A New Genus of Ectinosomatidae (Copepoda, Harpacticoida) Symbiont in the Digestive Tract of Eudistoma vannamei Millar, 1977 (Ascidia, Polycitoridae) †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Etymology
3.2. Genus Diagnosis
| Exopod | Endopod | |
|---|---|---|
| P1 | I-0, I-1, II + 2 + 1 | 0, 0-II-0 |
| P2 | I-1, I-1, II + 2 + 2 | 0, I, 2, 1 |
| P3 | I-1, I-1, II + 2 + 3 | 0, 0, 2, 1 |
| P4 | I-1, I-1, II + 2 + 2 | 0, 0, 2, 0 |
3.3. Putative Apomorphies of the Genus
3.4. Etymology
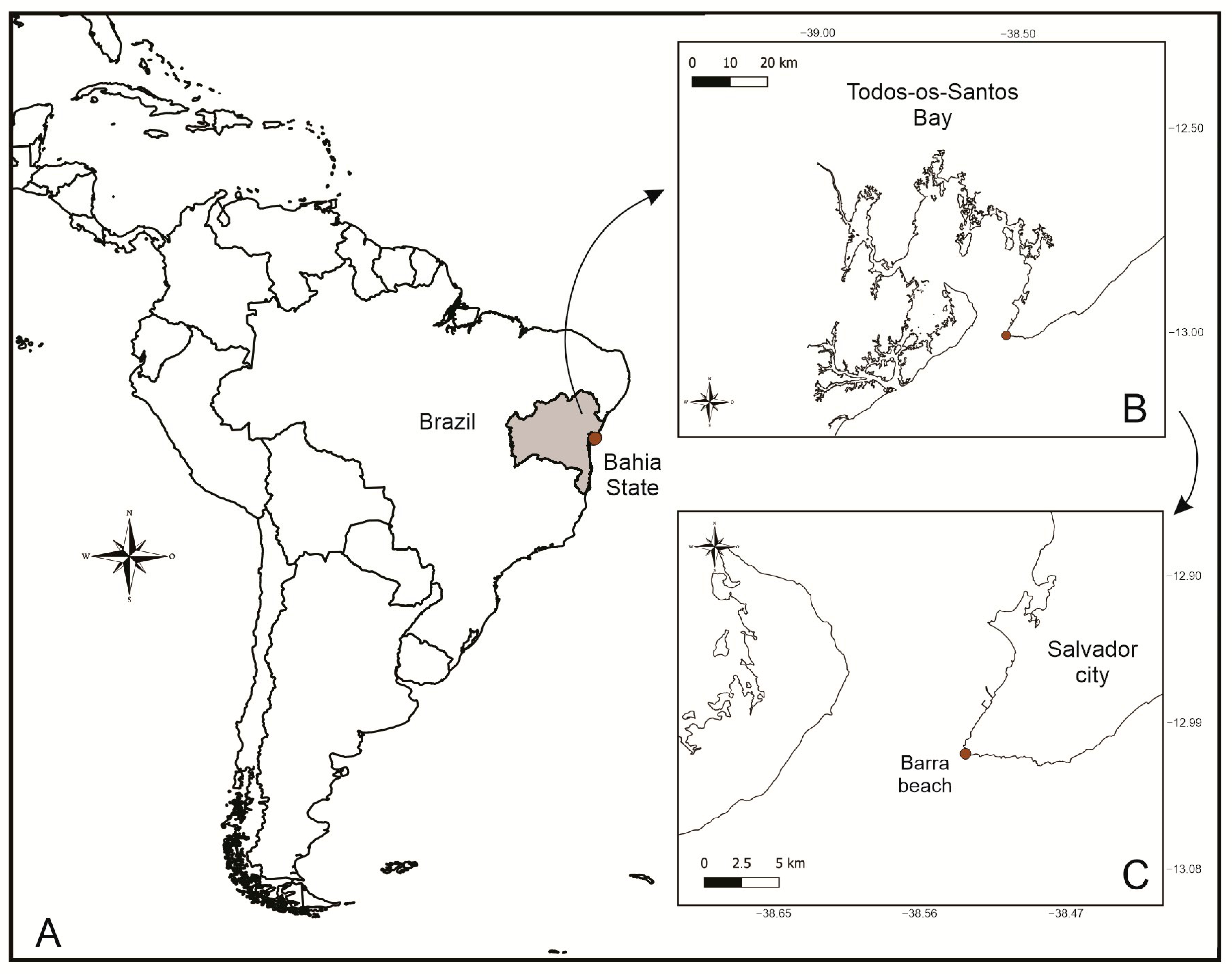
