Metabolomics-Driven Investigation of Harpin αβ and Laminarin Effects on Cannabis sativa L. Employing GC/EI/MS and 1H NMR Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Biological Material and Growth Conditions
2.3. Experimental Design and Sampling
2.4. Cannabis sativa L. GC/EI/MS & 1H NMR Metabolite Profiling
2.4.1. Extraction and Analysis of Cannabis Metabolites with GC/EI/MS
2.4.2. Extraction and Analysis of Cannabis Metabolites with 1H NMR
2.4.3. One Way-Analysis of Variance (ANOVA)
3. Results and Discussion
3.1. Overview of GC/EI/MS and 1H NMR Metabolomics Analyses
3.2. Treatments of Cannabis sativa L. Plants with Harpin (α,β) and Laminarin Substantially Alter Their Global Leaf Metabolism
3.3. Effect of Harpin (α.β) and Laminarin Treatments on the Content of Cannabis sativa L. Plants in Bioactive Metabolites and Metabolites That Play Key Roles in Their Metabolism
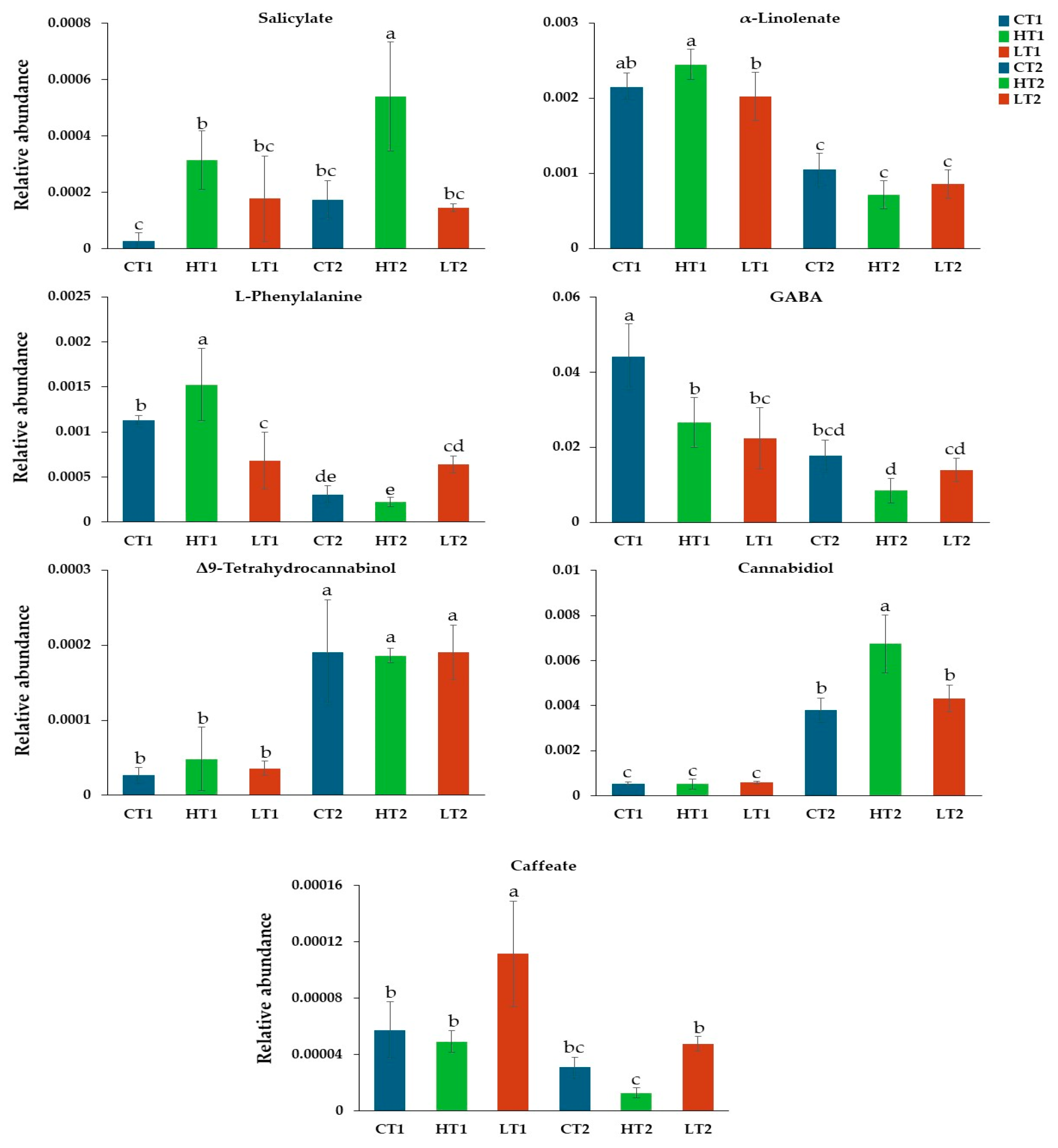
3.3.1. Harpin (α,β) and Laminarin Treatments Had a Variable Effect on the Content of Cannabis sativa L. Leaves in CBD and Δ9-THC
3.3.2. Harpin (αβ) and Laminarin Treatments Altered the Content of Cannabis sativa L. Leaves in Metabolites That Play a Key Role in Its Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef]
- Dal Martello, R.; Min, R.; Stevens, C.J.; Qin, L.; Fuller, D.Q. Morphometric approaches to Cannabis evolution and differentiation from archaeological sites: Interpreting the archaeobotanical evidence from bronze age Haimenkou, Yunnan. Veg. His. Archaeobot. 2024, 33, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Simiyu, D.C.; Jang, J.H.; Lee, O.R. Understanding Cannabis sativa L.: Current status of propagation, use, legalization, and haploid-inducer-mediated genetic engineering. Plants 2022, 11, 1236. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis systematics at the levels of family, genus, and species. Cannabis Ccannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Borroto Fernandez, E.; Peterseil, V.; Hackl, G.; Menges, S.; de Meijer, E.; Staginnus, C. Distribution of chemical phenotypes (Chemotypes) in European agricultural hemp (Cannabis sativa L.) cultivars. J. Forensic Sci. 2020, 65, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Cannabis Cult. 1975, 23, 21–38. