Abstract
Controversy over the oncogenicity of glyphosate-based herbicides (GBHs) persists seven years after a 2015 IARC Monograph classified glyphosate/GBHs as “probably carcinogenic” to humans. Most regulatory authorities have concluded that technical glyphosate poses little or no oncogenic risk via dietary exposure. The US EPA classified glyphosate as “not likely” to pose cancer risk in 1991, a decision reaffirmed in reports issued in 2017 and 2020. A Federal Circuit Court of Appeals in the US vacated EPA’s assessment of glyphosate human-health risks in 2022 and required EPA to revisit old and take into account new data in its forthcoming, possibly final glyphosate/GBH reregistration decision. Divergent assessments of GBH genotoxicity are the primary reason for differing conclusions regarding GBH oncogenic potential. We assessed whether assays published since completion of the EPA and IARC reviews shed new light on glyphosate/GBH genotoxicity. We found 94 such assays, 33 testing technical glyphosate (73% positive) and 61 on GBHs (95% positive). Seven of 7 in vivo human studies report positive results. In light of genotoxicity results published since 2015, the conclusion that GBHs pose no risk of cancer via a genotoxic mechanism is untenable.
1. Introduction
Measured as pounds of active ingredient applied, glyphosate-based herbicides (GBHs) remain the most heavily applied pesticide ever, both in the US and globally [1,2,3]. Further diversification continues in the places, reasons, and methods by which GBHs are applied and the ways people are exposed.
Unlike nearly all other pesticides, GBHs are widely used by farmers, ranchers, and forestry workers, as well as in non-agricultural treatments around homes, farmsteads, industrial facilities, rights-of-way, transportation corridors and a diversity of public spaces. For these reasons, there are numerous GBH application and exposure pathways and scenarios that sometimes lead to high dermal exposures [4,5,6,7,8,9] compared to typical dietary intake [10,11].
Non-agricultural applications with small-scale equipment (including a simple handheld wand) can lead to markedly higher applicator exposures per pound/kilogram of active ingredient applied (or per area treated) compared to GBH applications made by operators who drive machines equipped with enclosed, steel-glass cabs and active air-filtration systems.
Dietary exposure is now ubiquitous as evident in the high percent of urine samples found to contain glyphosate residues and its primary metabolite, aminomethylphosphonic acid (AMPA) [11,12,13,14,15,16,17,18]. It is highly likely that more people are exposed to GBHs occupationally, and from incidental contact with spray solution, than any other pesticide and these exposures almost certainly account for the majority of GBH high-exposure incidents worldwide.
The global commercialization of genetically engineered, Roundup Ready (RR) soybean, cotton, and corn seeds began in 1996. This was arguably the most rapid and successful major new technology-package launch in the history of agriculture [1,19]. Roundup Ready seeds simplified complex and challenging weed-management systems that were increasingly plagued by the spread of herbicide-resistant weeds. The RR system appeared in the early years to solve several problems: damage to subsequent crops from herbicide carryover in the soil, high costs, and poor or spotty control.
Just 5 years after market entry in the US, farmers planted GMO soybean and cotton seeds on ~90% of the acres planted to these two crops [1,20,21]. Widespread planting of GMO corn, alfalfa, and sugar beet seeds soon followed in many countries. By 2000, glyphosate had become the most heavily applied agricultural pesticide in the US, eclipsing atrazine [3]. Just 5 years later, glyphosate use had doubled (78.8 to 157.5 million pounds in 2005). It subsequently tripled by 2011 and continued to grow thereafter [1].
A growing body of evidence points to GBH exposure-driven adverse impacts on human reproduction [22,23,24,25,26,27,28,29] and upon the incidence of certain cancers, especially non-Hodgkin lymphoma [30,31,32,33,34,35,36,37,38,39,40,41,42,43]. The first evidence of adverse reproductive impacts emerged before the introduction of Roundup Ready seeds. Arbuckle et al. [25] (2001) reported an elevated odds ratio for late-term spontaneous abortions in women from farm families with preconception glyphosate exposure. Another study from 2002 documented increased risk of adverse neurobehavioral effects in children born to applicators of glyphosate [26]. Four birth cohort studies published since 2018 have reported associations between glyphosate and/or AMPA exposure and pre-term birth and/or low-birth weights [23,24,28,44]. Eaton et al. (2022) reported that elevated prenatal AMPA exposures were linked to increased risk of oxidative stress and inflammation during pregnancy [22]. Both of these conditions are known risk factors for pre-term birth.
Rising GBH use, coupled with recent studies reporting linkages between GBHs and certain adverse health outcomes in experimental animals and humans, have intensified scientific and regulatory scrutiny of GBH uses, exposures, and public-health outcomes.
Since 2015 litigation has been underway in the US pursued by individuals alleging their occupational, dermal exposures to Roundup and/or other GBHs caused or contributed to their non-Hodgkin lymphoma (NHL) [45]. This litigation has triggered an extensive reassessment of both published glyphosate studies and pesticide-registrant data. This reassessment focuses on GBH toxicity, exposure, metabolism, pharmacokinetics, genotoxicity, and cancer risk. In addition, the reassessment has brought into the public arena a large number of internal pesticide-manufacturer studies. These include regulatory submissions, emails, and planning and regulatory-response documents generally referred to as the “Monsanto Papers” [46]. Collectively, these documents provide a historical accounting of what Monsanto understood about Roundup toxicity and risks since first approval in 1974.
The authors of this paper have been involved in the Roundup-NHL litigation as testifying experts and/or consultants working with plaintiff law firms. The opportunity to serve in this capacity has afforded the time and resources to carry out a comprehensive appraisal of both published and proprietary, registrant-commissioned studies on GBH toxicity and cancer risk.
The Toxicology Branch within the Office of Pesticide Programs (OPP) in the US EPA (OPP/EPA) classified glyphosate as a “possible oncogen” in 1984–1985 based on a finding of renal tubular adenomas in male mice. This action by OPP set in motion a seven-year debate between OPP and Monsanto that came to focus on the presence of a single renal tumor in control mouse #1028. Several Monsanto scientists and consultants argued that a tumor in control mouse #1028 had been missed in the initial histopathological examination, an argument ultimately accepted by a Scientific Advisory Panel convened to assist OPP resolve the stalemate between Monsanto and OPP scientists [47].
In response to new data, the OPP/EPA reclassified glyphosate in 1991 as “not likely” to pose cancer risk, a decision reaffirmed in 2017 [3] and 2020 [48]. However, in 2022 the OPP/EPA withdrew its proposed interim glyphosate reregistration decision and its supporting human-health risk assessment after the 9th Circuit Court of Appeals vacated the glyphosate risk assessment and cancer classification decision [48,49,50]. The 9th Circuit explained in its order that in light of EPA cancer risk assessment guidelines, the panel of judges could not reconcile the substantial evidence of a link between GBH exposure and cancer (as discussed in the OPP Glyphosate Issue Paper) with the OPP decision to classify glyphosate as “not likely” to cause cancer.
In 2015, the International Agency for Research on Cancer (IARC) classified glyphosate/GBHs as a Group 2A “probable human carcinogen” [30,51]. Additionally, in 2015 at the request of the EPA Administrator, the agency’s Office of Research and Development (ORD) carried out an assessment of the data and basis of the OPP and IARC Working Group classification decisions [52]. The ORD ad hoc committee review focused on the degree to which the final classification judgements adhered to the respective criteria and guidelines governing EPA and IARC assessments of oncogenic potential.
In this paper we discuss OPP assessments and positions (OPP/EPA) in contrast to those of the Office of Research and Development committee (ORD/EPA). The results of the ORD/EPA assessment are recorded in two key documents [43,52] focusing on whether the OPP/EPA and IARC classification decisions adhered to EPA’s and IARC’s stated cancer risk-assessment guidelines and decision criteria.
The ORD committee concluded that neither the OPP/EPA nor the IARC Working Group supported classification of glyphosate/GBHs as “proven” carcinogens. The ORD scientists who conducted the review were divided on whether glyphosate should have been classified as a “possible” or “probable” oncogen in light of the evidence and EPA cancer risk-assessment guidelines [53].
Several published reviews address differences in the OPP/EPA and IARC assessments of glyphosate/GBH carcinogenicity [32,54,55,56]. Other reviews and meta-analyses focus on published epidemiology studies exploring whether dermal exposures to GBHs alter the risk of NHL [31,34,37].
The authors of this paper argue herein that the primary reason OPP/EPA and IARC reached markedly different cancer-classification decisions arises from divergent interpretations of glyphosate/GBH genotoxicity and mechanistic studies. The OPP/EPA focused primarily on genotoxic assays carried out on pure, technical glyphosate and placed little or no weight on published genotoxic studies on formulated GBHs, including several in vivo studies [45]. These were some of the studies upon which the IARC Working Group placed considerable weight in its final judgement that glyphosate/GBHs are “probably carcinogenic” [30].
As of late 2015, more than 70% of published genotoxicity assays reported one or more positive result, compared to about 1% of registrant-commissioned assays [54]. The stark disparity between registrant-commissioned genotoxicity assays and studies conducted by scientists not working on behalf of pesticide manufacturers raises questions about conflict-of-interest bias, the methodologies deployed, and the quality of data available to assess glyphosate/GBH genotoxicity.
Based on EPA cancer study and risk assessment guidelines [57], the OPP/EPA’s “not likely” to pose cancer-risk classification decision depends heavily on the absence of evidence of glyphosate/GBH mutagenic or genotoxic potential. This remains the case to this day.
The dozens of genotoxicity assays published since 2016 shed new light on the oncogenic potential of glyphosate-based herbicides. Other new science is deepening understanding of mechanisms. For example, a team composed of Centers for Disease Control and Prevention (CDC) and National Institutes of Health (NIH) scientists has linked glyphosate exposure levels in farmers to elevated markers of oxidative stress, concluding that their data “strengthen existing evidence from studies that have reported associations between glyphosate exposure and increased DNA damage” [58]. In addition, the CDC-NIH team suggests that “these [oxidative stress] effects may apply broadly to the general population who are primarily exposed through ingestion of contaminated food and water or residential applications”. The breadth and consistency of recently published mechanistic data warrants considerable weight as the Office of Pesticide Programs responds to shortcomings highlighted by the 9th Circuit in the interim 2020 reregistration decision. Here, we provide an overview of studies on glyphosate and/or GBH genotoxicity published since completion of the EPA and IARC reviews in 2015. The findings of new studies are reported and synthesized, with focus on their collective impact on a key question: “Does dermal exposure to a GBH pose a risk of damage to DNA, and hence possibly increase the risk of cancer among applicators and those occupationally exposed?”
