Regulatory Evolution of Neonicotinoid Insecticides as Plant Protection Active Substances in Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Legal Support
2.1.1. European Pesticides Database
2.1.2. Directives and Regulations
2.2. Definitions
2.2.1. General Definitions
2.2.2. Health and Safety Hazards/Risks Phrases
- H301: Toxic if swallowed.
- H302: Harmful if swallowed.
- H317: Skin Sens. 1
- H319: Causes serious eye irritation.
- H331: Toxic if inhaled.
- H332: Harmful if inhaled.
- H335: May cause respiratory irritation.
- H336: May cause drowsiness or dizziness.
- H351: Suspected of causing cancer.
- H360FD: May damage fertility or the unborn child.
- H400: Very toxic to aquatic life.
- H401: Toxic to aquatic life.
- H410: Very toxic to aquatic life with long-lasting effects.
- H411: Toxic to aquatic life with long-lasting effects.
3. Results
3.1. NN Active Substances
3.2. NN Insecticide Family in Europe
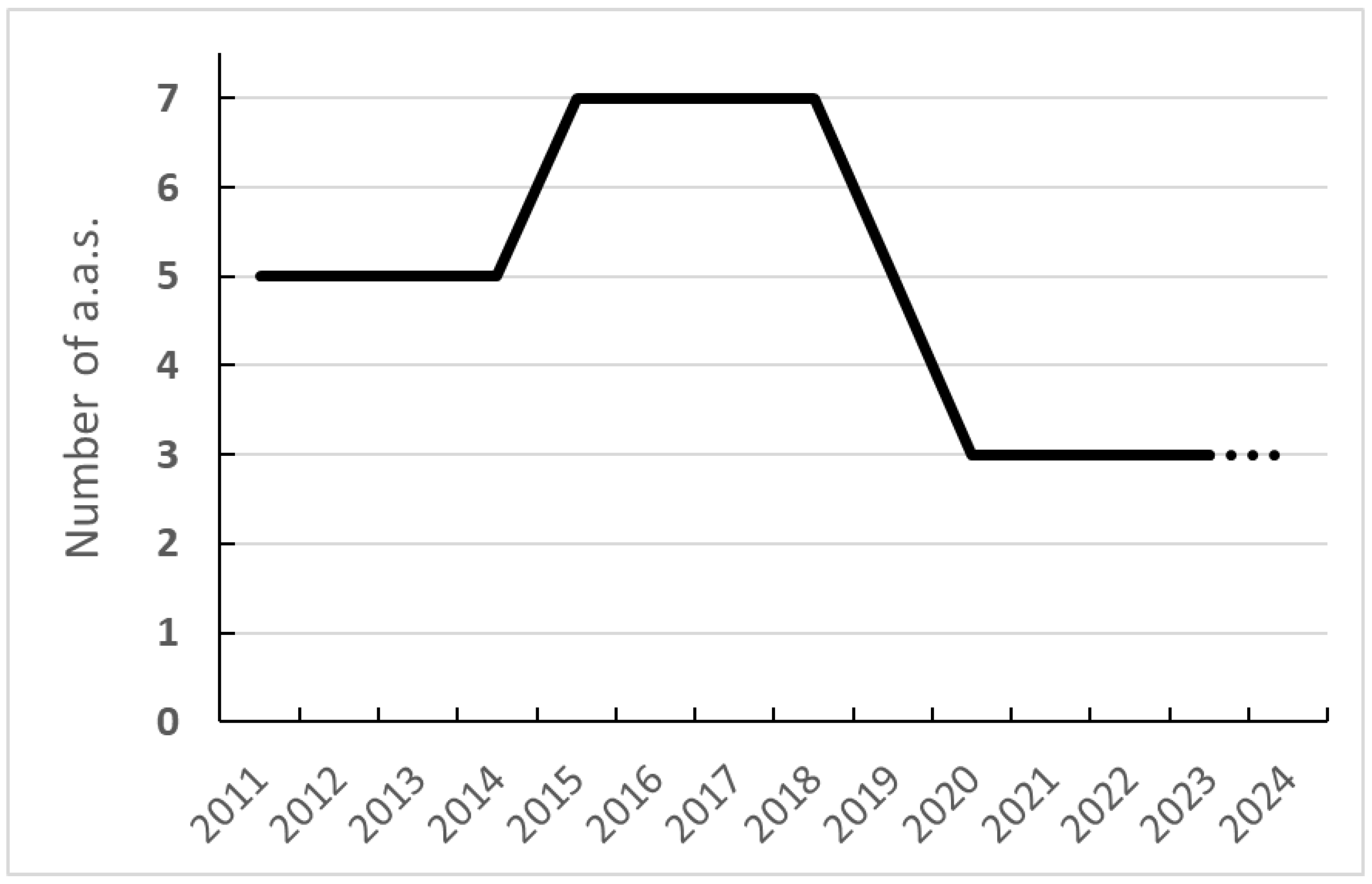
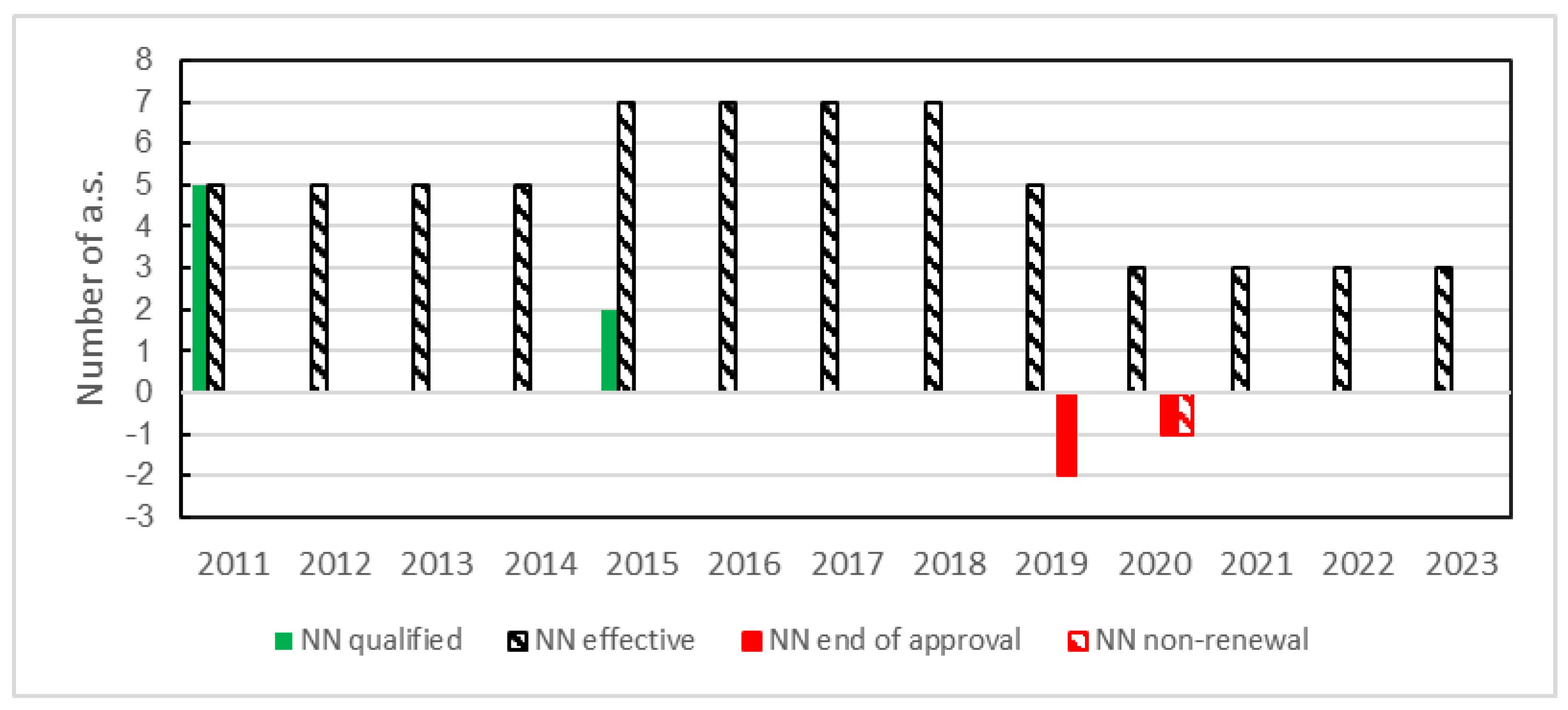
3.2.1. Evolution of the NN Active Substances in Europe since 2011
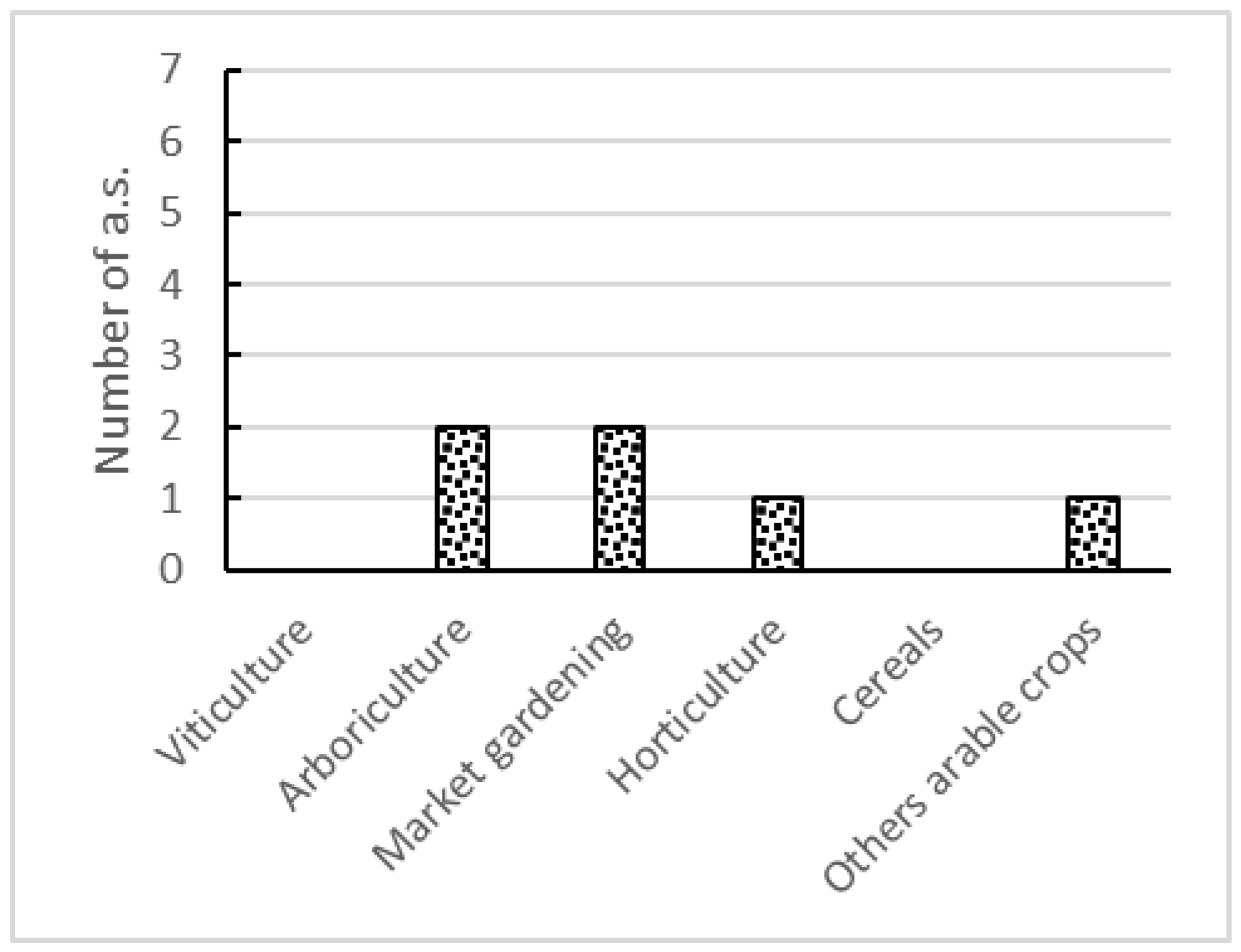
3.2.2. Consideration of EU NN Crop Usages
3.2.3. Residues of EU NN
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchand, P.A. EU chemical plant protection products in 2023: Current state and perspectives. Agrochemicals 2023, 2, 106–117. [Google Scholar] [CrossRef]
