Abstract
The search for fungicides of novel classes is the long-standing priority in crop protection due to the continuous development of fungal resistance against currently used types of active compounds. Recently, 4-nitropyrazolin-3-ones were discovered as highly potent fungicides, of which activity was believed to be strongly associated with the presence of a nitro group in the pyrazolone ring. In this paper, a series of 4-substituted pyrazolin-3-ones were synthesized and their fungicidal activity against an important species of phytopathogenic fungi (Venturia inaequalis, Rhizoctonia solani, Fusarium oxysporum, Fusarium moniliforme, Bipolaris sorokiniana, and Sclerotinia sclerotiorum) was tested in vitro. We discovered that 4-mono and 4,4-dihalogenated pyrazolin-3-ones demonstrate fungicidal activity comparable to that of 4-nitropyrazolin-3-ones and other modern fungicides (such as kresoxim methyl). This discovery indicates that NO2 moiety can be replaced by other groups of comparable size and electronic properties without the loss of fungicidal activity and significantly expands the scope of potent new fungicides based on a pyrazolin-3-one fragment.
1. Introduction
In today’s world, the problem of microbial contamination of agricultural crops is critical to providing food for a growing human population [1]. Fungi represent one of the most harmful groups of phytopathogens, which account for up to 80% of crop losses [2,3,4,5]. Moreover, phytopathogenic fungi produce toxic metabolites, which represent a serious risk for public health as food contaminants [6,7,8,9]. Besides, fungal pathogens can cause opportunistic fungal infections in humans and animals [10,11].
Despite active research [12,13,14], the output of new fungicides has been relatively constant for the past 20 years [15]. To date, only a few classes of compounds dominate the market (77% of sales in 2018 [15]): Quinone outside Inhibitors (QoI, strobilurins, C3), De-Methylation Inhibitors (DMI, triazoles and imidazoles, G1) [16], Succinate-dehydrogenase inhibitors (SDHI, C2) [17], Dithiocarbamates (M03), Chloronitriles (M05), Carboxylic Acid Amides (CAA, H5) and Phenyl Amides (PA, A1), which has created the basis for the development of pest resistance [18]. In addition, the use of fungicides with the same mechanism of action both for agricultural needs and in medicine can create conditions for outbreaks of human diseases caused by ant. imycotic-resistant strains of fungi [19,20]. In this regard, the development of novel types of fungicides (First-in-Class compounds) is a necessary and urgent task [21].
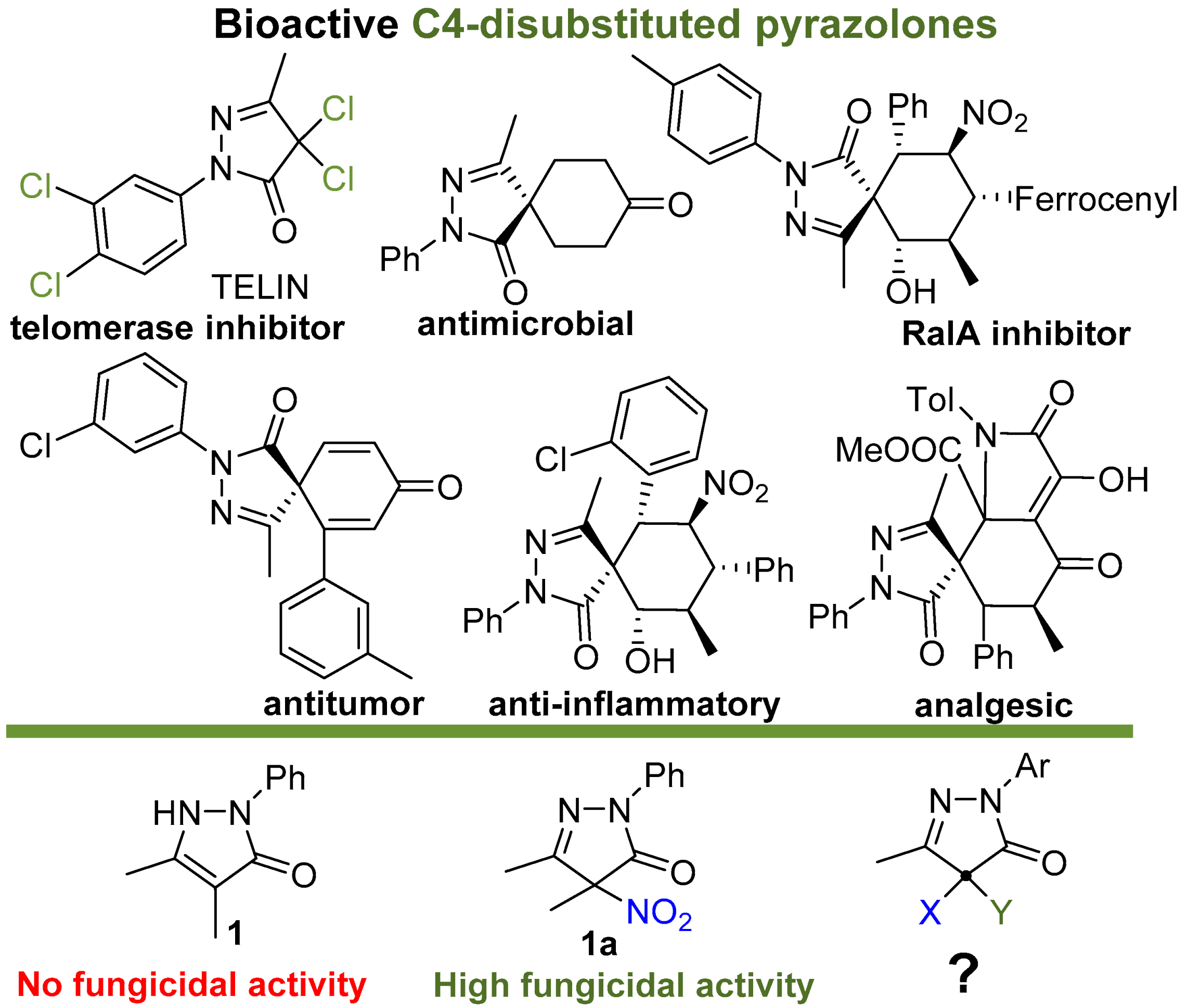
The five-membered pyrazolin-3-one (pyrazolone) ring is a privileged structural motif in medicinal chemistry with a wide range of biological activities [22] (Figure 1). The practical importance of pyrazolones attracts continuous interest to the development of their synthetic methodology and properties [23,24]. The history of the medical use of 4-unsubstituted pyrazolinones began as early as 1887 with the discovery of antipyrine (1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one), one of the first nonopioid analgesics and antipyretics. This discovery prompted the study of pyrazolone derivatives, including C4-monosubstituted pyrazolones, pyrazolone-based Schiff bases, and their metal complexes, which possess anti-inflammatory, antipyretic and analgesic [22,25,26,27], antitumor/cytotoxic [22,25,28,29,30], antimicrobial [22,25,31,32,33], antioxidant [22,26], and protein denaturation inhibiting [27] activities. The interest of medicinal chemists to pyrazolones has been maintained and is increasing at the present time [22]. Recently, C4-disubstituted pyrazolones, primarily spiropyrazolones, have also attracted increasing attention as biologically active compounds [34,35,36]. Due to the wide spectrum of the biological activity of disubstituted pyrazolones, new asymmetric methods for their synthesis are being actively developed [36,37,38,39,40,41]. C4-disubstituted pyrazolones are recognized as valuable antitumor agents [35,42,43,44,45,46], antimicrobial substances [47], inhibitors of trypanosomal phosphodiesterase B1 [48], RalA inhibitors [49], and miticides [50]. However, their potential as effective fungicides has not been expected.