2. Methods
This paper focuses on genotoxicity studies conducted since 2015 that were not referenced or relied upon in the 2015–2016 glyphosate/GBH oncogenicity reports issued by the OPP/EPA and IARC. We searched multiple databases to identify published papers assessing the genotoxicity of glyphosate/GBHs. Other mechanisms that can lead to cancer are beyond the scope of this review and include cell proliferation, immunologic, and epigenetic effects. Databases searched include PubMed, Web of Science, and Google Scholar.
Identified studies are incorporated in the master table of assays completed (Supplemental Table S5). Because of the size of the master table, Supplemental Tables S1–S4 divide the master table into four sections roughly aligned with the way OPP/EPA and IARC structured their respective genotoxicity reviews: Mammalian In vivo, Mammalian In vitro, Non-mammalian In Vivo, and Non-mammalian In vitro. Each row in Supplemental Tables S1–S5 covers the results of a single assay. Columns report the citation, test compound(s) and concentrations, test organism, assay or assays deployed, and primary findings. Tables in the body of this paper are largely derived from these Supplemental Tables.
We identified 80 studies published since 1/1/2016 reporting the results of genotoxicity assays on technical glyphosate or GBHs [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138]. Several studies reported results on both. For these studies, two results are recorded in Supplemental Tables S1–S5, one for glyphosate and one for GBHs. There are a total of 94 assay results on glyphosate and GBHs included in the master table. A few studies also report results for AMPA, glyphosate’s primary metabolite. A positive genotoxicity response in an assay testing AMPA is considered evidence of a positive response to a GBH/glyphosate.
Some reviews of the glyphosate/GBH genotoxicity database focus on the number of assays conducted and their results. Other reviews focus on the number of published studies reporting positive/negative results in one or more assays. Most published studies since 2015 have deployed multiple genotoxicity assays.
When the authors of a published study report one or more assay as indicative of a genotoxic response, the results of such an assay or assays are recorded as “Positive” in the present analysis. Likewise, assays regarded as negative for genotoxicity by authors are recorded as “Negative” in our tables. Some studies report both positive and negative assays, as well as some assays producing equivocal results.
Data and insights from unpublished, proprietary registrant-commissioned genotoxicity assays on glyphosate, AMPA, and GBHs are derived from four primary sources:
- Published reviews commissioned and paid for by Monsanto [139,140,141,142].
- The final OPP and IARC glyphosate/GBH oncogenicity review documents [3,30].
- “Glyphosate. Study summaries for genotoxicity studies” issued by OPP [143].
- Descriptions of Monsanto and other registrant-commissioned genotoxicity assays contained in expert reports, depositions and documents generated as a result of ongoing Roundup-NHL litigation.
The fourth category noted above includes information that has become publicly accessible as a consequence of the ongoing Roundup-NHL litigation via what is generally referred to as the “Monsanto Papers” [46].
3. Results
Table 1 provides an overview of the numbers of in vivo mammalian genotoxicity assays published since release of OPP’s 2016 Glyphosate Issue Paper and the IARC Working Group’s 2015 report. Supplemental Table S1 contains further details on individual study designs, assays deployed, test concentrations, and findings. There were 5 assays in which glyphosate technical was administered. A genotoxic response was reported in 4 of these 5 assays (80%).

Table 1.
Mammalian In Vivo Genotoxicity Assays Published Since the EPA and IARC Reviews of Glyphosate and GBH Oncogenicity.
Nine of 11 GBH in vivo assays in humans, rats, Holstein cows, and armadillos reported positive responses. To date, seven assays analyzed genotoxicity in humans exposed to a GBH, all of which reported positive responses (see Table 2). A biomonitoring and oxidative stress study of 177 school-age children in Cyprus was conducted as part of an EU-wide biomonitoring program [70]. The study reported a statistically significant association for AMPA and 8-OHdG, a marker of oxidative stress, but not for glyphosate.

Table 2.
Summary of Seven Human In Vivo Genotoxicity Studies.
AMPA is the primary metabolite of glyphosate in food. Some studies have reported statistically significant associations between AMPA and adverse health outcomes, but not to glyphosate [22]. Makris et al. stated, “The lack of significant associations between AMPA/GLY and lipid damage may suggest a biological mechanism of DNA damage for AMPA”. We regard Makris et al. (2022) [70] as one of the 7 positive in vivo human studies testing GBHs.
Kupske et al. (2021) studied 76 farmers/GBH applicators in Brazil using a micronuclei (MN) assay and oral mucosa epithelial cells [67]. Applicators had applied a GBH for a mean 5.5 eight-hour exposure days. Compared to unexposed controls, the 76 applicators revealed a greater frequency of micronuclei (p = 0.0002), as well as broken egg (p = 0.001), binucleation (p = 0.0001), and karyolysis (p = 0.0004). The buccal micronucleus cytome assay (BMCA) assessed DNA damage in a population of coffee workers in the Dominican Republic [66]. Several BCMA biomarkers showed statistically significant increases in the frequency of nuclear anomalies in the exposed versus control group.
A study published in 2019 by Leite et al. provides evidence of a genotoxic response as a result of GBH exposure [68]. This study tracked the impacts of aerial spraying of GBHs and organophosphate insecticides. The authors reported that GBH applications and volume applied accounted for most of the total pesticide use in the region.
Leite et al. evaluated human biomarkers within: (1) a community surrounded by transgenic Roundup Ready soybean crops, and (2) a control group of children born and residing in a community dedicated to family agriculture with predominantly biological control of pests and little or no use of GBHs. The study measured biomarker buccal micronucleus (with other nuclear abnormalities) together with a comet assay analysis in the exposed and unexposed groups to determine frequency of DNA and cellular damage. The study reported statistically significant differences between exposed and unexposed groups. Notably, higher frequencies were observed in the exposed groups for: micronucleus, increased binucleated, broken egg, karyorrhexis, karyolysis, pyknosis, and condensed chromatin. The study compared children exposed to pesticides to children largely unexposed to pesticides and found that the exposed group experienced greater DNA damage.
An epigenome-wide association study by Lucia et al. explored differences in methylation patterns among 299 post-menopausal women [69]. The SF3B2 gene plays a role in RNA splicing and DNA repair. The authors reported that glyphosate and AMPA altered methylation patterns near the SF3B2 and other genes associated with cancer and endocrine disruption in ways that elevate or promote cancer risk.
Both EPA and IARC consider in vivo genotoxicity assays in exposed humans to be the most relevant type of study for assessing a pesticide’s genotoxic potential in humans. Additional details are provided in Table 2 on 7 positive in vivo human studies published since 2007. Taken together, these in vivo human studies provide strong evidence that GBHs can damage DNA through multiple mechanisms. GBHs can also undermine repair of damaged DNA, and trigger new tumors or promote the growth and spread of existing tumors.
Table 3 covers in vitro mammalian assays using cells from a wide range of mammals. Details on each study and assay are presented in Supplemental Table S2. Thirteen of 19 assays testing technical glyphosate reported a positive response. One assay reported that technical glyphosate was approximately twice as genotoxic compared to AMPA in human peripheral blood mononuclear cells [88]. A second study by Wozniak et al. (2021) also reported that glyphosate triggered a genotoxic response in human peripheral blood mononuclear cells at a lower dose than AMPA [87].

Table 3.
Mammalian In Vitro Genotoxicity Assays Published Since the EPA and IARC Reviews of Glyphosate and GBH Oncogenicity.
All 12 mammalian in vitro assays done with formulated GBHs reported one or more positive result. Over the 31 mammalian in vitro assays, 25 reported one or more positive result (81%).
A total of 39 results from non-mammalian in vivo genotoxicity assays are summarized in Table 4. There were 33 assays using formulated GBHs and 6 focused on technical glyphosate. All 39 assays reported one or more positive response. Most of these results stem from a comet or micronucleus assay. Fish and reptile species were the most commonly tested. A variety of different GBH formulations were used in these experiments. Non-mammalian in vivo genotoxicity assays provide robust insights into the molecular mechanisms by which glyphosate can cause DNA damage. Interference with the mitochondrial respiratory chain, leading to an elevated production of reactive oxygen species, is the most frequently reported mechanism heightening risk of cancer [127]. This is corroborated by in vitro studies [136]. Glyphosate disturbs mitochondrial respiration by altering the function of the electron transport chain. Glyphosate mitochondrial toxicity was first described in 1979 [146,147].

Table 4.
Non-Mammalian In Vivo Genotoxicity Assays Published Since the EPA and IARC Reviews of Glyphosate and GBH Oncogenicity.
Table 5 summarizes the results of 8 in vitro assays in non-mammalian test systems using fish, frogs, snails, nematodes, and a plant-based system. One of 3 assays testing technical glyphosate was positive, while 4 of 5 assays were positive in the case of GBHs.

Table 5.
Non-mammalian In Vitro Genotoxicity Assays Published Since the EPA and IARC Reviews of Glyphosate and GBH Oncogenicity.
There are multiple mechanisms of GBH-induced oxidative stress, genotoxicity, and endocrine disruption among published studies of non-mammalian test systems. Over the years most formulations of GBHs sold in the US contain surfactants composed of various polyoxyethylene amine (POEA) compounds; POEA-based GBH formulations were banned and phased out in Europe by 2017 [148]. The primary mechanism described that is associated with the endocrine-disrupting effect of GBHs is the modulation of estrogen receptors and molecules involved in estrogenic pathways. Ingaramo et al. has documented the endocrine disrupting effects of exposure to glyphosate and GBHs at low or “environmentally relevant” doses in female reproductive tissues [149].
The impact of POEA on the freshwater teleost Prochilodus lineatus was assessed using the comet assay to measure DNA damage in blood cells, indicating significant genotoxicity [150]. The results of this study showed that POEA can affect various parameters such as hemolysis, DNA damage, and lipid peroxidation, which are directly related to an imbalance in the redox state of the fish. Studies of acute exposure of P. lineatus to Roundup also found liver-catalase activity inhibition. This suggests that both formulated Roundup and POEA interfere with the antioxidant defenses in fish. The authors also concluded exposure to POEA generates a condition of oxidative stress in fish. The comet assay used for analyzing DNA damage in blood cells indicated “the genotoxicity of the POEA surfactant at all concentrations tested”. The coherence between mammalian in vivo and non-mammalian in vitro findings across multiple organisms further enhances confidence that GBH exposures can lead to adverse genotoxic effects.
The results in Table 1 and Table 3, Table 4 and Table 5 are combined in Table 6. A total of 33 assays assessed the genotoxicity of technical glyphosate of which 24 were positive (73%). Fifty-eight of 61 assays were positive in the case of formulated GBH assays (95%). Across all 94 studies, 82 reported one or more positive genotoxic response (87%).

Table 6.
Overall Results of Genotoxicity Assays Published Since the 2015 EPA and IARC Reviews of Glyphosate and GBH Oncogenicity.