- Marchand, P.A. Evolution of plant protection active substances in Europe: Disappearance of chemicals in favour of biocontrol agents. Environ. Sci. Pollut. Res. 2023, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Charon, M.; Robin, D.; Marchand, P.A. The major interest for crop protection of agrochemical substances without maximum residue limit (MRL). Biotechnol. Agron. Soc. Environ. 2019, 23, 22–29. [Google Scholar] [CrossRef]
- Marchand, P.A. Synthetic Agrochemicals: A necessary clarification about their use exposure and impact in Crop Protection. Environ. Sci. Pollut. Res. 2019, 26, 17996–18000. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Marchand, P.A. Evolution of succinate dehydrogenase inhibitor (SDHI) fungicides as plant protection active substances in Europe. Arch. Crop Sci. 2022, 5, 193–198. [Google Scholar] [CrossRef]
- Charpentier, G.; Louat, F.; Bonmatin, J.-M.; Marchand, P.A.; Locker, D.; Decoville, M. Lethal and sublethal effects of Imidacloprid, after chronic exposure, on insect model Drosophila melanogaster. Environ. Sci. Technol. 2014, 48, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Giorio, C.; Safer, A.; Sánchez-Bayo, F.; Tapparo, A.; Lentola, A.; Girolami, V.; van Lexmond, M.B.; Bonmatin, J.M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: New molecules, metabolism, fate, and transport. Environ. Sci. Pollut. Res. 2021, 28, 11716–11748. [Google Scholar] [CrossRef]
