Seed Germination and Plant Growth under Drought Stress of Herbicide-Resistant and Herbicide-Susceptible Biotypes of Conyza Species and Smart Farming Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Statistical Analysis
3. Results
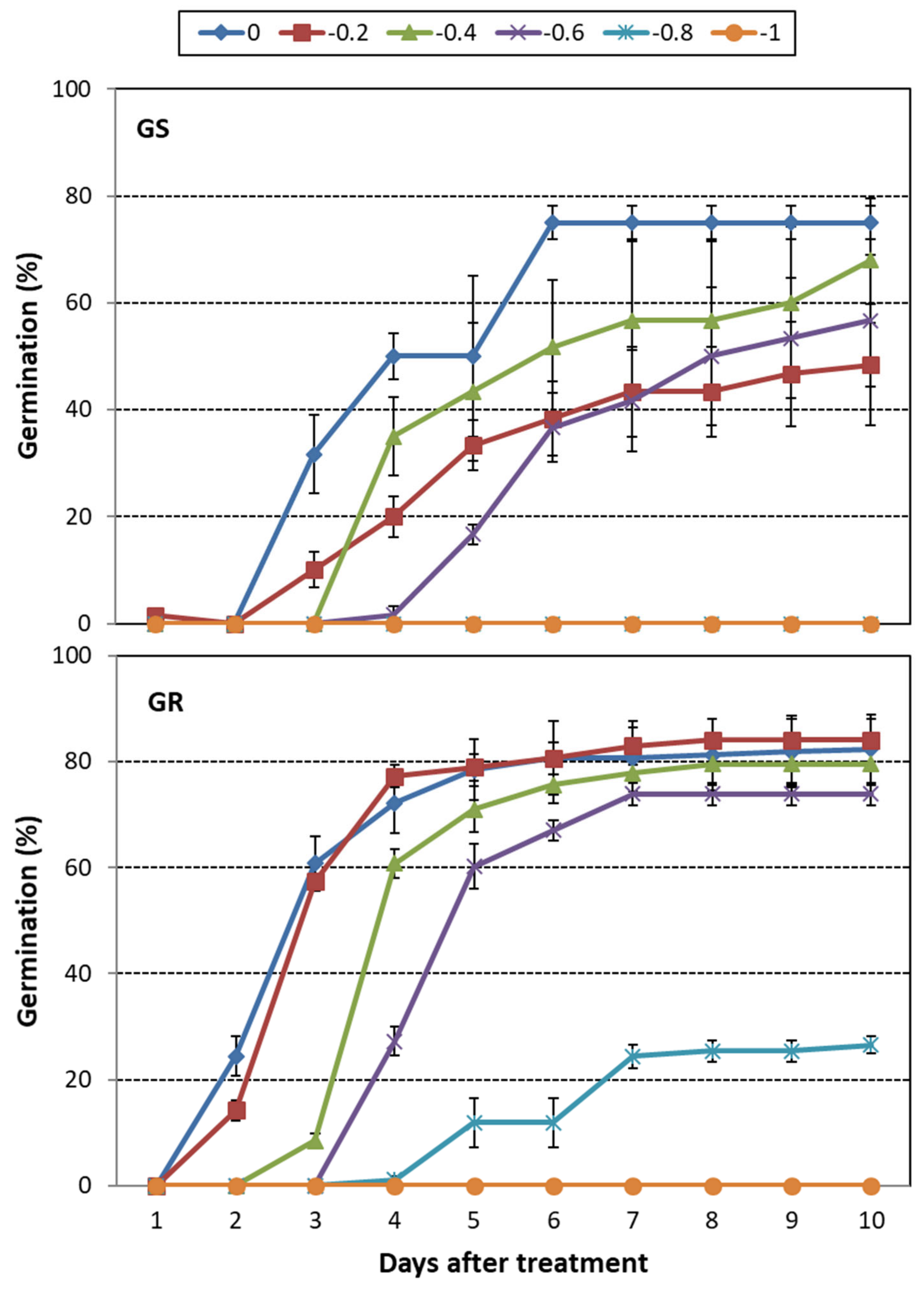
3.1. C. sumatrensis
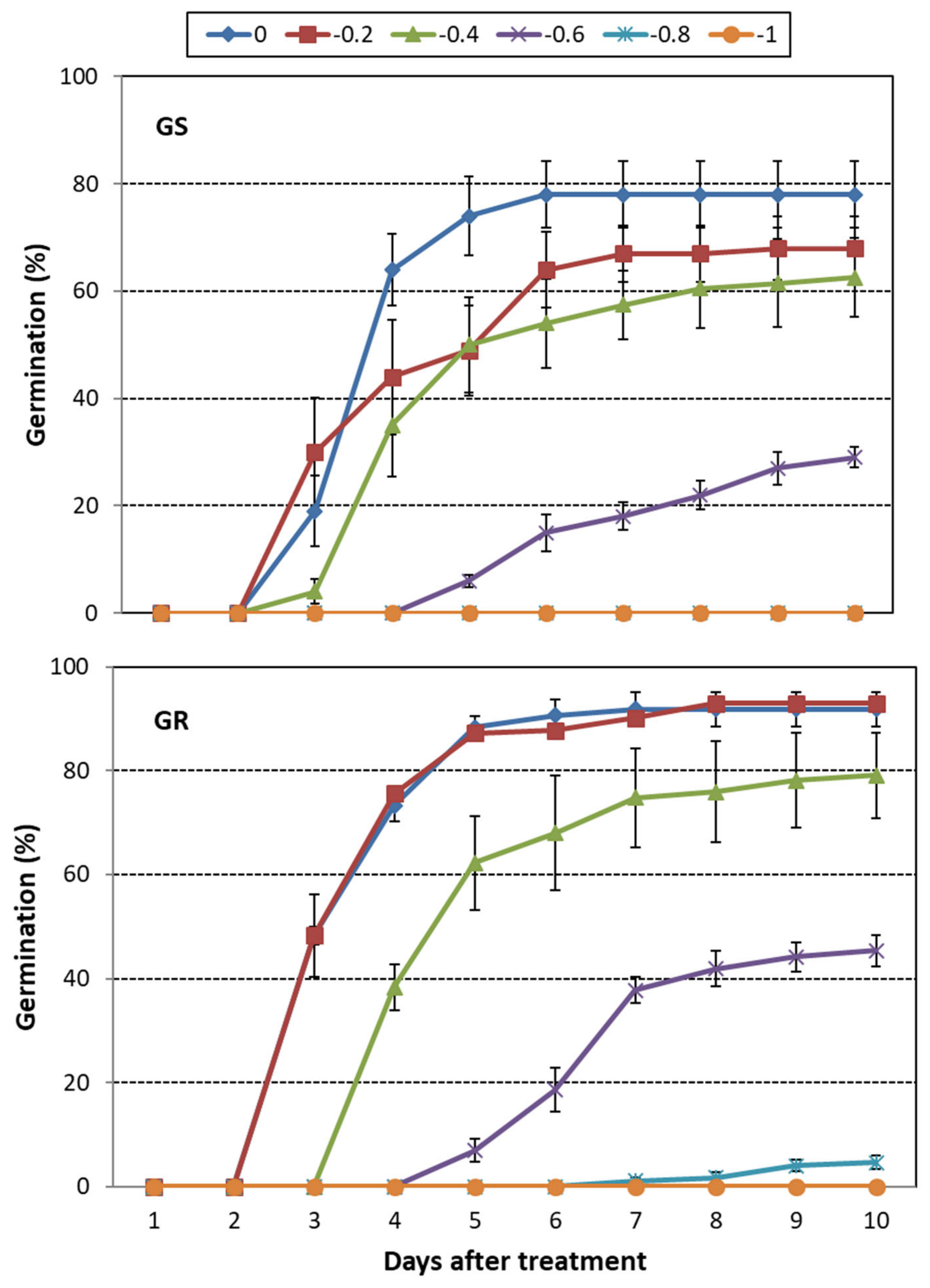
3.2. C. canadensis
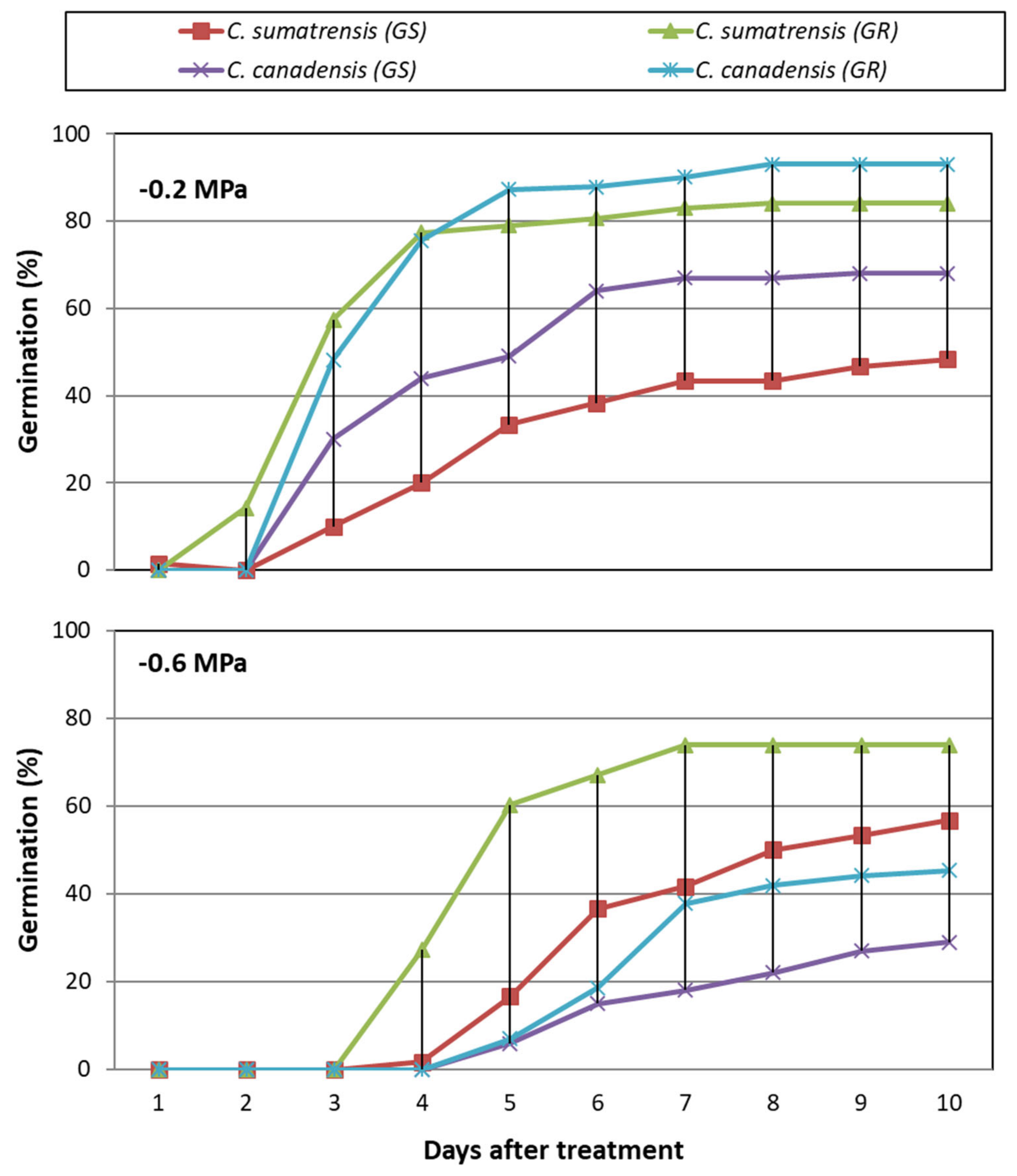
3.3. Overall Drought Stress Effect
3.4. Evaluation of Drought Response by Means of Remote Sensing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeffrey, C. Compositae: Introduction with key to tribes. In Flowering Plants, Eudicots, Asterales; Families and Genera of Vascular, Plants; Kadereit, J.W., Jeffrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume VIII, pp. 61–87. [Google Scholar]
- Travlos, I.S.; Chachalis, D. Glyphosate-Resistant Hairy Fleabane (Conyza bonariensis) Is Reported in Greece. Weed Technol. 2010, 24, 569–573. [Google Scholar] [CrossRef]
- Weaver, S.E. The biology of Canadian weeds. 115. Conyza canadensis. Can. J. Plant Sci. 2001, 81, 867–875. [Google Scholar] [CrossRef]
- Travlos, I.S.; Chachalis, D. Relative competitiveness of glyphosate-resistant and glyphosate-susceptible populations of hairy fleabane (Conyza bonariensis L.). J. Pest Sci. 2013, 86, 345–351. [Google Scholar] [CrossRef]
- Nandula, V.K.; Eubank, T.W.; Poston, D.H.; Koger, C.H.; Reddy, K.N. Factors affecting germination of horseweed (Conyza canadensis). Weed Sci. 2006, 54, 898–902. [Google Scholar] [CrossRef]
- Thebaud, C.; Abbott, R.J. Characterization of invasive Conyza species (Asteraceae) in Europe: Quantitative trait and isozyme analysis. Am. J. Bot. 1995, 82, 360–368. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to drought stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Travlos, I.S.; Liakopoulos, G.; Karabourniotis, G.; Fasseas, C.; Karamanos, A.I. Circadian leaflet movements of Tylosema esculentum (Burch) A. Schreib and relevant potassium effects. J. Arid Environ. 2008, 72, 1745–1750. [Google Scholar] [CrossRef]