While over two-thirds of the assays testing technical glyphosate reported one or more positive response, 95% did in the case of formulated GBHs—almost a 50% increase in the frequency of positive responses. Two conclusions seem clear from these data: (1) both glyphosate and GBHs are genotoxic across a wide array of organisms and test systems, and (2) the surfactants in formulated GBHs potentiate genotoxic responses through one or more mechanisms.
In the “Revised Glyphosate Issue Paper: Evaluation of Carcinogenic Potential” dated 12 December 2017 [3], the OPP/EPA focused its genotoxicity assessment on assays carried out on glyphosate technical. Tables 5.1 to 5.7 in the 2017 EPA report present the results of in vitro and in vivo assays testing glyphosate in a range of organisms and assay systems (see [3,54] for an accounting of these studies). In these 7 tables covering 52 assays carried out by GBH registrants testing glyphosate, 51 were reported as negative. All 43 genotoxicity assays carried out or contracted by registrants using a formulated GBH were reported as negative [3,54].
In Section 5.7 in the December 2017 glyphosate issue paper, OPP/EPA states its “Conclusion for Glyphosate” relative to the genotoxicity/mutagenicity of technical glyphosate:
“The overall weight of evidence indicates that there is no convincing evidence that glyphosate induces mutations in vivo via the oral route.”
Note this key OPP conclusion is silent on the genotoxicity of dermal exposures to formulated GBHs. Insight into why can be gained through assessment of an OPP memorandum dated 13 September 2016 [143]. This memo provides a summary of genotoxicity assays that OPP relied on in its assessment of the genotoxicity and oncogenicity of technical glyphosate.
Unlike Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 and their focus on genotoxicity assays published since 2015, Table 7 covers the results of this 2016 OPP assessment of mostly registrant-commissioned genotoxicity studies. Supplemental Table S6 lists the results of 65 assays on glyphosate technical reviewed by OPP, most of which were conducted in the 1980s and 1990s (some studies reported results for 2 or more assays). Supplemental Table S6 also records the gist of OPP “Reviewer comments” on the reliability of positive results noted by study authors [143]. All such comments cast doubt on some aspect of study design, data collection and interpretation, and/or question the biological significance of positive results.

Table 7.
OPP/EPA Evaluation of Glyphosate Genotoxicity Assays Referenced in the Glyphosate Oncogenicity Issue Paper (see notes).
In the case of the 37 genotoxicity assays conducted by registrants and included in this 2016 OPP review, 36 were reported as negative. In the 2016 memo, OPP/EPA reviewers offer no comments on study design, interpretation, or biological significance of these 36 negative assays; all are accepted as negative with no appraisal or comment.
Multiple published assays reviewed by OPP reported positive GBH results (20 of 28). Many of these GBH assays triggered positive responses in cases where technical glyphosate did not, or did so at lower glyphosate-equivalent exposure levels. OPP largely ignored these results in its review of glyphosate/GBH genotoxicity.
Almost 38% (14 out of 37) of registrant-commissioned, glyphosate technical genotoxicity assays utilized a bacterial reverse mutation (BRM) assay (e.g., an Ames test). Just over 1 in 5 of the registrant-commissioned assays were conducted since 2000 (8 out of 37). The most-recent registrant-commissioned study relied on by OPP in its evaluation of glyphosate genotoxicity was done by Sokolowski in 2010 (a negative BRM assay) [143].
A total of 28 published assays are included in the September 2016 OPP/EPA memo. Of these, the authors reported a positive result in 71% (20 out of 28). BRM studies made up only 14% of the published studies, less than one-half the share in the case of the registrant-commissioned studies.
4. Discussion
The evaluation of glyphosate and GBH oncogenicity draws upon three primary sources of data: (1) animal bioassays, (2) epidemiological findings, and (3) mechanistic studies assessing genotoxicity-induced damage to DNA. The cancer classification systems utilized by the US EPA, IARC, the European Food Standard Agency (EFSA) and most other regulatory authorities integrate qualitative assessments of animal, epidemiological, and mechanistic data into an overall weight-of-evidence conclusion. While terminology varies across classification systems, they most often include the following categories:
- Proven carcinogen or oncogenic;
- Probable carcinogen or probably oncogenic;
- Possible or possibly carcinogenic or oncogenic;
- Not likely to be carcinogenic or oncogenic;
- Inadequate data to support or reach a classification decision.
In the “Revised Glyphosate Issue Paper”, OPP evaluated the results of 9 oncogenicity bioassays in rats and 6 in the mouse fed technical glyphosate [3]. The OPP determined that 5 tumors in four of the mouse studies were elevated and in need of further assessment. In the 9 rat oncogenicity assays, OPP noted that 7 tumors were elevated in four of the studies. Across the 15 oncogenicity bioassays, OPP reviewed 12 tumor types in the “Glyphosate Issue Paper” that were statistically significant when evaluated in accord with EPA’s cancer risk assessment guidelines [58].
However, in each case the OPP determined the 12 tumors were “not treatment related” [3]. The common reasons cited included lack of a monotonic dose response curve, use of historical control data, lack of significance in pairwise tests, lack of evidence of progression from adenomas to carcinomas, no similar tumors in other studies, excessively high maximum dose levels, and adjustment for multiple comparisons. Across the 12 tumors, the OPP cites an average of about 3.5 of the above reasons in explaining their decision to discount the 12 statistically significant tumors. There are, to our knowledge, no instances in the OPP’s analysis of rodent bioassays on glyphosate where OPP invokes the same subjective factors (i.e., historical control data; consistency of response across multiple studies) to upgrade a marginally significant tumor dose–response to a significant response.
Both an OPP Scientific Advisory Panel [47] and the EPA’s ORD committee [52] have noted that the downgrading of many of these tumors by OPP to “not treatment related” relied upon judgements inconsistent with the data and EPA’s cancer risk assessment policies [57].
The IARC Working Group concluded that “There is sufficient evidence in experimental animals for the carcinogenicity of glyphosate”. The ORD/EPA committee noted that OPP “focused on pairwise comparisons (which were generally not significant), while IARC also uses trend tests, which yielded several significant results” [151]. ORD/EPA goes on to point out that the EPA’s cancer guidelines state that “Trend tests and pairwise comparison tests are the recommended tests… Significance in either kind of test is sufficient to reject the hypothesis that chance accounts for the result” (para 4, [151]).
An analysis by Portier (2019) [152] of glyphosate oncogenicity feeding studies focused on 13 of the 15 studies addressed in the “Glyphosate Issue Paper” (two studies OPP included in their analysis were regarded by Portier as low quality, and hence were not included in his analysis). Across the 13 bioassays Portier analyzed, 37 tumors were identified as elevated and statistically significant, including 25 tumors that the EPA does not address in the 2017 “Revised Glyphosate Issue Paper”. According to Portier, the 37 tumors reflect consistency across studies and particularly strong evidence of hemangiosarcomas, skin and kidney tumors, and malignant lymphomas.
Disagreement also exists in the interpretation of epidemiological studies focused on exposures to GBHs and cancer, and especially NHL. The “Revised Glyphosate Issue Paper” describes the epidemiological studies considered by OPP and assesses study quality. A total of 58 epidemiology papers covering various studies were identified by OPP, of which:
- 3 studies were judged to be “High” quality;
- 21 were regarded as “Moderate” quality; and
- 34 were determined to be of “low” quality.
OPP considered 6 studies in evaluating the association of GBHs and NHL, 5 of which report a positive association between GBH use and NHL [3]. After a critique of the 5 positive studies noting reasons why the reported associations might be spurious, the OPP concludes that:
“Based on the weight-of-the-evidence, the agency cannot exclude chance or bias as an explanation for the observed associations in the database… A conclusion regarding the association between glyphosate exposure and risk of NHL cannot be determined based on available data”.
The ORD/EPA assessment of the OPP and IARC classification decisions states that:
“ORD’s epidemiologists agree with IARC that there is ‘limited evidence’ of carcinogenicity in humans and understands IARC’s definition of ‘limited evidence’ as ‘a positive association has been observed’ for which a causal association is ‘credible, but chance, bias, or confounding could not be ruled out with reasonable confidence’” [43].
The IARC Working Group noted several studies reporting an association between GBH use/exposures and NHL and concluded that “There is limited evidence in humans for the carcinogenicity of glyphosate. A positive association has been observed for non-Hodgkin lymphoma” ([30], p. 78).
Several meta-analyses and reviews strive to explain what the epidemiological database shows. Most published studies done by scientists not affiliated with or funded by registrants of GHB herbicides conclude that there is limited-to-ample epidemiological data establishing an association between GBH use and NHL. For example, a team of scientists including three individuals that served on the December 2016 Scientific Advisory Panel assessing GBH oncogenicity concludes their meta-analysis by saying: “The overall evidence from human, animal, and mechanistic studies presented here supports a compelling link between exposures to GBHs and increased risk of NHL” [31].
Starkly Divergent Assessments of the Genotoxicity Database
Based on its assessment of glyphosate genotoxicity, the OPP concluded that there is “no convincing evidence that glyphosate induces mutations in vivo via the oral route”. Conversely, IARC concluded that there is “strong evidence” that exposures to GBHs are genotoxic through two modes of action: oxidative stress and damage to DNA.
While these two conclusions may seem irreconcilable, a careful reading of both reports, with focus on questions asked and answered and on what basis, reveals a plausible explanation of why these two reviews reached different conclusions.
As stated in its glyphosate issue paper, the OPP focused its review of GBH genotoxicity on assays using technical glyphosate. In addition as noted in Supplemental Table S6, the OPP discounted essentially all of the positive assay results in 18 published studies on glyphosate/GBH genotoxicity.
The IARC Working Group concluded that in addition to limited epidemiological evidence and sufficient animal bioassay data:
“… there is strong evidence that glyphosate can operate through two key [mechanistic] characteristics of known human carcinogens”. Specifically:
Thus, three primary reasons explain why OPP and IARC reached different conclusions on whether glyphosate/GBHs are genotoxic:
- OPP focused on studies conducted testing technical glyphosate, while IARC placed considerable weight on in vivo GBH studies focused on biomarkers of genotoxicity in exposed human populations and experimental animals.
- The significant potentiation of GBH genotoxicity caused by the coformulants in GBHs, coupled with the enhancement of dermal penetration brought about by the most common GBH surfactants (POEAs).
- OPP/EPA relied predominantly on inappropriate bacterial genotoxicity assays conducted by or for GBH registrants, almost all of which were negative, while the IARC Working Group relied on a larger body of mostly published genotoxicity assays, of which over 70% reported one or more positive response.
The factors noted in #2 above are important in accurately quantifying applicator exposures and risk yet played essentially no role in the OPP’s review of GBH oncogenicity among applicators, including those frequently exposed to relatively high levels of GBH spray solution.
