Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Electronic Databases
- “Septoria AND tritici AND blotch” (VOSviewer visualization can be seen in Figure 3)
- “Pyrenophora AND tritici-repentis” (VOSviewer visualization can be seen in Figure 4)
- “Septoria AND nodorum AND blotch” (VOSviewer visualization can be seen in Figure 5)
- “Climate change AND wheat AND diseases” (VOSviewer visualization can be seen in Figure 6)
- “wheat AND disease AND propiconazole” (VOSviewer visualization can be seen in Figure 7)
- “wheat AND disease AND prothioconazole” (VOSviewer visualization can be seen in Figure 8)
- “wheat AND diseases AND algorithm” (VOSviewer visualization can be seen in Figure 9)
2.2. Mapping SCOPUS Literature with VOSviewer
3. Results
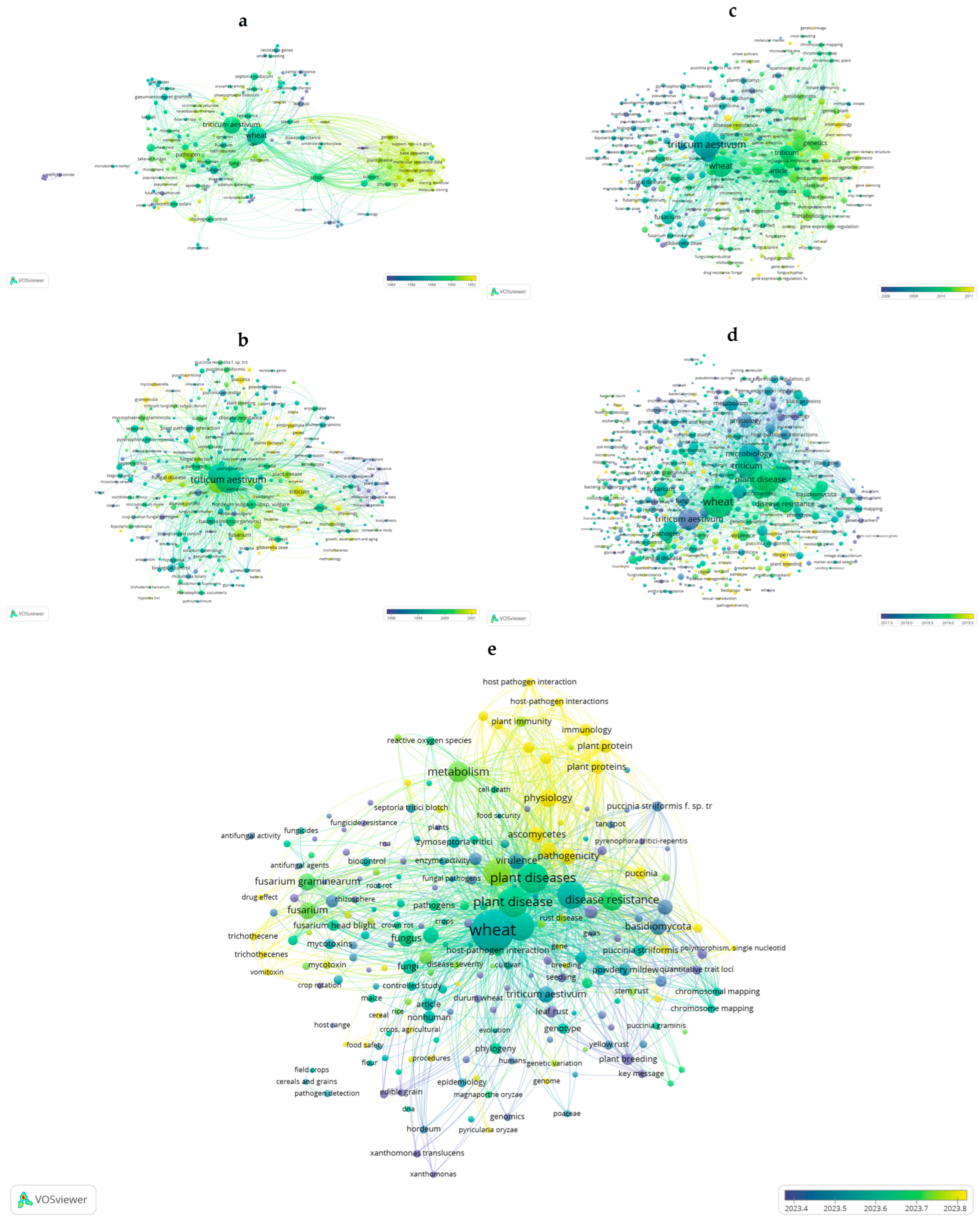
3.1. Mapping SCOPUS Literature with VOSviewer
3.1.1. Address the Most Important Pathogens That Are Threatening Winter Wheat Crop 1st Query
3.1.2. Address Wheat Diseases Management 2nd Query

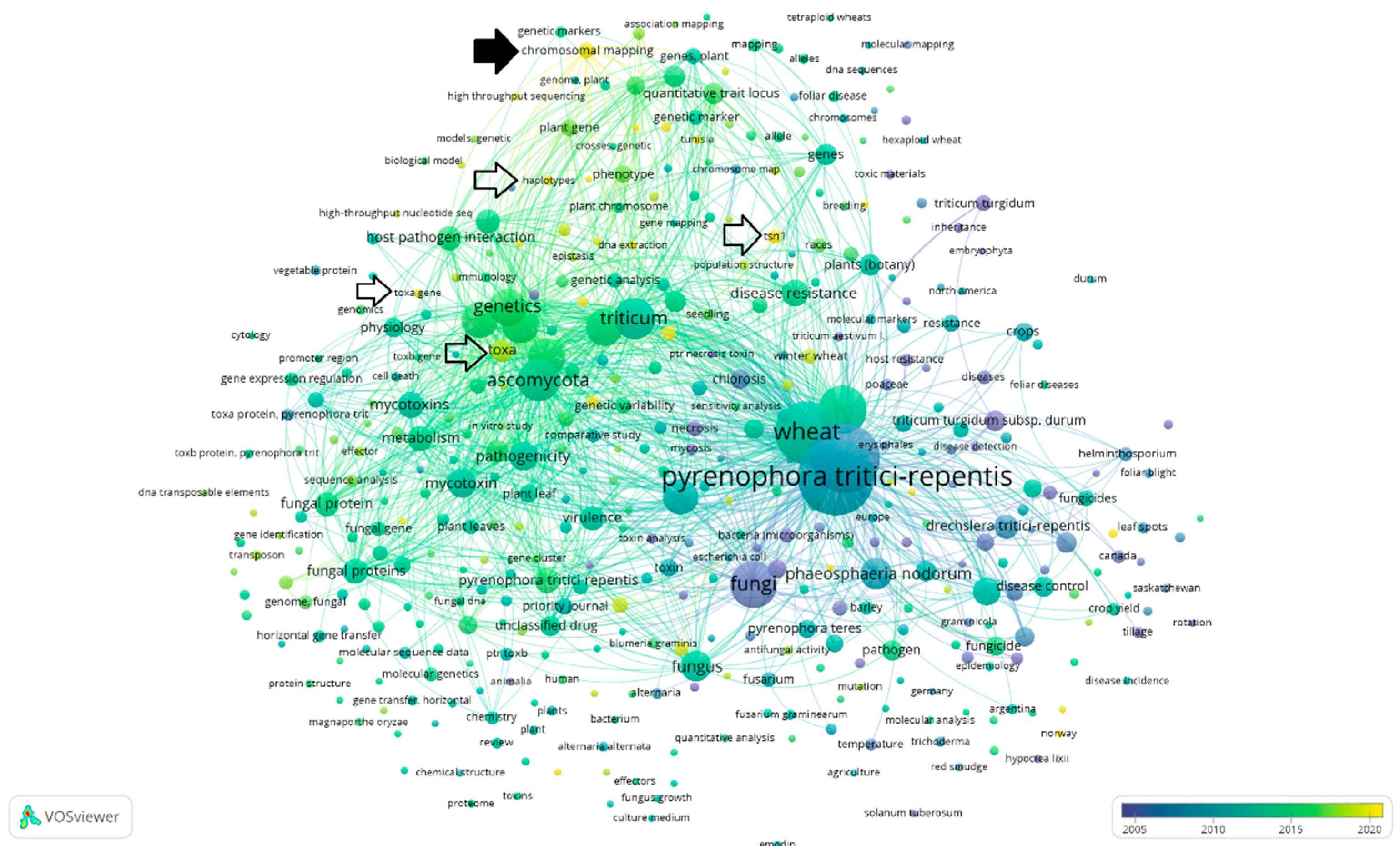
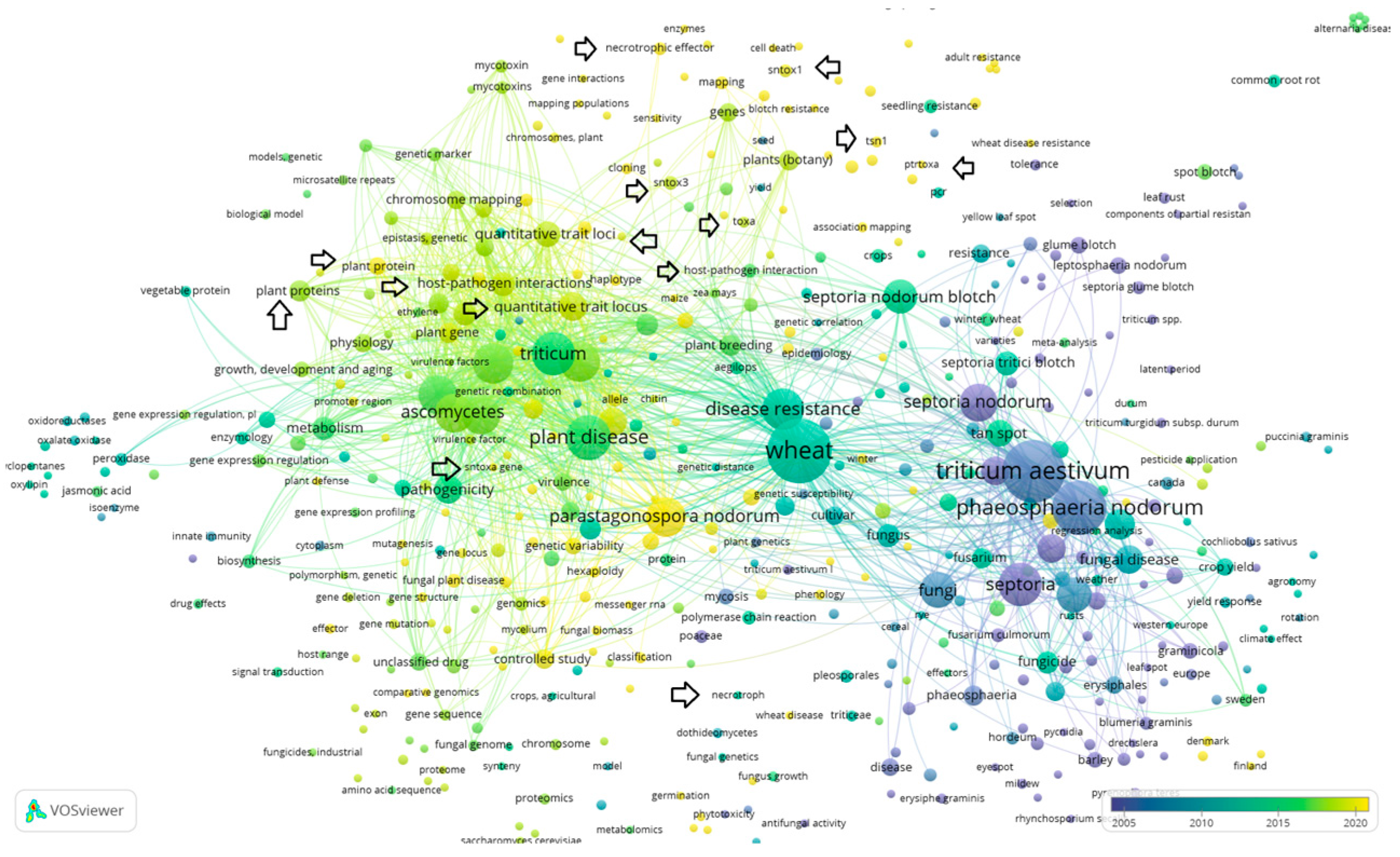
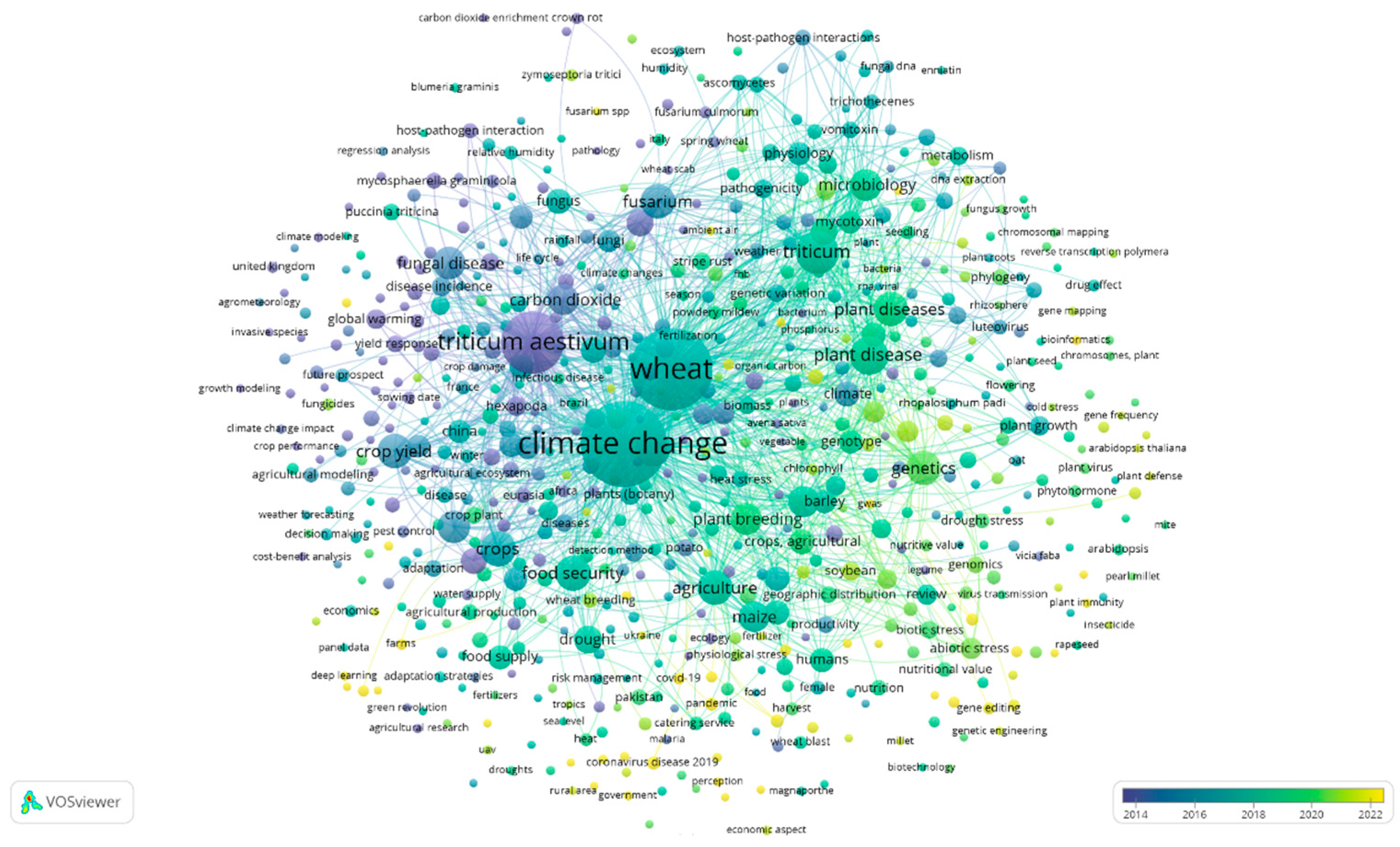
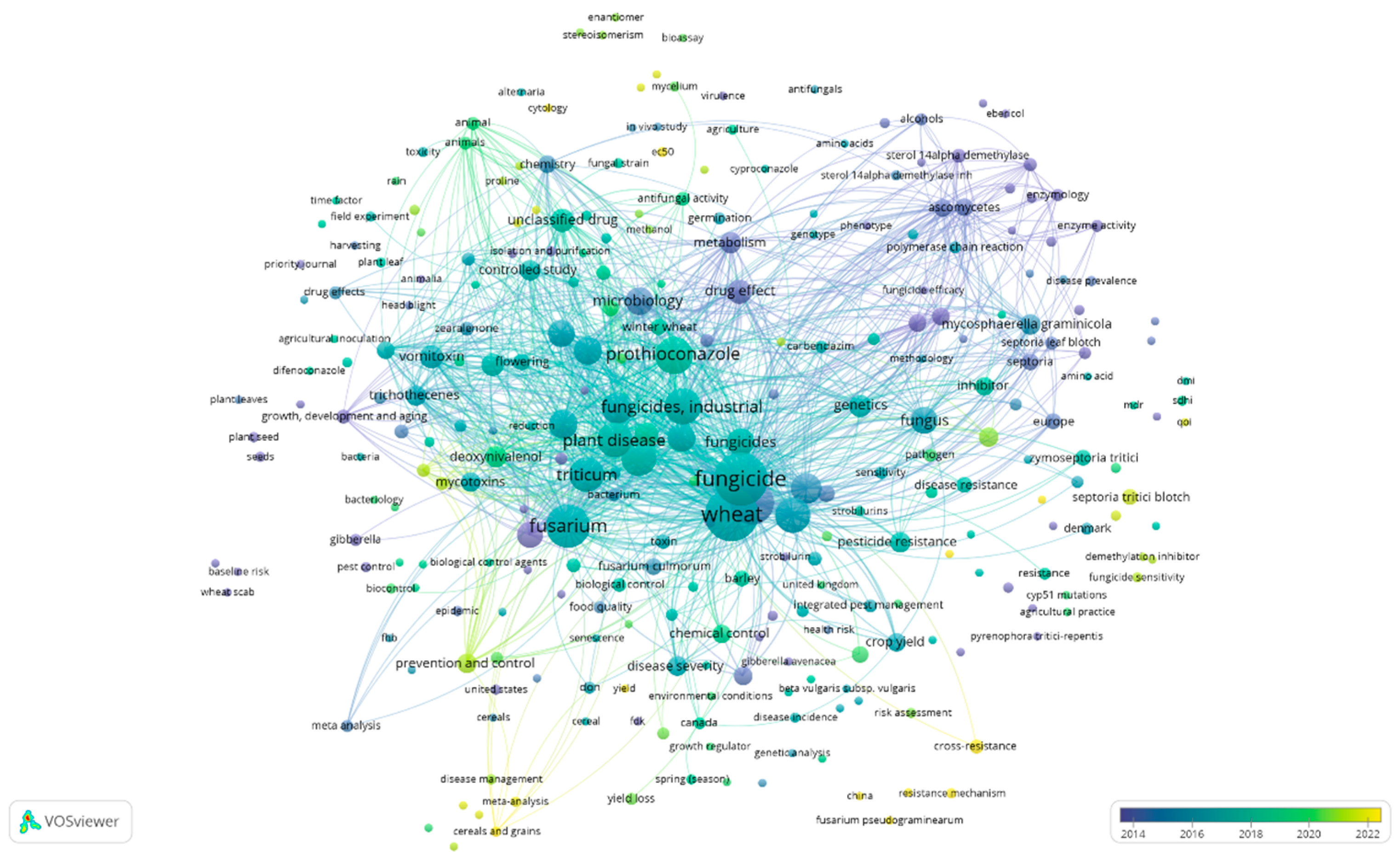

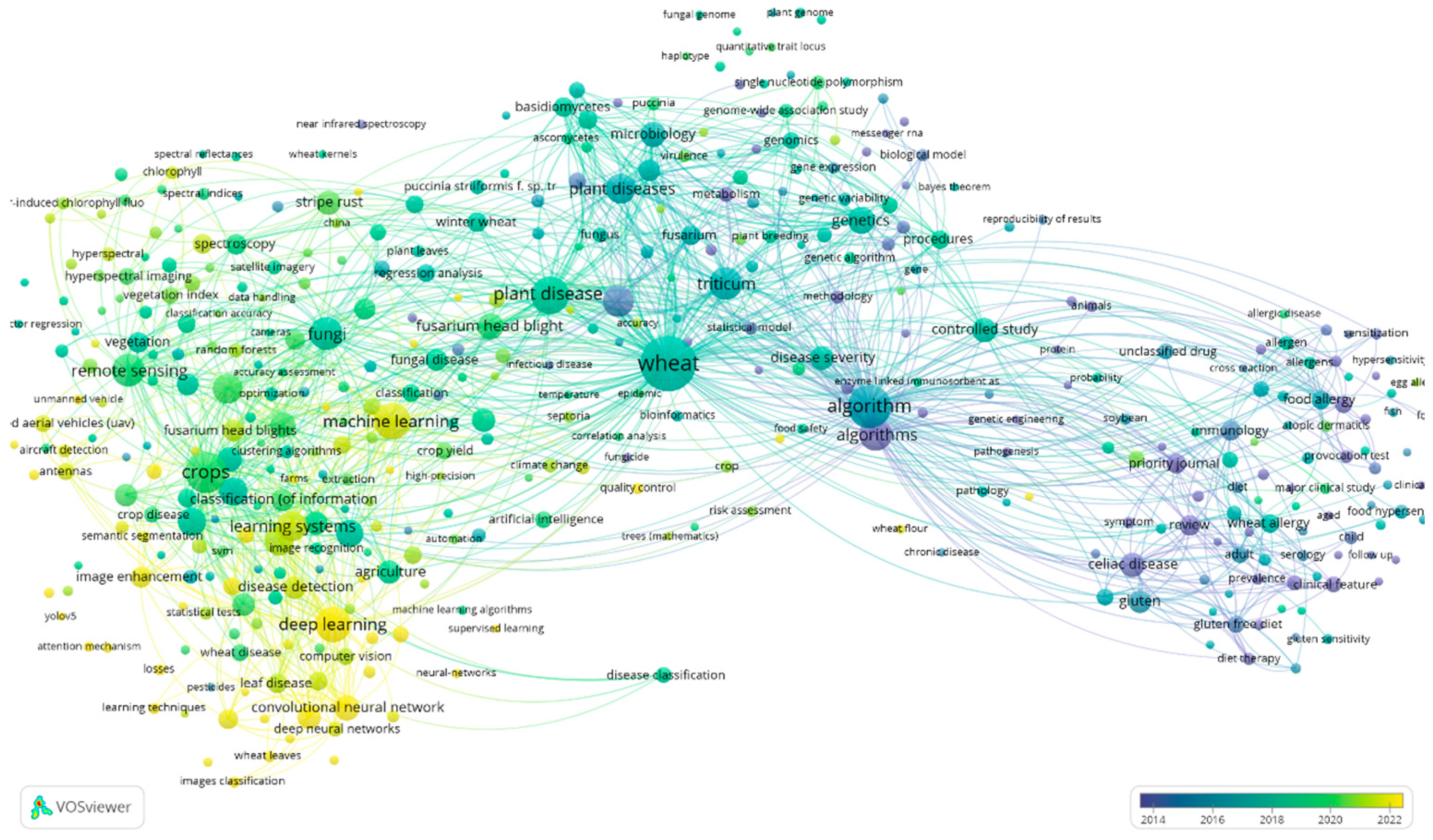
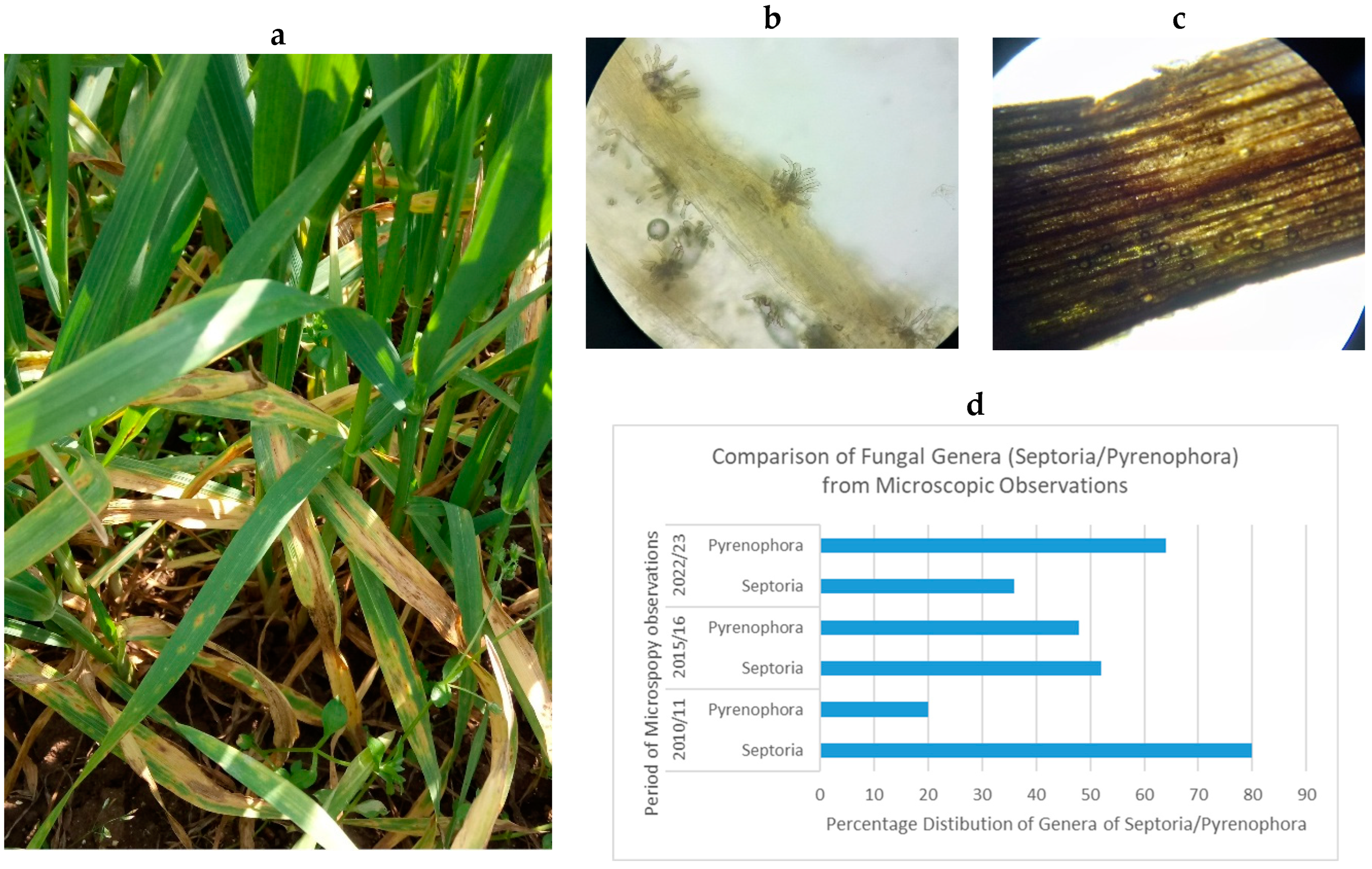
4. Discussion with Overview of the Collected Data
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dongen, P.V.; Groot, A.D. History of ergot alkaloids from ergotism to ergometrine. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 60, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.J. Compendium of Wheat Diseases Pests; Bockus, W.W., Bowden, R.L., Hunger, R.M., Murray, T.D., Smiley, R.W., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2010; pp. 30–32. [Google Scholar]
- Carbonell-Rozas, L.; Alabrese, A.; Meloni, R.; Righetti, L.; Blandino, M.; Dall’Asta, C. Occurrence of Ergot Alkaloids in Major and Minor Cereals from Northern Italy: A Three Harvesting Years Scenario. J. Agric. Food Chem. 2023, 71, 15821–15828. [Google Scholar] [CrossRef]
- Peloso, M.; Minkoumba Sonfack, G.; Prizio, I.; Baraldini Molgora, E.; Pedretti, G.; Fedrizzi, G.; Caprai, E. Climate Effects on Ergot and Ergot Alkaloids Occurrence in Italian Wheat. Foods 2024, 13, 1907. [Google Scholar] [CrossRef]
- Rollo, E.; Catellani, D.; Dall’Asta, C.; Dreolin, N.; Suman, M. Ergot alkaloids: Comparison of extraction efficiencies for their monitoring in several cereal-solvent combinations by UPLC-MS/MS. Mycotoxin Res. 2024, 41, 127–146. [Google Scholar] [CrossRef]
- Racca, P.; Kakau, J.; Kleinhenz, B.; Kuhn, C. Impact of Climate Change on the Phenological Development of Winter Wheat, Sugar Beet and Winter Oilseed Rape in Lower Saxony, Germany. J. Plant Dis. Prot. 2015, 122, 16–27. [Google Scholar] [CrossRef]
- Schultz, T.R.; Johnston, W.J.; Golob, C.T.; Maguire, J.D. Control of ergot in Kentucky bluegrass seed production using fungicides. Plant Dis. 1993, 77, 685–687. [Google Scholar] [CrossRef]
- Alaoufi, S.; Friskop, A.J.; Simsek, S. Effect of Field-applied Fungicides on Claviceps purpurea Sclerotia and Associated Toxins in Wheat. J. Food Prot. 2023, 86, 100046. [Google Scholar] [CrossRef]
- Vagelas, I.; Cavalaris, C.; Karapetsi, L.; Koukidis, C.; Servis, D.; Madesis, P. Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change. Agronomy 2022, 12, 2000. [Google Scholar] [CrossRef]
- Carignano, M.; Staggenborg, S.A.; Shroyer, J.P. Management Practices to Minimize Tan Spot in a Continuous Wheat Rotation. Agron. J. 2008, 100, 145–153. [Google Scholar] [CrossRef]
- Bockus, W.W.; Claassen, M.M. Effects of crop rotation and residue management practices on severity of tan spot of winter wheat. Plant Dis. 1992, 76, 633–636. [Google Scholar] [CrossRef]