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Charai, M.; Sghiouri, H.; Mezrhab, A.; Karkri, M. Thermal insulation potential of non-industrial hemp (Moroccan Cannabis sativa L.) fibers for green plaster-based building materials. J. Clean. Prod. 2021, 292, 126064. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to emerging industrial applications of cannabis (Cannabis sativa L.). Rend. Fis. Acc. Lincei 2021, 32, 233–243. [Google Scholar] [CrossRef]
- Parvez, A.M.; Lewis, J.D.; Afzal, M.T. Potential of industrial hemp (Cannabis sativa L.) for bioenergy production in Canada: Status, challenges and outlook. Renew. Sustain. Energy Rev. 2021, 141, 110784. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic use in clinical practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, A.M.; Vlachou, S.; Grintzalis, K. Efficacy of phytocannabinoids in epilepsy treatment: Novel approaches and recent advances. Int. J. Environ. Res. Public Health 2021, 18, 3993. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of metabolomics in cannabis (Cannabis sativa L.) research and development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef]
- Oregon Department of Agriculture. Available online: https://www.oregon.gov/oda/pesticides/pages/cannabis-and-pesticides.aspx (accessed on 7 August 2025).
- Government of Alberta. Available online: https://www.alberta.ca/registered-cannabis-pesticides.aspx (accessed on 7 August 2025).
- Hellenic Republic Ministry of Rural Develpoment and Food. Available online: https://1click.minagric.gr/oneClickUI/frmFytoPro.zul?lang=en (accessed on 7 August 2025).
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance). Off. J. Eur. Union 2019, L170, 1–114.