As noted in Williams et al. (2019), the Ames assay detects 93% of the known mutagens in a database of >10,000 compounds [153]. This is because the vast majority of mutagens induced gene mutations, the only class of mutations the Ames assay can detect. However, 7% of mutagens are solely clastogens that induce chromosomal mutations not detected by the Ames assay and, instead, require use of the comet assay or micronucleus assay [154]. Roughly one-half of the registrant-submitted glyphosate/GBH genotoxicity studies evaluated by OPP used the Ames assay. Heavy reliance by registrants and EPA on Ames-test results in assessing the genotoxicity of chromosomal mutagens such as glyphosate/GBHs has and continues to perpetuate the erroneous notion that glyphosate/GBHs are not genotoxic [55].
In addition, glyphosate can have antibiotic activity through inhibition of the shikimate pathway in bacteria [155]. For decades, use of the Ames test has not been recommended when testing compounds which can kill bacteria [156]. Bacteria also do not contain mitochondria, a common target of GBHs associated with genotoxic responses.
Figure 1 provides an overview of the results of glyphosate and GBH genotoxicity assays published since 2016 in each of the categories of assays covered in Table 1, Table 2, Table 3, Table 4 and Table 5.
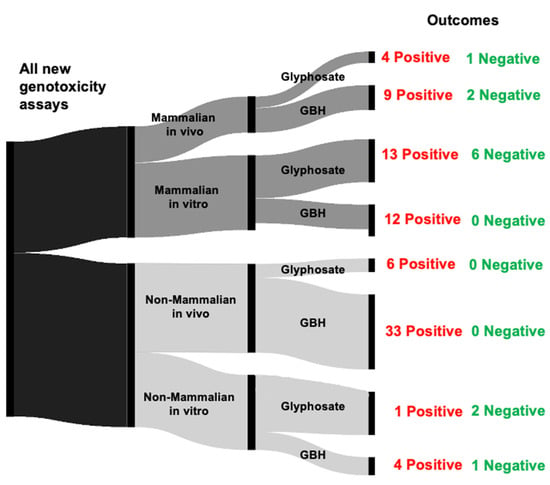
Figure 1.
Sankey chart summarizing the results of genotoxicity assays published since the EPA and IARC reviews of glyphosate and GBH oncogenicity, made with SankeyMATIC (https://sankeymatic.com, accessed on 10 October 2022).
5. Conclusions
The claimed absence of any credible evidence that exposure to glyphosate/GBHs can lead to damage to DNA via a genotoxic mechanism is one of the most important justifications cited over the last 40 years by regulators and GBH registrants in explaining why glyphosate/GBHs are not likely to be carcinogenic.
Over the years, the importance of the claim that glyphosate/GBHs are not genotoxic rose in step with the rising number and quality of animal bioassays and epidemiological studies pointing to links between GBH use, exposures, and cancer.
We conclude that the 80+ positive glyphosate/GBH genotoxicity assays published since 2016 provide clear and compelling evidence that both glyphosate and formulated GBHs are genotoxic. We also find that the POEA surfactants in many GBHs markedly increase genotoxic potency in contrast to glyphosate alone. Hence, the assertion of no credible data supporting the genotoxicity of GBHs is erroneous and no longer tenable.
Presumably the EPA’s cancer risk assessment guidelines will require the OPP to change its current glyphosate/GBH cancer classification. The OPP could change the GBH cancer classification to “probably” or “possibly” oncogenic in light of risks arising from dermal exposures to GBH spray solution. Alternatively, OPP could bifurcate the classification decision, retaining “not likely” for dietary exposures to technical glyphosate and reclassifying dermal exposures to GBHs as “probably” oncogenic for people occupationally exposed to relatively high levels of GBHs several times a year over a period of years.
In classifying GBH oncogenicity, we conclude that OPP and IARC asked and answered different questions, drew upon different datasets, and reached markedly divergent conclusions in the interpretation of studies within each of the three pillars supporting the classification decision.
Several factors led the OPP to its “not likely” conclusion in contrast to IARC’s “probably carcinogenic” decision. These include:
- In accord with US federal law, the OPP focused its review on dietary exposures to technical glyphosate (i.e., the “oral route” of exposure), and largely ignored dermal exposures to GBHs and the ways that the surfactants in GBHs increase applicator exposures and, consequently, increase cancer risk.
- The IARC Working Group placed heavy weight on published epidemiology and genotoxicity studies in human populations exposed to formulated GBHs, and especially studies involving people exposed to GBHs over a span of years. Conversely, OPP placed little or no weight on these key studies and/or raised questions over the conclusions articulated by study authors.
- GBH applicators are exposed to far more glyphosate in a day of spraying GBHs than from glyphosate residues in their diet. This is especially the case among applicators who apply a GBH with small-scale, handheld spray equipment.
- The OPP relied mostly on purportedly negative studies done by GBH registrants, while IARC relied primarily on published studies not commissioned or sponsored by manufacturers, of which more than 70% reported positive results.
Our analysis identified 80 studies published since 2016 reporting the results of 94 genotoxicity assays testing technical glyphosate or formulated GBHs. Some 87% of the 94 assays report one or more positive result.
The results of Table 7 are instructive in understanding the basis for OPP’s conclusion regarding glyphosate genotoxicity. This table summarizes the results of the OPP analysis of 65 genotoxicity assay results. Among the glyphosate genotoxicity assays commissioned by GBH registrants, only one assay reported a positive response. Among the 28 published genotoxicity assays the OPP reviewed, 71% reported one or more positive assays. In all 20 cases, the OPP notes reasons to ignore or place limited weight on the positive assays.
The lingering controversy over the risks and regulation of GBHs can be traced in large part to provisions in federal pesticide law dating back to 1972. Resolving systemic statutory problems in the pesticide risk assessment and regulatory process will require the US Congress to amend federal law [157]. Key statutory changes relevant to the OPP’s assessment of GBH oncogenicity include:
- (1)
- The identity and concentrations of all inert ingredients should be disclosed and listed on pesticide labels;
- (2)
- Applicator and farmworker dermal-exposure risk assessments should be markedly improved and new risk-mitigation measures and requirements codified in law;
- (3)
- The majority of the toxicological and exposure studies required by EPA prior to approval of new pesticide uses, or reregistration of existing uses, should be carried out on both active ingredients and selected, widely-sold formulated products; and
- (4)
- Most foundational pesticide toxicity and risk assessment studies should be conducted by scientists independent of the pesticide industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agrochemicals2010005/s1, Excel workbook with Supplemental Tables S1–S6.
Author Contributions
All authors contributed equally to conceptualizing the analysis and content of the paper. C.B. and R.M. compiled the studies evaluated in Table 1, Table 2, Table 3, Table 4 and Table 5 and Supplemental Tables S1–S5. C.B. compiled the studies in Table 7 and Supplemental Table S6. W.S. summarized the in vivo human studies in Table 2. C.B. wrote the initial draft, all three authors contributed through various revisions and in responding to reviewer comments. All authors have read and agreed to the published version of the manuscript.
Funding
This analysis and the writing of the paper received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated by this analysis and reported in the paper are in the Supplemental Table file and/or referenced documents and papers.
Acknowledgments
The authors thank Rachel Benbrook, from the Heartland Health Research Alliance, for her support in preparing the manuscript for submission and finalizing citations. C. Benbrook and W. Sawyer acknowledge financial support from plaintiff law firms in the Roundup/non-Hodgkin lymphoma litigation. This support made possible in-depth reviews, assessments, and synthesis of published and registrant-commissioned studies on the genotoxicity of glyphosate and GBHs.
Conflicts of Interest
C. Benbrook has published papers on the use, risks, and regulation of glyphosate/GBHs. R. Mesnage has led or participated on teams carrying out multiple genotoxicity assays of glyphosate/GBHs and published several glyphosate/GBH papers, including some in this paper’s Supplemental Tables. All authors have served as expert witnesses in litigation involving Roundup and non-Hodgkin lymphoma. Plaintiff law firms played no role in: (1) the decision to conduct the research and write the paper, (2) the focus and content of the tables and paper, and (3) our findings and conclusions.
References
- Benbrook, C. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.; Paisley-Jones, C. EPA Pesticides Industry Sales and Usage: 2008–2012 Market Estimates. 2017. Available online: https://www.epa.gov/pesticides/pesticides-industry-sales-and-usage-2008-2012-market-estimates (accessed on 10 October 2022).
- Environmental Protection Agency (US). Revised Glyphosate Issue Paper: Evaluation of Carcinogenic Potential. 2017. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2009-0361-0073 (accessed on 10 October 2022).
- Macfarlane, E.; Carey, R.; Keegel, T.; El-Zaemay, S.; Fritschi, L. Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf. Health Work 2013, 4, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Baldi, I.; Lebailly, P.; Jean, S.; Rougetet, L.; Dulaurent, S.; Marquet, P. Pesticide contamination of workers in vineyards in France. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lebailly, P.; Bouchart, V.; Baldi, I.; Lecluse, Y.; Heutte, N.; Gislard, A.; Malas, J.P. Exposure to pesticides in open-field farming in France. Ann. Occup. Hyg. 2009, 53, 69–81. [Google Scholar] [CrossRef]
- Connolly, A.; Coggins, M.A.; Galea, K.S.; Jones, K.; Kenny, L.; McGowan, P.; Basinas, I. Evaluating Glyphosate Exposure Routes and Their Contribution to Total Body Burden: A Study Among Amenity Horticulturalists. Ann. Work Expo. Health. 2019, 63, 133–147. [Google Scholar] [CrossRef]
- Dosemeci, M.; Alavanja, M.C.; Rowland, A.S.; Mage, D.; Zahm, S.H.; Rothman, N.; Lubin, J.H.; Hoppin, J.A.; Sandler, D.P.; Blair, A. A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann. Occup. Hyg. 2002, 46, 245–260. [Google Scholar] [CrossRef]
- Machado-Neto, J.G.; Bassini, A.J.; Aguiar, L.C. Safety of working conditions of glyphosate applicators on Eucalyptus forests using knapsack and tractor powered sprayers. Bull. Environ. Contam. Toxicol. 2000, 64, 309–315. [Google Scholar] [CrossRef]
- Stephenson, C.L.; Harris, C.A. An assessment of dietary exposure to glyphosate using refined deterministic and probabilistic methods. Food Chem. Toxicol. 2016, 95, 28–41. [Google Scholar] [CrossRef]
- Vicini, J.L.; Jensen, P.K.; Young, B.M.; Swarthout, J.T. Residues of glyphosate in food and dietary exposure. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5226–5257. [Google Scholar] [CrossRef]
- Benbrook, C. Tracking pesticide residues and risk levels in individual samples—Insights and applications. Environ. Sci. Eur. 2022, 34. [Google Scholar] [CrossRef]
- Centers for Disease Control (US). Urinary Glyphosate (N-(Phosphonomethyl)glycine) Data Tables Published. 2022. Available online: https://www.cdc.gov/exposurereport/whats_new_071922_1.html (accessed on 25 October 2022).
