Abstract
Adlay (Coix lacryma-jobi L.) is a cereal crop that has traditionally been used for medicinal purposes. It is processed into nutritious food in China and Southeast Asian countries. This study assesses the phytochemical constituents of this plant and their potential as antioxidants and crop protection agents. The methanolic extracts from seeds of Indonesian adlay (C. lacryma-jobi) varieties including Agrotis, Ma-yuen, and Aquatic, were tested against 2,2-diphnyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) to determine their free radical scavenging activity. The relationship between extraction solvents, phytochemical composition, and antioxidant activity was analyzed statistically using principal component analysis (PCA) to classify them based on the similarities among the components. The potential use of the phytochemicals as crop protection agents was also explored through a review of the literature. The Agrotis variety demonstrated the highest antioxidant activities (IC50 DPPH = 741.49 and ABTS =152.69 µg/mL). The ethyl acetate fraction of this variety showed the greatest antioxidant activity (IC50 DPPH and ABTS = 106.34 and 17.62 µg/mL, respectively), total phenolic content (275.16 mg GAE/g extract), and flavonoid content (37.41 mg QE/g extract). Fatty acids (FAs) and fatty acid methyl esters (FAMEs) accounted for 47.71 ± 0.02 and 41.73 ± 0.04%, respectively, and they were the major components of the extracts. The principal component analysis (PCA) revealed three different groups of phytochemical components in the seeds of Agrotis variety, including fatty acid methyl esters (FAMEs), such as methyl linoleate, methyl stearate, methyl vaccinates, and methyl palmitate, and fatty acids (FAs), including 7-hexadecanoid acid, bovinic acid, and 15-hydroxipentadecanoic acid. The final phytochemical group consisted of minor components, including uncategorized compounds such as decamethyl-tetrasiloxane and cycloalkenes. This study highlights the fact that C. lacrima-jobi is a promising source of natural antioxidants and agrochemicals.
1. Introduction
The search for natural products as crop protection agents is becoming increasingly important due to the growing global demand for organic products and the need for a sustainable food supply. Safe, selective, and cost-effective agrochemicals are needed to replace synthetic ones, which can have negative impacts on the environment [1]. Higher plants, including those that contain phytochemicals such as polyphenols, are promising sources of natural products that can be used as crop protection agents.
Plants are a significant source of phenolic compounds, which are synthesized as secondary metabolites during normal development in response to stressors such as wounding and exposure to UV radiation [2]. Some species of plants are invaluable resources that are useful in everyday life as alternative foods, food additives, herbs, and sources of aroma and color; they can also be used directly in pharmaceuticals [3]. These plant species contain phytochemical properties that have the potential to prevent or treat diseases and to be used for pest control [4].
Coix lacryma-jobi L., belonging to the Poaceae family in the form of panicoid grass, is a cereal crop commonly known as adlay. This annual plant grows both wild and cultivated in some regions in Indonesia, and is utilized as a popular nutritional supplement in traditional Chinese medicine. Traditionally, adlay has been used to treat bumps, neuralgia, and rheumatism. Modern research has shown that adlay seeds contain four primary fatty acids (linoleic, palmitic, stearic, and oleic acid) that have crucial effects in nutritional and clinical applications, including antitumor, anticancer, anti-inflammatory, and anti-aging activities, as well as neutralizing free radicals [5,6,7]. Furthermore, the extracts of adlay seeds provide health benefits from compounds such as fatty acids, phenolic compounds, triolein, and coixol [8,9]. The majority of fatty acids (FAs) are monounsaturated, and many studies have demonstrated that a high dietary intake of monounsaturated FAs can reduce the risk of cardiovascular disease through several mechanisms [10]. Phenolic compounds have a range of biological activities, including antioxidant activity and inhibition of xanthine oxidase activity [11]. However, environment conditions such as climate, geographical location, and the type of plant can affect the bioactive compound content in plants. While various studies have reported some phytochemicals in C. lacryma-jobi [12,13], the relationship between the different polarities of extracts and the chemical profile of C. lacryma-jobi, especially in certain varieties from Indonesia, remains unknown. This study was conducted to access the chemical constituents of different polarities of extracts from Indonesian adlay (C. lacryma-jobi) by screening for antioxidant activity in three varieties: Agrotis, Ma-yuen, and Aquatic. Principal component analysis (PCA) was applied to the GC-MS data to categorize the chemical constituents into homogenous groups. Additionally, a literature study was conducted to determine the potential use of phytochemicals from adlay as crop-protecting agents. This research provides valuable information for identifying the phytochemical content in this plant as potential source of natural agrochemicals to safeguard crops against pests, weeds, fungi, and bacteria.