- Travlos, I.S. Responses of invasive silverleaf nightshade (Solanum elaeagnifolium Cav.) populations to varying soil water availability. Phytoparasitica 2013, 43, 41–48. [Google Scholar] [CrossRef]
- Bellache, M.; Torres-Pagan, N.; Verdeguer, M.; Llinares, J.V.; Benfekih, L.A.; Sestras, R.E.; Vicente, O.; Sestras, A.F.; Boscaiu, M. Comparative Analysis of Tolerance to Salt Stress and Water Deficit in Two Invasive Weeds of the Genus Erigeron (Asteraceae). Plants 2022, 11, 2059. [Google Scholar] [CrossRef]
- Travlos, I.; De Prado, D.; Chachalis, D.; Bilalis, D.J. Herbicide resistance in weeds: Early detection, mechanisms, dispersal, new insights and management issues. Front. Ecol. Evolut. 2020, 8, 213. [Google Scholar] [CrossRef]
- Urbano, J.M.; Borrego, A.; Torres, V.; Leon, J.M.; Jimenez, C.; Dinelli, G.; Barnes, J. Glyphosate-resistant hairy fleabane (Conyza bonariensis) in Spain. Weed Technol. 2007, 21, 396–401. [Google Scholar] [CrossRef]
- Travlos, I.S.; Giannopolitis, C.; Economou, G. Diclofop resistance in sterile wild oat (Avena sterilis L.) in wheat fields in Greece and its management by other post-emergence herbicides. Crop Prot. 2011, 30, 1449–1454. [Google Scholar] [CrossRef]
- Travlos, I.S.; Chachalis, D. Assessment of glyphosate-resistant horseweed (Conyza canadensis L.) Cronq.) and fleabane (Conyza albida willd. ex spreng) populations from perennial crops in Greece. Int. J. Plant Prod. 2013, 7, 665–676. [Google Scholar]
- Travlos, I.S. Competition between ACC-inhibitor resistant and susceptible sterile wild oat (Avena sterilis L.) biotypes. Weed Sci. 2013, 61, 26–31. [Google Scholar] [CrossRef]
