Natural Herbicide Shows Cytotoxicity, Neurotoxicity, and Antioxidant System Alterations on SH-SY5Y and HaCaT Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Exposure
2.3. Cell Viability Assay
2.4. Cytotoxicity Assay
2.5. Acetylcholinesterase Enzyme Activity and Antioxidant System Assay
2.6. Statistical Analysis
3. Results
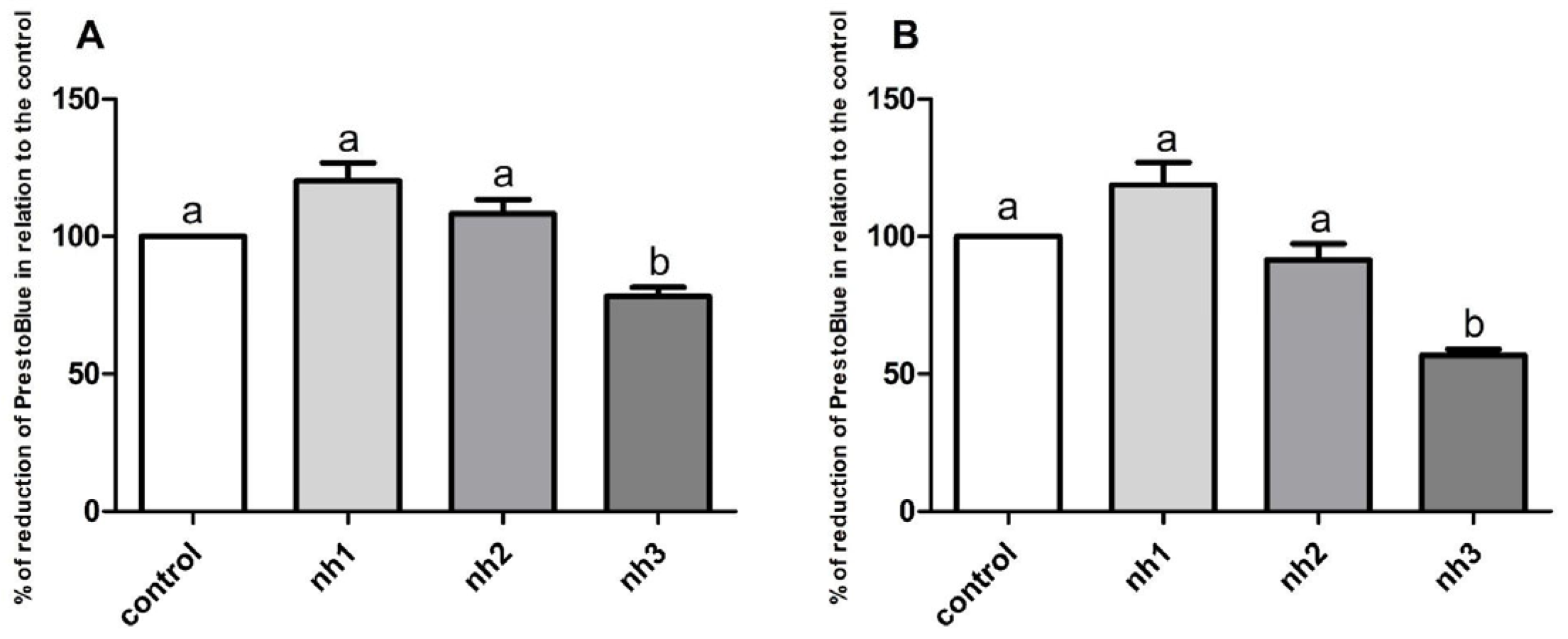
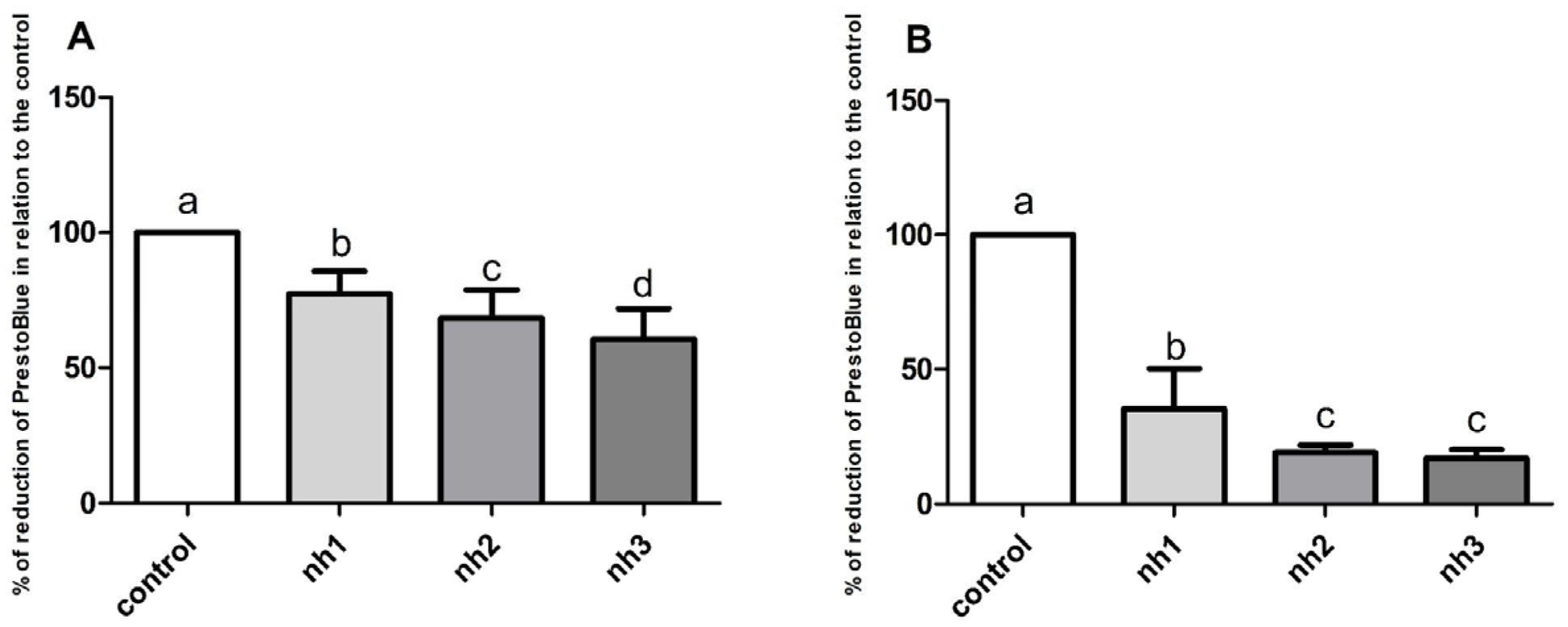
3.1. Cytotoxicity of NH on SH-SY5Y Cell Line
3.2. Cytotoxicity of NH on HaCaT Cell Line
3.3. Acetylcholinesterase Activity on SH-SY5Y Cell Line
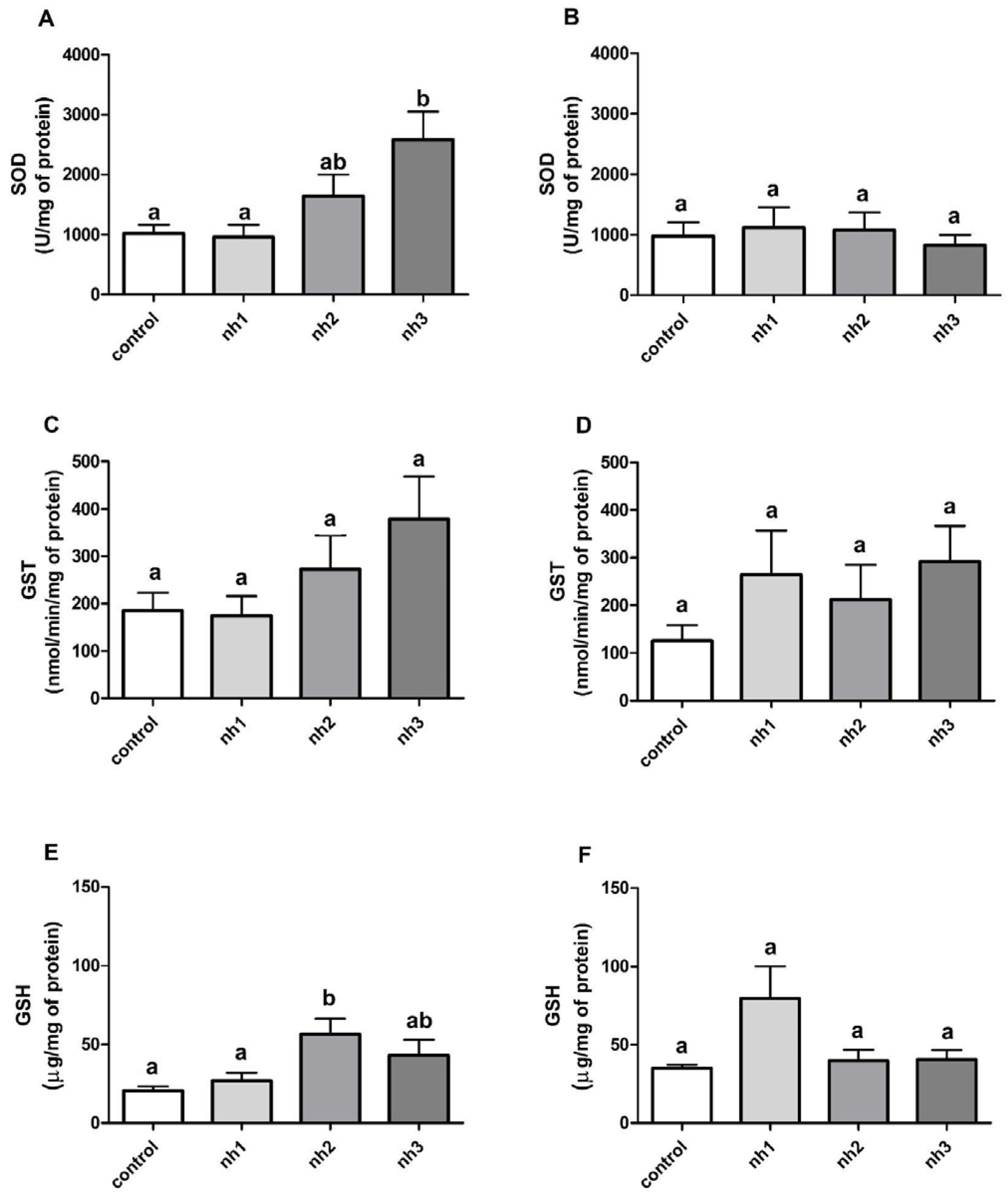
3.4. Effects of NH on Oxidative Stress Markers on SH-SY5Y Cell Line
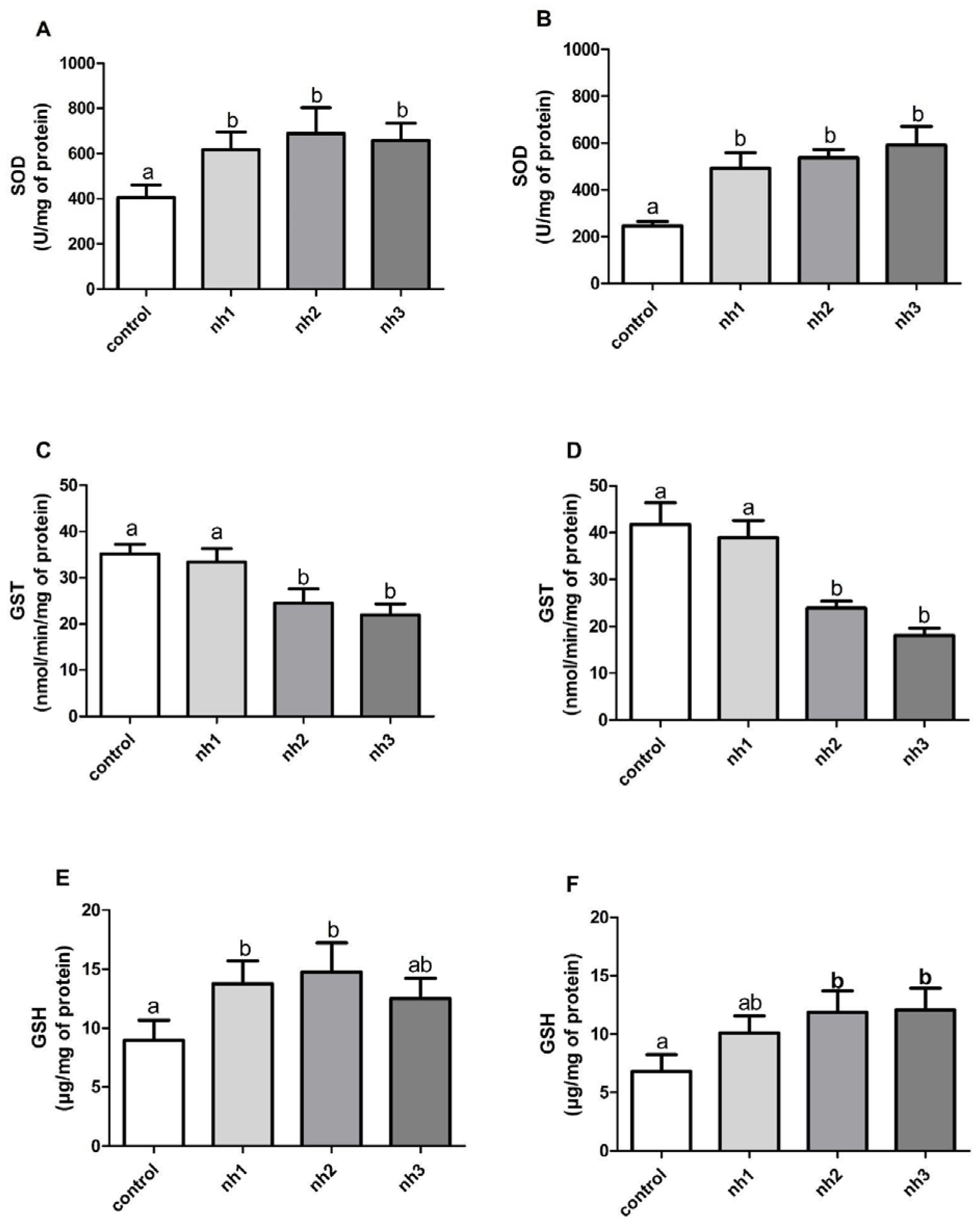
3.5. Effects of NH on Oxidative Stress Markers on HaCaT Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity analysis of endocrine disrupting pesticides on non-target organisms: A critical analysis on toxicity mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef] [PubMed]
- Costas-Ferreira, C.; Durán, D.; Faro, L.R.F. Toxic effects of glyphosate on the nervous system: A systematic review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, S.; Csomor, J.; Urbanek, P.; Pelclova, D. Toxic epidermal necrolysis after exposure to dithiocarbamate fungicide mancozeb. BCPT 2016, 118, 89–91. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure routes and health risks associated with pesticide application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Ghazi, R.M.; Yusoff, N.R.N.; Halim, N.S.A.; Rahayu, I.; Wahab, A.; Latif, N.A.; Hasmoni, S.H.; Zaini, M.A.A.; Zakari, Z.A. Health effects of herbicides and its current removal strategies. Bioengineered 2023, 14, 2259526. [Google Scholar] [CrossRef]