- Centers for Disease Control (US). National Health and Nutrition Examination Survey, 2013–2014 Data Documentation, Codebook, and Frequencies: Glyphosate (GLYP)—Urine (SSGLYP_H). 2022. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSGLYP_H.htm (accessed on 25 October 2022).
- Mills, P.K.; Kania-Korwle, I.; Fagan, J.; McEvoy, L.K.; Laughlin, G.A.; Barett-Connor, E. Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016. JAMA 2017, 318, 1610–1611. [Google Scholar] [CrossRef]
- Conrad, A.; Schroter-Kermani, C.; Hoppe, H.W.; Ruther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef]
- Grau, D.; Grau, N.; Gascuel, Q.; Paroissin, C.; Stratonovitch, C.; Lairon, D.; Devault, D.A.; Di Cristofaro, J. Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environ. Sci. Pollut. Res. Int. 2022, 29, 32882–32893. [Google Scholar] [CrossRef]
- Duke, S.O. Perspectives on transgenic, herbicide-resistant crops in the United States almost 20 years after introduction. Pest Manag. Sci. 2015, 71, 652–657. [Google Scholar] [CrossRef]
- Benbrook, C. Impacts of genetically engineered crops on pesticide use in the U.S.—The first sixteen years. Environ. Sci. Eur. 2012, 24. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Economic Research Service. Adoption of Genetically Engineered Crops in the U.S. n.d. Available online: https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-u-s/ (accessed on 15 November 2022).
- Eaton, J.L.; Cathey, A.L.; Fernandez, J.A.; Watkins, D.J.; Silver, M.K.; Milne, G.L.; Velez-Vega, C.; Rosario, Z.; Cordero, J.; Alshawabkeh, A.; et al. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicol. Environ. Saf. 2022, 233, 113300. [Google Scholar] [CrossRef]
- Silver, M.K.; Fernandez, J.; Tang, J.; McDade, A.; Sabino, J.; Rosario, Z.; Velez Vega, C.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Prenatal Exposure to Glyphosate and Its Environmental Degradate, Aminomethylphosphonic Acid (AMPA), and Preterm Birth: A Nested Case-Control Study in the PROTECT Cohort (Puerto Rico). Environ. Health Perspect. 2021, 129, 57011. [Google Scholar] [CrossRef]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 23. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Lin, Z.; Mery, L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect. 2001, 109, 851–857. [Google Scholar] [CrossRef]
- Garry, V.F.; Harkins, M.E.; Erickson, L.L.; Long-Simpson, L.K.; Holland, S.E.; Burroughs, B.L. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ. Health Perspect. 2002, 110, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Lesseur, C.; Pirrotte, P.; Pathak, K.V.; Manservisi, F.; Mandrioli, D.; Belpoggi, F.; Panzacchi, S.; Li, Q.; Barrett, E.S.; Nguyen, R.H.N.; et al. Maternal urinary levels of glyphosate during pregnancy and anogenital distance in newborns in a US multicenter pregnancy cohort. Environ. Pollut. 2021, 280, 117002. [Google Scholar] [CrossRef] [PubMed]
- Lesseur, C.; Pathak, K.V.; Pirrotte, P.; Martinez, M.N.; Ferguson, K.K.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Mandrioli, D.; Swan, S.H.; et al. Urinary glyphosate concentration in pregnant women in relation to length of gestation. Environ. Res. 2021, 203, 111811. [Google Scholar] [CrossRef] [PubMed]
- Kongtip, P.; Nankongnab, N.; Phupancharoensuk, R.; Palarach, C.; Sujirarat, D.; Sangprasert, S.; Sermsuk, M.; Sawattrakool, N.; Woskie, S.R. Glyphosate and Paraquat in Maternal and Fetal Serums in Thai Women. J. Agromedicine 2017, 22, 282–289. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Volume 112: Some Organophosphate Insecticides and Herbicides. 2017. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/07/mono112.pdf (accessed on 10 October 2022).
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to Glyphosate-Based Herbicides and Risk for Non-Hodgkin Lymphoma: A Meta-Analysis and Supporting Evidence. Mutat. Res./Rev. Mutat. Res. 2019, 781. [Google Scholar] [CrossRef]
- Portier, C.J.; Armstrong, B.K.; Baguley, B.C.; Baur, X.; Belyaev, I.; Belle, R.; Belpoggi, F.; Biggeri, A.; Bosland, M.C.; Bruzzi, P.; et al. Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J. Epidemiol. Community Health 2016, 70, 741–745. [Google Scholar] [CrossRef]
- Andreotti, G.; Koutros, S.; Hofmann, J.N.; Sandler, D.P.; Lubin, J.H.; Lynch, C.F.; Lerro, C.C.; De Roos, A.J.; Parks, C.G.; Alavanja, M.C.; et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J. Natl. Cancer Inst. 2018, 110, 509–516. [Google Scholar] [CrossRef]
- Chang, E.T.; Delzell, E. Systematic review and meta-analysis of glyphosate exposure and risk of lymphohematopoietic cancers. J. Environ. Sci. Health B 2016, 51, 402–434. [Google Scholar] [CrossRef]
- De Roos, A.J.; Blair, A.; Rusiecki, J.A.; Hoppin, J.A.; Svec, M.; Dosemeci, M.; Sandler, D.P.; Alavanja, M.C. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2005, 113, 49–54. [Google Scholar] [CrossRef]
- Hardell, L.; Eriksson, M.; Nordstrom, M. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: Pooled analysis of two Swedish case-control studies. Leuk. Lymphoma 2002, 43, 1043–1049. [Google Scholar] [CrossRef]
- Leon, M.E.; Schinasi, L.H.; Lebailly, P.; Beane Freeman, L.E.; Nordby, K.C.; Ferro, G.; Monnereau, A.; Brouwer, M.; Tual, S.; Baldi, I.; et al. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: A pooled analysis from the AGRICOH consortium. Int. J. Epidemiol. 2019, 48, 1519–1535. [Google Scholar] [CrossRef]
- Rana, I.; Taioli, E.; Zhang, L. Weeding out inaccurate information on glyphosate-based herbicides and risk of non-Hodgkin lymphoma. Environ. Res. 2020, 191, 110140. [Google Scholar] [CrossRef]
- Sheppard, L.; Shaffer, R.M. Re: Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J. Natl. Cancer Inst. 2019, 111, 214–215. [Google Scholar] [CrossRef]
- Stur, E.; Aristizabal-Pachon, A.F.; Peronni, K.C.; Agostini, L.P.; Waigel, S.; Chariker, J.; Miller, D.M.; Thomas, S.D.; Rezzoug, F.; Detogni, R.S.; et al. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS ONE 2019, 14, e0219610. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Avila-Vazquez, M.; Maturano, E.; Etchegoyen, A.; Difilippo, F.S.; Maclean, B. Association between Cancer and Environmental Exposure to Glyphosate. Int. J. Clin. Med. 2017, 8, 73–85. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US). Epidemiological Evidence. 2016. Available online: https://www.documentcloud.org/documents/20786671-doc101719 (accessed on 10 October 2022).
- Gerona, R.R.; Reiter, J.L.; Zakharevich, I.; Proctor, C.; Ying, J.; Mesnage, R.; Antoniou, M.; Winchester, P.D. Glyphosate exposure in early pregnancy and reduced fetal growth: A prospective observational study of high-risk pregnancies. Environ. Health 2022, 21, 95. [Google Scholar] [CrossRef]
- Benbrook, C. Shining a Light on Glyphosate-Based Herbicide Hazard, Exposures and Risk: Role of Non-Hodgkin Lymphoma Litigation in the USA. Eur. J. Risk Regul. 2020, 11, 498–519. [Google Scholar] [CrossRef]
- Baum Hedland. Monsanto Papers. n.d. Available online: https://www.baumhedlundlaw.com/toxic-tort-law/monsanto-roundup-lawsuit/monsanto-papers/ (accessed on 15 November 2022).
- Environmental Protection Agency (US). Memo: Transmission of Meeting Minutes and Final Report of the December 13-16, 2016 FIFRA SAP Meeting Held to Consider and Review Scientific Issues Associated with EPA’s Evaluation of the Carcinogenic Potential of Glyphosate. 2017. Available online: https://www.epa.gov/sites/default/files/2017-03/documents/december_13-16_2016_final_report_03162017.pdf (accessed on 10 October 2022).
- Environmental Protection Agency (US). Glyphosate: Interim Registration Review Decision Case Number 0178. 2020. Available online: https://www.epa.gov/sites/default/files/2020-01/documents/glyphosate-interim-reg-review-decision-case-num-0178.pdf (accessed on 10 October 2022).
- Ninth Circuit Court of Appeals (US). Order No. 20-70787, NRDC and Pesticide Action Network vs. Environmental Protection Agency. 2022. Available online: https://cdn.ca9.uscourts.gov/datastore/opinions/2022/06/17/20-70787.pdf (accessed on 10 October 2022).
- Environmental Protection Agency (US). EPA Withdraws Glyphosate Interim Decision. 2022. Available online: https://www.epa.gov/pesticides/epa-withdraws-glyphosate-interim-decision (accessed on 28 October 2022).
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US). Summary of ORD comments on OPP’s Glyphosate Cancer Assessment. 2015. Available online: https://usrtk.org/wp-content/uploads/2017/03/ORDcommentsonOPPglyphosate.pdf (accessed on 10 October 2022).
- Cogliano, V.J. Email “Subject: Re: Glyphosate follow up”, December 7, 2015, to Birchfield, N. 2015. Available online: https://www.thenewlede.org/wp-content/uploads/2022/10/Glyphosate-RUP-EPA-ORD-OPP-2015-12-7-ORD-email-re-evaluation-Cogliano-Memo-2.pdf (accessed on 10 October 2022).
- Benbrook, C. How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ. Sci. Eur. 2019, 31. [Google Scholar] [CrossRef]
- Davoren, M.J.; Schiestl, R.H. Glyphosate-based herbicides and cancer risk: A post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis 2018, 39, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Hendlin, Y.; Arcuri, A. Assessing the Safety of Glyphosate. Environ. Epidemiol. 2019, 3. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US). Guidelines for Carcinogen Risk Assessment. 2005. Available online: https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf (accessed on 10 October 2022).