- Ihara, M.; Buckingham, S.D.; Matsuda, K.; Sattelle, D.B. Modes of Action, Resistance and Toxicity of Insecticides Targeting Nicotinic Acetylcholine Receptors. Curr. Med. Chem. 2017, 24, 2925–2934. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X.; Zhang, T.; Song, S.; Zhu, H.; Lu, S.; Kannan, K.; Sun, H. Neonicotinoid Insecticides and Their Metabolites Can Pass through the Human Placenta Unimpeded. Environ. Sci. Technol. 2022, 56, 17143–17152. [Google Scholar] [CrossRef]
- Taira, K.; Fujioka, K.; Aebi, A.; Glauser, G.; Mitchell, E.A.D.; Ikenaka, Y.; Sanchez-Bayo, F.; Tapparo, A.; Lumawig-Heitzmann, E.; Bonmatin, J.-M.; et al. Contrasted concentrations of neonicotinoids in human hair and urine suggest long-term vs short-term exposure in two observational studies, international academics and rural population of the Philippines. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Stark, J.D.; Jepson, P.C.; Mayer, D.F. Limitations to Use of Topical Toxicity Data for Predictions of Pesticide Side Effects in the Field. J. Econom. Entomol. 1995, 88, 1081–1088. [Google Scholar] [CrossRef]
- Delbeke, F.; Vercruysse, P.; Tirry, L.; De Clercq, P.; Degheele, D. Toxicity of diflubenzuron, pyriproxyfen, imidacloprid and diafenthiuron to the predatory bug Orius laevigatus (Het.: Anthocoridae). BioControl 1997, 42, 349–358. [Google Scholar] [CrossRef]
- Mestres Lóbez, I. Lethality of Imidacloprid and Fipronil on Apis mellifera: A retrospective on the French case. Jul. Kühn Arch. 2020, 465, 59–62. [Google Scholar] [CrossRef]