- Favaretto, A.; Cantrell, C.L.; Fronczek, F.R.; Duke, S.O.; Wedge, D.E.; Ali, A.; Scheffer-Basso, S.M. New phytotoxic cassane-like diterpenoids from Eragrostis plana. J. Agric. Food Chem. 2019, 67, 1973–1981. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic effects of essential oil from Cymbopogon citratus and its physiological mechanisms on barnyardgrass (Echinochloa crus-Galli). Ind. Crops Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Refifa, T.; Chahdoura, H.; Flamini, G.; Adouni, K.; Hammami, M.; Helal, A.N. Allelopathic potential of Pinus halepensis needles. Allelopath. J. 2016, 38, 193–214. [Google Scholar]
- Haas, P.; Kuhn, D.; Cordeiro, S.G.; Schweizer, Y.A.; Costa, B.; Hoehne, L. Phytotoxic effect of Pinus elliottii extracts on invasive plants from agricultural cultivation systems. Rev. Ibero-Am. Ciênc. Ambie. 2021, 12, 121–131. [Google Scholar] [CrossRef]
- Al-Naib, F.A.G.; Rice, E.L. Allelopathic effects of Platanus occidentalis. Bull. Torrey Bot. Club. 1971, 98, 75–82. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Hiroyuki, T.; Mitsuhiro, M.; Khanh, T.D.; Chung, I.M. Evaluation on phytotoxicity of neem (Azadirachta indica. A. Juss) to crops and weeds. Crop Prot. 2004, 23, 335–345. [Google Scholar] [CrossRef]
- Lee, G.H.; Choi, K.C. Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef]
