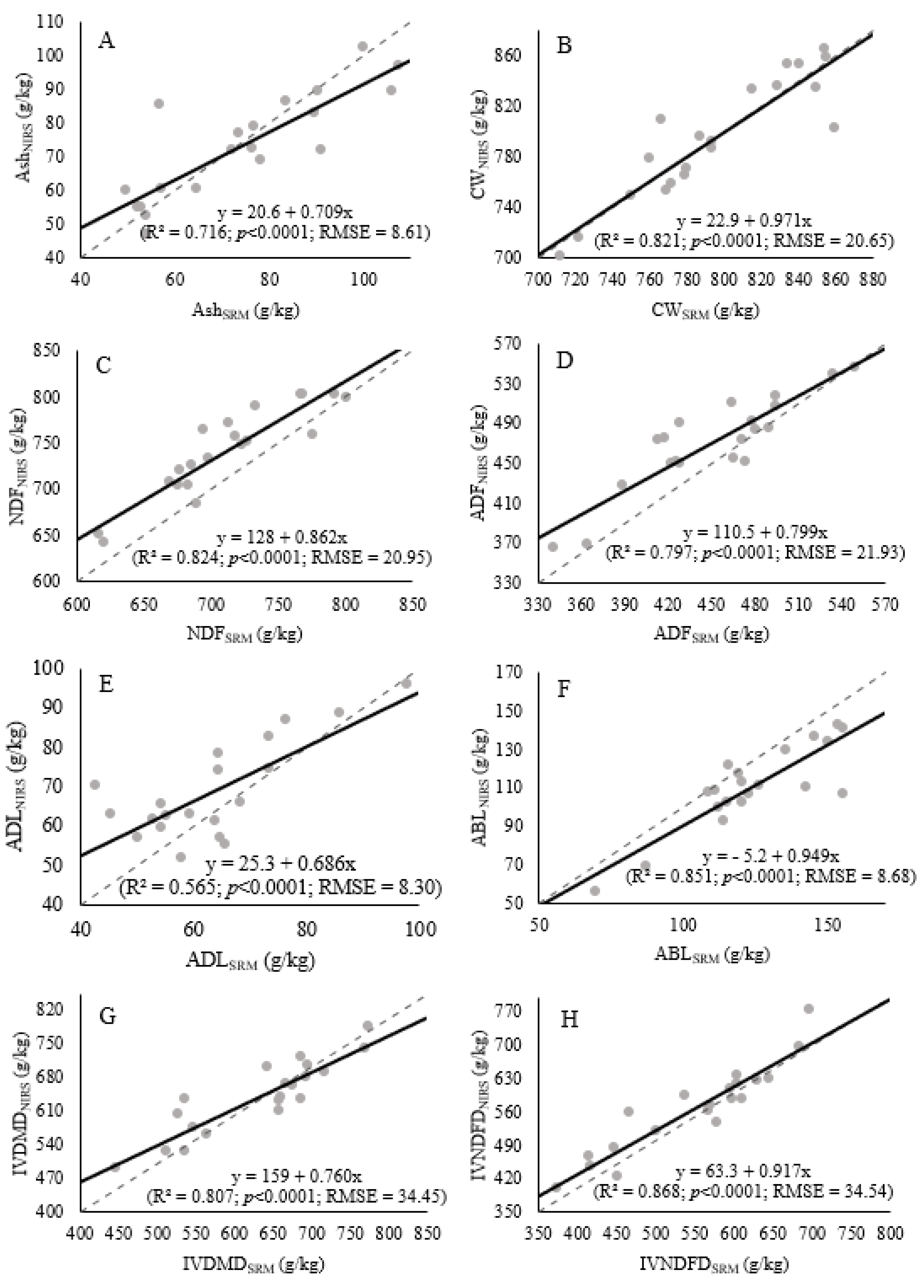
| R2 3 | 0.7162 | 0.8213 | 0.8243 | 0.7966 | 0.5651 | 0.8514 | 0.8072 | 0.8675 |
| R2adjusted | 0.7004 | 0.8113 | 0.8146 | 0.7853 | 0.541 | 0.8432 | 0.7965 | 0.8602 |
| RMSE 4 | 8.61 | 20.6 | 20.9 | 21.9 | 8.30 | 8.67 | 34.4 | 34.5 |
| Coefficients | ||||||||
| Intercept | 20.60 | 22.93 | 128.00 | 110.48 | 25.26 | −5.21 | 158.94 | 632.709 |
| p-value | 0.019 | 0.790 | 0.071 | 0.019 | 0.013 | 0.657 | 0.010 | 0.195 |
| Slope 5 | 0.709 | 0.971 | 0.862 | 0.798 | 0.686 | 0.949 | 0.759 | 0.917 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Item | Lignin Methods | |||
|---|---|---|---|---|
| ADL | ADLNIRS | ABL | ABLNIRS | |
| IVDMD | −0.7691 ** | −0.6685 * | −0.8887 ** | −0.8830 ** |
| IVNDFD | −0.7630 ** | −0.6896 * | −0.8935 ** | −0.8863 ** |
| IVDMDNIRS | −0.7563 * | −0.5816 * | −0.8075 * | −0.9041 ** |
| IVNDFDNIRS | −0.7860 ** | −0.6444 * | −0.9096 ** | −0.9683 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, I.C.d.S.B.; da Silva, T.H.; Picchi, C.C.; Fukushima, R.S. Assessing the Repeatability and Reliability of NIRS to Predict Nutritional Values and to Evaluate Two Lignin Methods in Urochloa spp. Grasses. Grasses 2023, 2, 112-126. https://doi.org/10.3390/grasses2020010
Guimarães ICdSB, da Silva TH, Picchi CC, Fukushima RS. Assessing the Repeatability and Reliability of NIRS to Predict Nutritional Values and to Evaluate Two Lignin Methods in Urochloa spp. Grasses. Grasses. 2023; 2(2):112-126. https://doi.org/10.3390/grasses2020010
Chicago/Turabian StyleGuimarães, Iuli Caetano da Silva Brandão, Thiago Henrique da Silva, Cristina Cirino Picchi, and Romualdo Shigueo Fukushima. 2023. "Assessing the Repeatability and Reliability of NIRS to Predict Nutritional Values and to Evaluate Two Lignin Methods in Urochloa spp. Grasses" Grasses 2, no. 2: 112-126. https://doi.org/10.3390/grasses2020010
APA StyleGuimarães, I. C. d. S. B., da Silva, T. H., Picchi, C. C., & Fukushima, R. S. (2023). Assessing the Repeatability and Reliability of NIRS to Predict Nutritional Values and to Evaluate Two Lignin Methods in Urochloa spp. Grasses. Grasses, 2(2), 112-126. https://doi.org/10.3390/grasses2020010