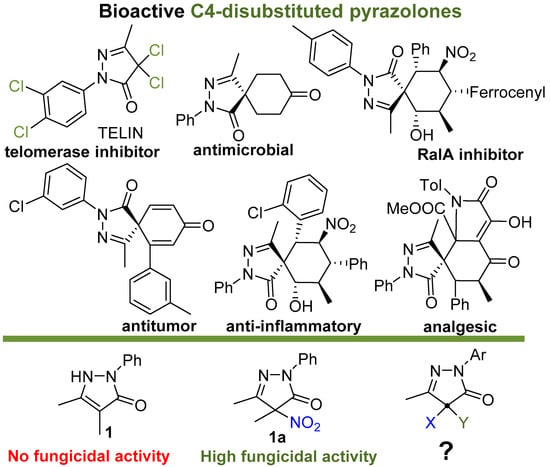
Figure 1.
Selected examples of biologically active C4-disubtituted pyrazolin-3-ones.
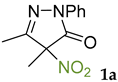
In our previous reports, 4-nitropyrazoline-3-ones were discovered as the novel class of highly potent broad spectrum fungicides [51,52]. However, the understanding of the underlying modes of action was lacking. To shed light on this issue, it is desirable to know how the structure of pyrazolone derivatives affects their fungicidal activity. Our previous study [51] of the structure–activity relationship revealed the importance of the aromatic substituent at N2, a small alkyl substituents at the C4 and C5 atoms of the pyrazolone ring, and the C(sp3)-hybridized C4 atom for high fungicidal activity [51]. Since the unnitrated pyrazolone (1) did not exhibit significant activity [52] (Figure 1), fungicidal properties were believed to be associated with the presence of a nitro group in the pyrazolone ring. In the present work, we aimed to synthesize and study other pyrazolones with a C(sp3)-hybridized C4 atom containing no nitro-group to reveal the role of substituents at this position for the fungicidal activity.
2. Results and Discussion
2.1. The Synthesis of the 4,4-Disubstituted Pyrazolone Derivatives
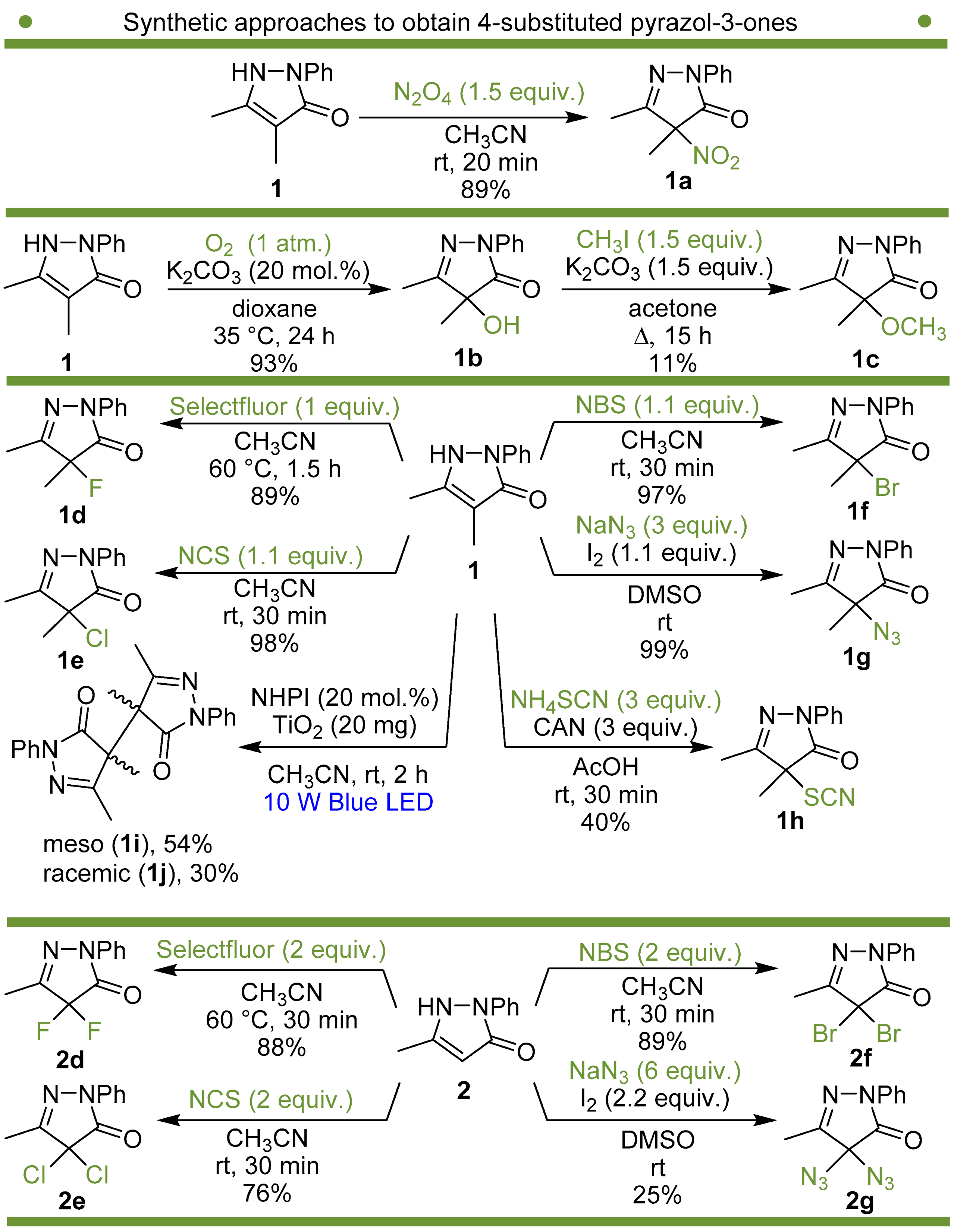
We have synthesized and tested a series of pyrazolones with variable substituent at position 4 instead of the NO2 group to reveal the role of this substituent. The structure of one of the most active nitropyrazolones, 4,5-dimethyl-4-nitro-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (1a, Scheme 1), was used as the reference [51,52].
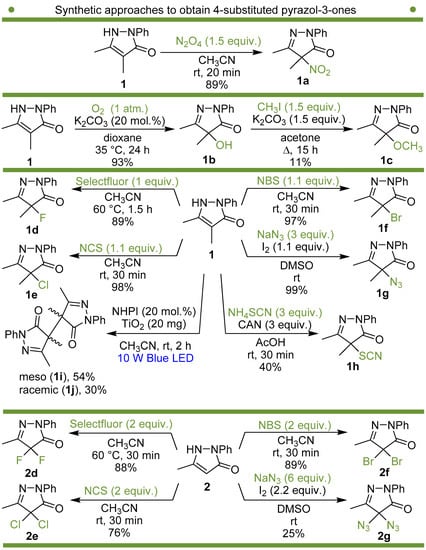
Scheme 1.
Synthetic approaches to 4-substituted pyrazolin-3-ones used in the present study.