- Wu, L.; Gao, X.; Xia, F.; Joshi, J.; Borza, T.; Wang-Pruski, G. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiol. Mol. Plant Pathol. 2019, 106, 49–56. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-based protein hydrolysate improves salinity tolerance in Hemp: Agronomical and physiological aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar] [CrossRef]
- Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant biostimulants to enhance abiotic stress resilience in crops. Int. J. Mol. Sci. 2025, 26, 1129. [Google Scholar] [CrossRef]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The futuristic sustainable approach for alleviating crop productivity and abiotic stress tolerance. J. Plant Growth Reg. 2024, 43, 659–674. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Francioso, O.; Sanchez-Cortes, S.; Foggia, M.D.; Schiavon, M. Effects of two protein hydrolysates obtained from Chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) plants. Front. Plant Sci. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Soleymani, A. The roles of plant-growth-promoting rhizobacteria (PGPR)-based biostimulants for agricultural production systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and root applications of vegetal-derived protein hydrolysates differentially enhance the yield and qualitative attributes of two lettuce cultivars grown in floating system. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, J.; Zheng, J.; Zhao, J.; Qiu, C.; Xiao, D.; Mu, L.; Liu, X. Arsenate phytotoxicity regulation by humic acid and related metabolic mechanisms. Ecotoxicol. Environ. Saf. 2021, 207, 111379. [Google Scholar] [CrossRef]
- Monterisi, S.; Zhang, L.; Garcia-Perez, P.; Alzate Zuluaga, M.Y.; Ciriello, M.; El-Nakhel, C.; Buffagni, V.; Cardarelli, M.; Colla, G.; Rouphael, Y. Integrated multi-omic approach reveals the effect of a Graminaceae-derived biostimulant and its lighter fraction on salt-stressed lettuce plants. Sci. Rep. 2024, 14, 10710. [Google Scholar] [CrossRef] [PubMed]