- Chang, V.C.; Andreotti, G.; Ospina, M.; Parks, C.G.; Liu, D.; Shearer, J.J.; Rothman, N.; Silverman, D.T.; Sandler, D.P.; Calafat, A.M.; et al. Glyphosate Exposure and Urinary Oxidative Stress Biomarkers in the Agricultural Health Study. J. Natl. Cancer Inst. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kasuba, V.; Milic, M.; Rozgaj, R.; Kopjar, N.; Mladinic, M.; Zunec, S.; Vrdoljak, A.L.; Pavicic, I.; Cermak, A.M.M.; Pizent, A.; et al. Effects of low doses of glyphosate on DNA damage, cell proliferation and oxidative stress in the HepG2 cell line. Environ. Sci. Pollut. Res. 2017, 24, 19267–19281. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Christensen, T.B.; Hojberg, O.; Sorensen, M.T. Exposure of pigs to glyphosate affects gene-specific DNA methylation and gene expression. Toxicol. Rep. 2022, 9, 298–310. [Google Scholar] [CrossRef]
- Mesnage, R.; Ibragim, M.; Mandrioli, D.; Falcioni, L.; Tibaldi, E.; Belpoggi, F.; Brandsma, I.; Bourne, E.; Savage, E.; Mein, C.A.; et al. Comparative Toxicogenomics of Glyphosate and Roundup Herbicides by Mammalian Stem Cell-Based Genotoxicity Assays and Molecular Profiling in Sprague-Dawley Rats. Toxicol. Sci. 2022, 186, 83–101. [Google Scholar] [CrossRef]
- Milic, M.; Zunec, S.; Micek, V.; Kasuba, V.; Mikolic, A.; Lovakovic, B.T.; Semren, T.Z.; Pavicic, I.; Cermak, A.M.M.; Pizent, A.; et al. Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate. Arch. Ind. Hyg. Toxicol. 2018, 69, 154–168. [Google Scholar] [CrossRef]
- Tarboush, N.A.; Almomani, D.H.; Khabour, O.F.; Azzam, M.I. Genotoxicity of Glyphosate on Cultured Human Lymphocytes. Int. J. Toxicol. 2022, 41, 126–131. [Google Scholar] [CrossRef]
- Avdatek, F.; Birdane, Y.O.; Turkmen, R.; Demirel, H.H. Ameliorative effect of resveratrol on testicular oxidative stress, spermatological parameters and DNA damage in glyphosate-based herbicide-exposed rats. Andrologia 2018, 50, e13036. [Google Scholar] [CrossRef]
- De Maria Serra, F.; Parizi, J.L.S.; Odorizzi, G.; Sato, G.; Patrão, I.B.; Chagas, P.H.N.; de Azevedo Mello, F.; Nai, G.A.-O. Subchronic exposure to a glyphosate-based herbicide causes dysplasia in the digestive tract of Wistar rats. Environ. Sci. Pollut. Res. 2021, 28, 61477–61496. [Google Scholar] [CrossRef]
- Hutter, H.P.; Khan, A.W.; Lemmerer, K.; Wallner, P.; Kundi, M.; Moshammer, H. Cytotoxic and Genotoxic Effects of Pesticide Exposure in Male Coffee Farmworkers of the Jarabacoa Region, Dominican Republic. Int. J. Environ. Res. Public Health 2018, 15, 81641. [Google Scholar] [CrossRef]
- Kupske, C.; Baroni, S.; Zamin, L.L. Cellular Changes in Buccal Mucosa from Farmers Exposed to Glyphosate/Alterações Celulares na Mucosa Bucal de Agricultores Expostos ao Glifosato. Braz. J. Dev. 2021, 7, 105242–105257. [Google Scholar] [CrossRef]
- Leite, S.B.; Franco de Diana, D.M.; Segovia Abreu, J.A.; Avalos, D.S.; Denis, M.A.; Ovelar, C.C.; Samaniego Royg, M.J.; Thielmann Arbo, B.A.; Corvalan, R. DNA damage induced by exposure to pesticides in children of rural areas in Paraguay. Indian J. Med. Res. 2019, 150, 290–296. [Google Scholar] [CrossRef]
- Lucia, R.M.; Huang, W.L.; Pathak, K.V.; McGilvrey, M.; David-Dirgo, V.; Alvarez, A.; Goodman, D.; Masunaka, I.; Odegaard, A.O.; Ziogas, A.; et al. Association of Glyphosate Exposure with Blood DNA Methylation in a Cross-Sectional Study of Postmenopausal Women. Environ. Health Perspect. 2022, 130, 47001. [Google Scholar] [CrossRef]
- Makris, K.C.; Efthymiou, N.; Konstantinou, C.; Anastasi, E.; Schoeters, G.; Kolossa-Gehring, M.; Katsonouri, A. Oxidative stress of glyphosate, AMPA and metabolites of pyrethroids and chlorpyrifos pesticides among primary school children in Cyprus. Environ. Res. 2022, 212, 113316. [Google Scholar] [CrossRef]
- Rossi, L.F.; Luaces, J.P.; Palermo, A.M.; Merani, M.S.; Mudry, M.D. Cytogenetic damage in peripheral blood cultures of Chaetophractus villosus exposed in vivo to a glyphosate formulation (Roundup). Ecotoxicol. Environ. Saf. 2018, 157, 121–127. [Google Scholar] [CrossRef]
- Schnabel, K.; Schmitz, R.; Frahm, J.; Meyer, U.; Breves, G.; Danicke, S. Functionality and DNA-damage properties of blood cells in lactating cows exposed to glyphosate contaminated feed at different feed energy levels. Arch. Anim. Nutr. 2020, 74, 87–106. [Google Scholar] [CrossRef]
- Soudani, N.; Chaabane, M.; Ghorbel, I.; Elwej, A.; Boudawara, T.; Zeghal, N. Glyphosate disrupts redox status and up-regulates metallothionein I and II genes expression in the liver of adult rats. Alleviation by quercetin. Gen. Physiol. Biophys. 2019. [Google Scholar] [CrossRef]
- Alvarez-Moya, C.; Samano-Leon, A.G.; Reynoso-Silva, M.; Ramirez-Velasco, R.; Ruiz-Lopez, M.A.; Villalobos-Arambula, A.R. Antigenotoxic Effect of Ascorbic Acid and Resveratrol in Erythrocytes of Ambystoma mexicanum, Oreochromis niloticus and Human Lymphocytes Exposed to Glyphosate. Curr. Issues Mol. Biol. 2022, 44, 2230–2242. [Google Scholar] [CrossRef]
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The Effect of Glyphosate on Human Sperm Motility and Sperm DNA Fragmentation. Int. J. Environ. Res. Public Health 2018, 15, 61117. [Google Scholar] [CrossRef]
- Barron Cuenca, J.; de Oliveira Galvao, M.F.; Unlu Endirlik, B.; Tirado, N.; Dreij, K. In vitro cytotoxicity and genotoxicity of single and combined pesticides used by Bolivian farmers. Environ. Mol. Mutagen 2022, 63, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Cheng, J.; Xu, W.; Xu, Z.; Gao, J.; Tao, L. Adjuvant contributes Roundup’s unexpected effects on A549 cells. Environ. Res. 2020, 184, 109306. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Reszka, E.; Wozniak, K.; Jablonska, E.; Michalowicz, J.; Bukowska, B. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Ferguson, S.; Brandsma, I.; Moelijker, N.; Zhang, G.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Antoniou, M.N. The surfactant co-formulant POEA in the glyphosate-based herbicide RangerPro but not glyphosate alone causes necrosis in Caco-2 and HepG2 human cell lines and ER stress in the ToxTracker assay. Food Chem. Toxicol. 2022, 168, 113380. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Argaw Tessema, R.; Szasz, I.; Smeirat, T.; Al Rajo, A.; Adam, B. Micronucleus Formation Induced by Glyphosate and Glyphosate-Based Herbicides in Human Peripheral White Blood Cells. Front. Public Health 2021, 9, 639143. [Google Scholar] [CrossRef]
- Olah, M.; Farkas, E.; Szekacs, I.; Horvath, R.; Szekacs, A. Cytotoxic effects of Roundup Classic and its components on NE-4C and MC3T3-E1 cell lines determined by biochemical and flow cytometric assays. Toxicol. Rep. 2022, 9, 914–926. [Google Scholar] [CrossRef]
- Rice, J.R.; Dunlap, P.; Ramaiahgari, S.; Ferguson, S.; Smith-Roe, S.L.; DeVito, M. Poster: Effects of Glyphosate and its Formulations on Markers of Oxidative Stress and Cell Viability in HepaRG and HaCaT Cell Lines. 2018. Available online: https://ntp.niehs.nih.gov/ntp/results/pubs/posters/rice_sot20190300.pdf (accessed on 10 October 2022).
