1. Introduction
Immunoblasts are activated mature B- or T-cell lineage lymphocytes characterized by intermediate-to-large cell size, enlarged nuclei with vesicular chromatin, prominent nucleoli, and variably abundant cytoplasm. During B-cell activation, immunoblasts commonly downregulate CD20 and may acquire CD30 expression, particularly secondary to infections or inflammation [
1,
2,
3].
Reactive ILVIP is an uncommon finding in gastrointestinal specimens from patients with both acute and chronic inflammatory conditions, characterized by proliferation of immunoblasts within lymphovascular spaces [
4]. B-cell lineage immunoblastic proliferations have been also described in lymph node sinuses [
5]. Isolated reports have linked intralymphovascular immunoblastic proliferations to gastric mucosa-associated lymphoid tissue (MALT) lymphoma [
6], as well as atypical CD30-positive T-cell immunoblastic proliferations identified in conjunction with lichen sclerosis and an endometrial polyp [
7,
8,
9]. B-cell lineage ILVIP shows polytypic light chain expression and lacks immunoglobulin gene rearrangement, supporting a reactive nature. However, rare cases with T-cell immunoblastic proliferations have shown immunoglobulin gene rearrangement, a finding of uncertain clinical significance [
9]. Although considered reactive, ILVIP can morphologically mimic intravascular lymphoma, posing a significant diagnostic pitfall with critical therapeutic implications.
In this study, we describe clinicopathologic features of reactive B-cell ILVIP incidentally identified in two patients who presented with small bowel obstruction requiring right hemicolectomy (patient 1) or small bowel resection (patient 2). However, patient #2 was erroneously diagnosed with intravascular large B-cell lymphoma and referred to our institution for further evaluation, highlighting the diagnostic challenge posed by ILVIP and the potential consequences of misinterpretation, including unwarranted workup, undue patient anxiety, and possible overtreatment.