- Reichel, P.; Munz, S.; Hartung, J.; Präger, A.; Kotiranta, S.; Burgel, L.; Schober, T.; Graeff-Hönninger, S. Impact of three different light spectra on the yield, morphology and growth trajectory of three different Cannabis sativa L. strains. Plants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A. Effect of colonization of Trichoderma harzianum on growth development and CBD content of hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Fiorentino, N.; Di Mola, I.; Iaccarino, N.; Gargiulo, E.; Chianese, G. Effect of saline irrigation and plant-based biostimulant application on fiber hemp (Cannabis sativa L.) growth and phytocannabinoid composition. Front. Plant Sci. 2024, 15, 1293184. [Google Scholar] [CrossRef] [PubMed]
- Daler, S.; Karaca, I.; Delavar, H.; Kaya, O. Harnessing the potential of harpin proteins: Elicitation strategies for enhanced secondary metabolite accumulation in grapevine callus cultures. Processes 2024, 12, 1416. [Google Scholar] [CrossRef]
- Sands, L.B.; Cheek, T.; Reynolds, J.; Ma, Y.; Berkowitz, G.A. Effects of Harpin and Flg22 on growth enhancement and pathogen defense in Cannabis sativa seedlings. Plants 2022, 11, 1178. [Google Scholar] [CrossRef]
- de Borba, M.C.; Velho, A.C.; de Freitas, M.B.; Holvoet, M.; Maia-Grondard, A.; Baltenweck, R.; Magnin-Robert, M.; Randoux, B.; Hilbert, J.-L.; Reignault, P. A laminarin-based formulation protects wheat against Zymoseptoria tritici via direct antifungal activity and elicitation of host defense-related genes. Plant Dis. 2022, 106, 1408–1418. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. GC/EI/MS and 1H NMR metabolomics reveal the effect of an olive tree endophytic Bacillus sp. lipopeptide extract on the metabolism of Colletotrichum acutatum. Metabolites 2023, 13, 462. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Giaki, K.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics 2022, 10, 494. [Google Scholar] [CrossRef]
- Franzin, M.; Di Lenardo, R.; Ruoso, R.; Addobbati, R. Incomplete Decarboxylation of Acidic Cannabinoids in GC-MS Leads to Underestimation of the Total Cannabinoid Content in Cannabis Oils Without Derivatization. Pharmaceutics 2025, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Copley, T.R.; Aliferis, K.A.; Kliebenstein, D.J.; Jabaji, S.H. An integrated RNAseq–1H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Livaja, M.; Palmieri, M.C.; von Rad, U.; Durner, J. The effect of the bacterial effector protein harpin on transcriptional profile and mitochondrial proteins of Arabidopsis thaliana. J. Proteom. 