- Davis, V.M.; Kruger, G.R.; Stachler, J.M.; Loux, M.M.; Johnson, W.G. Growth and seed production of horseweed (Conyza canadensis) populations resistant to glyphosate, ALS-imhibiting, and multiple (glyphosate + ALS-inhibiting) herbicides. Weed Sci. 2009, 57, 494–504. [Google Scholar] [CrossRef]
- Shrestha, A.; Hanson, B.D.; Fidelibus, M.W.; Alcorta, M. Growth, phenology, and intraspecific competition between glyphosate-resistant and glyphosate-susceptible horseweed (Conyza canadensis) in the San Joaquin valley of California. Weed Sci. 2010, 58, 147–153. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G. Multi-temporal wheat disease detection by multi-spectral remote sensing. Precis. Agric. 2007, 8, 161–172. [Google Scholar] [CrossRef]
- Naser, M.A.; Khosla, R.; Longchamps, L.; Dahal, S. Using NDVI to differentiate wheat genotypes productivity under dryland and irrigated conditions. Remote Sens. 2020, 12, 824. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Ma, B.L.; Belec, C.; Vigneault, P.A. Comparison of crop data measured by two commercial sensors for variable-rate nitrogen application. Precis. Agric. 2009, 10, 145–161. [Google Scholar] [CrossRef]
- Steinmaus, S.J.; Prather, T.S.; Holt, J.S. Estimation of base temperatures for nine weed species. J. Exp. Bot. 2000, 51, 275–286. [Google Scholar] [CrossRef]
- Travlos, I.; Tsekoura, A.; Antonopoulos, N.; Kanatas, P.; Gazoulis, I. Novel sensor-based method (quick test) for the in-season rapid evaluation of herbicide efficacy under real field conditions in durum wheat. Weed Sci. 2021, 69, 147–160. [Google Scholar] [CrossRef]
- Mahajan, G.; Prasad, A.; Chauhan, B. Seed germination ecology of Sumatran fleabane (Conyza sumatrensis) in relations to various environmental parameters. Weed Sci. 2021, 69, 687–694. [Google Scholar] [CrossRef]