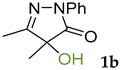
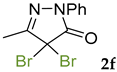
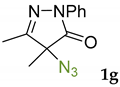
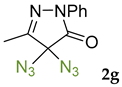
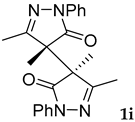
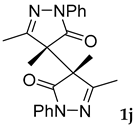
Synthetic approaches to the target compounds 1b–1j and 2d–g are shown in Scheme 1. A previously developed synthetic procedure using N2O4 as a nitrating agent [51] was used for the synthesis of 1a. The advantages of this method are the absence of metal-containing reagents in contrast to the metal salt/NaNO2 system [52], high selectivity, and scalability up to multigram quantities without the yield drop and without the need for chromatographic purification of the target product. 4-Methyl-4-hydroxypyrazolone (1b) was synthesized by aerobic oxidation of 4-methyl-2-phenyl-5-dimethyl-pyrazolin-3-one (1) under basic conditions (K2CO3) [53]. The further methylation step gave 4-methoxypyrazolone (1c). Monohalogenated fluoro-, chloro- and bromopyrazolones (1d–f) were synthesized by the halogenation of pyrazolone 1 with Selectfluor™, N-chlorosuccinimide (NCS), and N-bromosuccinimide (NBS), respectively. It should be noted that these procedures provided up to quantitative yields. 4,4-Dihalogenated pyrazolones (2d–f) were synthesized similarly from 2-phenyl-5-dimethyl-pyrazol-3-one (2) employing two equivalents of halogenating agents. 4-Azido-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (1g) was synthesized with nearly quantitative yield (99%) according to the modified literature procedure [54]. However, the same procedure was less effective for the synthesis of diazide (2g) from 2, where only 25% yield of 2g was obtained. Thiocyanate (1h) was synthesized according to the previously developed procedure for the thiocyanation of the CH-acidic substrates [55]. Dimers (1i and 1j) were synthesized by dehydrogenative dimerization of 1 employing mixed heterogeneous photocatalysis and homogeneous organocatalysis in photooxidative system N-hydroxyphthalimide (NHPI)/TiO2 [56]. The reaction proceeded under air as the terminal oxidant to obtain the mixture of diastereomeric dimers with a total yield of 84%.
2.2. Study of the Fungicidal Activity of 4,4-Disubstituted Pyrazolones
In the next step we tested the fungicidal activity of the synthesized 4-disubstituted 2-phenyl-5-methylpyrazolin-3-ones against six species of phytopathogenic fungi characterized by high impact on crop production: Venturia inaequalis, Rhizoctonia solani, Fusarium oxysporum, Fusarium moniliforme, Bipolaris sorokiniana, and Sclerotinia sclerotiorum. Tests were performed by using the mycelium radial growth inhibition method in Petri dishes at a concentration of 10 mg/L and 5 mg/L in the culture medium. Kresoxim-methyl and triadimefon were used as the reference compounds (Table 1).

Table 1.
In vitro fungicidal activity of the 4-substituted pyrazolin-3-ones *.
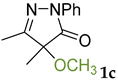
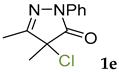
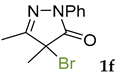
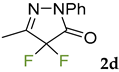
The results of fungicidal tests showed that the nitro group in the fourth position of the pyrazolone ring is not the principal structural element responsible for the manifestation of fungicidal activity, as it was previously assumed [51,52]. The most effective derivatives were revealed: 4-methyl-4-chloropyrazolone (1e), 4-methyl-4-bromopyrazolone (1f), 4,4-dichloropyrazolone (2e), 4,4-dibromopyrazolone (2f) and 4,4-diazidopyrazolone (2g). Fluorinated derivatives (1d and 2d) were moderately active. Monoazide derivative (1g) and thiocyanate (1h) showed low activity. The least active are pyrazolones with hydroxy and methoxy substituents (1b and 1c), as well as dimeric compounds (1i and 1j).
It should be noted that the synthesized C4-disubstituted pyrazolones have a different spectrum of activity than kresoxim-methyl. Pyrazolones 1a, 1d, 1f, 2e, 2f and 2g are more active against F. moniliforme, and 4,4-dibromopyrazolone 2f also showed outstanding activity against S. sclerotiorum, the least affected by kresoxim-methyl, even at concentrations as low as 5 mg/L. Pyrazolones 1a, 1d, 1e, 2e, 2f and 2g showed activity at or above the kresoxim-methyl level against B. sorokiniana, with nitropyrazolone 1a being the most active on the list. Pyrazolones 1a, 1e, 1f, 2e, 2f and 2g have excellent activity against V. inaequalis and R. solani: up to 100% mycelium growth inhibition at a concentration of 10 mg/L or 5 mg/L in the case of dihalogen pyrazolones 2e and 2f. Thus, the newly discovered fungicidal compounds can serve as a useful addition to the current range of agricultural fungicides for the control of fungi that are the least susceptible to existing fungicides.
Most of the previously known biologically active C4-disubstituted pyrazolones, with the exception of TELIN [43], belong to the class of spiro compounds bearing two C—C bonds at C4. Thus, the main structural feature that distinguishes the pyrazolones tested by us from other biologically active C4-disubstituted pyrazolones is the presence of a C4-heteroatom bond, which may be a key aspect for the manifestation of fungicidal activity.
3. Materials and Methods
K2CO3 (98%, extra pure, anhydrous, Thermo Scientific, Waltham, MA, USA), CH3I (99.5%, Acros Organics, Geel, Belgium), Selectfluor (95%, Acros Organics), N-chlorosuccinimide (98%, Acros Organics), N-bromosuccinimide (99%, Acros Organics), NaN3 (99.5%, Acros Organics), I2 (99.8%, Acros Organics), NH4SCN (97.5%, Acros Organics), Ce(NH4)2(NO2)6 (CAN, 98.5%, Thermo Scientific), N-hydroxypthalimide (NHPI, 98%, Acros Organics) were used as is. Hombikat UV 100 (anatase, specific surface area, BET: 300 m2·g−1, primary crystal size according to Scherrer < 10 nm) was used as is. 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 [51] and 4,5-dimethyl-4-nitro-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1a [51] were synthesized according to the literature procedures. CH3CN was distilled over P2O5, acetone was distilled over KMnO4. 1,4-dioxane, DMSO, and glacial AcOH were used as is from commercial sources.
In all experiments, see Supplementary File, RT stands for 22–25 °C. 1H and 13C NMR spectra were recorded on a Bruker AVANCE II 300 and Bruker Fourier 300HD (300.13 and 75.47 MHz, respectively) spectrometers in CDCl3 and DMSO-D6. FT-IR spectra were recorded on Bruker Alpha instrument. High-resolution mass spectra (HR-MS) were measured on a Bruker maXis instrument using electrospray ionization (ESI). The measurements were performed in a positive ion mode (interface capillary voltage—4500 V); mass range from m/z 50 to m/z 3000 Da; external calibration with Electrospray Calibrant Solution (Fluka). A syringe injection was used for all acetonitrile solutions (flow rate 3 µL/min). Nitrogen was applied as a dry gas; the interface temperature was set at 180 °C.
For investigation of fungicidal activity, aseptic polystyrene Petri dishes (90 × 17 mm) were used. All glassware used for addition and mixing of acetone solutions of the tested compounds with agar medium were sterilized before usage. Experiments were performed in a laminar flow cabinet.
3.1. 4-Hydroxy-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1b [57]
4-Hydroxy-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1b was synthesized according to the literature procedure [53]. K2CO3 (20 mol.%, 0.4 mmol, 55 mg) was added to a solution of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (2 mmol, 376 mg) in dioxane (30 mL). Then the flask was evacuated and filled with oxygen three times. The reaction mixture was stirred at 35 °C; for 24 h. Then the solution was evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 3/1 to afford 1b as white crystals (377 mg, 1.85 mmol, 93%). Mp = 111–112 °C (lit. Mp = 113 °C [57]). 1H NMR (300.13 MHz, CDCl3): δ = 7.84 (m, 2H, ArH), 7.38 (m, 2H, ArH), 7.18 (m, 1H, ArH), 4.58 (bs, 1H, OH), 2.18 (s, 3H), 1.53 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 174.8, 163.4, 133.7, 129.0, 125.5, 119.0, 77.4, 22.3, 12.7.