- Cotuna, O.; Paraschivu, M.; Paraschivu, A.M.; Sărățeanu, V. The influence of tillage, crop rotation and residue management on tan spot (Drechslera tritici repentis Died. Shoemaker) in winter wheat. Res. J. Agric. Sci. 2015, 47, 13–21. [Google Scholar]
- Cox, C.M.; Garrett, K.A.; Bowden, R.L.; Fritz, A.K.; Dendy, S.P.; Heer, W.F. Cultivar mixtures for the simultaneous management of multiple diseases: Tan spot and leaf rust of wheat. Phytopathology 2004, 94, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Sehgal, S.K.; Ali, S.; Liatukas, Ž.; Ittu, M.; Kaur, N. Characterization of Pyrenophora tritici-repentis (Tan Spot of Wheat) Races in Baltic States and Romania. Plant Pathol. J. 2017, 33, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Geiger, H.H. Biology, Genetics, and Management of Ergot (Claviceps spp.) in Rye, Sorghum, and Pearl Millet. Toxins 2015, 7, 659–678. [Google Scholar] [CrossRef]
- Basnet, B.; Juliana, P.; Bhattarai, K.; Upreti, U. A Review on Major Rust Resistance Gene and Amino Acid Changes on Wheat (Triticum aestivum L). Adv. Agric. 2022, 2022, 7419326. [Google Scholar] [CrossRef]
- Bajoriya, D.K.; Mishra, K.K.; Yadav, V.K. Evaluation of newer fungicides for the management of wheat stem rust caused by (Puccinia graminis f. sp. tritici). J. Cereal Res. 2023, 15, 277–283. [Google Scholar] [CrossRef]
- Svarta, A.; Bimsteine, G. Winter wheat leaf diseases and several steps included in their integrated control: A review. Res. Rural Dev. 2019, 2, 55–62. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Zwingman, M.V.; Breathnach, J.A.; Baenziger, S. Economic returns from fungicide application to control foliar fungal diseases in winter wheat. Crop Prot. 2011, 30, 685–692. [Google Scholar] [CrossRef]
- Watson, B.H.; Hunger, R.M.; Marburger, D.A. Economic returns of one versus two fungicide applications in oklahoma winter wheat. Crop Sci. 2020, 60, 441–453. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. TAG. Theor. Appl. Genetics. Theor. Und Angew. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef]
- Yang, L.; Gao, F.; Shang, L.; Zhan, J.; McDonald, B.A. Association between Virulence and Triazole Tolerance in the Phytopathogenic Fungus Mycosphaerella graminicola. PLoS ONE 2013, 8, e59568. [Google Scholar] [CrossRef] [PubMed]
- Moya-Elizondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Duveiller, E.; Sharma, R.C. Effects of seed treatment and foliar fungicides on Helminthosporium leaf blight and on performance of wheat in warmer growing conditions. J. Phytopathol. 2005, 153, 401–408. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Zhao, X.; Zhang, S.; Ma, H. Exploring and applying genes to enhance the resistance to Fusarium head blight in wheat. Front. Plant Sci. 2022, 13, 1026611. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á. What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review. Toxins 2024, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Q.; Wang, S.; Wang, M.; Wang, C.; Tian, Z.; Liu, X.; Ji, W.; Zhang, H. Gene co-expression network analysis provides a novel insight into the dynamic response of wheat to powdery mildew stress. J. Genet. 2020, 99, 44. [Google Scholar] [CrossRef]
- Wang, B.; Meng, T.; Xiao, B.; Yu, T.; Yue, T.; Jin, Y.; Ma, P. Fighting wheat powdery mildew: From genes to fields. Theor. Appl. Genet. 2023, 136, 196. [Google Scholar] [CrossRef]
- Esmail, S.M.; Draz, I.S. An active role of systemic fungicides to curb wheat powdery mildew caused by Blumeria graminis f. sp. tritici. Agric. Eng. Int. CIGR J. 2018, 19, 315–322. [Google Scholar]
- Chandra, S.; Kazmi, A.Z.; Ahmed, Z.G.; Roychowdhury, G.; Kumari, V.; Kumar, M.; Mukhopadhyay, K. Genome-wide identification and characterization of NB-ARC resistant genes in wheat (Triticum aestivum L.) and their expression during leaf rust infection. Plant Cell Rep. 2017, 36, 1097–1112. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Y.; Zhou, M.; Zeng, L.; Liu, R.; Li, Y.; Liu, Z.; Zhang, C.; Lu, L.; Zhang, L. Characterization and diagnostic marker development for Yr28-rga1 conferring stripe rust resistance in wheat. Eur. J. Plant Pathol. 2019, 156, 623–634. [Google Scholar] [CrossRef]