- Yang, B.; Lin, X.; Yu, N.; Ga, H.; Zhang, Y.; Liu, W.; Liu, Z. Contribution of Glutathione S-Transferases to Imidacloprid Resistance in Nilaparvata lugens. J. Agric. Food Chem. 2020, 68, 15403–15408. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, S.; Wei, X. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) 2023/1536 of 25 July 2023 amending Annex III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for nicotine in or on certain products. Off. J. Eur. Union 2023, L187, 6–25. [Google Scholar]
- Bonmatin, J.-M.; Marchand, P.A.; Charvet, R.; Moineau, I.; Bengsh, E.R.; Colin, M.E. Quantification of imidacloprid uptake in maize crops. J. Agric. Food Chem. 2005, 53, 5336–5341. [Google Scholar] [CrossRef]
- Pisa, L.; Goulson, D.; Yang, E.C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef]
- Robin, D.; Marchand, P.A. Evolution of Regulation (EU) No 540/2011 since its entry into force. J. Regul. Sci. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- EU. EU Pesticides Database v3.1. 2023. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 17 July 2023).
- EC. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, L70, 1–16. [Google Scholar]
- EC. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- EU. Judgment of the Court (First Chamber) of 19 January 2023. Off. J. Eur. Union 2023, C162, 1–13, Document 62021CJ0162. [Google Scholar]