2. Materials and Methods
2.1. Plant Materials and Chemicals
The seeds from three varieties, Agrotis (Batu), Ma-yuen (Pulut), and Aquatic (Unpad) (Figure 1), were sourced from an adlay farm in Sumedang, West Java, Indonesia. The mature seeds of C. lacryma-jobi seeds were collected in March 2021 from more than 30 plants and served as replicates. Methanol, hexane, ethyl acetate, chloroform, DPPH (2,2-diphenyl-1-picrylhydrazyl), acetic acid, sodium acetate anhydrous, ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), potassium persulfate, Folin–Ciocalteu reagent, aluminum chloride, and quercetin were procured from Sigma-Aldrich (Saint Louis, MO, USA).

Figure 1.
Indonesian adlay (C. lacryma-jobi) seeds of different varieties; (a) Agrotis, (b) Aquatic, and (c) Ma-yuen.
2.2. Seeds Extraction for Screening Antioxidant Potentials
The antioxidant potential of three varieties of C. lacryma-jobi (Ma-yuen, Agrotis, and Aquatic) was screened using the procedure outlined in Figure 2. The seeds were dried at 40 °C in an oven and then ground into fine powder. After that, 5 g of each variety of pulverized powder was soaked in 170 mL of methanol, stirred for 24 h, filtered, and evaporated using a rotary evaporator at 30 °C (QR 2005-S, Shimadzu, Tokyo, Japan) to obtain the extract. Methanol was chosen due its high polarity, which allows for a high extraction yield and extracts a diverse range of compounds, as reported by Truong et al. [14]. All extracts were then tested for their antioxidant activity against DPPH and ABTS free radicals. The adlay variety with the highest antioxidant activity was selected for further screening in a fractionation procedure using different polar solvents (hexane, chloroform, ethyl acetate, and water).
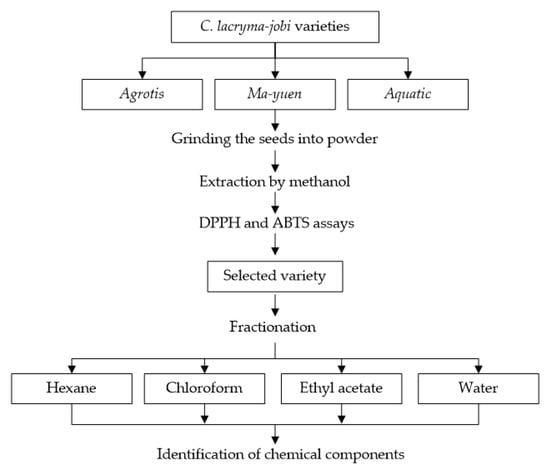
Figure 2.
Screening of the antioxidant potential of adlay (C. lacryma-jobi).
2.3. Fractionation of Agrotis Variety from C. lacryma-jobi
A total of 1365 g of seed powder of the selected variety of adlay was soaked in 2625 mL methanol for nine days under ambient conditions, followed by filtration. The residue was soaked with the same volume of methanol 4 times until a final methanol extract of 10.95 g was obtained. The filtrate was then evaporated under vacuum at 40 °C using a rotary evaporator (QR 2005-S, Shimadzu, Tokyo, Japan. The yields obtained were 0.72, 0.78, 0.31, and 3.72 g for the hexane, chloroform, ethyl acetate, and water extracts, respectively. After obtaining the dried extracts or fractions, all samples were dissolved in methanol to prepare a stock solution, then stored at 4 °C for future analysis.