2008, 71, 148–159. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A disease resistance elicitor laminarin enhances tea defense against a piercing herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef]
- Yao, X.; Yao, Y.; An, L.; Li, X.; Bai, Y.; Cui, Y.; Wu, K. Accumulation and regulation of anthocyanins in white and purple Tibetan Hulless Barley (Hordeum vulgare L. var. nudum Hook. f.) revealed by combined de novo transcriptomics and metabolomics. BMC Plant Biol. 2022, 22, 391. [Google Scholar] [CrossRef]
- Clark, T.J.; Guo, L.; Morgan, J.; Schwender, J. Modeling plant metabolism: From network reconstruction to mechanistic models. Ann. Rev. Plant Biol. 2020, 71, 303–326. [Google Scholar] [CrossRef]
- Wanke, A.; Rovenich, H.; Schwanke, F.; Velte, S.; Becker, S.; Hehemann, J.H.; Wawra, S.; Zuccaro, A. Plant species-specific recognition of long and short β-1, 3-linked glucans is mediated by different receptor systems. Plant J. 2020, 102, 1142–1156. [Google Scholar] [CrossRef]
- Lyu, D.; Ruan, E.D.; Backer, R.; Gagné-Bourque, F.; Smith, D.L. Changes in morpho-physiological traits and phytochemical composition of Cannabis sativa L. treated with microbial biostimulants across different substrates. Ind. Crop. Prod. 2025, 224, 120377. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pak. J. Agric. Res. 2019, 32, 8. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, X.; Liu, W.; Huang, J.; Liu, Q.; Sun, J.; Cai, X.; Miao, W. HpaXpm, a novel harpin of Xanthomonas phaseoli pv. manihotis, acts as an elicitor with high thermal stability, reduces disease, and promotes plant growth. BMC Microbiol. 2020, 20, 4. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
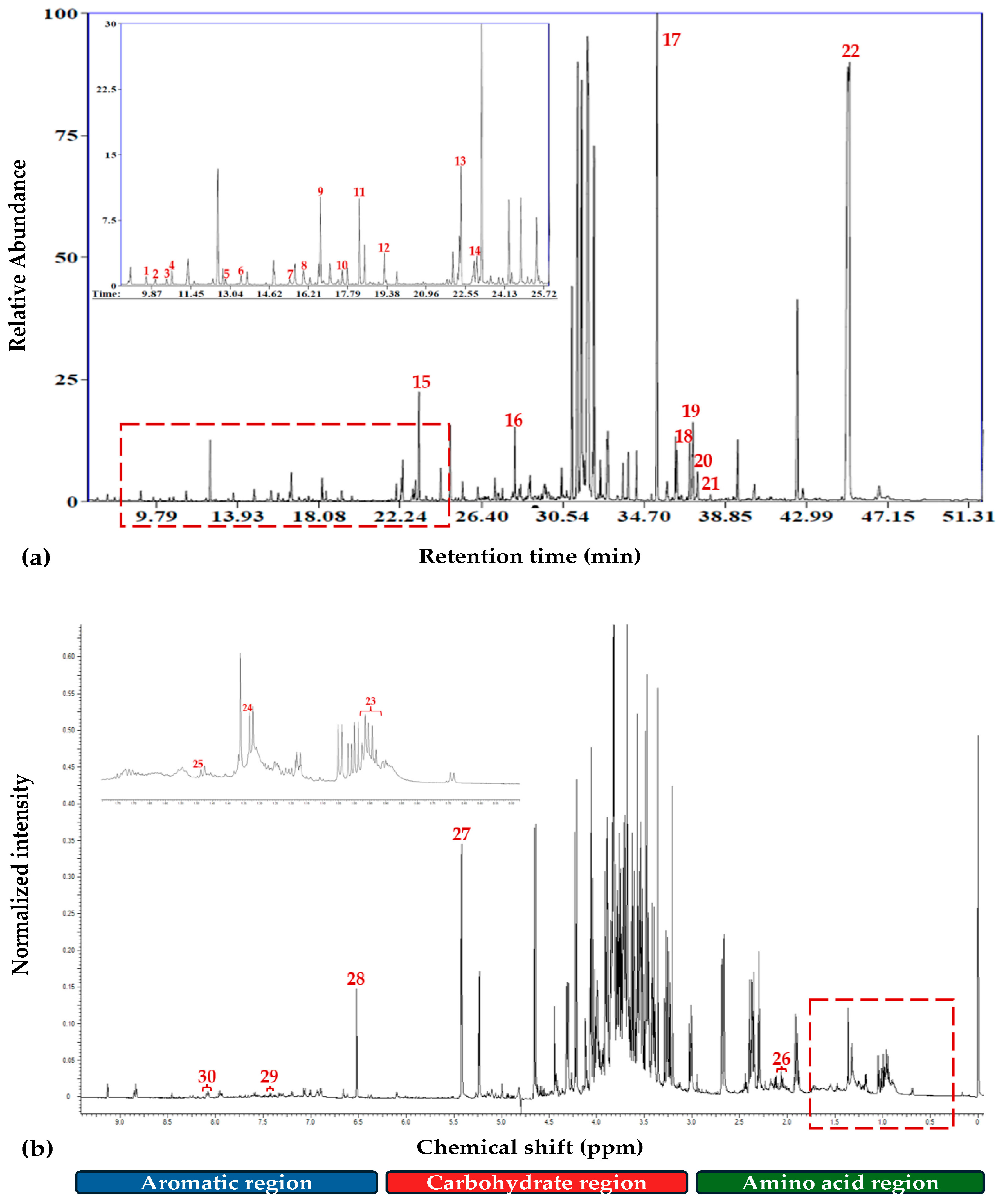
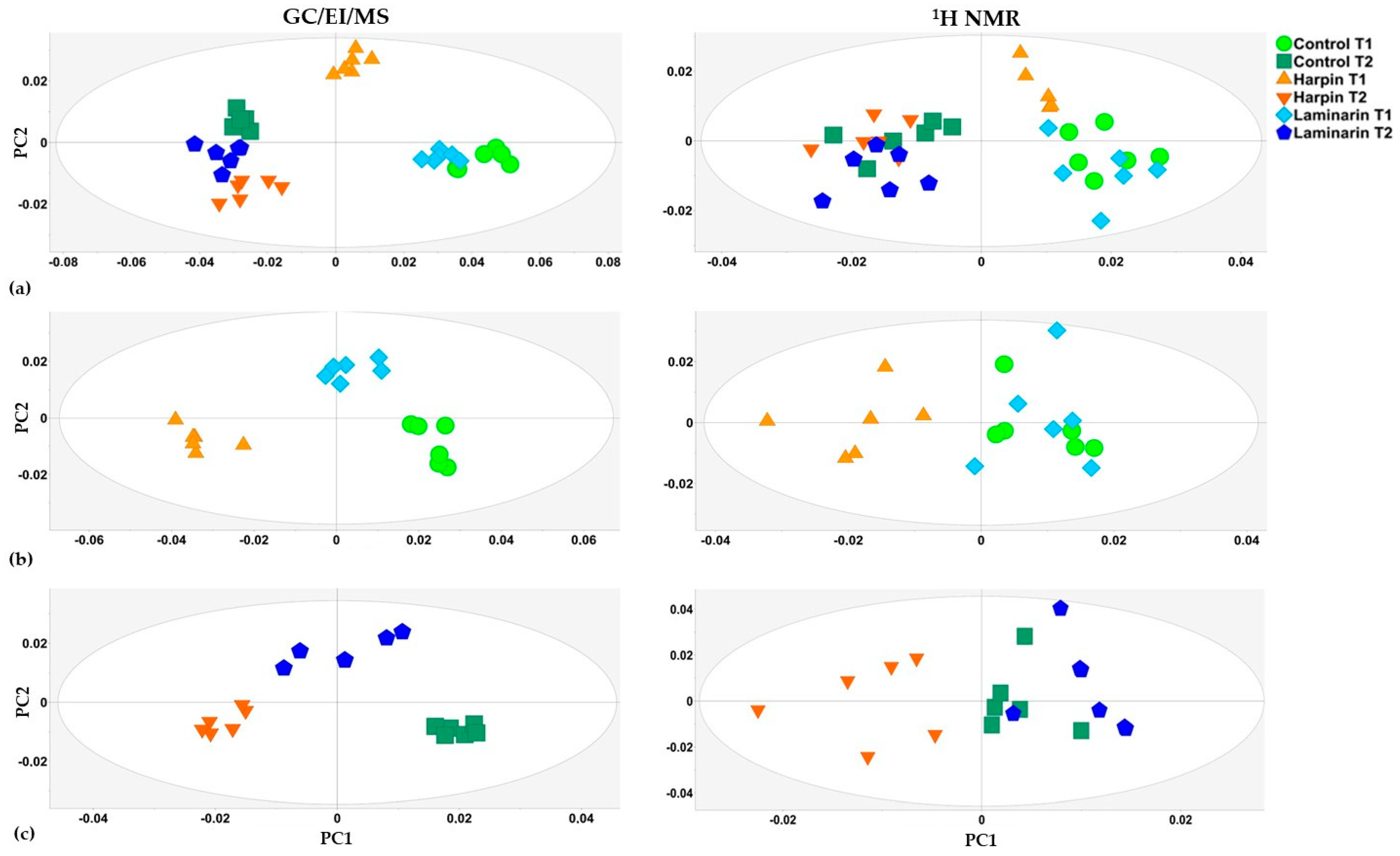