2. Case Reports
Patient 1, a 71-year-old female, presented with abdominal pain following planned perineal hernia repair. Laboratory evaluation showed a white blood cell count of 7.9 K/µL with relative neutrophilia (86%). There was no lymphadenopathy, hepatosplenomegaly, fever, or night sweats. Abdominal imaging demonstrated small bowel obstruction with colonic ischemia requiring ileocolonic resection.
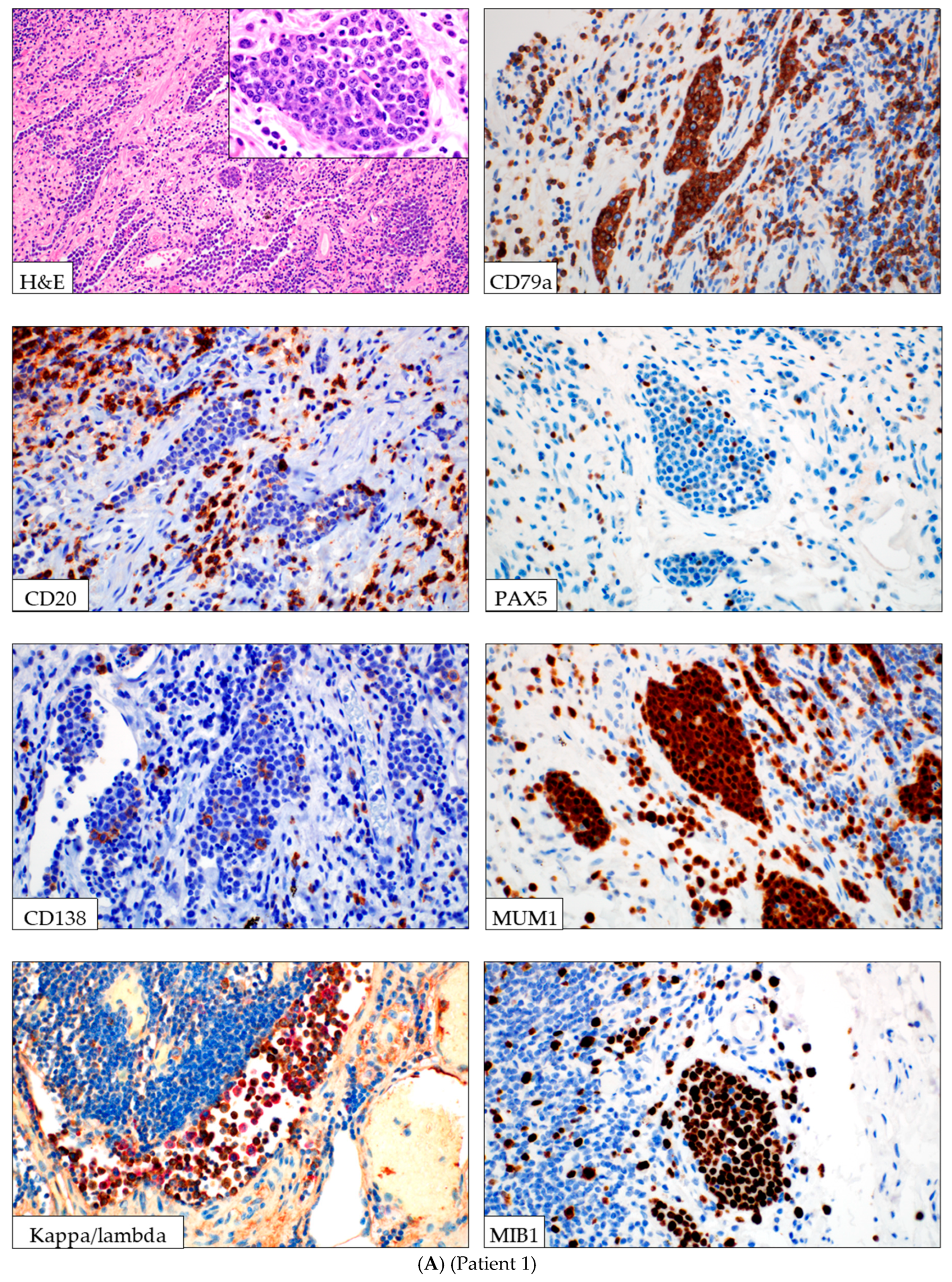
Histologic evaluation of the resected colon revealed perforated ischemic colitis with serosal adhesions. Additionally, a focal intralymphovascular proliferation of atypical lymphoid cells was observed. These cells were intermediate to large in size, with round to slightly irregular nuclei, vesicular chromatin, variably prominent nucleoli, and frequent apoptotic bodies (
Figure 1A,
Table 1). Immunohistochemical studies confirmed B-cell lineage, with strong expression of CD45, CD19, CD79a, and MUM1 with polytypic kappa and lambda light chain expression. The cells were negative for CD3, CD20, CD30, CD138, CD10, ALK1, and TDT. HHV8 and EBV (by in-situ hybridization) were negative. The proliferation index was high (100% by MIB1). The endothelial cells were positive for CD31 and D2-40, suggestive of a lymphatic vessel. PCR-based clonality testing performed on the section of colon with immunoblastic proliferation demonstrated no clonal IGH or IGK gene rearrangements. Flow cytometry was not performed. Unfortunately, despite treatment with antibiotic therapy, the patient died several weeks later from sepsis-related complications.
Patient 2, an 85-year-old female, presented with a small bowel obstruction secondary to an incarcerated ventral hernia. Laboratory evaluation showed mild leukocytosis (white blood cell count 11.5 k/μL) with absolute neutrophilia. There was no lymphadenopathy, hepatosplenomegaly, fever, chills, weight loss, or night sweats. The patient underwent surgical repair with small bowel resection.