- Sirin, G.S.; Zhang, Y. How is acetylcholinesterase phosphonylated by soman? An ab initio QM/MM molecular dynamics study. J. Phys. Chem. A 2014, 118, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Lechinovski, L.; Bados, M.; Rosa, J.; Moda, D.B.; Krawczyk, A.C.D.B. Ecotoxicological effects of conventional herbicides and a natural herbicide on freshwater fish (Danio rerio). J. Environ. Sci. Health Part B 2022, 57, 812–820. [Google Scholar] [CrossRef]
- Kandárová, H.; Letašiová, S. Alternative methods in toxicology: Pre-validated and validated methods. Interdiscip. Toxicol. 2011, 4, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Martínez, M.A.; Rodríguez, J.L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Maximiliano, J.E.; Anadón, A.; Ares, I. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2020, 231, 105414. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. JCB 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Gehin, A.; Guyon, C.; Nicod, L. Glyphosate-induced antioxidant imbalance in HaCaT: The protective effect of vitamins C and E. Environ. Toxicol Pharmacol. 2006, 22, 27–34. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, A.Y.; Jeong, S.H.; Park, K.H.; Paik, M.K.; Cho, N.J.; Kim, J.E.; Cho, M.H. Chlorpyrifos induces NLRP3 inflammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells. Toxicology 2015, 338, 37–46. [Google Scholar] [CrossRef]
- Sawickia, K.; Czajkaa, M.; Matysiak-Kuchareka, M.; Kruszewskia, M.; Skawińskia, W.; Brzóskac, K.; Kapka-Skrzypczaka, L. Chlorpyrifos stimulates expression of vitamin D3 receptor in skin cells irradiated with UVB. Pestic. Biochem. Physiol. 2019, 154, 17–22. [Google Scholar] [CrossRef]