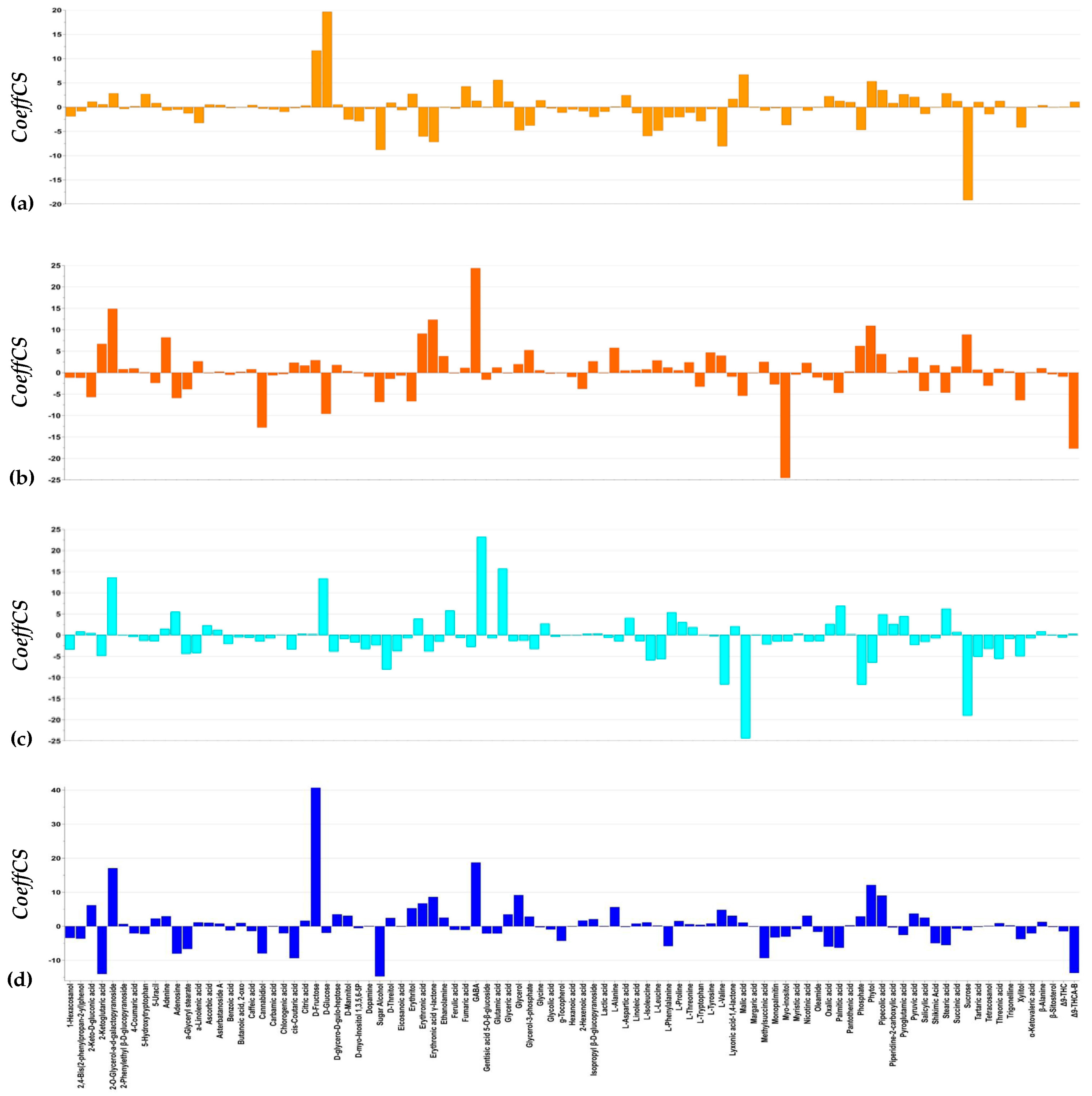
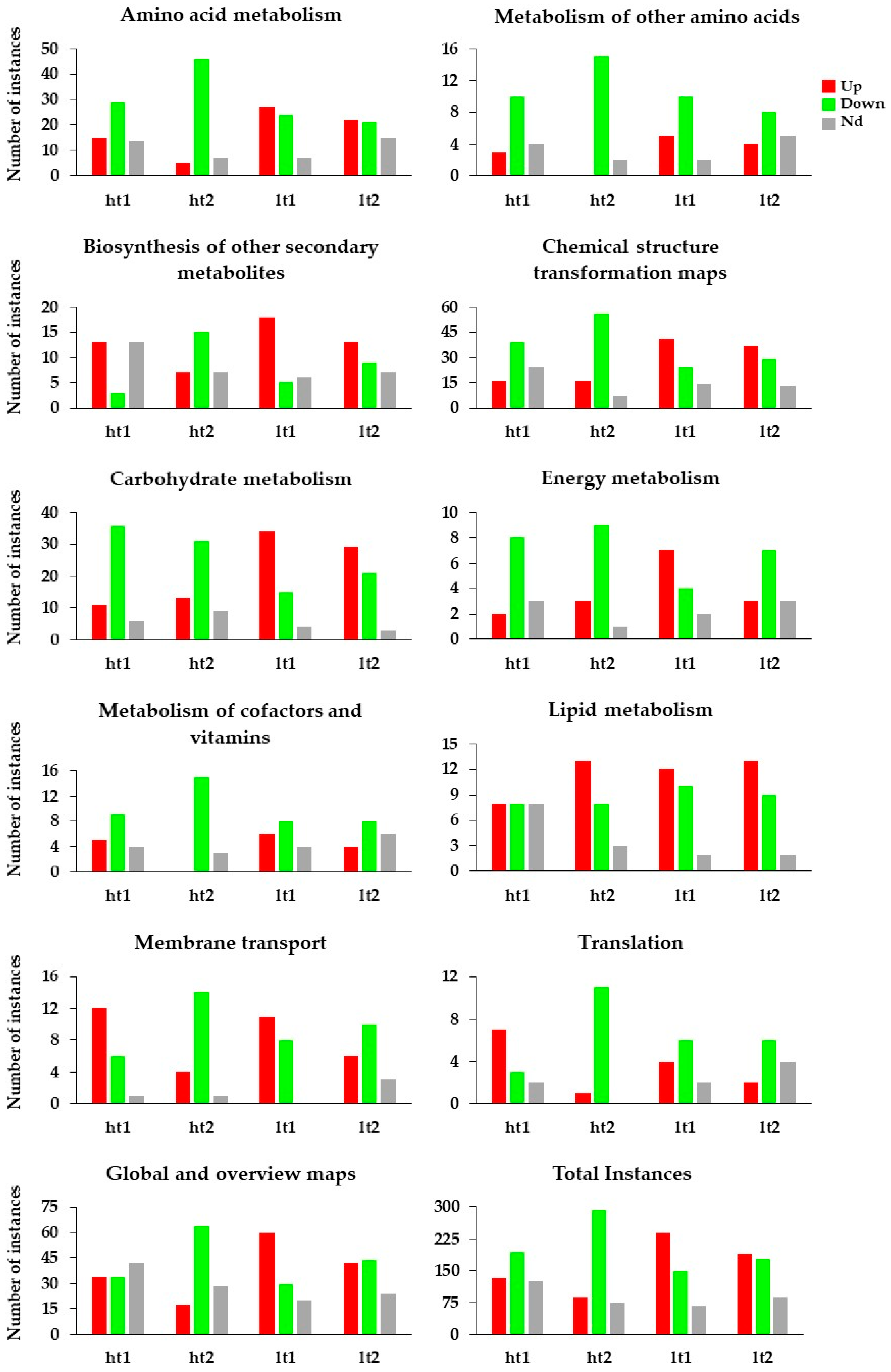

| Biosynthetic Pathway | ht1U | ht1D | ht1Nd | ht2U | ht2D | ht2Nd | lt1U | lt1D | lt1Nd | lt2U | lt2D | lt2Nd | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| map00400 Phenylalanine, tyrosine and tryptophan biosynthesis | 2 | 0 | 2 | 1 | 3 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | Amino acid metabolism |
| map00290 Valine leucine and isoleucine biosynthesis | 4 | 0 | 1 | 0 | 4 | 1 | 4 | 1 | 0 | 0 | 4 | 1 | Amino acid metabolism |
| map00350 Tyrosine metabolism | 0 | 2 | 3 | 1 | 4 | 0 | 3 | 1 | 1 | 3 | 1 | 1 | Amino acid metabolism |
| map00260 Glycine, serine and threonine metabolism | 2 | 3 | 1 | 1 | 3 | 2 | 2 | 3 | 1 | 0 | 3 | 3 | Amino acid metabolism |
| map00360 Phenylalanine metabolism | 2 | 2 | 3 | 1 | 5 | 1 | 4 | 2 | 1 | 5 | 2 | 0 | Amino acid metabolism |
| map00250 Alanine, aspartate and glutamate metabolism | 0 | 6 | 2 | 0 | 8 | 0 | 3 | 4 | 1 | 4 | 3 | 1 | Amino acid metabolism |
| Varia Amino acid metabolism | 5 | 12 | 2 | 1 | 15 | 3 | 8 | 10 | 1 | 5 | 7 | 7 | Amino acid metabolism |
| map00940 Phenylpropanoid biosynthesis | 2 | 0 | 4 | 0 | 4 | 2 | 4 | 1 | 1 | 5 | 1 | 0 | Biosynthesis of other secondary metabolites |
| map00960 Tropane, piperidine and pyridine alkaloid biosynthesis | 3 | 2 | 1 | 0 | 4 | 2 | 3 | 3 | 0 | 1 | 3 | 2 | Biosynthesis of other secondary metabolites |
| map00966 Glucosinolate biosynthesis | 5 | 0 | 1 | 1 | 5 | 0 | 3 | 1 | 2 | 1 | 3 | 2 | Biosynthesis of other secondary metabolites |
| Varia Biosynthesis of other secondary metabolites | 3 | 1 | 7 | 6 | 2 | 3 | 8 | 0 | 3 | 6 | 2 | 3 | Biosynthesis of other secondary metabolites |
| map00020 Citrate cycle (TCA cycle) | 0 | 4 | 1 | 1 | 4 | 0 | 3 | 1 | 1 | 3 | 2 | 0 | Carbohydrate metabolism |
| map00630 Glyoxylate and dicarboxylate metabolism | 0 | 8 | 2 | 2 | 6 | 2 | 4 | 4 | 2 | 5 | 3 | 2 | Carbohydrate metabolism |
| Varia Carbohydrate metabolism | 6 | 12 | 1 | 7 | 8 | 4 | 15 | 3 | 1 | 11 | 8 | 0 | Carbohydrate metabolism |