- Robin, D.C.; Marchand, P.A. The slow decrease of the active substances candidates for substitution in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Eur. J. Risk Regul. 2022, 13, 1–22. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Moineau, I.; Charvet, R.; Colin, M.E.; Fleche, C.; Bengsch, E.R. Behaviour of Imidacloprid in Fields. Toxicity for Honey Bees. In Environmental Chemistry; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 483–494. [Google Scholar] [CrossRef]
- Cabrera, C.L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, M.P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, 7939–8028. [Google Scholar] [CrossRef]
- Bellisai, G.; Bernasconi, G.; Brancato, A.; Cabrera, C.L.; Castellan, I.; Ferreira, L.; Giner, G.; Greco, L.; Jarrah, S.; Leuschner, R.; et al. Reasoned Opinion on the modification of the existing maximum residue levels for sulfoxaflor in various crops. EFSA J. 2022, 20, 7283–7314. [Google Scholar] [CrossRef]
- Bellisai, G.; Bernasconi, G.; Brancato, A.; Cabrera, C.L.; Castellan, I.; Ferreira, L.; Santonja, G.G.; Greco, L.; Jarrah, S.; Leuschner, R.; et al. Reasoned Opinion on the setting of import tolerances for sulfoxaflor in various crops. EFSA J. 2023, 21, 8062–8106. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Kaur, P.; Gan, J. A critical review on the accumulation of neonicotinoid insecticides in pollen and nectar: Influencing factors and implications for pollinator exposure. Sci. Total Environ. 2023, 899, 165670. [Google Scholar] [CrossRef]
- Abdelgader, H. Improving seed treatment methods: A key factor to reduce the risk to Honey bees and other pollinators to maintain biodiversity. J. Agric. Mar. Sci. 2023, 28, 60. [Google Scholar]