- Mentz, L.A.; Petrovick, P.R. Farmacognosia: Da Planta ao Medicamento; Editora da UFSC: Florianópolis, Brazil, 2003. [Google Scholar]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2001, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Zheng, W.; Wei, X.; Wu, W.; Duysen, E.G.; Jiang, W. Study of acetylcholinesterase activity and apoptosis in SH-SY5Y cells and mice exposed to ethanol. Toxicology 2017, 384, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huchthausen, J.; Schlichting, R.; Scholz, S.; Henneberger, L.; Escher, B.I. Validation of an SH-SY5Y Cell–Based Acetylcholinesterase Inhibition Assay for Water Quality Assessment. Environ. Toxicol. Chem. 2022, 41, 3046–3057. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectroch. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Singh, V.K.; Pathak, A.K.; Bala, L. Serum deprivation enhanced monocrotophos mediated cellular damages in human lung carcinoma and skin keratinocyte. Gene Rep. 2022, 27, 101562. [Google Scholar] [CrossRef]
- Lasalvia, M.; Perna, G.; Capozzi, V. Raman spectroscopy of human neuronal and epidermal cells exposed to an insecticide mixture of chlorpyrifos and deltamethrin. Appl. Spectrosc. 2014, 68, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Farombi, E.O. Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic. Biochem. Physiol. 2015, 118, 90–98. [Google Scholar] [CrossRef]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. pH-responsive eco-friendly chitosan modified cenosphere/alginate composite hydrogel beads as carrier for controlled release of Imidacloprid towards sustainable pest control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- Heu, C.; Berquand, A.; Elie-Caille, C.; Nicod, L. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Biol. 2012, 178, 1–7. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Lin, H.; Gu, K.; Feng, F. Recent progress in the development of small molecule Nrf2 modulators: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 763–785. [Google Scholar] [CrossRef]