- Chandra, S.; Satapathy, L.; Basu, S.; Jha, S.K.; Kumar, M.; Mukhopadhyay, K. Characterization of the leaf rust responsive ARF genes in wheat (Triticum aestivum L.). Plant Cell Rep. 2020, 39, 1639–1654. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Backhouse, D.; Ponte, E.M. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Melloy, P.; Hollaway, G.J.; Luck, J.; Norton, R.M.; Aitken, E.A.; Chakraborty, S. Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Glob. Chang. Biol. 2010, 16, 3363–3373. [Google Scholar] [CrossRef]
- Bouktila, D.; Khalfallah, Y.; Habachi-Houimli, Y.; Mezghani-Khemakhem, M.; Makni, M.; Makni, H. Full-genome identification and characterization of NBS-encoding disease resistance genes in wheat. Mol. Genet. Genom. 2015, 290, 257–271. [Google Scholar] [CrossRef]
- Adhikari, U.; Cowger, C.; Ojiambo, P.S. Evaluation of a Model for Predicting Onset of Septoria nodorum blotch in Winter Wheat. Plant Dis. 2022, 107, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Vagelas, I.; Leontopoulos, S. A Bibliometric Analysis and a Citation Mapping Process for the Role of Soil Recycled Organic Matter and Microbe Interaction due to Climate Change Using Scopus Database. AgriEngineering 2023, 5, 581–610. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Gohari, A.M.; Mehrabi, R.; Kilaru, S.; Steinberg, G.; Ali, S.; Bailey, A.M.; Hammond-Kosack, K.E.; Kema, G.H.; Rudd, J.J. Phosphopantetheinyl transferase (Ppt)-mediated biosynthesis of lysine, but not siderophores or DHN melanin, is required for virulence of Zymoseptoria tritici on wheat. Sci. Rep. 2018, 8, 17069. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Vazquez, Y.; Adams, I.P.; McGreig, S.; Walshaw, J.; van den Berg, F.; Sanderson, R.A.; Pufal, H.; Conyers, C.; Langton, D.W.; Broadhead, R.; et al. Profiling azole resistant haplotypes within Zymoseptoria tritici populations using nanopore sequencing. Front. Agron. 2022, 4, 943440. [Google Scholar] [CrossRef]
- Vestergård, N.F.; Jørgensen, L.N.; Hellin, P.; Heick, T.M. Fungicide spraying intensity in the field drives the selection of amino acid alteration conferring resistance in Zymoseptoria tritici. Eur. J. Plant Pathol. 2023, 166, 385–401. [Google Scholar] [CrossRef]
- Pedersen, T.B.; Nielsen, M.R.; Kristensen, S.B.; Spedtsberg, E.M.; Sørensen, T.; Petersen, C.; Muff, J.; Sondergaard, T.E.; Nielsen, K.L.; Wimmer, R.; et al. Speed dating for enzymes! Finding the perfect phosphopantetheinyl transferase partner for your polyketide synthase. Microb. Cell Factories 2022, 21, 9. [Google Scholar] [CrossRef]
- Tiley, M.M.A.; Lawless, C.; Pilo, P.; Karki, S.J.; Lu, J.; Long, Z.; Gibriel, H.A.; Bailey, A.M.; Feechan, A. The Zymoseptoria tritici white collar-1 gene, ZtWco-1, is required for development and virulence on wheat. Fungal Genet. Biol. 2022, 161, 103715. [Google Scholar] [CrossRef]
- Muduli, S.; Karmakar, S.; Mishra, S. The coordinated action of the enzymes in the L-lysine biosynthetic pathway and how to inhibit it for antibiotic targets. Biochim. Et Biophys. Acta. Gen. Subj. 2023, 1867, 130320. [Google Scholar] [CrossRef]
- Yang, N.; Ovenden, B.; Baxter, B.; McDonald, M.C.; Solomon, P.S.; Milgate, A.W. Multi-stage resistance to Zymoseptoria tritici revealed by GWAS in an Australian bread wheat diversity panel. Front. Plant Sci. 2022, 13, 990915. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; KockAppelgren, P.; Hehir, J.G.; Kang, J.; Meade, F.; Cockram, J.; Milbourne, D.; Spink, J.H.; Mullins, E.; Byrne, S.L. Genetic Analysis Using a Multi-Parent Wheat Population Identifies Novel Sources of Septoria Tritici Blotch Resistance. Genes 2020, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.E.; Sackett, K.E.; Anderson, N.P.; Flowers, M.D.; Mundt, C.C. Evidence of Selection for Fungicide Resistance in Zymoseptoria tritici Populations on Wheat in Western Oregon. Plant Dis. 2016, 100, 483–489. [Google Scholar] [CrossRef]