3.5. Type Locality
3.6. Material Examined
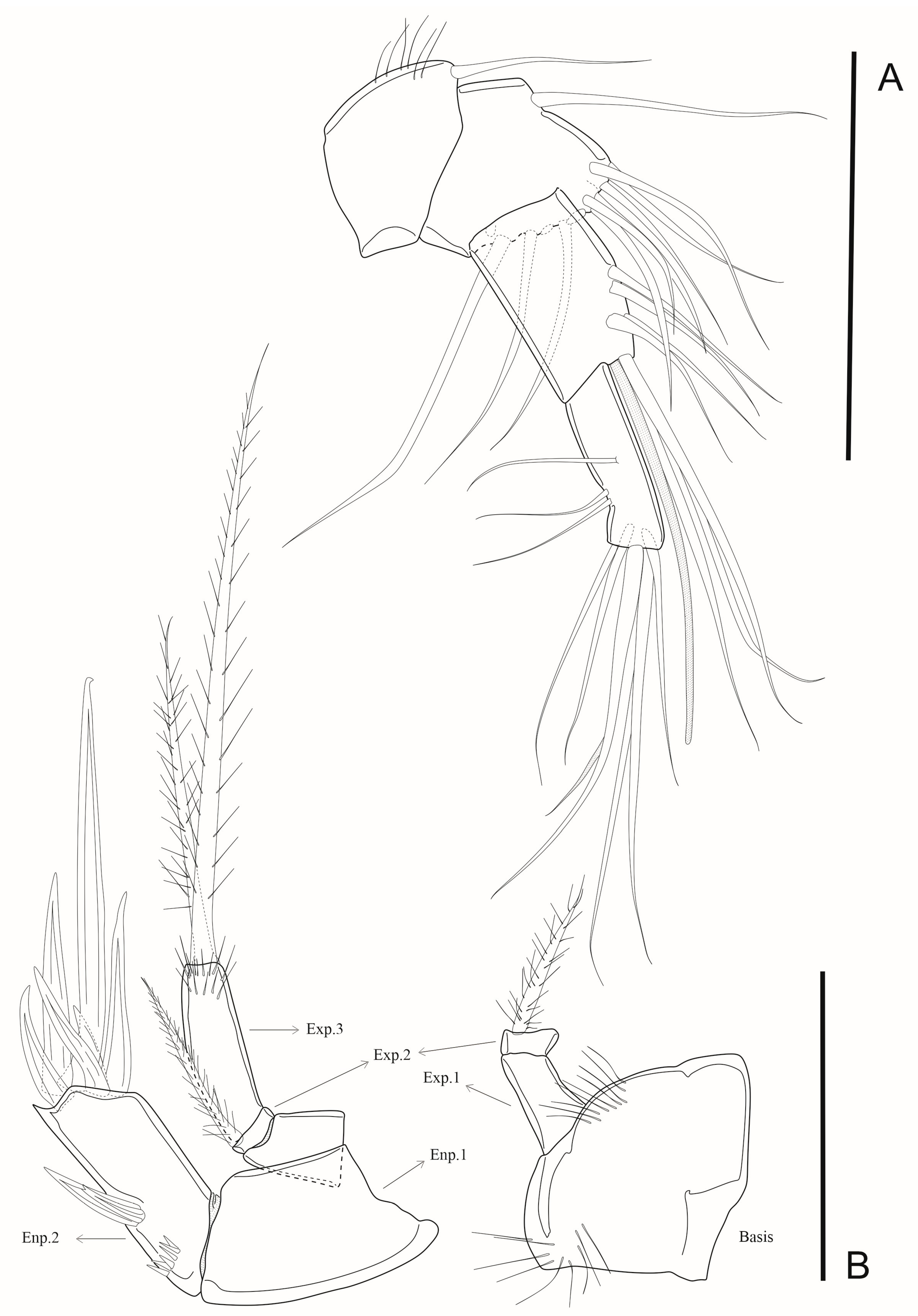
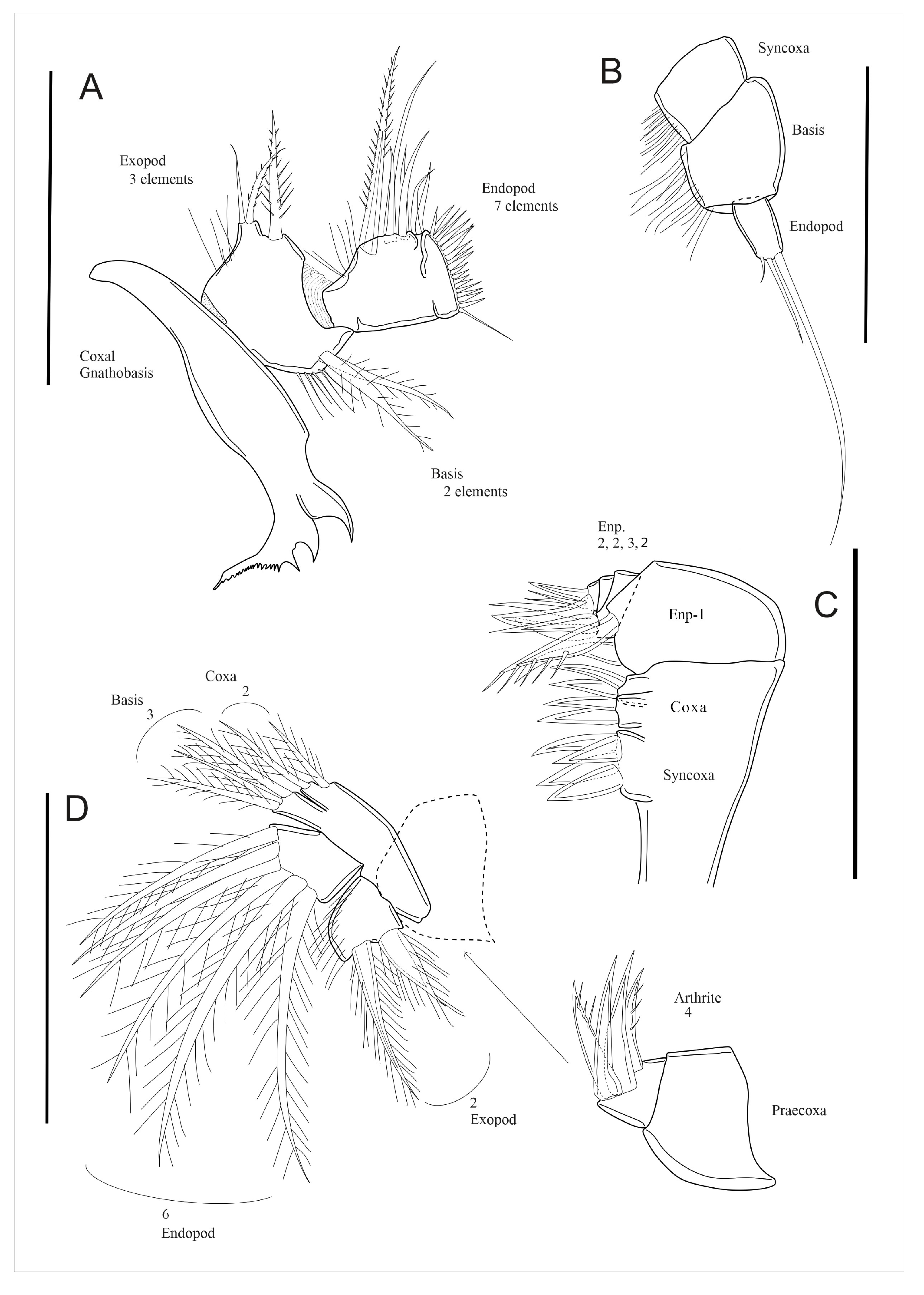
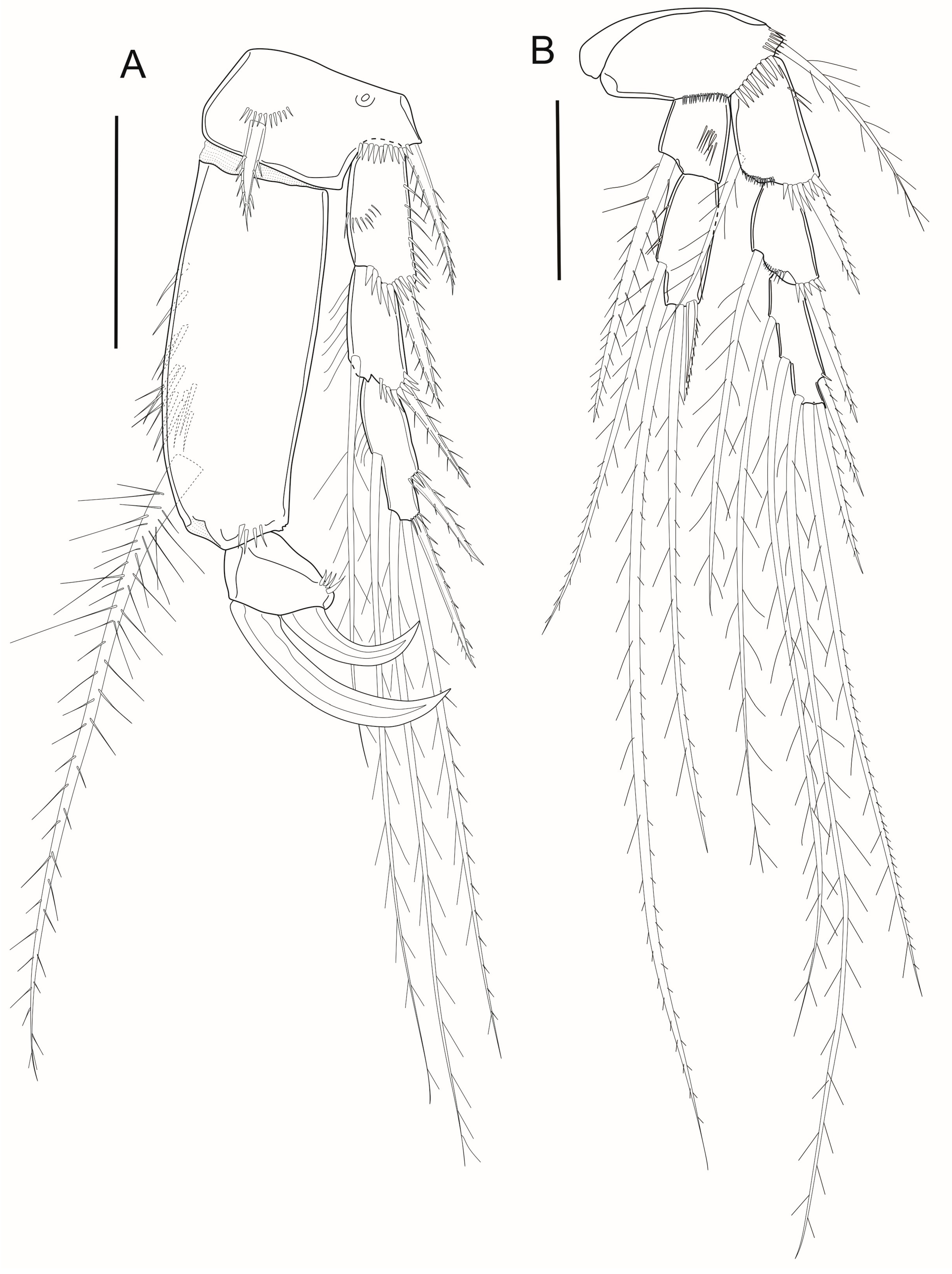