- Mota, W.M.; Barros, M.L.; Cunha, P.E.L.; Santana, M.V.A.; Stevam, C.S.; Leopoldo, P.T.G.; Fernandes, R.P.M. Avaliação da inibição da acetilcolinesterase por extratos de plantas medicinais. Rev. Bras. Plantas Med. 2012, 14, 624–628. [Google Scholar] [CrossRef]
- Fernandez, M.; Ríos, J.C.; Jos, A.; Repetto, G. Comparative Cytotoxicity of Alachlor on RTG-2 Trout and SH-SY5Y Human Cells. Arch. Environ. Contam. Toxicol. 2006, 51, 515–520. [Google Scholar] [CrossRef]
- Modesto, K.A.; Martinez, C.B.R. Effects of Roundup Transorb on fish: Hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 2010, 81, 781–787. [Google Scholar] [CrossRef]
- Zhang, X.J.; Greenberg, D.S. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? JMAJ 2002, 47, 271–276. [Google Scholar]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide radicals in the execution of cell death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tiffany-Castiglioni, E. The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: Relevance to the dopaminergic pathogenesis. J. Toxicol. Environ. Health Part A 2005, 68, 1939–1961. [Google Scholar] [CrossRef]
- Diken, M.E.; Dorgan, S.; Dorgan, M.; Turhan, Y. In vitro effects of some pesticides on glutathione-s transferase activity. Fresenius Environ. Bull. 2017, 26, 8023–8029. [Google Scholar]