- McDonald, M.C.; Renkin, M.; Spackman, M.E.; Orchard, B.A.; Croll, D.; Solomon, P.S.; Milgate, A.W. Rapid Parallel Evolution of Azole Fungicide Resistance in Australian Populations of the Wheat Pathogen Zymoseptoria tritici. Appl. Environ. Microbiol. 2018, 85, e01908-18. [Google Scholar] [CrossRef] [PubMed]
- Lavrukaitė, K.; Heick, T.M.; Ramanauskienė, J.; Armonienė, R.; Ronis, A. Fungicide sensitivity levels in the Lithuanian Zymoseptoria tritici population in 2021. Front. Plant Sci. 2023, 13, 1075038. [Google Scholar] [CrossRef]
- Lavrukaitė, K.; Almogdad, M.; Ramanauskienė, J.; Sabeckis, A. Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides. Agronomy 2024, 14, 813. [Google Scholar] [CrossRef]
- Kildea, S.; Hellin, P.; Heick, T.M.; Byrne, S.; Hutton, F. Mefentrifluconazole sensitivity amongst European Zymoseptoria tritici populations and potential implications for its field efficacy. Pest Manag. Sci. 2024, 80, 533–543. [Google Scholar] [CrossRef]
- Qu, J.; Li, S.; Yu, D. Detection of complex chromosome rearrangements using optical genome mapping. Gene 2023, 884, 147688. [Google Scholar] [CrossRef]
- Corsi, B.; Percival-Alwyn, L.; Downie, R.C.; Venturini, L.; Iagallo, E.M.; Campos Mantello, C.; McCormick-Barnes, C.; See, P.T.; Oliver, R.P.; Moffat, C.S.; et al. Genetic analysis of wheat sensitivity to the ToxB fungal effector from Pyrenophora tritici-repentis, the causal agent of tan spot. TAG. Theor. Appl. Genetics. Theor. Und Angew. Genet. 2020, 133, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, G.K.; Wyatt, N.A.; Shi, G.; Liu, S.; Yan, C.; Ma, Y.; Zhong, S.; Rasmussen, J.B.; Moolhuijzen, P.M.; Moffat, C.S.; et al. A genome-wide genetic linkage map and reference quality genome sequence for a new race in the wheat pathogen Pyrenophora tritici-repentis. Fungal Genet. Biol. 2021, 152, 103571. [Google Scholar] [CrossRef]
- Moffat, C.S.; See, P.T.; Oliver, R.P. Generation of a ToxA knockout strain of the wheat tan spot pathogen Pyrenophora tritici-repentis. Mol. Plant Pathol. 2014, 15, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Cherif, M.; Hafez, M.; Despins, T.; Aboukhaddour, R. Pyrenophora tritici–repentis in Tunisia: Race Structure and Effector Genes. Front. Plant Sci. 2019, 10, 1562. [Google Scholar] [CrossRef]
- Leišová-Svobodová, L.; Hanzalová, A.; Kučera, L.J. Expansion and variability of the PTR TOX A gene in populations of Pyrenophora tritici-repentis and Pyrenophora Teres. J. Plant Pathol. 2010, 92, 729–735. [Google Scholar]
- Mironenko, N.V.; Orina, A.S.; Kovalenko, N.M. Nuclear Genetic Polymorphism in Pyrenophora tritici-repentis Strains for ToxA and ToxB Effector Genes. Russ. J. Genet. 2021, 57, 533–539. [Google Scholar] [CrossRef]
- Kaneps, J.; Bankina, B.; Moročko-Bičevska, I. Virulence of Pyrenophora tritici-repentis: A minireview. Res. Rural Dev. 2021, 36, 21–28. [Google Scholar] [CrossRef]
- Yudin, N.; Barkhash, A.V.; Maksimov, V.N.; Ignatieva, E.V.; Romaschenko, A.G. Human Genetic Predisposition to Diseases Caused by Viruses from Flaviviridae Family. Mol. Biol. 2018, 52, 165–181. [Google Scholar] [CrossRef]
- Peters Haugrud, A.R.; Zhang, Z.; Friesen, T.L.; Faris, J.D. Genetics of resistance to septoria nodorum blotch in wheat. Theor. Appl. Genet. 2022, 135, 3685–3707. [Google Scholar] [CrossRef]