2.4. DPPH Free Radical Scavenging Activity
The determination of DPPH free radical scavenging activity from adlay extract was performed using the method reported by Andriana et al. [15], with slight modifications. A volume of 1.0 mL of the sample (10–3000 µg/mL in methanol) or quercetin (1–20 µg/mL) as a standard was mixed with 0.5 mL of 0.5 mM DPPH and 1 mL of acetate buffer 0.1 M (pH 5.5), then incubated in the dark at 26 °C for 30 min. The absorbance of the mixture was recorded at a wavelength of 517 nm with the use of a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan). The inhibition level was calculated using the following formula:
where Acontrol refers to the absorbance of the reaction without sample and Asample represents the absorbance of the reaction with the sample.
% DPPH = [(Acontrol − Asample)]/Acontrol] × 100
2.5. ABTS Free Radical Scavenging Activity
The determination of ABTS free radical scavenging activity was performed using the method described by Andriana et al. [16] with a minor modification. An ABTS+ solution was created by mixing 25 mL of a 7 mM ABTS solution and 25 mL of a 2.45 mM potassium persulfate. The mixture was left to incubate at room temperature in the dark for 16 h. Prior to the test, the ABTS+ solution was diluted with methanol to achieve an absorbance of 0.70 ± 0.05 at 734 nm. A total of 2 mL of ABTS+ methanol solution was combined with 0.4 mL of the sample (10–3000 µg/mL in methanol), and the mixture was stored under ambient conditions for 30 min. The absorbance of the mixture was then measured with a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan) at a wavelength of 734 nm. Quercetin solutions (1–20 µg/mL) were used as standard solutions. The level of antioxidant activity was obtained by the following formula:
where Acontrol is the absorbance of the reaction without the sample and Asample is the absorbance of the reaction with the sample.
% ABTS = [(Acontrol − Asample)/Acontrol] × 100
2.6. Total Phenolic Contents (TPC)
The total phenolic contents of extracts were evaluated by using the Folin–Ciocalteu’s method, following the previous study reported by Iwansyah et al. [17] with some modifications. A volume of 0.2 mL sample (1000 µg/mL) was mixed with 1 mL of 10% Folin–Ciocalteu reagent. The mixture was vortexed; then, 0.8 mL of 5% sodium carbonate solution was added. The reaction mixture was incubated in a dark room for 30 min. The absorbance was measured at a wavelength of 750 nm by using a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan). The total phenolic contents were expressed as mg gallic acid equivalent (GAE)/g of extract.
2.7. Total Flavonoid Content (TFC)
The total flavonoid content of the extracts was determined using the method reported previously by Minh et al. [18], with a minor modification. A sample of 1 mL volume (1000 µg/mL) was mixed with 1 mL aluminum chloride–methanol solution (2%). The mixture was then left to incubate for 15 min at room temperature. The absorbance was recorded at a wavelength of 430 nm using a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan). The total flavonoid content was expressed as the equivalent of mg of quercetin in g of extract.
2.8. Identification of Chemicals Constituent of C. lacrima-joby var. Agrotis by Gas Chromatography-Mass Spectrometry (GC-MS)
The Agilent GC-MS system (Agilent 7890B/MSD 5977 A, Agilent Technology, Inc., Santa Clara, CA, USA) was employed to detect chemical constituents of ethyl acetate extract of the Agrotis variety. The volume of the injected sample was 1 µL. The Agilent HP-5MS (19091S-433:93.92873) (Agilent Technology, Inc., J&W Scientific Products, Santa Clara, CA, USA), with dimensions of 30 m in length, 250 μm internal diameter, and 0.25 μm in thickness, was used as the column. Helium was chosen as the carrier gas. The mode of the helium inlet was spitless, with a heater temperature of 250 °C, a pressure of 7.0699 psi, and a total flow of 104 mL/min. The operating conditions of the GC oven temperature were maintained as follows: The initial temperature = 40 °C with 1 min of hold time, which increased by the programmed rate = 10 °C/min up to a final temperature of 325 °C, with 4 min of hold time. The mass range scanned from 122–1021 amu. Electron ionization (EI) was employed as an ion source, and positive ions were collected in the MS analyses. The control of the GC-MS system and the data peak processing were carried out using the Agilent ChemStation software featured with the NIST mass spectral library (Agilent Technology, Inc., J&W Scientific Products, Santa Clara, CA, USA).