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Bautista, A.; Mills, N.J. Neonicotinoid Sunflower Seed Treatment, While Not Detected in Pollen and Nectar, Still Impacts Wild Bees and Crop Yield. Agrochemicals 2023, 2, 279–295. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Mitchell, E.A.D.; Glauser, G.; Lumawig-Heitzman, E.; Claveria, F.; Bijleveld van Lexmond, M.; Taira, K.; Sánchez-Bayo, F. Residues of neonicotinoids in soil, water and people's hair: A case study from three agricultural regions of the Philippines. Sci. Total Environ. 2022, 757, 143822. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Implementing Regulation (EU) 2022/686 of 28 April 2022 amending Implementing Regulations (EU) 2015/1295 and (EU) No 540/2011 as regards the conditions of approval of the active substance sulfoxaflor. Off. J. Eur. Union 2022, L126, 18–22. [Google Scholar]
- EU. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union 2018, L132, 31–34. [Google Scholar]
- EU. Commission Implementing Regulation (EU) 2018/784 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance clothianidin. Off. J. Eur. Union 2018, L132, 35–39. [Google Scholar]
- EU. Commission Implementing Regulation (EU) 2018/785 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam. Off. J. Eur. Union 2018, L132, 40–44. [Google Scholar]
- Furlan, L.; Pozzebon, A.; Duso, C.; Simon-Delso, N.; Sánchez-Bayo, F.; Marchand, P.A.; Bijleveld van Lexmond, M.; Bonmatin, J.M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 3: Alternatives to systemic insecticides. Environ. Sci. Pollut. Res. 2021, 28, 11798–11820. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Uneme, H.; Benet-Buchholz, J.; Stölting, J.; Sirges, W.; Beck, M.E.; Etzel, W. Clothianidin (TI-435)—The third member of the chloronicotinyl insecticide (CNI™) family. Pflanzenschutz-Nachrichten Bayer 2003, 56, 5–25. [Google Scholar]
- EU. Communication from the Commission on the European Citizens’ Initiative (ECI) ‘Save bees and farmers! Towards a bee-friendly agriculture for a healthy environment’ 2023/C 148/01. Off. J. Eur. Union 2023, C148, 1–12. [Google Scholar]

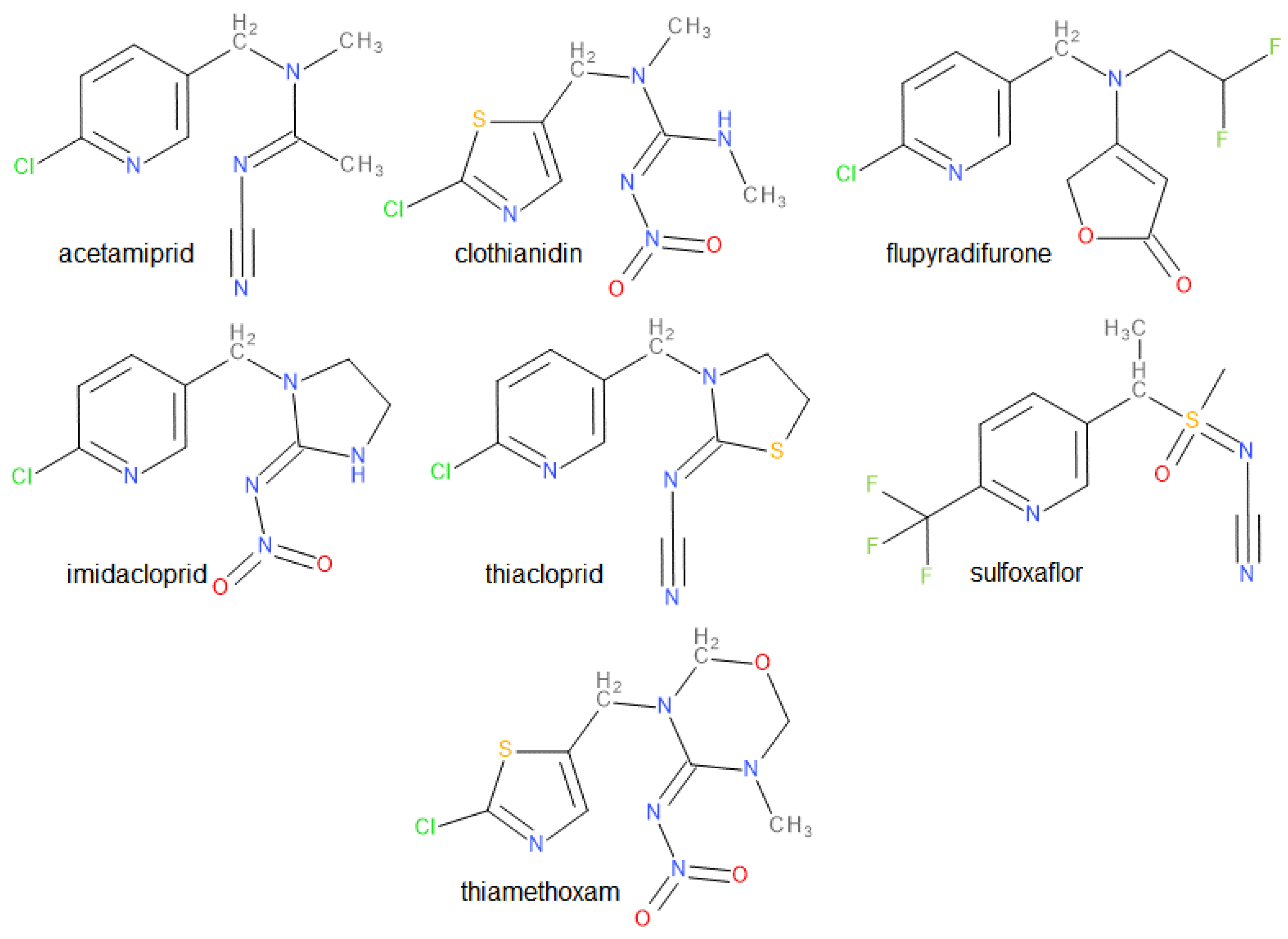

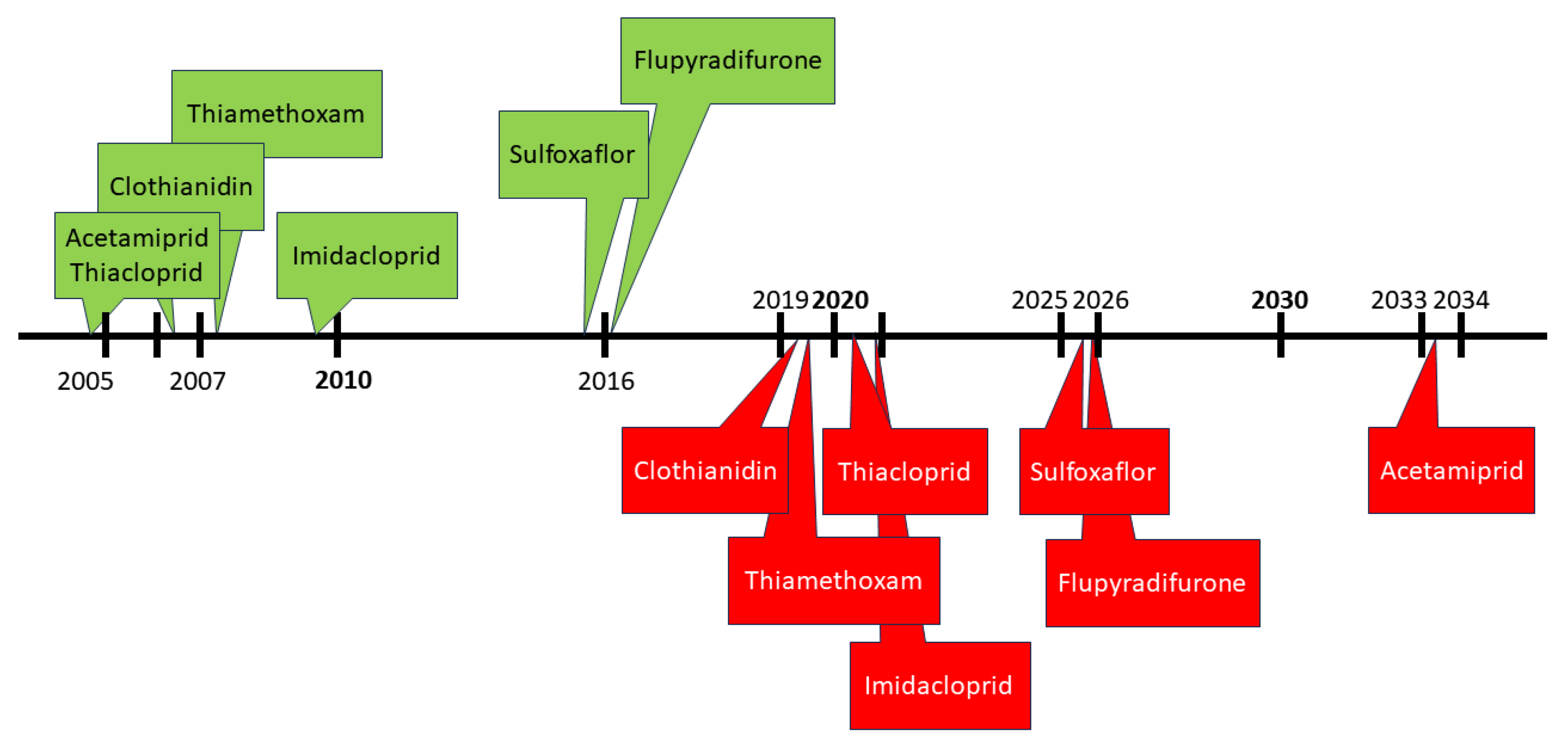

| EU Active Substance | Type | MRL 1 Annex | Tox Ecotox | Nb of Extension/s.a (2023) | Part (Reg. 540) | End of Approval | AIR Program |
|---|---|---|---|---|---|---|---|
| acetamiprid | cyano imidamide | II | Low ADI H302 H400 | 2 | B | 2033 | III |
| clothianidin | nitro guanidine | II | Low ADI H302 H317 H400 H401 H410 | 2 | A | 2019 | III |
| flupyradifurone | butenolide | II, IIIA | H332 H317 H410 | 0 | B | 2025 | VI |
| imidacloprid | nitro guanidine | II, IIIA | H302 H400 H410 | 1 | A | 2020 | VI |
| sulfoxaflor | sulfo-ximine | II | H302 H401 H411 | 0 | B | 2025 | VI |
| thiacloprid | cyano imidamide | II | Low ADI H302 H317 H318 H332 H336 H351 H360FD H400 H401 H410 | 4 | A | 2020 | III |