- Nuzhnaya, T.; Veselova, S.V.; Burkhanova, G.F.; Maksimov, I.V. Virulence Factors of the Fungal Pathogen Stagonospora nodorum Manipulate Hormonal Signaling Pathways in Triticum aestivum L. by Regulating Host Plant MicroRNA Expressions. Front. Biosci. 2023, 15, 22. [Google Scholar] [CrossRef]
- Adhikari, U.; Brown, J.K.; Ojiambo, P.S.; Cowger, C. Effects of Host and Weather Factors on the Growth Rate of Septoria nodorum blotch Lesions on Winter Wheat. Phytopathology 2023, 113, 1898–1907. [Google Scholar] [CrossRef]
- Chhabra, B.; Thrasu, S.; Wallace, S.; Schoen, A.; Shahoveisi, F.; Dong, Y.; Tiwari, V.; Rawat, N. Evaluation of speed breeding conditions for accelerating Fusarium head blight and deoxynivalenol screening in wheat. Crop Sci. 2024, 64, 1586–1594. [Google Scholar] [CrossRef]
- Liu, J.; Cobertera, D.C.; Zemetra, R.S.; Mundt, C.C. Identification of quantitative trait loci for resistance to wheat sharp eyespot in the Einstein × Tubbs recombinant inbred line population. Plant Dis. 2022, 107, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Jabran, M.; Ali, M.A.; Zahoor, A.; Muhae-Ud-Din, G.; Liu, T.; Chen, W.; Gao, L. Intelligent reprogramming of wheat for enhancement of fungal and nematode disease resistance using advanced molecular techniques. Front. Plant Sci. 2023, 14, 1132699. [Google Scholar] [CrossRef]
- Neog, P.P.; Batra, S.; Saraswat, S.; Sharma, E.L.; Kumar, P.P.; Pandey, A.K. A Brief Overview of Deep Learning based Techniques for the Detection of Wheat Leaf Disease: A Recent Study. In Proceedings of the 2023 7th International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 17–19 May 2023; pp. 209–217. [Google Scholar] [CrossRef]
- Talas, F.; McDonald, B.A. Significant variation in sensitivity to a DMI fungicide in field populations of Fusarium graminearum. Plant Pathol. 2015, 64, 664–670. [Google Scholar] [CrossRef]
- Ma, S.; Xin, H.; Zhao, P.; Feng, S.; Chen, J.; Yin, S.; Wei, Y.; Shi, Y.; Jin, G.; Di, X.; et al. Comprehensive Stereoselectivity Assessment of Toxicokinetics, Tissue Distribution, Cytotoxicity, and Environmental Fate of Chiral Pesticide Propiconazole. J. Agric. Food Chem. 2023, 71, 19760–19771. [Google Scholar] [CrossRef]
- Chen, B.; Shen, X.; Li, Z.; Wang, J.; Li, X.; Xu, Z.; Shen, Y.; Lei, Y.; Huang, X.; Wang, X.; et al. Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable. Foods 2022, 11, 324. [Google Scholar] [CrossRef]
- Qian, H.; Du, J.; Chi, M.; Sun, X.; Liang, W.; Huang, J.; Li, B. The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag. Sci. 2018, 74, 1472–1477. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Fan, Z.; Wu, J.; Wang, L.; He, D.; Mohamed, S.R.; Dawood, D.H.; Shi, J.R.; Gao, T.; et al. Antifungal activities of metconazole against the emerging wheat pathogen Fusarium pseudograminearum. Pestic. Biochem. Physiol. 2023, 190, 105298. [Google Scholar] [CrossRef]
- Glaab, A.; Weilacher, X.; Hoffmeister, M.; Strobel, D.; Stammler, G. Occurrence and distribution of CYP51 haplotypes of Zymoseptoria tritici in recent years in Europe. J. Plant Dis. Prot. 2024, 131, 1187–1194. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Li, H.; Yin, S. WeChat mini program for wheat diseases recognition based on VGG-16 convolutional neural network. Int. J. Appl. Sci. Eng. 2023, 20, 2023067. [Google Scholar] [CrossRef] [PubMed]
- Therasa, P.; Hemalatha, R.; Sivajothi, E.; Jayashankari, J.; Princy, B.A. Identification of Wheat Leaf Diseases Based on Deep Learning Algorithms. In Proceedings of the 2023 7th International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 22–24 November 2023; pp. 721–725. [Google Scholar] [CrossRef]
- Sheenam, S.; Khattar, S.; Verma, T. Automated Wheat Plant Disease Detection using Deep Learning: A Multi-Class Classification Approach. In Proceedings of the 2023 3rd International Conference on Intelligent Technologies (CONIT), Hubli, India, 23–25 June 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, M.; Chen, J.; Maimaiti, M.; Liu, Q. Recognition of Wheat Leaf Diseases Using Lightweight Convolutional Neural Networks against Complex Backgrounds. Life 2023, 13, 2125. [Google Scholar] [CrossRef]