3.2. 4-Methoxy-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1c
CH3I (1.5 mmol, 213 mg) was added to a stirred solution of 4-hydroxy-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1 (1 mmol, 204 mg) and K2CO3 (1.5 mmol, 207 mg) in acetone (10 mL). The reaction mixture was refluxed for 15 h, and the product formation was monitored by TLC (eluent Petroleum Ether/EtOAc = 5/1, Rf = 0.5). Then the reaction mixture was diluted with CH2Cl2 (20 mL) and water (20 mL), the CH2Cl2 layer was separated, and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). Then, all organic extracts were combined, washed with water (2 × 20 mL), and dried over MgSO4. The solvent was rotary evaporated and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 5/1 to afford 1c as a slightly yellow liquid (23 mg, 0.11 mmol, 11%). 1H NMR (300.13 MHz, CDCl3): δ = 7.92 (m, 2H), 7.41 (m, 2H), 7.20 (m, 1H), 3.20 (s, 3H), 2.15 (s, 3H), 1.47 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 171.6, 161.3, 137.9, 129.0, 125.4, 118.6, 82.8, 54.3, 20.7, 13.1. FT-IR (thin layer): νmax = 1596, 1500, 1398, 1364, 1304, 1241, 1136, 1058, 759, 693 cm−1. HR-MS (ESI): m/z = 236.1397, calcd. for C12H14N2O2+NH4+: 236.1394
3.3. 4-Fluoro-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1d
SelectfluorTM (177 mg, 0.5 mmol) was added to a solution of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (94 mg, 0.5 mmol) in CH3CN (5 mL) at 60 °C. The reaction mixture was stirred for 1.5 h at 60 °C, then diluted with water (20 mL) and CH2Cl2 (10 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with brine (20 mL), and dried over MgSO4. Then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the CH2Cl2 eluent to afford 1d as yellow liquid (92 mg, 89%). 1H NMR (300.13 MHz, CDCl3): δ = 7.94–7.80 (m, 2H), 7.46–7.34 (m, 2H), 7.25–7.14 (m, 1H), 2.19 (d, J = 1.6 Hz, 2H), 1.65 (d, J = 23.3 Hz, 2H). 13C NMR (75.47 MHz, CDCl3): δ = 168.2 (d, J = 21.5 Hz), 158.1 (d, J = 16.6 Hz), 137.4, 129.0, 125.5, 118.5, 92.1 (d, J = 191.0 Hz), 18.8 (d, J = 27.0 Hz), 12.66 (d, J = 1.2 Hz). 19F NMR (282.47 MHz, CDCl3): δ = −166.72 (q, J = 23.1 Hz). FT-IR (thin layer): νmax = 1736, 1598, 1501, 1368, 1144, 1117, 758. HR-MS (ESI): m/z = 229.0744, calcd. for C11H11FN2O+Na+: 229.0748
3.4. 4-Chloro-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1e [58]
The solution of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (1 mmol, 188 mg) in CH3CN (20 mL) was added to a solution of N-chlorosuccinimide (NCS, 1.1 mmol, 147 mg) in CH3CN (5 mL) dropwise in 5 min at room temperature. The reaction mixture was stirred for another 30 min, then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 5/1 to afford 1e as white crystals (218 mg, 0.98 mmol, 98%). Mp = 68–69 °C (lit. Mp = 68 °C [58]). 1H NMR (300.13 MHz, CDCl3): δ = 7.89 (m, 2H), 7.41 (m, 2H), 7.21 (m, 1H), 2.24 (s, 3H), 1.77 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 169.8, 159.5, 137.6, 129.1, 125.6, 118.9, 62.7, 22.5, 12.9.
3.5. 4-Bromo-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1f [58]
The solution of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (1 mmol, 188 mg) in CH3CN (20 mL) was added to a solution of N-bromosuccinimide (NBS, 1.1 mmol, 196 mg) in CH3CN (5 mL) dropwise in 5 min at room temperature. The reaction mixture was stirred for another 30 min, then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 10/1 to afford 1f (259 mg, 0.97 mmol, 97%) as yellow crystals. Mp = 81–82 °C (lit. Mp = 83 °C [58]). 1H NMR (300 MHz, Chloroform-d) δ 7.93–7.86 (m, 2H), 7.45–7.37 (m, 2H), 7.25–7.17 (m, 1H), 2.29 (s, 3H), 1.87 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 170.3, 159.3, 137.7, 129.1, 125.6, 118.8, 52.7, 22.5, 13.2. FT-IR (thin layer): νmax = 1716, 1621, 1594, 1442, 1397, 1366, 1304, 1129, 767, 696, 635, 574, 511 cm−1. HR-MS (ESI): m/z = 268.0515, calcd. for C11H11BrN2O + H: 268.0506.
3.6. 4,4-Difluoro-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 2d [59]
Selectfluor (354 mg, 1 mmol) was added to a solution of 5-methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 2 (0.5 mmol, 87 mg) in CH3CN (5 mL) under heating (60 °C). The reaction mixture was stirred at 60 °C for 30 min. Then the reaction mixture was diluted with water (20 mL) and CH2Cl2 (10 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with brine (20 mL), and dried over MgSO4. Then the solvent was rotary evaporated to afford 2d as a yellow liquid (93 mg, 88%). 1H NMR (300.13 MHz, CDCl3): δ = 7.87–7.68 (m, 2H), 7.44–7.30 (m, 2H), 7.25–7.15 (m, 1H), 2.23 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 159.2 (t, J = 29.9 Hz), 152.0 (t, J = 23.0 Hz), 136.8, 129.2, 126.3, 118.6, 108.2 (t, J = 256.6 Hz), 11.8. 19F NMR (282.39 MHz, CDCl3): δ = -123.1. FT-IR (thin layer): νmax = 1751, 1597, 1501, 1255, 1148, 1114, 756. HR-MS (ESI): m/z = 211.0684, calcd. for C10H8F2N2O + H+: 211.0677.
3.7. 4,4-Dichloro-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 2e [60]
N-chlorosuccinimide (NCS, 2 mmol, 267 mg) was added in portions to a solution of 5-methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 2 (1 mmol, 174 mg) in CH3CN (10 mL). The reaction mixture was stirred at room temperature for 30 min, then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the CH2Cl2 eluent to afford 2e as yellow crystals (185 mg, 0.76 mmol, 76%). Mp = 61–62 °C (lit. Mp = 61.5–63°C [60]). 1H NMR (300.13 MHz, CDCl3): δ = 7.93–7.81 (m, 2H), 7.52–7.41 (m, 2H), 7.33–7.20 (m, 1H), 2.39 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 164.1, 155.6, 137.0, 129.2, 126.3, 119.0, 73.5, 12.2. FT-IR (thin layer): νmax = cm−1. HR-MS (ESI): m/z = 243.0085, calcd. for C10H11Cl2N2O + H+: 243.0086.
3.8. 4,4-Dibromo-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 2f [61]
N-bromosuccinimide (NBS, 2 mmol, 356 mg) was added in portions to a solution of 5-methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 2 (1 mmol, 174 mg) in CH3CN (10 mL). The reaction mixture was stirred at room temperature for 30 min, then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using eluent Petroleum Ether/EtOAc = 10/1 to afford 2f as yellow crystals (297 mg, 0.89 mmol, 89%). Mp = 76–78 °C (lit. Mp = 80–82 °C [61]). 1H NMR (300.13 MHz, CDCl3): δ = 7.93–7.82 (m, 2H), 7.49–7.36 (m, 2H), 7.33–7.19 (m, 1H), 2.44 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 165.3, 156.1, 137.1, 129.2, 126.2, 119.0, 46.2, 13.3. FT-IR (thin layer): νmax = 1722, 1592, 1491, 1364, 1283, 948, 804, 765, 694, 659, 632, 510 cm−1. HR-MS (ESI): m/z = 330.9068, calcd. for C10H8Br2N2O + H: 330.9076.