- Shaikh, S.B.; Nicholson, L.F. Effects of chronic low dose rotenone treatment on human microglial cells. Mol. Neurodegener. 2009, 4, 55. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A. A comparison of the persistence, toxicity, and exposure to high-volume natural plant-derived and synthetic pesticides. Toxicol. Res. Appl. 2020, 4, 1–4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nominato-Oliveira, L.; Silva, J.F.d.; Milhorini, S.d.S.; Lechinovski, L.; Krawczyk, A.C.d.D.B.; Guiloski, I.C. Natural Herbicide Shows Cytotoxicity, Neurotoxicity, and Antioxidant System Alterations on SH-SY5Y and HaCaT Cell Lines. Agrochemicals 2025, 4, 17. https://doi.org/10.3390/agrochemicals4030017
Nominato-Oliveira L, Silva JFd, Milhorini SdS, Lechinovski L, Krawczyk ACdDB, Guiloski IC. Natural Herbicide Shows Cytotoxicity, Neurotoxicity, and Antioxidant System Alterations on SH-SY5Y and HaCaT Cell Lines. Agrochemicals. 2025; 4(3):17. https://doi.org/10.3390/agrochemicals4030017
Chicago/Turabian StyleNominato-Oliveira, Leticia, Juliana Ferreira da Silva, Shayane da Silva Milhorini, Larissa Lechinovski, Ana Carolina de Deus Bueno Krawczyk, and Izonete Cristina Guiloski. 2025. "Natural Herbicide Shows Cytotoxicity, Neurotoxicity, and Antioxidant System Alterations on SH-SY5Y and HaCaT Cell Lines" Agrochemicals 4, no. 3: 17. https://doi.org/10.3390/agrochemicals4030017
APA StyleNominato-Oliveira, L., Silva, J. F. d., Milhorini, S. d. S., Lechinovski, L., Krawczyk, A. C. d. D. B., & Guiloski, I. C. (2025). Natural Herbicide Shows Cytotoxicity, Neurotoxicity, and Antioxidant System Alterations on SH-SY5Y and HaCaT Cell Lines. Agrochemicals, 4(3), 17. https://doi.org/10.3390/agrochemicals4030017