- Khan, A.A.; Raza, S.; Qureshi, M.F.; Mushtaq, Z.; Taha, M.; Amin, F. Deep Learning-Based Classification of Wheat Leaf Diseases for Edge Devices. In Proceedings of the 2023 2nd International Conference on Emerging Trends in Electrical, Control, and Telecommunication Engineering (ETECTE), Lahore, Pakistan, 27–29 November 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Etaati, S.; Khoramdel, J.; Najafi, E. Automated Wheat Disease Detection using Deep Learning: An Object Detection and Classification Approach. In Proceedings of the 2023 11th RSI International Conference on Robotics and Mechatronics (ICRoM), Tehran, Iran, 19–21 December 2023; pp. 116–121. [Google Scholar] [CrossRef]
- Alharbi, A.; Khan, M.U.; Tayyaba, B. Wheat Disease Classification Using Continual Learning. IEEE Access 2023, 11, 90016–90026. [Google Scholar] [CrossRef]
- Shobhit; Kaur, E.G.; Singh, P.; Ansari, A.; Dongre, P.; Chandel, R. Hybrid Deep Learning for Wheat Bunt Disease Severity Assessment. In Proceedings of the 2023 International Conference on Advanced Computing & Communication Technologies (ICACCTech), Banur, India, 23–24 December 2023; pp. 736–742. [Google Scholar] [CrossRef]
- Banerjee, D.; Kukreja, V.; Chandran, N.; Garg, N. Precision Diagnosis of Wheat Powdery Mildew Using CNN and Random Forest. In Proceedings of the 2024 International Conference on Emerging Smart Computing and Informatics (ESCI), Pune, India, 5–7 March 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Goyal, L.; Sharma, C.M.; Singh, A.; Singh, P.K. Leaf and spike wheat disease detection & classification using an improved deep convolutional architecture. Inform. Med. Unlocked 2021, 25, 100642. [Google Scholar] [CrossRef]
- Abdalla, M.; Mohamed, O.; Azmi, E.M. Adaptive Learning Model for Detecting Wheat Diseases. Int. J. Adv. Comput. Sci. Appl. 2024, 15, 1287–1298. [Google Scholar] [CrossRef]
- Nigam, S.; Jain, R.; Marwaha, S.; Arora, A.; Haque, M.A.; Dheeraj, A.; Singh, V.K. Deep transfer learning model for disease identification in wheat crop. Ecol. Inform. 2023, 75, 102068. [Google Scholar] [CrossRef]
- Yadao, G.G.; Julkipli, O.M.; Manlises, C.O. Performance Analysis of EfficientNet for Rice Grain Quality Control-An Evaluation against YOLOv7 and YOLOv8. In Proceedings of the 2024 7th International Conference on Information and Computer Technologies (ICICT), Honolulu, HI, USA, 15–17 March 2024; pp. 93–98. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Xu, X.; Sun, C. Precision detection of crop diseases based on improved YOLOv5 model. Front. Plant Sci. 2023, 13, 1066835. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, W.; Yang, C.; Gong, Z.; Yue, J.; Fu, Y.; Feng, H. A fast and lightweight detection model for wheat fusarium head blight spikes in natural environments. Comput. Electron. Agric. 2024, 216, 108484. [Google Scholar] [CrossRef]
- Kim, Y.M.; Bouras, N.; Kav, N.N.; Strelkov, S.E. Inhibition of photosynthesis and modification of the wheat leaf proteome by Ptr ToxB: A host-specific toxin from the fungal pathogen Pyrenophora tritici-repentis. Proteomics 2010, 10, 2911–2926. [Google Scholar] [CrossRef]
- Figueroa, M.; Manning, V.A.; Pandelova, I.; Ciuffetti, L.M. Persistence of the Host-Selective Toxin Ptr ToxB in the Apoplast. Mol. Plant-Microbe Interact. MPMI 2015, 28, 1082–1090. [Google Scholar] [CrossRef]
- Tran, V.A.; Aboukhaddour, R.; Strelkov, I.S.; Bouras, N.; Spaner, D.M.; Strelkov, S.E. The sensitivity of Canadian wheat genotypes to the necrotrophic effectors produced by Pyrenophora tritici-repentis. Can. J. Plant Pathol. 2017, 39, 149–162. [Google Scholar] [CrossRef]