3.9. 4-Azido-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1g
4-azido-4,5-dimethyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 1g was synthesized according to the modified literature procedure [54]. I2 (1.1 mmol, 279 mg) was added to a suspension of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (1 mmol, 188 mg) and NaN3 (3 mmol, 195 mg) in DMSO (10 mL). The reaction mixture was stirred at room temperature, and the formation of the product was monitored by TLC (eluent Petroleum Ether/EtOAc = 5/1, Rf = 0.6). Then the reaction mixture was diluted with Na2S2O3 solution (0.1 M, 30 mL) and CH2Cl2 (10 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with water (2 × 20 mL), and dried over MgSO4. Then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 5/1 to afford 1g as a colorless liquid (226 mg, 0.99 mmol, 99%). 1H NMR (300.13 MHz, CDCl3): δ = 7.88 (m, 2H), 7.41 (m, 2H), 7.21 (m, 1H), 2.14 (s, 3H), 1.63 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 170.7, 159.6, 137.6, 129.1, 125.6, 118.8, 66.0, 18.7, 13.2. FT-IR (thin layer): νmax = 2109, 1719, 1597, 1501, 1399, 1366, 1312, 1246, 1137, 756, 692 cm−1. HR-MS (ESI): m/z = 252.0860, calcd. for C11H11N5O+Na+: 252.0856.
3.10. 4,4-Diazido-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 2g [54]
4,4-diazido-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 2g was synthesized according to the literature procedure [54]. I2 (2.2 mmol, 558 mg) was added to a suspension of 5-methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 2 (1 mmol, 174 mg) and NaN3 (6 mmol, 390 mg) in DMSO (10 mL). The reaction mixture was stirred at room temperature, and the formation of the product was monitored by TLC (eluent Petroleum Ether/EtOAc = 20/1, Rf = 0.5). Then the reaction mixture was diluted with Na2S2O3 solution (0.1 M, 30 mL) and CH2Cl2 (10 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with water (2 × 20 mL), and dried over MgSO4. Then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 20/1 to afford 2g as brown liquid (62 mg, 0.25 mmol, 25%). 1H NMR (300 MHz, Chloroform-d) δ 7.96–7.85 (m, 2H), 7.53–7.39 (m, 2H), 7.34–7.21 (m, 1H), 2.18 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 163.9, 155.6, 136.8, 129.2, 126.2, 118.8, 13.0.
3.11. 4,5-Dimethyl-2-phenyl-4-thiocyanato-2,4-dihydro-3H-pyrazol-3-one 1h [55]
4,5-dimethyl-2-phenyl-4-thiocyanato-2,4-dihydro-3H-pyrazol-3-one 1h was synthesized according to the literature procedure [55]. Ce(NH4)2(NO2)6 (CAN, 3 mmol, 1644 mg) was added in portions to a solution of 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (1 mmol, 188 mg) and NH4SCN (3 mmol, 228 mg) in AcOH (10 mL) within 30 min. The reaction mixture was stirred at room temperature, and the formation of the product was monitored by TLC (eluent Petroleum Ether/EtOAc = 5/1). Then the reaction mixture was diluted with CH2Cl2 (20 mL) and water (20 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with water (2 × 20 mL), and dried over MgSO4. Then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent Petroleum Ether/EtOAc = 5/1 to afford 1h as slightly yellow crystals (97 mg, 0.40 mmol, 40%). Mp = 99–100 °C (lit. Mp = 97–99 °C [55]). 1H NMR (300.13 MHz, CDCl3): δ = 7.92 (m, 2H), 7.48 (m, 2H), 7.29 (m 1H), 2.35 (s, 3H), 1.76 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 169.4, 157.6, 137.1, 129.1, 126.1, 119.3, 107.2, 56.8, 18.7, 13.4. FT-IR (thin layer): νmax = cm−1. HR-MS (ESI): m/z = 268.0506, calcd. for C12H11N3OS+Na+: 268.0515.
3.12. 4,4’,5,5’-Tetramethyl-2,2’-diphenyl-2,2’,4,4’-tetrahydro-3H,3’H-[4,4’-bipyrazole]-3,3’-dione, meso 1i and racemic 1j [62]
Dimers were synthesized according to the modified literature procedure [56]. To a 50 mL round-bottomed flask 4,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one 1 (1 mmol, 188 mg), TiO2 Hombikat UV 100 (20 mg), N-hydroxyphthalimide (20 mol%, 0.2 mmol, 32.6 mg) and a solvent (CH3CN, 2 mL) were placed. The resulting suspension was sonicated for 1 min. The stirred reaction mixture was irradiated with 10 W Blue LED (443 nm) at room temperature until full conversion of 1 (2 h, monitored by TLC, eluent CH2Cl2). Upon completion, the reaction mixture was diluted with CH2Cl2 (20 mL) and water (20 mL). The CH2Cl2 layer was separated and the water layer was additionally extracted with CH2Cl2 (2 × 10 mL). All organic extracts were combined, washed with NaHCO3 saturated solution (20 mL) and water (20 mL), and dried over MgSO4. Then the solvent was rotary evaporated, and the residue was purified by column chromatography on silica gel using the eluent CH2Cl2/EtOAc = 40/1 to afford diastereomeric dimers 1i (54%, 0.27 mmol, 100 mg) and 1j (30%, 0.15 mmol, 56 mg) as white crystals.
Meso-3,3’,4,4’-tetramethyl-1,1’-diphenyl-[4,4’-bipyrazol]-5,5’-dione, 1i Mp = 161–162 °C (Lit. Mp = 163–164 °C [62]); 1H NMR (300.13 MHz, CDCl3): δ = 7.93–7.87 (m, 2H), 7.46–7.38 (m, J = 10.8, 5.1 Hz, 2H), 7.22 (t, J = 7.4 Hz, 1H), 1.93 (s, 6H), 1.73 (s, 6H); 13C NMR (75.47 MHz, CDCl3): δ = 173.1, 161.9, 137.6, 129.1, 125.7, 119.0, 54.4, 14.7, 14.6.
Racemic-3,3’,4,4’-tetramethyl-1,1’-diphenyl-[4,4’-bipyrazol]-5,5’-dione, 1j Mp = 141–142 °C (Lit. Mp = 140–141 °C [62]); 1H NMR (300.13 MHz, CDCl3): δ = 7.85 (d, J = 7.9 Hz, 4H), 7.45–7.31 (m, 4H), 7.18 (t, J = 7.3 Hz, 2H), 2.19 (s, 6H), 1.60 (s, 6H); 13C NMR (75.47 MHz, CDCl3): δ = 173.1, 159.8, 137.7, 129.0, 125.4, 119.3, 55.7, 16.0, 15.4.