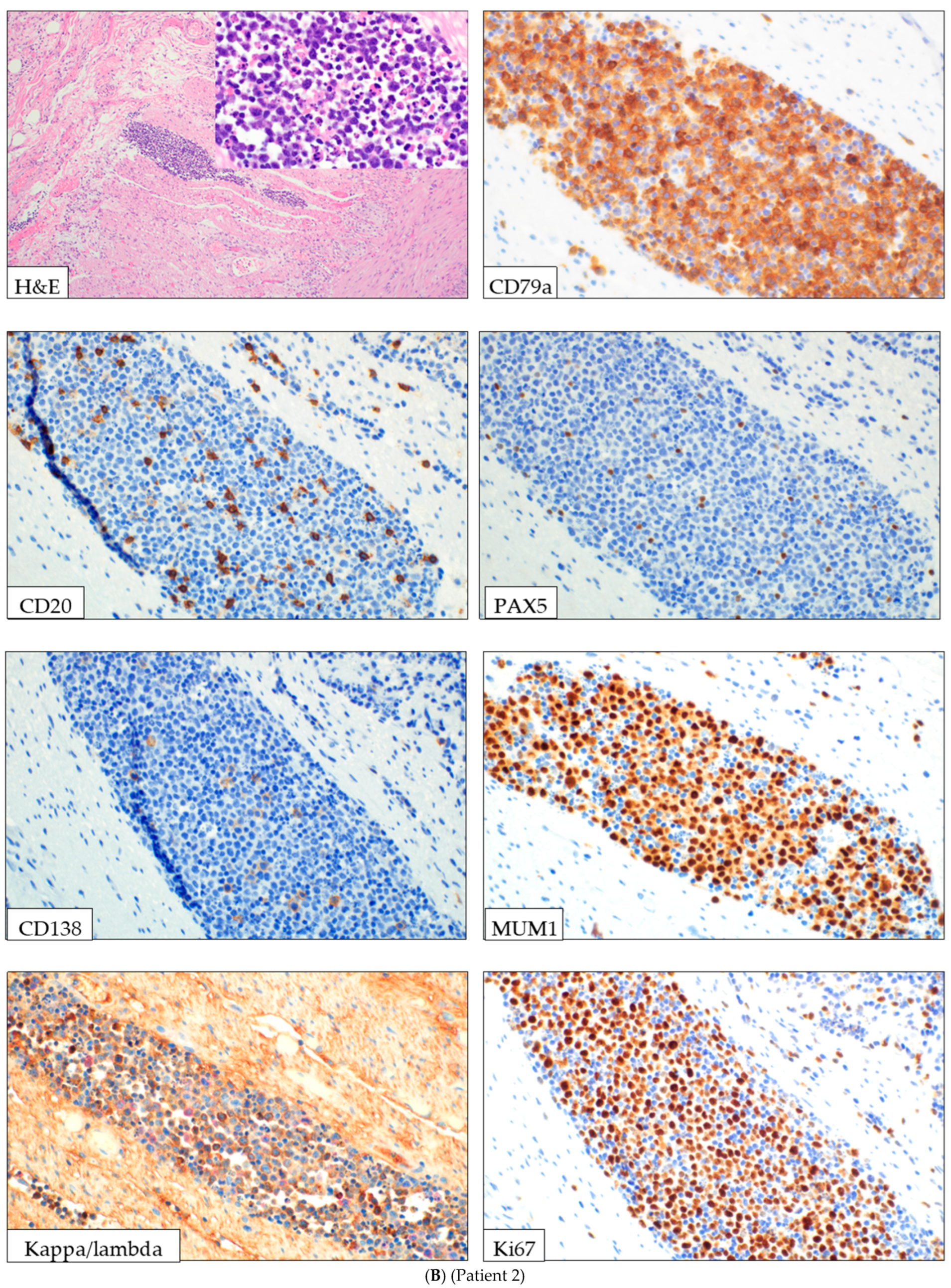
Histologic evaluation of the small bowel demonstrated a focal intravascular proliferation of atypical lymphoid cells. The cells were intermediate to large in size, with enlarged irregular nuclei, variably prominent nucleoli, and abundant apoptotic debris. Immunohistochemical studies supported B-cell lineage with expression of CD45, CD79a, CD38, MUM1, and polytypic immunoglobulin kappa and lambda light chains (confirmed by immunohistochemistry and in situ hybridization). A minor subset of cells showed CD3 and weak CD30 expression. The cells lacked expression of CD20, PAX5, CD10, BCL-6, CD138, Cyclin D1, Sox11, C-MYC, TdT, CD34, and ALK1. EBV and HHV8 were negative. Ki67 showed a high proliferative index (80%) (
Figure 1B,
Table 1). The vascular lining was not apparently positive for D2-40 and CD34, not conclusive for lymphatic or blood vessel origin. Flow cytometry and PCR clonality testing were not performed on the lesional tissue.