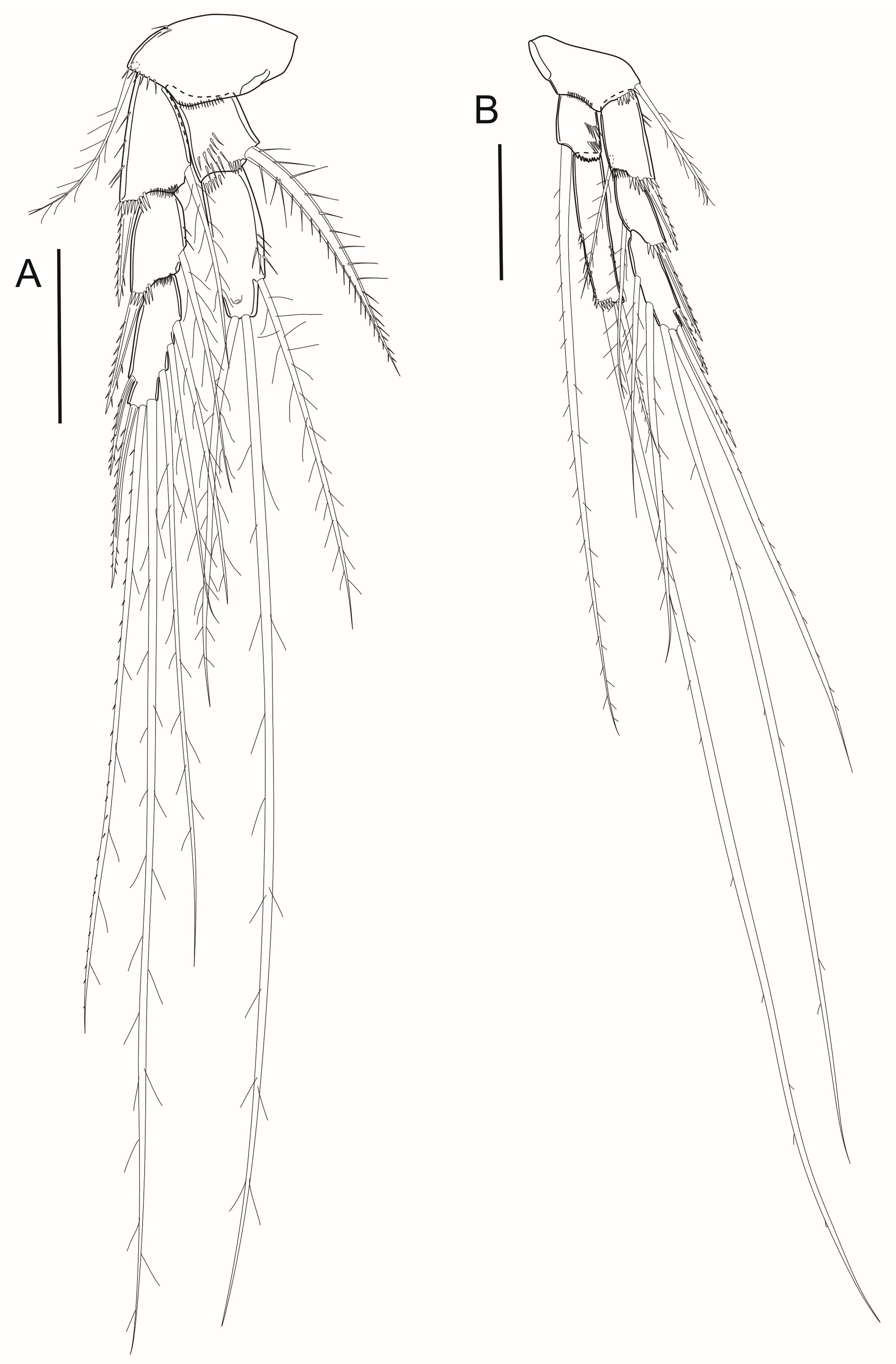
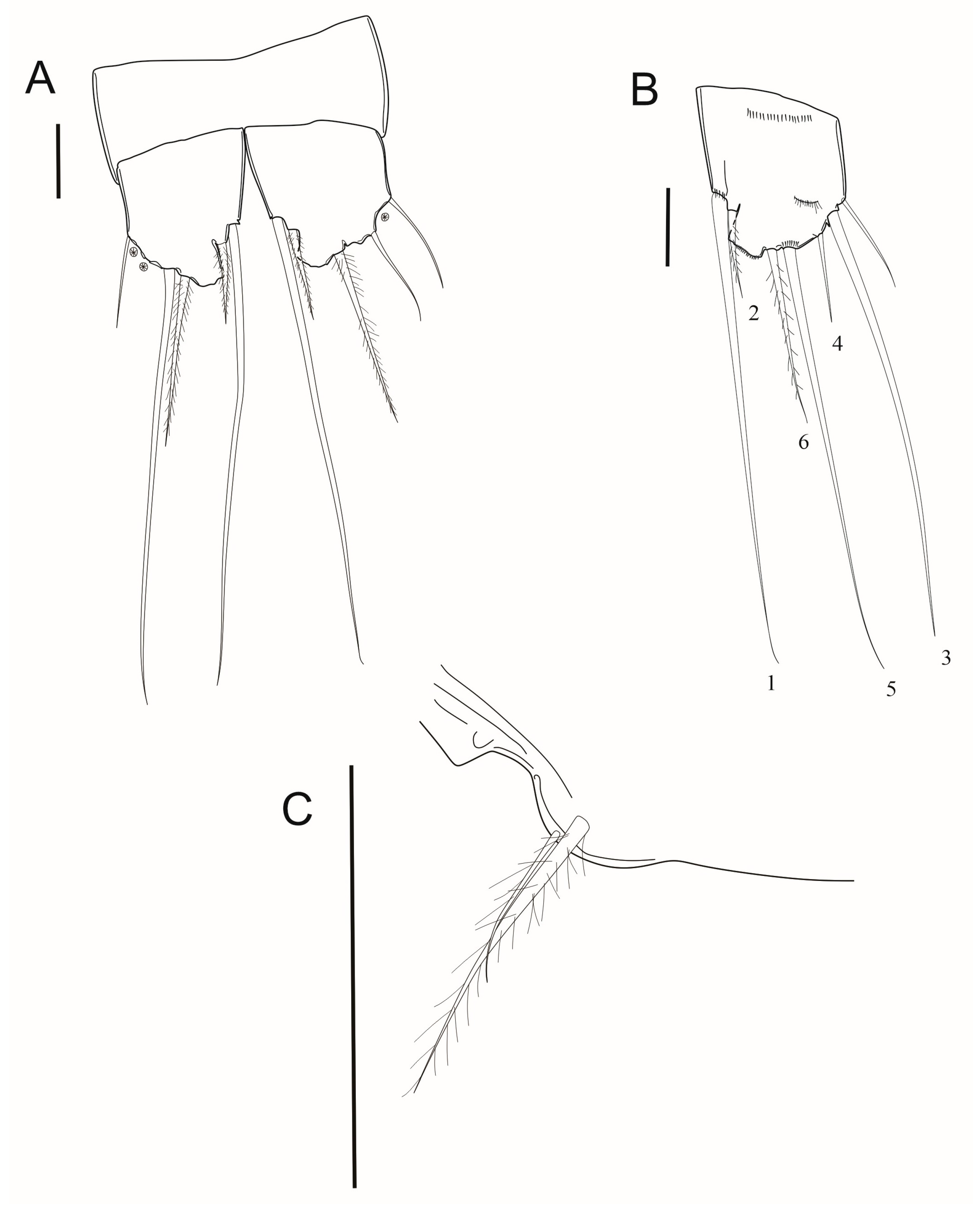
3.7. Description
4. Discussion
5. Key to the Genera of Ectinosomatidae (Adapted from Kihara and Huys, 2009)
- 1.