- Weith, S.; Ridgway, H.J.; Jones, E.E. Determining the presence of host specific toxin genes, ToxA and ToxB, in New Zealand Pyrenophora tritici-repentis isolates, and susceptibility of wheat cultivars. N. Z. Plant Prot. 2021, 74, 20–29. [Google Scholar] [CrossRef]
- Simón, M.R.; Fleitas, M.C.; Castro, A.C.; Schierenbeck, M. How Foliar Fungal Diseases Affect Nitrogen Dynamics, Milling, and End-Use Quality of Wheat. Front. Plant Sci. 2020, 11, 569401. [Google Scholar] [CrossRef]
- Motta-Romero, H.; Niyongira, F.; Boehm, J.D.; Rose, D.J. Effects of foliar fungicide on yield, micronutrients, and cadmium in grains from historical and modern hard winter wheat genotypes. PLoS ONE 2021, 16, e0247809. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, W.; Nowicki, B.; Bełka, D.; Kazimierowicz, A.; Kulwicki, M.; Grzebisz, W. Effect of Foliar Application of Micronutrients and Fungicides on the Nitrogen Use Efficiency in Winter Wheat. Agronomy 2022, 12, 257. [Google Scholar] [CrossRef]
- Spitters, C.J.; Roermund, H.V.; Nassau, H.; Schepers, J.S.; Mesdag, J. Genetic variation in partial resistance to leaf rust in winter wheat: Disease progress, foliage senescence and yield reduction. Neth. J. Plant Pathol. 2005, 96, 3–15. [Google Scholar] [CrossRef]
- Hafez, M.; Gourlie, R.; McDonald, M.C.; Telfer, M.; Carmona, M.; Sautua, F.J.; Moffat, C.S.; Moolhuijzen, P.M.; See, P.T.; Aboukhaddour, R. Evolution of the ToxB gene in Pyrenophora tritici-repentis and related species. Mol. Plant-Microbe Interact. MPMI 2023, 37, 327–337. [Google Scholar] [CrossRef]
- Dimmock, J.; Gooding, M. The effects of fungicides on rate and duration of grain filling in winter wheat in relation to maintenance of flag leaf green area. J. Agric. Sci. 2002, 138, 1–16. [Google Scholar] [CrossRef]
- Rangan, P.; Furtado, A.; Henry, R.J. New evidence for grain specific C4 photosynthesis in wheat. Sci. Rep. 2016, 6, 31721. [Google Scholar] [CrossRef]
- Serrago, R.A.; Lo Valvo, P.J.; Miralles, D.J. Is the source-sink ratio at anthesis a driver to avoid yield reductions caused by late foliar disease in wheat? Field Crops Res. 2019, 235, 11–17. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Li, C.; McHugh, A.D.; Li, M.; Xiong, T.; Liu, Y.; Tang, Y. Source–sink relations and responses to sink–source manipulations during grain filling in wheat. J. Integr. Agric. 2022, 21, 1593–1605. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Heick, T.M.; Havis, N.; Holdgate, S.; Clark, B.; Blake, J.; Głazek, M.; Korbas, M.; Danielewicz, J.; et al. Decreasing azole sensitivity of Z. tritici in Europe contributes to reduced and varying field efficacy. J. Plant Dis. Prot. 2020, 128, 287–301. [Google Scholar] [CrossRef]
- Gourlie, R.; McDonald, M.C.; Hafez, M.; Ortega-Polo, R.; Low, K.E.; Abbott, D.W.; Strelkov, S.E.; Daayf, F.; Aboukhaddour, R. The pangenome of the wheat pathogen Pyrenophora tritici-repentis reveals novel transposons associated with necrotrophic effectors ToxA and ToxB. BMC Biol. 2022, 20, 239. [Google Scholar] [CrossRef]
- Veselova, S.; Nuzhnaya, T.; Burkhanova, G.; Rumyantsev, S.; Maksimov, I. Necrotrophic Effectors SnTox from the Stagonospora nodorum (Berk.) Manipulate the Redox Metabolism of the Host Plant to Hijack Its Early Defense Response. Biol. Life Sci. Forum 2021, 4, 106. [Google Scholar]
- Veselova, S.; Nuzhnaya, T.; Burkhanova, G.; Rumyantsev, S.; Maksimov, I. Reactive Oxygen Species in Host Plant Are Required for an Early Defense Response against Attack of Stagonospora nodorum Berk. Necrotrophic Effectors SnTox. Plants 2021, 10, 1586. [Google Scholar] [CrossRef]
- Petronaitis, T.; Forknall, C.R.; Simpfendorfer, S.; Backhouse, D.; Flavel, R.J. Stubble trouble! Moisture, pathogen fitness and cereal type drive colonisation of cereal stubble by three fungal pathogens. Australas. Plant Pathol. 2022, 51, 363–368. [Google Scholar] [CrossRef]