2.9. Statistical Analysis
Data analysis was performed using the one-way ANOVA and R Software (R Core Team, Vienna, Austria, 2020), and each assay was conducted in triplicate. Data were presented as mean ± standard deviation. A Duncan’s test was used to determine the significant difference between the treated samples and the controls at a confidence level of 95% (p < 0.05). A multivariate analysis was conducted by performing principal component analysis (PCA) using R software [19] to determine the relationship between the extracting solvents and the concentration of compounds contained in the extracts.
3. Results
3.1. Screening Antioxidant Potentials from Adlay (C. lacryma-jobi) Varieties
The DPPH and ABTS radical scavenging activity assays revealed that the antioxidant potential of three varieties of adlay (C. lacryma-jobi) were significantly different, according to Duncan’s test (p < 0.05). The Agrotis variety exhibited the highest antioxidant activity (IC50 DPPH and ABTS = 741.39 and 152.69 µg/mL, respectively) followed by the Aquatic and Ma-yuen cultivars (Table 1). However, when compared to the positive control, quercetin, these results were still lower. The Agrotis variety appeared to have higher antioxidant capacities to compare to other varieties, making it the preferred option for further fractionation.

Table 1.
Antioxidant activity of Agrotis, Ma-yuen, and Aquatic varieties from C. lacryma-jobi.
3.2. Antioxidant Potentials from C. lacryma-jobi var. Agrotis Extracts
Since the Agrotis variety demonstrated the highest antioxidant activity, its methanol extract was fractionated using solvents of varying polarities. Table 2 displays the antioxidant activity of the extracts obtained from the Agrotis variety. The type of solvent influenced the IC50 of the DPPH and ABTS assays of the Agrotis variety of adlay, and the results were found to be significantly different according to Duncan’s test (p < 0.05). Ethyl acetate extract displayed the strongest antioxidant activity (IC50 DPPH and ABTS= 106.34 and 38.20 µg/mL, respectively) followed by chloroform, water, and hexane extracts. However, when compared to quercetin as the positive control (IC50 DPPH and ABTS = 6.55 and 9.24 µg/mL, respectively), the extract still showed a lower level of activity.

Table 2.
Antioxidant activity of different extracts of the Agrotis variety.
3.3. Total Phenolic and Flavonoid Contents (TPC and TFC)
Figure 3 and Figure 4 indicate the total phenolic and flavonoid content of several seed extracts of the Agrotis variety. The results revealed that the polarities of the solvents significantly impacted the total phenolic and flavonoid content of the Agrotis variety, according to Duncan’s test (p < 0.05). When ethyl acetate was used as the extracting solvent, the highest quantities of phenolic compounds (275.18 ± 16.71 mg GAE/g extract) and flavonoids (37.91 ± 0.68 mg QE/g extract) were found. On the other hand, the hexane fraction yielded the lowest amounts of phenols and flavonoids (35.18 ± 2.19 mg GAE/g extract and 3.96 ± 0.01 mg QE/g extract, respectively).
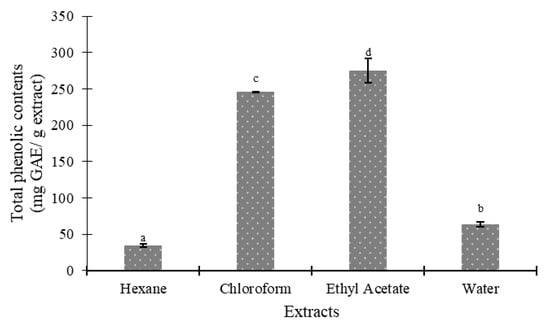
Figure 3.
Total phenolics of various fractions of Agrotis variety. Data are presented as mean ± SD (standard deviation). Values marked with different letters show significant differences according to Duncan’s test (p < 0.05).