- Body cylindrical with cephalothorax rectangular in dorsal view; body the same width throughout its length ….……………………...…………………………………………2
- -
- Body fusiform with cephalothorax sub-triangular in dorsal view; greatest body width usually at posterior margin of cephalothorax; urosome gradually tapering towards the posterior end…………………………………………….……………………………7
- -
- Body with dorsoventrally depressed prosome, clearly wider than urosome .....................................................................……… Peltobradya Médioni and Soyer, 1968
- 2.
- Antennary exp two-segmented; maxilla prehensile, with major articulation between elongate syncoxa and elongate allobasis .............……………… Noodtiella Wells, 1965
- -
- Antennary exp one or three-segmented; maxilla not prehensile, with at most a slight angle between syncoxa and allobasis ......................................................……………… 3
- 3.
- Enps P2–P4 2-segmented ..................…………………… Ectinosomoides Nicholls, 1945
- -
- Enps P2–P4 3-segmented .....…..................................................................……………… 4
- 4.
- Anal somite with dorsal armature of claws, lappets or spiniform processes around anal opening; P5 exp with three marginal and one surface seta ………… ………..............................................…………………………… Arenosetella Wilson, 1932
- -
- Anal somite without such ornamentation........................................................…………5
- 5.
- Antennary exp one-segmented, enp one-segmented .................. Tetanopsis Brady, 1910
- -
- Antennary exp one-segmented, enp two-segmented…. Parahalectinosoma George and Schwabe, 2019
- -
- Antennary exp three-segmented.........................................................................………… 6
- 6.
- Female P5 with foliaceous setae on exp and benp, exp with three marginal and no surface setae; male P5 exp with four normal marginal setae ………………………………………………………...……………… Oikopus Wells, 1967
- -
- P5 with normal setae on exp and benp in both sexes, exp with 3 marginal and typically a surface seta [absent in Hastigerella noodti Soyer, 1974 = G. soyeri (Bodin, 1976)] ................................……………………………………………… Glabrotelson Huys, 2009
- 7.
- P1–P4 enps 2-segmented .............................……………… Pseudectinosoma Kunz, 1935
- -
- P1 enp 2- or 3-segmented, P2–P4 enps 3-segmented......................…………………… 8
- 8.
- P1 enp prehensile ……….....................................................................…………………… 9
- -
- P1 enp not prehensile .........................................................................…………………… 12
- 9.
- P1 enp 2-segmented............................................................................…………………… 10
- -
- P1 enp 3-segmented .....................…………………… Klieosoma Hicks & Schriever, 1985
- 10.
- P1–P2 exp-3 with two outer elements.........................................................…………… 11
- -
- P1–P2 exp-3 with three outer elements ...............………… Halophytophilus Brian, 1919
- 11.
- Antennule with large spine on segment 2 (and often segments 1 and 3); antennary exp rudimentary, with 1–3 small setae; P1 enp-2 with 4 elements (1–2 pinnate and claw-like) ........................................……………………………… Bradyellopsis Brian, 1925
- -
- Armature elements on antennulary segments 1–3 setiform; antennary exp well developed and 3-segmented; P1 enp-2 with six elements (outer one bifid and claw-like) ...........................................…………………………… Chaulionyx Kihara and Huys, 2009
- -
- P1 enp-2 P1 with two claws; P5 completely fused without evidence of the borders between exp and baseoendopod; marine, found in digestive tract of ascidian………... Cruscollatus gen. nov.
- 12.
- Maxilla prehensile, with syncoxa and allobasis forming right angle; P5 exo-pod poorly developed, short, fused to benp in female and distinct in male, with 3 marginal and no surface setae; body very small (< 300 μm) .................... Sigmatidium Giesbrecht, 1881
- -
- These characters not combined..............................................................………………… 13
- 13.
- P5 exp and benp fused, forming a single plate in both sexes….…………………..… 14
- -
- P5 exp and benp at least partly discrete............................……………………………… 15
- 14.
- P1–P4 exp-3 with 5, 6, 6, 6 elements, respectively; male P6 unarmed; body of female small (< 400 μm); continental groundwater……….. Rangabradya Karanovic and Pesce, 2001
- -
- P1–P4 exp-3 with 6, 7, 8, 8 elements, respectively; male P6 with two setae; body of female large (≥1200 μm); marine, usually deepwater ....................................Parabradya Lang, 1944
- 15.