3.13. Investigation of Fungicidal Activity (Table 1)
The fungicidal activity was investigated according to a standard procedure [63,64,65,66,67,68,69]. The strains for fungicidal studies were obtained from the working collection of the All-Russian Research Institute for Phytopathology (B. Vyazemy, Moscow reg., Russia): Venturia inaequalis (V.i.) MRA-16-2, Rhizoctonia solani (R.s.) 100063, Fusarium oxysporum (F.o.) FO-8, Fusarium moniliforme (F.m.) 100146, Bipolaris sorokiniana (B.s.) MRB(V)-1, Sclerotinia sclerotiorum (S.s.) 100033. The test substances preliminarily dissolved in acetone (concentration 1 or 0.5 mg mL−1) were introduced into liquid potato sugar agar having a temperature of 50–55 °C so that the final concentration of the substance in the nutrient medium was 10 mg L−1 or 5 mg L−1 (0.9 mL of solution in acetone per 90 mL of agar). After mixing, the agar was poured into sterile Petri dishes and cooled to room temperature. Pieces of mycelium from the peripheral growth zone of mycelium culture incubated for 3–5 days were transferred to Petri dishes with diluted tested compounds using a needle. The control was a colony grown in the same nutrient medium without the addition of the active substance (acetone without substance was added). After inoculation for 72 h, the diameters of the formed fungal colonies were measured. The indicator of fungicidal activity was the suppression of mycelium growth in comparison with the control, calculated as [(Dc−Ds)/Dc] × 100 %, in which Dc is the diameter of the colony of fungus in the control medium and Ds is the diameter of the colony in the medium with the test substance added.
4. Conclusions
Pronounced broad spectrum fungicidal activity was found to be characteristic for a wide range of C4-disubstituted pyrazolin-3-ones. This discovery shows that 4-nitropyrazolin-3-ones, previously reported as a novel class of fungicides, are actually the subgroup of a more diverse type of fungicidal structures, which can be explored further. Currently, the most active compounds are 4,4-dichloro-, 4,4-dibromo pyrazolin-3-ones 2e and 2f. 4-Methyl-4-chloro-, 4-methyl-4-bromo- and 4,4-diazidopyrazolones showed somewhat lower activity. For the most active structures, efficient synthesis procedures have been proposed that allow for obtaining substances with high to quantitative yields. The ease of synthesis, the availability of reagents, and high fungicidal activity comparable to that of commercial fungicides, make the discovered 4-disubstituted pyrazolin-3-ones attractive candidates for the role of a new class of fungicidal compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agrochemicals2010004/s1, 1H and 13C NMR spectra of the synthesized compounds, FT-IR and HRMS data for new compounds.
Author Contributions
Conceptualization, I.B.K. and A.O.T.; investigation, E.R.L., A.S.B., A.L.A. and I.B.K.; writing—original draft preparation, E.R.L.; writing—review and editing, I.B.K., A.S.B. and A.O.T.; supervision, A.I.I., A.P.G. and A.O.T.; project administration, A.I.I. and A.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 19-73-20190.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rauzan, B.M.; Lorsbach, B.A. Designing Sustainable Crop Protection Actives. In ACS Symposium Series; Rauzan, B.M., Lorsbach, B.A., Eds.; American Chemical Society: Washington, DC, USA, 2021; Volume 1390, pp. 1–9. ISBN 978-0-8412-9821-7. [Google Scholar]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Willocquet, L.; Meza, W.R.; Dumont, B.; Klocke, B.; Feike, T.; Kersebaum, K.C.; Meriggi, P.; Rossi, V.; Ficke, A.; Djurle, A.; et al. An Outlook on Wheat Health in Europe from a Network of Field Experiments. Crop Prot. 2021, 139, 105335. [Google Scholar] [CrossRef]
- Goudarzi, A.; Bagheri, A.; Hajebi, A. Aspergillus Niger Causes Black Mould Disease on Piarom Dates, the Most Economically Valuable Export Date Cultivar in Southern Iran. Crop Prot. 2022, 160, 106047. [Google Scholar] [CrossRef]
- Ayofemi Olalekan Adeyeye, S. Aflatoxigenic Fungi and Mycotoxins in Food: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 709–721. [Google Scholar] [CrossRef]
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem. In Mycotoxigenic Fungi; Moretti, A., Susca, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1542, pp. 3–12. ISBN 978-1-4939-6705-6. [Google Scholar]
- Iqbal, S.Z. Mycotoxins in Food, Recent Development in Food Analysis and Future Challenges; a Review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Imade, F.; Ankwasa, E.M.; Geng, H.; Ullah, S.; Ahmad, T.; Wang, G.; Zhang, C.; Dada, O.; Xing, F.; Zheng, Y.; et al. Updates on Food and Feed Mycotoxin Contamination and Safety in Africa with Special Reference to Nigeria. Mycology 2021, 12, 245–260. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A Global View on Fungal Infections in Humans and Animals: Opportunistic Infections and Microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113. [Google Scholar] [CrossRef]
- van Rhijn, N.; Bromley, M. The Consequences of Our Changing Environment on Life Threatening and Debilitating Fungal Diseases in Humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef]
- Stierli, D.; Eberle, M.; Lamberth, C.; Jacob, O.; Balmer, D.; Gulder, T. Quarternary α-Cyanobenzylsulfonamides: A New Subclass of CAA Fungicides with Excellent Anti-Oomycetes Activity. Bioorg. Med. Chem. 2021, 30, 115965. [Google Scholar] [CrossRef]
- Winter, C.; Fehr, M. Discovery of the Trifluoromethyloxadiazoles—A New Class of Fungicides with a Novel Mode-of-Action. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 401–423. ISBN 978-0-12-821035-2. [Google Scholar]
- Yan, X.; Chen, S.; Sun, W.; Zhou, X.; Yang, D.; Yuan, H.; Wang, D. Primary Mode of Action of the Novel Sulfonamide Fungicide against Botrytis Cinerea and Field Control Effect on Tomato Gray Mold. Int. J. Mol. Sci. 2022, 23, 1526. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Crop Protection Compounds—Trends and Perspective. Pest Manag. Sci. 2021, 77, 3608–3616. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of Triazole Fungicides on Soil Microbiota and on the Activities of Enzymes Found in Soil: A Review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The Research Progress in and Perspective of Potential Fungicides: Succinate Dehydrogenase Inhibitors. Bioorg. Med. Chem. 2021, 50, 116476. [Google Scholar] [CrossRef]
- Thind, T.S. New Insights into Fungicide Resistance: A Growing Challenge in Crop Protection. Indian Phytopathol. 2022, 75, 927–939. [Google Scholar] [CrossRef]