| map01070 Biosynthesis of plant hormones | 3 | 3 | 2 | 2 | 6 | 0 | 4 | 3 | 1 | 3 | 4 | 1 | Chemical structure transformation maps |
| map01064 Biosynthesis of alkaloids derived from ornithine, lysine and nicotinic acid | 2 | 8 | 1 | 1 | 9 | 1 | 4 | 6 | 1 | 5 | 4 | 2 | Chemical structure transformation maps |
| map01061 Biosynthesis of phenylpropanoids | 3 | 4 | 5 | 3 | 8 | 1 | 8 | 2 | 2 | 8 | 3 | 1 | Chemical structure transformation maps |
| map01063 Biosynthesis of alkaloids derived from shikimate pathway | 3 | 5 | 5 | 3 | 8 | 2 | 8 | 2 | 3 | 7 | 4 | 2 | Chemical structure transformation maps |
| map01060 Biosynthesis of plant secondary metabolites | 4 | 7 | 7 | 4 | 12 | 2 | 7 | 8 | 3 | 5 | 8 | 5 | Chemical structure transformation maps |
| Varia Sum Energy metabolism | 2 | 5 | 2 | 2 | 7 | 0 | 5 | 3 | 1 | 3 | 4 | 2 | Energy metabolism |
| map01220 Degradation of aromatic compounds | 2 | 1 | 3 | 1 | 3 | 2 | 6 | 0 | 0 | 3 | 2 | 1 | Global and overview maps |
| map01200 Carbon metabolism | 0 | 4 | 3 | 1 | 5 | 1 | 3 | 2 | 2 | 2 | 3 | 2 | Global and overview maps |
| map01210 2-Oxocarboxylic acid metabolism | 5 | 3 | 3 | 1 | 9 | 1 | 5 | 3 | 3 | 3 | 5 | 3 | Global and overview maps |
| map01230 Biosynthesis of amino acids | 7 | 4 | 5 | 1 | 14 | 1 | 7 | 6 | 3 | 4 | 8 | 4 | Global and overview maps |
| map00061 Fatty acid biosynthesis | 0 | 2 | 2 | 3 | 0 | 1 | 1 | 2 | 1 | 4 | 0 | 0 | Lipid metabolism |
| map01040 Biosynthesis of unsaturated fatty acids | 3 | 2 | 1 | 4 | 2 | 0 | 3 | 3 | 0 | 3 | 2 | 1 | Lipid metabolism |
| Varia Sum Lipid metabolism | 5 | 4 | 5 | 6 | 6 | 2 | 8 | 5 | 1 | 6 | 7 | 1 | Lipid metabolism |
| map02010 ABC transporters | 12 | 6 | 1 | 4 | 14 | 1 | 11 | 8 | 0 | 6 | 10 | 3 | Membrane transport |
| map00760 Nicotinate and nicotinamide metabolism | 2 | 5 | 0 | 0 | 6 | 1 | 4 | 3 | 0 | 2 | 3 | 2 | Metabolism of cofactors and vitamins |
| Varia Metabolism of cofactors and vitamins | 2 | 2 | 3 | 0 | 6 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | Metabolism of cofactors and vitamins |
| Varia Metabolism of other amino acids | 0 | 1 | 2 | 0 | 3 | 0 | 3 | 0 | 0 | 1 | 2 | 0 | Metabolism of other amino acids |