- Integument of somites with distinctive subrectangular pores; P5 exp with four marginal setae…………………………..…………………………… Ectinosoma Boeck, 1865
- -
- Integument of somites without distinctive subrectangular pores; P5 exp with three marginal setae and one seta on anterior surface………… …………………………16
- 16.
- Mandible with rudimentary gnathobasis, elongate basis and filiform exp and enp, each terminating in 2–3 setae; antennary exp without lateral spines ………… …………...........................……………………………………… Ectinosomella Sars, 1910
- -
- These characters not combined……....................................................……………… 17
- 17.
- Third segment of female antennule three times as long as wide; mandibular enp with one strong seta laterally; P1–P4 exp-3 with 2 outer spines; planktonic (occasionally in sediment) ………………………………….… Microsetella Brady and Robertson, 1873
- -
- These characters not combined….........................................................……………… 18
- 18.
- Body comparatively robust with prosome-urosome separation usually distinct (exception: B. kurtschminkei Seifried and Martínez Arbizu, 2008; with dorsoventrally flattened habitus); antenna with two setae on proximal exp segment and one seta on proximal enp segment; mandibular exp with at least five setae; maxilliped robust with short enp usually fused at an angle with basis and bearing four conspicuous setae ....…………………………………………………...………… Bradya Boeck, 1973
- -
- Body comparatively slender with no sharp separation between prosome and urosome; antenna with less than 2 setae on proximal exp segment (except Pseudobradya ambigua Sars, 1920 with 2) and no seta on proximal enp segment; mandibular exp generally with fewer than 5 setae; Maxilliped usually slender and straight with discrete enp bearing 1 small and 4 conspicuous setae…………………………………19
- 19.
- Antennule with or without dark pigment spot within the proximal three segments: maxilla prehensile, allobasis usually truncate distally and carrying 3-segmented enp (although enp sometimes exceedingly small and segmentation difficult to discern; reduced to a narrow three-segmented cylinder in P. leptognatha Sars, 1920); maxilliped short and robust……………………………………...…… Pseudobradya Sars, 1904
- -
- Antennule without pigment spot; maxilla with at most a slight angle between syncoxa and allobasis, the latter attenuating distally, enp three-segmented but always small, its morphology not clearly discernible; maxilliped slender ……………………… ………………....………………… Halectinosoma Vervoort, 1962
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huys, R.; Gee, J.M.; Moore, C.G.; Hamond, R. Marine and Brackish Water Harpacticoid Copepods; The Linnean Society of London and Estuarine and Coastal Sciences Association by Field Studies Council: Shrewsbury, UK; London, UK, 1996; 323p. [Google Scholar]
- Kihara, T.C.; Huys, R. A new genus of Ectinosomatidae (Copepoda, Harpacticoida) from sublittoral sediments in Ubatuba, São Paulo State (Brazil), an updated key to genera and notes on Noodtiella Wells, 1965. ZooKeys 2009, 17, 57–88. [Google Scholar] [CrossRef]
- Sars, G.O. Copepoda Harpacticoida. Parts III & IV. Ectinosomidae, Harpacticidae (Part). An Account of the Crustacea of Norway, with Short Descriptions and Figs. of All the Species 5; Bergen Museum: Bergen, Norway, 1904; pp. 29–56, pls. 17–32. [Google Scholar]
- Moore, C.G. An emendation of the family name Ectinosomidae Sars to Ectinosomatidae (Copepoda, Harpacticoida). Crustaceana 1978, 34, 111. [Google Scholar] [CrossRef]
- Ho, J.-S. Why do symbiotic copepods matter? Hydrobiologia 2001, 453/454, 1–7. [Google Scholar] [CrossRef]
- Huys, R. Harpacticoid copepods—Their symbiotic associations and biogenic substrata: A review. Zootaxa 2016, 4174, 448–729. [Google Scholar] [CrossRef] [PubMed]
- Illg, P.L.; Dudley, P.L. The family Ascidicolidae and its subfamilies (Copepoda, Cyclopoida), with descriptions of new species. Mém. Mus. Hist. Nat. Sér. A Zool. 1980, 117, 1–192. [Google Scholar]
- Johnsson, R.; Bahia, C.; Neves, E. A new genus of Asterocheridae (Copepoda: Siphonostomatoida) ectoassociate of the ascidian Eudistoma vannamei Millar, 1977 (Polycitoridae) from Brazil. Zootaxa 2016, 4114, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Humes, A.G. Cnidarians and copepods: A success story. Trans. Am. Micros. Soc. 1985, 104, 313–320. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991; 468p. [Google Scholar]
- Johnsson, R.; Neves, E. Siphonostomatoid copepods (Crustacea) associated with marine invertebrates and algae in Brazil: A review and future considerations. Zoosymposia 2012, 8, 69–80. [Google Scholar] [CrossRef]
- Lotufo. T.M.C. Ascidiacea (Chordata: Tunicata) do Litoral Tropical Brasileiro. Ph.D. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 2002.