- Ishii, H.; Bryson, P.K.; Kayamori, M.; Miyamoto, T.; Yamaoka, Y.; Schnabel, G. Cross-Resistance to the New Fungicide Mefentrifluconazole in DMI-Resistant Fungal Pathogens. Pestic. Biochem. Physiol. 2021, 171, 104737. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Goldman, G.H.; Santos, D.A. Fungicide Effects on Human Fungal Pathogens: Cross-Resistance to Medical Drugs and Beyond. PLOS Pathog. 2021, 17, e1010073. [Google Scholar] [CrossRef]
- Thind, T.S. Changing Trends in Discovery of New Fungicides: A Perspective. Indian Phytopathol. 2021, 74, 875–883. [Google Scholar] [CrossRef]
- Mustafa, G.; Zia-ur-Rehman, M.; Sumrra, S.H.; Ashfaq, M.; Zafar, W.; Ashfaq, M. A Critical Review on Recent Trends on Pharmacological Applications of Pyrazolone Endowed Derivatives. J. Mol. Struct. 2022, 1262, 133044. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.K.; Jasiński, R. Experimental and Theoretical Mechanistic Study on the Thermal Decomposition of 3,3-Diphenyl-4-(Trichloromethyl)-5-Nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef] [PubMed]
- Mandha, S.R.; Siliveri, S.; Alla, M.; Bommena, V.R.; Bommineni, M.R.; Balasubramanian, S. Eco-Friendly Synthesis and Biological Evaluation of Substituted Pyrano[2,3-c]Pyrazoles. Bioorg. Med. Chem. Lett. 2012, 22, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Pasin, J.S.M.; Ferreira, A.P.O.; Saraiva, A.L.L.; Ratzlaff, V.; Andrighetto, R.; Machado, P.; Marchesan, S.; Zanette, R.A.; Bonacorso, H.G.; Zanatta, N.; et al. Antipyretic and Antioxidant Activities of 5-Trifluoromethyl-4,5-Dihydro-1H-Pyrazoles in Rats. Braz. J. Med. Biol. Res. 2010, 43, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.; Shastri, L.A.; Gudimani, P.; Joshi, S.; Sunagar, V. Synthesis, Characterization and Molecular Docking of Novel Lonazolac Analogues 3-(3-Hydroxy-5-Methyl-1H-Pyrazol-4-Yl)-3-Arylpropanoic Acid Derivatives: Highly Potential COX-1/COX-2, Matrix Metalloproteinase and Protein Denaturation Inhibitors. J. Mol. Struct. 2022, 1260, 132782. [Google Scholar] [CrossRef]
- Mingoia, F.; Panzeca, G.; Vitale, M.C.; Monica, G.L.; Bono, A.; Lauria, A.; Martorana, A. One Pot-like Regiospecific Access to 1-Aryl-1H-Pyrazol-3(2H)-One Derivatives and Evaluation of the Anticancer Activity. Arkivoc 2022, 2022, 191–203. [Google Scholar] [CrossRef]
- Marković, V.; Erić, S.; Juranić, Z.D.; Stanojković, T.; Joksović, L.; Ranković, B.; Kosanić, M.; Joksović, M.D. Synthesis, Antitumor Activity and QSAR Studies of Some 4-Aminomethylidene Derivatives of Edaravone. Bioorganic Chem. 2011, 39, 18–27. [Google Scholar] [CrossRef]
- Kshatriya, R.; Shelke, P.; Mali, S.; Yashwantrao, G.; Pratap, A.; Saha, S. Synthesis and Evaluation of Anticancer Activity of Pyrazolone Appended Triarylmethanes (TRAMs). ChemistrySelect 2021, 6, 6230–6239. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, R.; Dua, N.; Sharma, C.; Pundeer, R. Some New Pyrazolyl Pyrazolones and Cyanopyrazolyl Acrylates: Design, Synthesis and Biological Evaluation. ChemistrySelect 2019, 4, 6849–6853. [Google Scholar] [CrossRef]
- Rasapalli, S.; Fan, Y.; Yu, M.; Rees, C.; Harris, J.T.; Golen, J.A.; Jasinski, J.P.; Rheingold, A.L.; Kwasny, S.M.; Opperman, T.J. Detour of Prenostodione Synthesis towards Pyrazolones for Antibacterial Activity. Bioorg. Med. Chem. Lett. 2013, 23, 3235–3238. [Google Scholar] [CrossRef]
- Alam, F.; Amin, R. Synthesis and Pharmacological Activity of Some Pyrazolone Derivatives. J. Pharm. Res. Int. 2020, 9, 46–55. [Google Scholar] [CrossRef]
- Xie, X.; Xiang, L.; Peng, C.; Han, B. Catalytic Asymmetric Synthesis of Spiropyrazolones and Their Application in Medicinal Chemistry. Chem. Rec. 2019, 19, 2209–2235. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Ni, T.; Shi, W.; Guo, Y.; Li, K.; Shi, A.; Wu, S.; Sheng, C. Discovery of Pyrazolone Spirocyclohexadienone Derivatives with Potent Antitumor Activity. Bioorg. Med. Chem. Lett. 2020, 30, 126662. [Google Scholar] [CrossRef]
- Carceller-Ferrer, L.; Blay, G.; Pedro, J.R.; Vila, C. Recent Advances in Catalytic Enantioselective Synthesis of Pyrazolones with a Tetrasubstituted Stereogenic Center at the 4-Position. Synthesis 2021, 53, 215–237. [Google Scholar] [CrossRef]
- Li, Z.-F.; He, H.-J.; Wang, R.-H.; Zhou, L.-Y.; Xiao, Y.-C.; Chen, F.-E. Copper-Catalyzed Asymmetric Alkynylation of Pyrazole-4,5-Diones Using Chloramphenicol Base-Derived Hydroxyl Oxazoline Ligands. Org. Chem. Front. 2022, 9, 2792–2796. [Google Scholar] [CrossRef]
- Bao, X.; Wei, S.; Zou, L.; He, Y.; Xue, F.; Qu, J.; Wang, B. Asymmetric Chlorination of 4-Substituted Pyrazolones Catalyzed by Natural Cinchona Alkaloid. Chem. Commun. 2016, 52, 11426–11429. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Enders, D. Asymmetric Synthesis of Pyrazoles and Pyrazolones Employing the Reactivity of Pyrazolin-5-One Derivatives. Chem. Commun. 2015, 51, 12890–12907. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Sun, Z.; Li, P.; Ding, T. Highly Site- and Enantioselective N—H Functionalization ofN -Monosubstituted Aniline Derivatives Affording Pyrazolones Bearing a Quaternary Stereocenter. Chin. J. Chem. 2022, 40, 1144–1148. [Google Scholar] [CrossRef]
- Bao, X.; Wang, B.; Cui, L.; Zhu, G.; He, Y.; Qu, J.; Song, Y. An Organocatalytic Asymmetric Friedel–Crafts Addition/Fluorination Sequence: Construction of Oxindole–Pyrazolone Conjugates Bearing Vicinal Tetrasubstituted Stereocenters. Org. Lett. 2015, 17, 5168–5171. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Sasaki, N.; Satoh-Masuoka, M.; Murofushi, H.; Murakami-Murofushi, K. A Novel Pyrazolone, 4,4-Dichloro-1-(2,4-Dichlorophenyl)-3-Methyl-5-Pyrazolone, as a Potent Catalytic Inhibitor of Human Telomerase. Biochem. Biophys. Res. Commun. 2004, 320, 1351–1358. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Oyama, M.; Nakatake, M.; Okamoto, Y.; Kai, H.; Arima, H.; Murofushi, H.; Murakami-Murofushi, K. Inhibition of Human Tumor Cell Proliferation by the Telomerase Inhibitor TELIN. Cytologia 2010, 75, 177–183. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Xu, G.; Chen, S.; Zhang, Y.; Liu, N.; Dong, G.; Miao, C.; Su, H.; Zhang, W.; et al. Novel Spiropyrazolone Antitumor Scaffold with Potent Activity: Design, Synthesis and Structure–Activity Relationship. Eur. J. Med. Chem. 2016, 115, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, F.-Y.; Kang, J.-W.; Zhou, J.; Peng, C.; Huang, W.; Zhou, M.-K.; He, G.; Han, B. Stereoselective Assembly of Multifunctional Spirocyclohexene Pyrazolones That Induce Autophagy-Dependent Apoptosis in Colorectal Cancer Cells. J. Org. Chem. 2019, 84, 9138–9150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, S.; Wang, S.; Fang, K.; Dong, G.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; et al. Divergent Cascade Construction of Skeletally Diverse “Privileged” Pyrazole-Derived Molecular Architectures: Pyrazole-Derived Molecular Architectures. Eur. J. Org. Chem. 2015, 2015, 2030–2037. [Google Scholar] [CrossRef]