- Loura, D.; Sahil, F.S.; Chauhan, B. Germination ecology of hairy fleabane (Conyza bonariensis) and its implications for weed management. Weed Sci. 2020, 68, 411–417. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Khalofah, A.; Zuan, A.T.K.; Ullah, R.; El-Shehawi, A.M. Seed germination ecology of Conyza sumatrensis populations stemming from different habitats and implications for management. PLoS ONE 2021, 16, e0260674. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; DeSouza, L.; Yang, P.; Sosnoskie, L.; Hanson, B. Differential tolerance of glyphosate-susceptible and glyphosate-resistant biotypes of junglerice (Echinochloa colona) to environments during germination, growth, and intraspecific competition. Weed Sci. 2018, 66, 340–346. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B. Seed germination response to high temperature and drought stress in three invasive Asteraceae weeds from Xishuangbanna, SW China. PLoS ONE 2018, 13, e0191710. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Ruthling, M.; Thill, D.C.; Shafii, B. Differential competitiveness of sulfonylurea resistant and susceptible prickly lettuce (Lactuca serriola). Weed Technol. 1992, 6, 303–309. [Google Scholar] [CrossRef]
- Tataridas, A.; Jabran, K.; Kanatas, P.; Oliveira, R.S.; Freitas, H.; Travlos, I. Early detection, herbicide resistance screening, and integrated management of invasive plant species: A review. Pest Manag. Sci. 2022, 78(, 3957–3972. [Google Scholar] [CrossRef]
- Nansen, C.; Macedo, T.; Swanson, R.; Weaver, D.K. Use of spatial structure analysis of hyperspectral data cubes for detection of insect-induced stress in wheat plants. Int. J. Remote Sens. 2009, 30, 2447–2464. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Feistkorn, D. Monitoring growth status of winter oilseed rape by NDVI and NDYI derived from UAV-based Red-Green-Blue Imagery. Agronomy 2022, 12, 2212. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Dai, C.; Fang, S.; Gong, Y.; Wu, X.; Zhu, R.; Liu, K. Remote prediction of yield based on LAI estimation in oilseed rape under different planting methods and nitrogen fertilizer applications. Agric. For. Meteorol. 2019, 271, 116–125. [Google Scholar] [CrossRef]
- Rousta, I.; Olafsson, H.; Moniruzzaman, M.; Zhang, H.; Liou, Y.A.; Darlington Mushore, T.; Gupta, A. Impact of drought on vegetation assessed by vegetation indices and meteorological factors in Afghanistan. Remote Sens. 2020, 12, 2433. [Google Scholar] [CrossRef]
- Caturegli, L.; Matteoli, S.; Gaetani, M.; Grossi, N.; Magni, S.; Minelli, A.; Corsini, G.; Remorini, D.; Volterrani, M. Effects of drought stress on spectral reflectance of bermudagrass. Sci. Rep. 2020, 10, 15055. [Google Scholar] [CrossRef]
| Region | Positions | Crops | C. canadensis | C. sumatrensis |
|---|---|---|---|---|
| Pelion | 39°26′19″ N, 23°2′47″ E | Orchards | 2 (S and R) | - |
| Domokos | 39°08′ N, 22°18′ E | Orchards, vineyards | - | 1 (S and R) |
| Water Potential (MPa) | g L−1 |
|---|---|
| −0.2 | 112.2 |
| −0.4 | 169.4 |
| −0.6 | 213.6 |
| −0.8 | 251.0 |
| −1 | 284.0 |
| Source of Variation | Df | Sum of Squares | Mean of Squares | F Ratio | p Value |
|---|---|---|---|---|---|
| SP | 1 | 2161.972 | 2161.972 | 18.844 | <0.001 * |
| BI | 1 | 2585.891 | 2585.891 | 22.539 | <0.001 * |
| CO | 5 | 85,158.082 | 17,031.616 | 148.45 | <0.001 * |
| SP × BI | 1 | 2462.83 | 2462.83 | 21.466 | <0.001 * |
| SP × CO | 5 | 5141.643 | 1028.329 | 8.963 | <0.001 * |
| BI × CO | 5 | 1161.694 | 232.339 | 2.025 | 0.085 |
| SP × BI × CO | 5 | 1157.709 | 231.542 | 2.018 | 0.086 |
| C. sumatrensis | C. canadensis | |||
|---|---|---|---|---|