| map00410 beta-Alanine metabolism | 0 | 3 | 1 | 0 | 3 | 1 | 0 | 3 | 1 | 0 | 2 | 2 | Metabolism of other amino acids |
| map00480 Glutathione metabolism | 0 | 4 | 0 | 0 | 3 | 1 | 0 | 4 | 0 | 2 | 1 | 1 | Metabolism of other amino acids |
| map00460 Cyanoamino acid metabolism | 3 | 2 | 1 | 0 | 6 | 0 | 2 | 3 | 1 | 1 | 3 | 2 | Metabolism of other amino acids |
| Varia Nucleotide metabolism | 2 | 1 | 2 | 2 | 3 | 0 | 1 | 3 | 1 | 1 | 2 | 2 | Nucleotide metabolism |
| Varia Signal transduction | 5 | 2 | 1 | 4 | 3 | 1 | 7 | 1 | 0 | 4 | 3 | 1 | Signal transduction |
| map00970 Aminoacyl-tRNA biosynthesis | 7 | 3 | 2 | 1 | 11 | 0 | 4 | 6 | 2 | 2 | 6 | 4 | Translation |
| TOTAL MAPS INSTANCES | 134 | 193 | 127 | 88 | 293 | 73 | 239 | 149 | 66 | 188 | 178 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerezoudis, C.N.; Zervou, M.; Matzapetakis, M.; Bilalis, D.; Aliferis, K.A. Metabolomics-Driven Investigation of Harpin αβ and Laminarin Effects on Cannabis sativa L. Employing GC/EI/MS and 1H NMR Metabolomics. Agrochemicals 2025, 4, 16. https://doi.org/10.3390/agrochemicals4030016
Kerezoudis CN, Zervou M, Matzapetakis M, Bilalis D, Aliferis KA. Metabolomics-Driven Investigation of Harpin αβ and Laminarin Effects on Cannabis sativa L. Employing GC/EI/MS and 1H NMR Metabolomics. Agrochemicals. 2025; 4(3):16. https://doi.org/10.3390/agrochemicals4030016
Chicago/Turabian StyleKerezoudis, Christos N., Maria Zervou, Manolis Matzapetakis, Dimitrios Bilalis, and Konstantinos A. Aliferis. 2025. "Metabolomics-Driven Investigation of Harpin αβ and Laminarin Effects on Cannabis sativa L. Employing GC/EI/MS and 1H NMR Metabolomics" Agrochemicals 4, no. 3: 16. https://doi.org/10.3390/agrochemicals4030016
APA StyleKerezoudis, C. N., Zervou, M., Matzapetakis, M., Bilalis, D., & Aliferis, K. A. (2025). Metabolomics-Driven Investigation of Harpin αβ and Laminarin Effects on Cannabis sativa L. Employing GC/EI/MS and 1H NMR Metabolomics. Agrochemicals, 4(3), 16. https://doi.org/10.3390/agrochemicals4030016