The patient was initially diagnosed with IVLBCL at the referring institution and underwent PET/CT, bone marrow biopsy, and brain/spine MRI, all of which showed no evidence of lymphoma. Review at our institution supported a diagnosis of reactive ILVIP. The patient was observed without systemic therapy and remains healthy.
3. Discussion
ILVIP is a rare, reactive intralymphovascular proliferation of intermediate-to-large immunoblasts most commonly identified in gastrointestinal specimens from patients with acute inflammation, infection, or mechanical obstruction without evidence of lymphoma [
4]. These immunoblasts most often exhibit B-cell lineage with postgerminal center immunophenotype. However, less common T-cell immunoblastic variants have also been described [
7,
8,
9]. The morphologic and immunophenotypic features of ILVIP may closely overlap with intravascular lymphoma or leukemia, with IVLBCL most concerning in immunoblasts with B-cell immunophenotype. Distinguishing ILVIP from IVLBCL is critical, as the latter is an aggressive malignancy requiring intensive chemotherapy and is associated with a poor prognosis, with a five-year overall survival rate of approximately 50% [
10].
To date, the two largest series of ILVIP describe eight cases of B-cell lineage immunoblastic proliferation in gastrointestinal resection specimens and twelve cases involving lymph node sinuses [
4,
5]. Our two cases share a similar clinical presentation that parallels prior observations that ILVIP occurs most often in gastrointestinal specimens associated with infection, inflammation, or mechanical obstruction, suggesting that they represent a localized reactive response [
4]. The mechanism underlying intralymphovascular accumulation of immunoblasts remains incompletely understood, although alterations in adhesion molecule expression (LFA-1, ICAM-1) or intestinal lymphatic obstruction have been proposed to play a role [
11].
In both our cases, ILVIP was identified as a focal finding. The immunoblasts exhibited a post-germinal center B-cell immunophenotype, characterized by the expression of CD45, CD79a, and MUM1, and the absence of CD10, CD138, CD20, and PAX5. Immunoglobulin light chain expression was polytypic in both cases, and B-cell clonality by PCR was negative for immunoglobulin rearrangement in patient 1. Unlike previously reported series, our cases lacked significant CD30 expression and were negative for definitive PAX5 and CD20 expression [
4]. A small subset of CD3-positive immunoblasts was present in Patient 2, consistent with a prior report describing mixed B- and T-immunoblastic proliferation [
4]. Other markers, such as ALK1, EBV, and HHV-8 were negative.
These features contrast with intravascular large B-cell lymphoma, which typically shows extensive intravascular involvement, strong expression of B-cell markers such as CD20 and PAX5, light chain restriction, and clonal immunoglobulin rearrangement. CD30, EBV, and HHV8 are usually negative in IVLBCL.
Our findings add to the growing recognition that ILVIP is an incidental, reactive finding identified in gastrointestinal resection specimens in patients with bowel obstruction without systemic symptoms of lymphoma. Immunoblasts in this setting exhibit a postgerminal center B-cell immunophenotype with a high proliferation index and no evidence of clonality, as judged by polytypic expression of kappa and lambda light chains and without clonal immunoglobulin gene rearrangement.
Importantly, erroneous diagnosis of IVLBCL in Patient 2 prompted extensive, ultimately unnecessary evaluation. Therefore, pathologists and clinicians should maintain a high index of suspicion for ILVIP when interpreting findings in specimens with intralymphovascular immunoblastic proliferations, especially when the clinical presentation lacks features of lymphoma.