- Santovito, A.; Ruberto, S.; Gendusa, C.; Cervella, P. In vitro evaluation of genomic damage induced by glyphosate on human lymphocytes. Environ. Sci. Pollut. Res. Int. 2018, 25, 34693–34700. [Google Scholar] [CrossRef]
- Suarez-Larios, K.; Salazar-Martinez, A.M.; Montero-Montoya, R. Screening of Pesticides with the Potential of Inducing DSB and Successive Recombinational Repair. J. Toxicol. 2017, 2017, 3574840. [Google Scholar] [CrossRef]
- Szepanowski, F.; Szepanowski, L.P.; Mausberg, A.K.; Albrecht, P.; Kleinschnitz, C.; Kieseier, B.C.; Stettner, M. Differential impact of pure glyphosate and glyphosate-based herbicide in a model of peripheral nervous system myelination. Acta Neuropatholologica 2018, 136, 979–982. [Google Scholar] [CrossRef]
- Townsend, M.; Peck, C.; Meng, W.; Heaton, M.; Robison, R.; O’Neill, K. Evaluation of various glyphosate concentrations on DNA damage in human Raji cells and its impact on cytotoxicity. Regul. Toxicol. Pharmacol. 2017, 85, 79–85. [Google Scholar] [CrossRef]
- Wozniak, E.; Reszka, E.; Jablonska, E.; Michalowicz, J.; Huras, B.; Bukowska, B. Glyphosate and AMPA Induce Alterations in Expression of Genes Involved in Chromatin Architecture in Human Peripheral Blood Mononuclear Cells (In Vitro). Int. J. Mol. Sci. 2021, 22, 62966. [Google Scholar] [CrossRef]
- Wozniak, E.; Sicinska, P.; Michalowicz, J.; Wozniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells — genotoxic risk assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Xu, D.-Q.; Feng, X.-Z. The toxic effects and possible mechanisms of glyphosate on mouse oocytes. Chemosphere 2019, 237, 124435. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Mittal, M.; Saraf, P. Effective attenuation of glyphosate-induced oxidative stress and granulosa cell apoptosis by vitamins C and E in caprines. Mol. Reprod. Dev. 2019, 86, 42–52. [Google Scholar] [CrossRef]
- De Almeida, L.K.S.; Pletschke, B.I.; Frost, C.L. Moderate levels of glyphosate and its formulations vary in their cytotoxicity and genotoxicity in a whole blood model and in human cell lines with different estrogen receptor status. 3 Biotech 2018, 8, 438. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, W.; Gao, J.; Zhang, Y.; Yang, Y.; Tao, L. Roundup-Induced AMPK/mTOR-Mediated Autophagy in Human A549 Cells. J. Agric. Food Chem. 2019, 67, 11364–11372. [Google Scholar] [CrossRef]
- Luaces, J.P.; Rossi, L.F.; Chirino, M.G.; Browne, M.; Merani, M.S.; Mudry, M.D. Genotoxic effects of Roundup Full II® on lymphocytes of Chaetophractus villosus (Xenarthra, Mammalia): In vitro studies. PLoS ONE 2017, 12, e0182911. [Google Scholar] [CrossRef]
- Luo, L.; Wang, F.; Zhang, Y.; Zeng, M.; Zhong, C.; Xiao, F. In vitro cytotoxicity assessment of roundup (glyphosate) in L-02 hepatocytes. J. Environ. Sci. Health Part B 2017, 52, 410–417. [Google Scholar] [CrossRef]
- De Brito Rodrigues, L.; Gonçalves Costa, G.; Lundgren Thá, E.; da Silva, L.R.; de Oliveira, R.; Morais Leme, D.; Cestari, M.M.; Koppe Grisolia, C.; Campos Valadares, M.; de Oliveira, G.A.R. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2019, 842, 94–101. [Google Scholar] [CrossRef]
- Giommi, C.; Ladisa, C.; Carnevali, O.; Maradonna, F.; Habibi, H.R. Metabolomic and Transcript Analysis Revealed a Sex-Specific Effect of Glyphosate in Zebrafish Liver. Int. J. Mol. Sci. 2022, 23, 52724. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, X.; Yan, G.; Huang, Y.; Zuo, F.; Shen, Y.; Ding, Y.; Cheng, Y. Effects of glyphosate on immune responses and haemocyte DNA damage of Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2017, 71, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Benvindo-Souza, M.; Carvalho, W.F.; Nunes, H.F.; de Lima, P.N.; Costa, M.S.; Benetti, E.J.; Guerra, V.; Saboia-Morais, S.M.T.; Santos, C.E.; et al. Evaluation of the genotoxic, mutagenic, and histopathological hepatic effects of polyoxyethylene amine (POEA) and glyphosate on Dendropsophus minutus tadpoles. Environ. Pollut. 2021, 289, 117911. [Google Scholar] [CrossRef] [PubMed]
- Marçal, R.; Pacheco, M.; Guilherme, S. DNA of crayfish spermatozoa as a target of waterborne pesticides—An ex vivo approach as a tool to short-term spermiotoxicity screening. J. Hazard. Mater. 2020, 400, 123300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jia, R.; Cao, L.; Du, J.; Gu, Z.; He, Q.; Xu, P.; Yin, G. Effects of chronic glyphosate exposure on antioxdative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108878. [Google Scholar] [CrossRef] [PubMed]
- Acar, Ü.; İnanan, B.E.; Navruz, F.Z.; Yılmaz, S. Alterations in blood parameters, DNA damage, oxidative stress and antioxidant enzymes and immune-related genes expression in Nile tilapia (Oreochromis niloticus) exposed to glyphosate-based herbicide. Comp. Biochem. Physiol. C Toxicol. Pharmacol 2021, 249, 109147. [Google Scholar] [CrossRef]
- Akça, A.; Kocabaş, M.; Kutluyer, F. Glyphosate disrupts sperm quality and induced DNA damage of rainbow trout (Oncorhynchus mykiss) sperm. J. Environ. Sci. Health C Toxicol. Carcinog. 2021, 39, 413–422. [Google Scholar] [CrossRef]
- Aribisala, O.A.; Sogbanmu, T.O.; Kemabonta, K.A. Genotoxic, biochemical and histological biomarkers of subacute concentrations of paraquat and glyphosate in Nile Tilapia. Environ. Anal. Health Toxicol. 2022, 37, e2022012. [Google Scholar] [CrossRef]
- Ayanda, O.I.; Tolulope, A.; Oniye, S.J. Mutagenicity and genotoxicity in juvenile African catfish, Clarias gariepinus exposed to formulations of glyphosate and paraquat. Sci. Prog. 2021, 104, 1–15. [Google Scholar] [CrossRef]
- Baurand, P.E.; Capelli, N.; de Vaufleury, A. Genotoxicity assessment of pesticides on terrestrial snail embryos by analysis of random amplified polymorphic DNA profiles. J. Hazard. Mat. 2015, 298, 320–327. [Google Scholar] [CrossRef]
- Braz-Mota, S.; Sadauskas-Henrique, H.; Duarte, R.M.; Val, A.L.; Almeida-Val, V.M. Roundup(R) exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere 2015, 135, 53–60. [Google Scholar] [CrossRef]
- Burella, P.M.; Simoniello, M.F.; Poletta, G.L. Evaluation of Stage-Dependent Genotoxic Effect of Roundup((R)) (Glyphosate) on Caiman latirostris Embryos. Arch. Environ. Contam. Toxicol. 2017, 72, 50–57. [Google Scholar] [CrossRef]
- Burella, P.M.; Odetti, L.M.; Simoniello, M.F.; Poletta, G.L. Oxidative damage and antioxidant defense in Caiman latirostris (Broad-snouted caiman) exposed in ovo to pesticide formulations. Ecotoxicol. Environ. Saf. 2018, 161, 437–443. [Google Scholar] [CrossRef]
- Carvalho, W.F.; Ruiz de Arcaute, C.; Perez-Iglesias, J.M.; Laborde, M.R.R.; Soloneski, S.; Larramendy, M.L. DNA damage exerted by mixtures of commercial formulations of glyphosate and imazethapyr herbicides in Rhinella arenarum (Anura, Bufonidae) tadpoles. Ecotoxicology 2019, 28, 367–377. [Google Scholar] [CrossRef]
- Carvalho, W.F.; Ruiz de Arcaute, C.; Torres, L.; de Melo, E.S.D.; Soloneski, S.; Larramendy, M.L. Genotoxicity of mixtures of glyphosate with 2,4-dichlorophenoxyacetic acid chemical forms towards Cnesterodon decemmaculatus (Pisces, Poeciliidae). Environ. Sci. Pollut. Res. Int. 2020, 27, 6515–6525. [Google Scholar] [CrossRef]
- Silva, G.S.d.; Matos, L.V.d.; Freitas, J.O.d.S.; Campos, D.F.d.; Almeida e Val, V.M.F.d. Gene expression, genotoxicity, and physiological responses in an Amazonian fish, Colossoma macropomum (CUVIER 1818), exposed to Roundup® and subsequent acute hypoxia. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 49–58. [Google Scholar] [CrossRef]
- Santo, G.D.; Grotto, A.; Boligon, A.A.; Da Costa, B.; Rambo, C.L.; Fantini, E.A.; Sauer, E.; Lazzarotto, L.M.V.; Bertoncello, K.T.; Junior, O.T.; et al. Protective effect of Uncaria tomentosa extract against oxidative stress and genotoxicity induced by glyphosate-Roundup(R) using zebrafish (Danio rerio) as a model. Environ. Sci. Pollut. Res. Int. 2018, 25, 11703–11715. [Google Scholar] [CrossRef]
- De Melo, M.S.; Nazari, E.M.; Müller, Y.M.R.; Gismondi, E. Modulation of antioxidant gene expressions by Roundup® exposure in the decapod Macrobrachium potiuna. Ecotoxicol. Environ. Saf. 2020, 190, 110086. [Google Scholar] [CrossRef]
- De Moura, F.R.; da Silva Lima, R.R.; da Cunha, A.P.S.; da Costa Marisco, P.; Aguiar, D.H.; Sugui, M.M.; Sinhorin, A.P.; Sinhorin, V.D.G. Effects of glyphosate-based herbicide on pintado da Amazonia: Hematology, histological aspects, metabolic parameters and genotoxic potential. Environ. Toxicol. Pharmacol. 2017, 56, 241–248. [Google Scholar] [CrossRef]
- De Oliveira, F.G.; Lirola, J.R.; Salgado, L.D.; de Marchi, G.H.; Mela, M.; Padial, A.A.; Guimarães, A.T.B.; Cestari, M.M.; Silva de Assis, H.C. Toxicological effects of anthropogenic activities in Geophagus brasiliensis from a coastal river of southern Brazil: A biomarker approach. Sci. Total Environ. 2019, 667, 371–383. [Google Scholar] [CrossRef]
- De Oliveira, J.S.P.; Vieira, L.G.; Carvalho, W.F.; de Souza, M.B.; de Lima Rodrigues, A.S.; Simões, K.; de Melo De Silva, D.; dos Santos Mendonça, J.; Hirano, L.Q.L.; Santos, A.L.Q.; et al. Mutagenic, genotoxic and morphotoxic potential of different pesticides in the erythrocytes of Podocnemis expansa neonates. Sci. Total Environ. 2020, 737, 140304. [Google Scholar] [CrossRef]
- Herek, J.S.; Vargas, L.; Trindade, S.A.R.; Rutkoski, C.F.; Macagnan, N.; Hartmann, P.A.; Hartmann, M.T. Can environmental concentrations of glyphosate affect survival and cause malformation in amphibians? Effects from a glyphosate-based herbicide on Physalaemus cuvieri and P. gracilis (Anura: Leptodactylidae). Environ. Sci. Pollut. Res. 2020, 27, 22619–22630. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, X.; Huang, Y.; Yan, G.; Cheng, Y. Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide Roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere 2018, 210, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kumar, V.; Srivastava, A.; Saxena, G.; Verma, P.C. Biomarker-based evaluation of cytogenotoxic potential of glyphosate in Vigna mungo (L.) Hepper genotypes. Environ. Monit. Assess. 2021, 193, 73. [Google Scholar] [CrossRef] [PubMed]