- Chande, M.S.; Barve, P.A.; Suryanarayan, V. Synthesis and Antimicrobial Activity of Novel Spirocompounds with Pyrazolone and Pyrazolthione Moiety. J. Heterocycl. Chem. 2007, 44, 49–53. [Google Scholar] [CrossRef]
- Amata, E.; Bland, N.D.; Campbell, R.K.; Pollastri, M.P. Evaluation of Pyrrolidine and Pyrazolone Derivatives as Inhibitors of Trypanosomal Phosphodiesterase B1 (TbrPDEB1). Tetrahedron Lett. 2015, 56, 2832–2835. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Huang, W.; Haruehanroengra, P.; Peng, C.; Sheng, J.; Han, B.; He, G. Application of Organocatalysis in Bioorganometallic Chemistry: Asymmetric Synthesis of Multifunctionalized Spirocyclic Pyrazolone–Ferrocene Hybrids as Novel RalA Inhibitors. Org. Chem. Front. 2018, 5, 2229–2233. [Google Scholar] [CrossRef]
- Tada, I.; Motoki, M.; Takahashi, N.; Miyata, T.; Takechi, T.; Uchida, T.; Takagi, Y. Synthesis and Structure-Activity Relationships of Miticidal 4,5-Dihydropyrazole-5-Thiones. Pestic. Sci. 1996, 48, 165–173. [Google Scholar] [CrossRef]
- Budnikov, A.S.; Lopat’eva, E.R.; Krylov, I.B.; Segida, O.O.; Lastovko, A.V.; Ilovaisky, A.I.; Nikishin, G.I.; Glinushkin, A.P.; Terent’ev, A.O. 4-Nitropyrazolin-5-Ones as Readily Available Fungicides of the Novel Structural Type for Crop Protection: Atom-Efficient Scalable Synthesis and Key Structural Features Responsible for Activity. J. Agric. Food Chem. 2022, 70, 4572–4581. [Google Scholar] [CrossRef]
- Krylov, I.B.; Budnikov, A.S.; Lopat’eva, E.R.; Nikishin, G.I.; Terent’ev, A.O. Mild Nitration of Pyrazolin-5-ones by a Combination of Fe(NO3)3 and NaNO2: Discovery of a New Readily Available Class of Fungicides, 4-Nitropyrazolin-5-ones. Chem. Eur. J. 2019, 25, 5922–5933. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, J.; Yang, H.; Jiang, G. Tunable Aerobic Oxidative Hydroxylation/Dehydrogenative Homocoupling of Pyrazol-5-Ones under Transition-Metal-Free Conditions. Org. Lett. 2017, 19, 2618–2621. [Google Scholar] [CrossRef]
- Holzschneider, K.; Tong, M.L.; Mohr, F.; Kirsch, S.F. A Synthetic Route Toward Tetrazoles: The Thermolysis of Geminal Diazides. Chem. Eur. J. 2019, 25, 11725–11733. [Google Scholar] [CrossRef]
- Sharipov, M.Y.; Krylov, I.B.; Karpov, I.D.; Vasilkova, O.V.; Oleksiienko, A.-M.V.; Terent’ev, A.O. NaSCN–(NH4)2Ce(NO3)6 System in Heterocycle Thiocyanation: Synthesis of Novel Highly Potent Broad-Spectrum Fungicides for Crop Protection. Chem. Heterocycl. Compd. 2021, 57, 531–537. [Google Scholar] [CrossRef]
- Krylov, I.B.; Lopat’eva, E.R.; Subbotina, I.R.; Nikishin, G.I.; Yu, B.; Terent’ev, A.O. Mixed Hetero-/Homogeneous TiO2/N-Hydroxyimide Photocatalysis in Visible-Light-Induced Controllable Benzylic Oxidation by Molecular Oxygen. Chin. J. Catal. 2021, 42, 1700–1711. [Google Scholar] [CrossRef]
- Veibel, S.; Westöö, G.; Jensen, K.A.; Schønfeldt, E.; Steensgaard, I.; Rosenberg, T. Pyrazole Studies. V. The Oxidation by Air of Some 3,4-Dialkylsubstituted 1-Phenylpyrazolones-5. Acta Chem. Scand. 1953, 7, 119–127. [Google Scholar] [CrossRef]
- Westöö, G.; Virtanen, A.I.; Lunde, K. Studies on Pyrazolones. I. Light Absorption and Constitution of Certain 4-Halo-5-Pyrazolones. Acta Chem. Scand. 1952, 6, 1499–1515. [Google Scholar] [CrossRef]
- Howard, J.L.; Nicholson, W.; Sagatov, Y.; Browne, D.L. One-Pot Multistep Mechanochemical Synthesis of Fluorinated Pyrazolones. Beilstein J. Org. Chem. 2017, 13, 1950–1956. [Google Scholar] [CrossRef]
- Smith, P.A.S.; Breen, G.J.W.; Hajek, M.K.; Awang, D.V.C. Isolation of Primary Decomposition Products of Azides. II. Azidopyrazoles. J. Org. Chem. 1970, 35, 2215–2221. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, H.-C.; Cheng, K.-M.; Su, W.-N.; Sung, K.-C.; Lin, T.-P.; Huang, J.-J.; Lin, S.-K.; Wong, F.F. Efficient Di-Bromination of 5-Pyrazolones and 5-Hydroxypyrazoles by N-Bromobenzamide. Tetrahedron 2009, 65, 9592–9597. [Google Scholar] [CrossRef]
- Veibel, S.; Meza, S.; Haug, A.; Songstad, J.; Pilotti, Å. Pyrazole Studies. XIII. Oxidation by Air of 4-Substituted Pyrazole-5-Ones and Stereochemistry of the Oxidation Products. Acta Chem. Scand. 1972, 26, 3685–3690. [Google Scholar] [CrossRef]
- Ncama, K.; Mditshwa, A.; Tesfay, S.Z.; Mbili, N.C.; Magwaza, L.S. Topical Procedures Adopted in Testing and Application of Plant-Based Extracts as Bio-Fungicides in Controlling Postharvest Decay of Fresh Produce. Crop Prot. 2019, 115, 142–151. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Gong, C.; Jin, H.; Qin, B. Synthesis of N-Substituted Phthalimides and Their Antifungal Activity against Alternaria Solani and Botrytis Cinerea. Microb. Pathog. 2016, 95, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Popkov, S.V.; Kovalenko, L.V.; Bobylev, M.M.; Molchanov, O.Y.; Krimer, M.Z.; Tashchi, V.P.; Putsykin, Y.G. The Synthesis and Fungicidal Activity of 2-Substituted 1-Azol-1-Ylmethyl-6-Arylidenecyclohexanols. Pestic. Sci. 1997, 49, 125–129. [Google Scholar] [CrossRef]
- Xia, D.; Cheng, X.; Liu, X.; Zhang, C.; Wang, Y.; Liu, Q.; Zeng, Q.; Huang, N.; Cheng, Y.; Lv, X. Discovery of Novel Pyrazole Carboxylate Derivatives Containing Thiazole as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 8358–8365. [Google Scholar] [CrossRef] [PubMed]
- Obydennov, K.L.; Kalinina, T.A.; Galieva, N.A.; Beryozkina, T.V.; Zhang, Y.; Fan, Z.; Glukhareva, T.V.; Bakulev, V.A. Synthesis, Fungicidal Activity, and Molecular Docking of 2-Acylamino and 2-Thioacylamino Derivatives of 1H-Benzo[d]Imidazoles as Anti-Tubulin Agents. J. Agric. Food Chem. 2021, 69, 12048–12062. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Anikina, L.V.; Nechaeva, T.V.; Pukhov, S.A.; Karpova, T.B.; Popkov, S.V.; Nelyubina, Y.V.; Kolotyrkina, N.G.; Kravchenko, A.N. Synthesis and Biological Evaluation of New Substituted Thioglycolurils, Their Analogues and Derivatives. Eur. J. Med. Chem. 2017, 140, 141–154. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral Inhibits Mycelial Growth of Penicillium Italicum by a Membrane Damage Mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).