- Médioni, A.; Soyer, J. Copépodes Harpacticoïdes de Banyuls-sur-Mer. 6. Nouvelles formes associées à des Bryozoaires. Vie et Milieu 1968, 18, 317–343. [Google Scholar]
- Snelgrove, P.V.R.; Lewis, J.B. Response of a coral-associated crustacean community to eutrophication. Mar. Biol. 1989, 101, 249–257. [Google Scholar] [CrossRef]
- George, K.H.; Schwabe, E. A new species of Ectinosomatidae Sars (Copepoda, Harpacticoida) associated with Pseudoikedella achaeta (Zenkevitch, 1958) (Echiura, Bonelliida). Prog. Oceanogr. 2019, 173, 1–8. [Google Scholar] [CrossRef]
- Michels, J.; Büntzow, M. Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. J. Microsc. 2010, 238, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.D.; Ivanenko, V.N. The identity of protopodal segments and the ramus of maxilla 2 of copepods (Copepoda). Crustaceana 2008, 81, 823–835. [Google Scholar] [CrossRef]
- Sewell, R.B.S. The littoral and semi-parasitic Cyclopoida, the Monstrilloida and Notodelphyoida. Sci. Rep. John Murray Exped. Publ. Br. Mus. 1949, 9, 17–199. [Google Scholar]
- Lang, K. Die während der schwedischen Expedition nach Spitzbergen 1898 und nach Grönland 1899 eingesammelten Harpacticiden. K. Sven. Vetensk. Hand. 1936, 15, 1–55. [Google Scholar]
- Klie, W. Harpacticoida (Cop.) aus dem Bereich von Helgoland und der Kieler Bucht. 1. Kiel. Meeresforsch. 1949, 6, 90–128. [Google Scholar]
- Karanovic, T.; Pesce, G.L. A new genus and species of the family Ectinosomatidae (Crustacea: Copepoda: Harpacticoida) from the groundwaters of India. Ann. Limnol. 2001, 37, 281–292. [Google Scholar] [CrossRef]
- Watkins, R.L. Description of new species of Bradyellopsis and Perissocope (Copepoda: Harpacticoida) from the California coast with revised keys to the genera. J. Crust. Biol. 1987, 7, 380–395. [Google Scholar] [CrossRef]
- Brian, A. Descrizione di un nuove genere di Copepodo Arpacticoide dell’ Adriatico. [Description of a new genus of Copepoda Harpacticoida from the Adriatic Sea.]. Atti Soc. Ligustica 1925, 4, 116–122. [Google Scholar]




| Exopod | Endopod | |
|---|---|---|
| P1 | I-0, I-1, II + 2 + 1 | 0, 0-II-0 |
| P2 | I-1, I-1, II + 2 + 2 | 0, I, 2, 1 |
| P3 | I-1, I-1, II + 2 + 3 | 0, 0, 2, 1 |
| P4 | I-1, I-1, II + 2 + 2 | 0, 0, 2, 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corgosinho, P.H.; Kihara, T.C.; Farias, A.; Schizas, N.; Neves, E.; Johnsson, R. A New Genus of Ectinosomatidae (Copepoda, Harpacticoida) Symbiont in the Digestive Tract of Eudistoma vannamei Millar, 1977 (Ascidia, Polycitoridae). Arthropoda 2025, 3, 8. https://doi.org/10.3390/arthropoda3020008
Corgosinho PH, Kihara TC, Farias A, Schizas N, Neves E, Johnsson R. A New Genus of Ectinosomatidae (Copepoda, Harpacticoida) Symbiont in the Digestive Tract of Eudistoma vannamei Millar, 1977 (Ascidia, Polycitoridae). Arthropoda. 2025; 3(2):8. https://doi.org/10.3390/arthropoda3020008
Chicago/Turabian StyleCorgosinho, Paulo H., Terue C. Kihara, Amilcar Farias, Nikolaos Schizas, Elizabeth Neves, and Rodrigo Johnsson. 2025. "A New Genus of Ectinosomatidae (Copepoda, Harpacticoida) Symbiont in the Digestive Tract of Eudistoma vannamei Millar, 1977 (Ascidia, Polycitoridae)" Arthropoda 3, no. 2: 8. https://doi.org/10.3390/arthropoda3020008
APA StyleCorgosinho, P. H., Kihara, T. C., Farias, A., Schizas, N., Neves, E., & Johnsson, R. (2025). A New Genus of Ectinosomatidae (Copepoda, Harpacticoida) Symbiont in the Digestive Tract of Eudistoma vannamei Millar, 1977 (Ascidia, Polycitoridae). Arthropoda, 3(2), 8. https://doi.org/10.3390/arthropoda3020008