| GS | GR | GS | GR | |
| No drought stress | 0.78 a | 0.75 a | 0.82 a | 0.77 a |
| Moderate drought stress | 0.45 c | 0.53 b | 0.42 c | 0.51 b |
| High drought stress | 0.36 f | 0.49 d | 0.27 g | 0.42 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanatas, P.; Ntaoulis, V.; Gazoulis, I.; Andreou, A.; Danaskos, M.; Mpounanos, D.; Karanika, E.-A.; Papastylianou, P.; Travlos, I. Seed Germination and Plant Growth under Drought Stress of Herbicide-Resistant and Herbicide-Susceptible Biotypes of Conyza Species and Smart Farming Approaches. Agrochemicals 2023, 2, 436-445. https://doi.org/10.3390/agrochemicals2030024
Kanatas P, Ntaoulis V, Gazoulis I, Andreou A, Danaskos M, Mpounanos D, Karanika E-A, Papastylianou P, Travlos I. Seed Germination and Plant Growth under Drought Stress of Herbicide-Resistant and Herbicide-Susceptible Biotypes of Conyza Species and Smart Farming Approaches. Agrochemicals. 2023; 2(3):436-445. https://doi.org/10.3390/agrochemicals2030024
Chicago/Turabian StyleKanatas, Panagiotis, Vasilis Ntaoulis, Ioannis Gazoulis, Athanasios Andreou, Marios Danaskos, Dimitrios Mpounanos, Eleni-Anna Karanika, Panayiota Papastylianou, and Ilias Travlos. 2023. "Seed Germination and Plant Growth under Drought Stress of Herbicide-Resistant and Herbicide-Susceptible Biotypes of Conyza Species and Smart Farming Approaches" Agrochemicals 2, no. 3: 436-445. https://doi.org/10.3390/agrochemicals2030024
APA StyleKanatas, P., Ntaoulis, V., Gazoulis, I., Andreou, A., Danaskos, M., Mpounanos, D., Karanika, E.-A., Papastylianou, P., & Travlos, I. (2023). Seed Germination and Plant Growth under Drought Stress of Herbicide-Resistant and Herbicide-Susceptible Biotypes of Conyza Species and Smart Farming Approaches. Agrochemicals, 2(3), 436-445. https://doi.org/10.3390/agrochemicals2030024