| thiamethoxam | nitroguanidine | II | H400H401H410 | 2 | A | 2019 | III |
| Active Substance | Type | Tox 1 |
|---|---|---|
| cycloxaprid | nitromethylene | £ |
| dicloromezotiaz | mesoionic | £ |
| dinotefuran | nitroguanidine | £ |
| fenmezoditiaz | mesoionic | H400, H410 |
| flupyrimin | pyridylidene | H315, H319, H351, H361 |
| imidaclothiz | nitroguanidine | £ |
| nitenpyram | nitromethylene | H302, H319 |
| nithiazine | nitromethylene | H302, H312, H315, H319 H332, H355 |
| paichongding | nitromethylene | £ |
| triflumezopyrim | mesoionic | £ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchand, P.A. Regulatory Evolution of Neonicotinoid Insecticides as Plant Protection Active Substances in Europe. Agrochemicals 2023, 2, 446-457. https://doi.org/10.3390/agrochemicals2030025
Marchand PA. Regulatory Evolution of Neonicotinoid Insecticides as Plant Protection Active Substances in Europe. Agrochemicals. 2023; 2(3):446-457. https://doi.org/10.3390/agrochemicals2030025
Chicago/Turabian StyleMarchand, Patrice A. 2023. "Regulatory Evolution of Neonicotinoid Insecticides as Plant Protection Active Substances in Europe" Agrochemicals 2, no. 3: 446-457. https://doi.org/10.3390/agrochemicals2030025
APA StyleMarchand, P. A. (2023). Regulatory Evolution of Neonicotinoid Insecticides as Plant Protection Active Substances in Europe. Agrochemicals, 2(3), 446-457. https://doi.org/10.3390/agrochemicals2030025