- Lajmanovich, R.C.; Peltzer, P.M.; Attademo, A.M.; Martinuzzi, C.S.; Simoniello, M.F.; Colussi, C.L.; Cuzziol Boccioni, A.P.; Sigrist, M. First evaluation of novel potential synergistic effects of glyphosate and arsenic mixture on Rhinella arenarum (Anura: Bufonidae) tadpoles. Heliyon 2019, 5, e02601. [Google Scholar] [CrossRef] [PubMed]
- Lopez Gonzalez, E.C.; Larriera, A.; Siroski, P.A.; Poletta, G.L. Micronuclei and other nuclear abnormalities on Caiman latirostris (Broad-snouted caiman) hatchlings after embryonic exposure to different pesticide formulations. Ecotoxicol. Environ. Saf. 2017, 136, 84–91. [Google Scholar] [CrossRef]
- López González, E.C.; Siroski, P.A.; Poletta, G.L. Genotoxicity induced by widely used pesticide binary mixtures on Caiman latirostris (broad-snouted caiman). Chemosphere 2019, 232, 337–344. [Google Scholar] [CrossRef]
- Lopez Gonzalez, E.C.; Odetti, L.M.; Latorre, M.A.; Avila, O.B.; Contini, L.E.; Siroski, P.A.; Poletta, G.L. A comprehensive approach using multiple biomarkers to detect damage induced by pesticides in broad-snouted caiman (Caiman latirostris) under ex-situ conditions. Heliyon 2022, 8, e08667. [Google Scholar] [CrossRef]
- Martins, A.W.S.; Silveira, T.L.R.; Remião, M.H.; Domingues, W.B.; Dellagostin, E.N.; Junior, A.S.V.; Corcini, C.D.; Costa, P.G.; Bianchini, A.; Somoza, G.M.; et al. Acute exposition to Roundup Transorb® induces systemic oxidative stress and alterations in the expression of newly sequenced genes in silverside fish (Odontesthes humensis). Environ. Sci. Pollut. Res. Int. 2021, 28, 65127–65139. [Google Scholar] [CrossRef]
- Odetti, L.M.; Lopez Gonzalez, E.C.; Romito, M.L.; Simoniello, M.F.; Poletta, G.L. Genotoxicity and oxidative stress in Caiman latirostris hatchlings exposed to pesticide formulations and their mixtures during incubation period. Ecotoxicol. Environ. Saf. 2020, 193, 110312. [Google Scholar] [CrossRef]
- Pavan, F.A.; Samojeden, C.G.; Rutkoski, C.F.; Folador, A.; Da Fré, S.P.; Müller, C.; Hartmann, P.A.; Hartmann, M.T. Morphological, behavioral and genotoxic effects of glyphosate and 2,4-D mixture in tadpoles of two native species of South American amphibians. Environ. Toxicol. Pharmacol. 2021, 85, 103637. [Google Scholar] [CrossRef]
- Pereira, A.G.; Jaramillo, M.L.; Remor, A.P.; Latini, A.; Davico, C.E.; da Silva, M.L.; Muller, Y.M.R.; Ammar, D.; Nazari, E.M. Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere 2018, 209, 353–362. [Google Scholar] [CrossRef]
- Santovito, A.; Audisio, M.; Bonelli, S. A micronucleus assay detects genotoxic effects of herbicide exposure in a protected butterfly species. Ecotoxicology 2020, 29, 1390–1398. [Google Scholar] [CrossRef]
- Schaumburg, L.G.; Siroski, P.A.; Poletta, G.L.; Mudry, M.D. Genotoxicity induced by Roundup® (Glyphosate) in tegu lizard (Salvator merianae) embryos. Pestic. Biochem. Physiol. 2016, 130, 71–78. [Google Scholar] [CrossRef]
- Soloneski, S.; Ruiz de Arcaute, C.; Larramendy, M.L. Genotoxic effect of a binary mixture of dicamba- and glyphosate-based commercial herbicide formulations on Rhinella arenarum (Hensel, 1867) (Anura, Bufonidae) late-stage larvae. Environ. Sci. Pollut. Res. 2016, 23, 17811–17821. [Google Scholar] [CrossRef]
- Trigueiro, N.S.S.; Gonçalves, B.B.; Dias, F.C.; de Oliveira Lima, E.C.; Rocha, T.L.; Sabóia-Morais, S.M.T. Co-exposure of iron oxide nanoparticles and glyphosate-based herbicide induces DNA damage and mutagenic effects in the guppy (Poecilia reticulata). Environ. Toxicol. Pharmacol. 2021, 81, 103521. [Google Scholar] [CrossRef]
- Vieira, C.E.; Costa, P.G.; Lunardelli, B.; de Oliveira, L.F.; Cabrera Lda, C.; Risso, W.E.; Primel, E.G.; Meletti, P.C.; Fillmann, G.; Martinez, C.B. Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in Southern Brazil. Sci. Total Environ. 2016, 542, 44–56. [Google Scholar] [CrossRef]
- Congur, G. Electrochemical investigation of the interaction of 2,4-D and double stranded DNA using pencil graphite electrodes. Turk. J. Chem. 2021, 45, 600–615. [Google Scholar] [CrossRef]
- Da Silva, N.D.G.; Carneiro, C.E.A.; Campos, E.V.R.; de Oliveira, J.L.; Risso, W.E.; Fraceto, L.F.; Zaia, D.A.M.; Martinez, C.B.R. Interference of goethite in the effects of glyphosate and Roundup® on ZFL cell line. Toxicol. Vitro 2020, 65, 104755. [Google Scholar] [CrossRef]
- Perez-Iglesias, J.M.; Franco-Belussi, L.; Moreno, L.; Tripole, S.; de Oliveira, C.; Natale, G.S. Effects of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ. Sci. Pollut. Res. Int. 2016, 23, 9852–9861. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.C.; Todt, C.E.; Burchfield, S.L.; Pressley, A.S.; Denney, R.D.; Snapp, I.B.; Negga, R.; Traynor, W.L.; Fitsanakis, V.A. Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2018, 57, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Bollani, S.; de Cabo, L.; Chagas, C.; Moretton, J.; Weigandt, C.; de Iorio, A.F.; Magdaleno, A. Genotoxicity of water samples from an area of the Pampean region (Argentina) impacted by agricultural and livestock activities. Environ. Sci. Pollut. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Brito Rodrigues, L.; de Oliveira, R.; Abe, F.R.; Brito, L.B.; Moura, D.S.; Valadares, M.C.; Grisolia, C.K.; de Oliveira, D.P.; de Oliveira, G.A.R. Ecotoxicological assessment of glyphosate-based herbicides: Effects on different organisms. Environ. Toxicol. Chem. 2017, 36, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef]
- Kier, L.D.; Kirkland, D.J. Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit. Rev. Toxicol. 2013, 43, 283–315. [Google Scholar] [CrossRef]
- McClellan, R.O. Evaluating the potential carcinogenic hazard of glyphosate. Crit. Rev. Toxicol. 2016, 46, 1–2. [Google Scholar] [CrossRef]
- Brusick, D.; Aardema, M.; Kier, L.; Kirkland, D.; Williams, G. Genotoxicity Expert Panel review: Weight of evidence evaluation of the genotoxicity of glyphosate, glyphosate-based formulations, and aminomethylphosphonic acid. Crit. Rev. Toxicol. 2016, 46, 56–74. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US). Memo: Glyphosate. Study Summaries for Genotoxicity Studies. 2016. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2016-0385-0098 (accessed on 10 October 2022).
- Bolognesi, C.; Carrasquilla, G.; Volpi, S.; Solomon, K.R.; Marshall, E.J. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: Association to occupational exposure to glyphosate. J. Toxicol. Environ. Health Part A 2009, 72, 986–997. [Google Scholar] [CrossRef]
- Paz-y-Miño, C.; Sánchez, M.E.; Arévalo, M.; Muñoz, M.J.; Witte, T.; De-la-Carrera, G.O.; Leone, P.E. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Genet. Mol. Biol. 2007, 30, 456–460. [Google Scholar] [CrossRef]
- Olorunsogo, O.O. Modification of the transport of protons and Ca2+ ions across mitochondrial coupling membrane by N-(phosphonomethyl)glycine. Toxicology 1990, 61, 205–209. [Google Scholar] [CrossRef]
- Olorunsogo, O.O.; Bababunmi, E.A.; Bassir, O. Effect of glyphosate on rat liver mitochondria in vivo. Bull. Environ. Contam. Toxicol. 1979, 22, 357–364. [Google Scholar] [CrossRef]
- European Commission. Regulations: Commission Implementing Regulation (EU) 2016/1313 of 1 August 2016 amending Implementation Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance glyphosate. Off. J. Eur. Union 2016, 208, 1–3. [Google Scholar]
- Ingaramo, P.; Alarcon, R.; Munoz-de-Toro, M.; Luque, E.H. Are glyphosate and glyphosate-based herbicides endocrine disruptors that alter female fertility? Mol. Cell. Endocrinol. 2020, 518, 110934. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.D.C.; Martinez, C.B.R. Effects of the surfactant polyoxyethylene amine (POEA) on genotoxic, biochemical and physiological parameters of the freshwater teleost Prochilodus lineatus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 165, 83–90. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US). Memo: Review of the Office of Pesticide Programs (OPP) Draft Glyphosate Risk Assessment and the Cancer Assessment Review Committee (CARe) Final Report on the Carcinogenic Potential of Glyphosate. 2015. Available online: https://hh-ra.org/wp-content/uploads/2022/10/ehegy-review-of-OPP-report.pdf (accessed on 10 October 2022).
- Portier, C.J. A comprehensive analysis of the animal carcinogenicity data for glyphosate from chronic exposure rodent carcinogenicity studies. Environ. Health 2020, 19, 18. [Google Scholar] [CrossRef]
- Williams, R.V.; DeMarini, D.M.; Stankowski, L.F.; Escobar, P.A.; Zeiger, E.; Howe, J.; Elespuru, R.; Cross, K.P. Are all bacterial strains required by OECD mutagenicity test guideline TG471 needed? Mutat. Res. Genet. Toxicol. Environ. Mutagen 2019, 848, 503081. [Google Scholar] [CrossRef]
- Walmsley, R.M.; Billinton, N. How accurate is in vitro prediction of carcinogenicity? Br. J. Pharmacol. 2011, 162, 1250–1258. [Google Scholar] [CrossRef]
- Mesnage, R.; Teixeira, M.; Mandrioli, D.; Falcioni, L.; Ducarmon, Q.R.; Zwittink, R.D.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Amiel, C.; et al. Use of Shotgun Metagenomics and Metabolomics to Evaluate the Impact of Glyphosate or Roundup MON 52276 on the Gut Microbiota and Serum Metabolome of Sprague-Dawley Rats. Environ. Health Perspect. 2021, 129, 17005. [Google Scholar] [CrossRef]
- Lowe, D. The Ames Test and the Real World. 2022. Available online: https://www.science.org/content/blog-post/ames-test-and-real-world (accessed on 10 October 2022).
- Benbrook, C.; Perry, M.J.; Belpoggi, F.; Landrigan, P.J.; Perro, M.; Mandrioli, D.; Antoniou, M.N.; Winchester, P.; Mesnage, R. Commentary: Novel strategies and new tools to curtail the health effects of pesticides. Environ. Health 2021, 20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).