Author Contributions
Conceptualization, A.S. and A.R.; writing—original draft preparation, N.U.E. and S.K.; writing—review and editing, A.S., A.R., A.A., N.U.E. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Utah (Approval Number: 00077285, Approval Date: 23 May 2025).
Informed Consent Statement
Patient consent is waived under this study because it is a retrospective analysis of archived specimens that are de-identified and present no risk to the subjects.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to the patients and the clinical team.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SBO | small bowel obstruction |
| IVLBCL | Intravascular large B-cell lymphoma |
| ILVIP | intralymphovascular immunoblastic proliferation |
References
- Treetipsatit, J.; Rimzsa, L.; Grogan, T.; Warnke, R.A.; Natkunam, Y. Variable expression of B-cell transcription factors in reactive immunoblastic proliferations: A potential mimic of classical Hodgkin lymphoma. Am. J. Surg. Pathol. 2014, 38, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Anolik, J.; Looney, R.J.; Bottaro, A.; Sanz, I.; Young, F. Down-regulation of CD20 on B cells upon CD40 activation. Eur. J. Immunol. 2003, 33, 2398–2409. [Google Scholar] [CrossRef] [PubMed]
- Oflazoglu, E.; Grewal, I.S.; Gerber, H. Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Adv. Exp. Med. Biol. 2009, 647, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, W.; Zhang, L.; Shen, Q.; Yuan, J.; Reichard, K.K.; Hu, Z.; Medeiros, L.J. Reactive Intralymphovascular Immunoblastic Proliferations Mimicking Aggressive Lymphomas. Am. J. Surg. Pathol. 2022, 46, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Parisi, X.; Wang, W.; Liu, W.; Loghavi, S.; Hu, S.; Jelloul, F.; Medeiros, L.J.; Fang, H. Reactive Intra-sinusoidal Immunoblastic Proliferations in Lymph Nodes: A Diagnostic Pitfall for Diffuse Large B-Cell Lymphoma. Lab. Investig. 2024, 104, S1462–S1463. [Google Scholar]
- Zhong, L.L.; Tang, Z.P.; Zhang, H.P.; Zhong, S.; Huang, Y.W.; Huang, G.X. Reactive Intravascular Plasmablastic/Immunoblastic Proliferation in a Patient with Concurrent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma and Tuberculosis Potentially Mimicking Aggressive Intravascular Lymphoma: A Rare Case Report. Case Rep. Gastroenterol. 2025, 19, 581–589. [Google Scholar] [PubMed]
- Kempf, W.; Keller, K.; John, H.; Dommann-Scherrer, C. Benign Atypical Intravascular CD30+ T-Cell Proliferation: A Recently Described Reactive Lymphoproliferative Process and Simulator of Intravascular Lymphoma: Report of a Case Associated with Lichen Sclerosus and Review of the Literature. Am. J. Clin. Pathol. 2014, 142, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Falkenbach, E.; Fernández-Figueras, M.T.; Rodríguez-Peralto, J.L. Benign atypical intravascular CD30+ T-cell proliferation: A reactive condition mimicking intravascular lymphoma. Am. J. Dermatopathol. 2013, 35, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.; Lawton, H.; Al-Talib, R.; Wright, D.H.; Theaker, J.M. Intravascular proliferation of reactive lymphoid blasts mimicking intravascular lymphoma—A diagnostic pitfall. Histopathology 2007, 51, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.; Bossard, C.; Gabellier, L.; Rohmer, J.; Laghmari, O.; Parrens, M.; Sarkozy, C.; Dulery, R.; Roland, V.; Llamas-Gutierrez, F.; et al. Clinical presentation, outcome, and prognostic markers in patients with intravascular large B-cell lymphoma, a lymphoma study association (LYSA) retrospective study. Cancer Med. 2022, 11, 3602–3611. [Google Scholar] [CrossRef] [PubMed]
- Milićević, N.M.; Milićević, Z.; Westermann, J. Lymphocyte function-associated antigen-1 and intercellular adhesion molecule-1 expression on B-cell subsets and the effects of splenectomy-experimental studies. Leuk. Lymphoma 2002, 43, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).