- Sawińska, Z.; Małecka, I. Effect of seed treatment and foliar protection with fungicides on health status of winter wheat. Plant Prot. Sci. 2018, 43, 13–18. [Google Scholar] [CrossRef]
- Vagelas, I. Important Foliar Wheat Diseases and their Management: Field Studies in Greece. Mod. Concepts Dev. Agron. 2021, 8, 783–786. [Google Scholar] [CrossRef]
- Harvey, I.C.; Craigie, R.; McCloy, B.L. The control of tan spot of wheat (caused by Pyrenophora tritici-repentis): A possible emerging disease in New Zealand. N. Z. Plant Prot. 2015, 68, 428–433. [Google Scholar] [CrossRef]
- MacLean, D.; Aboukhaddour, R.; Tran, V.A.; Askarian, H.; Strelkov, S.E.; Turkington, T.K.; Kutcher, H.R. Race characterization of Pyrenophora tritici-repentis and sensitivity to propiconazole and pyraclostrobin fungicides. Can. J. Plant Pathol. 2017, 39, 433–443. [Google Scholar] [CrossRef]
- Murphy, L.A.; Moore, T.M.; Nesnow, S. Propiconazole-enhanced hepatic cell proliferation is associated with dysregulation of the cholesterol biosynthesis pathway leading to activation of Erk1/2 through Ras farnesylation. Toxicol. Appl. Pharmacol. 2012, 260, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Skolness, S.Y.; Blanksma, C.A.; Cavallin, J.E.; Churchill, J.J.; Durhan, E.J.; Jensen, K.M.; Johnson, R.; Kahl, M.D.; Makynen, E.A.; Villeneuve, D.L.; et al. Propiconazole inhibits steroidogenesis and reproduction in the fathead minnow (Pimephales promelas). Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 132, 284–297. [Google Scholar] [CrossRef]
- Schierenbeck, M.; Fleitas, M.C.; Simón, M.R. The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat. Plants 2023, 12, 212. [Google Scholar] [CrossRef]
- Rösch, A.; Gottardi, M.; Vignet, C.; Cedergreen, N.; Hollender, J. Mechanistic Understanding of the Synergistic Potential of Azole Fungicides in the Aquatic Invertebrate Gammarus pulex. Environ. Sci. Technol. 2017, 51, 12784–12795. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, Y.; He, J.; Cheng, H.; Martyniuk, C.J. Endocrine disruption by azole fungicides in fish: A review of the evidence. Sci. Total Environ. 2022, 822, 153412. [Google Scholar] [CrossRef]
- Gottardi, M.; Tyzack, J.D.; Bender, A.; Cedergreen, N. Can the inhibition of cytochrome P450 in aquatic invertebrates due to azole fungicides be estimated with in silico and in vitro models and extrapolated between species? Aquat. Toxicol. 2018, 201, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, P.F.; Yin, X.; Zhu, G.; Brock, T. Aquatic and sediment ecotoxicity data of difenoconazole and its potential environmental risks in ponds bordering rice paddies. Ecotoxicol. Environ. Saf. 2024, 273, 116135. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhen, T.; Li, Z. Lightweight Multiscale CNN Model for Wheat Disease Detection. Appl. Sci. 2023, 13, 5801. [Google Scholar] [CrossRef]
- Vagelas, I.; Papadimos, A.; Lykas, C. Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor. Agronomy 2021, 11, 1682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I. Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research. Agrochemicals 2025, 4, 4. https://doi.org/10.3390/agrochemicals4010004
Vagelas I. Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research. Agrochemicals. 2025; 4(1):4. https://doi.org/10.3390/agrochemicals4010004
Chicago/Turabian StyleVagelas, Ioannis. 2025. "Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research" Agrochemicals 4, no. 1: 4. https://doi.org/10.3390/agrochemicals4010004
APA StyleVagelas, I. (2025). Effective Strategies for Managing Wheat Diseases: Mapping Academic Literature Utilizing VOSviewer and Insights from Our 15 Years of Research. Agrochemicals, 4(1), 4. https://doi.org/10.3390/agrochemicals4010004