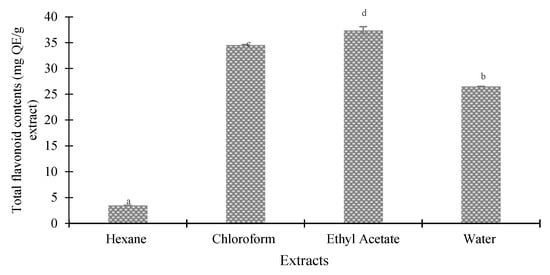
Figure 4.
Total flavonoids in different extracts of Agrotis variety. Data are presented as mean ± SD (standard deviation). Values marked with different letters show significant differences according to Duncan’s test (p < 0.05).
3.4. Identification of Phytochemical Constituents Adlay (C. lacryma-jobi) var. Agrotis by Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS analysis was performed to identify and quantify the phytochemical constituents of adlay (C. lacryma-jobi) var. Agotis. The GC-MS chromatograms of its extracts are presented in Figure 5, and the chemical constituents are listed in Table 3. A total of 20 compounds in various categories, such as cycloalkane, amides, alkanes, fatty acid methyl esters (FAMEs), fatty acids, polycyclic aromatic hydrocarbons (PAHs), alcohols, aldehydes, cyclosiloxanes, and others, were identified. The highest number of detected compounds was found in hexane, followed by the water, ethyl acetate, and chloroform extracts. FAME was determined to be the most dominant chemical group. The highest concentration of a component was found for linoelaidic acid (38.27%), followed by methyl linoleate (14.07%), palmitic acid (9.66%), methyl elaidate (9.51%), and the rest (<9.5%).
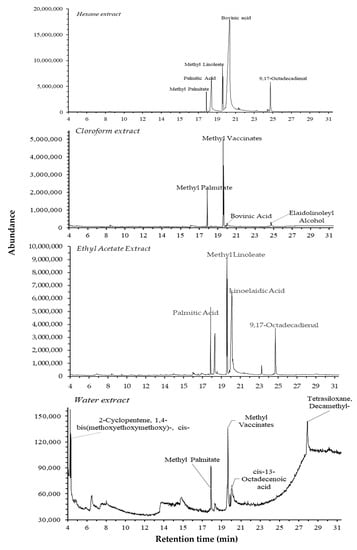
Figure 5.
The total ion chromatograms of different extracts of C. lacryma-jobi var. Agrotis.

Table 3.
Chemical constituent of different extracts of C. lacryma-jobi var. Agrotis.
3.5. Relationship of Extracting Solvents to Antioxidant Activity and Phytochemical Constituents of Adlay (C. lacryma-jobi) var. Agrotis by Principal Component Analysis (PCA)
The score plot of concentrations of phytochemicals from the Agrotis variety was created using PCA, as illustrated in Figure 6. The detected compounds were classified into three different groups. The compounds extracted from chloroform and ethyl acetate were grouped together in a similar quadrant, as they were largely similar. Figure 6 also shows the differentiation between the identified compounds present in the C. lacryma-jobi var. Agrotis extracts along the first two principal components, PC1 and PC2, in the scatter plot. The explained variances for PC1 and PC2 were 41.7% and 38.0%, respectively. The cumulative contribution rates of PC1 and PC2 reached a high level, which was enough to explain the maximum variation of compounds and to classify them in PCA. As shown in the scatterplot of PC1 and PC2, different extracts of C. lacryma-jobi seeds were clearly distributed in distinct regions of the PCA plot (quadrants I, II, and IV). The first group (quadrant I) consisted of six compounds (3−acetoxypentadecane, 7−hexadecenoic acid, bovinic acid, 2−methyl−Z, Z−3,13−octadecadienol, 15−hydroxypentadecanoic acid, and linoleyl aldehyde). The second group (quadrat II) included nine compounds (methyl palmitate, palmitic acid, methyl linoleate, methyl vaccinates, methyl stearate, cis−13−octadecenoic acid, linoelaidic acid, elaidolinoleyl alcohol, and 9,17−octadecadienal). The third group (quadrant IV) comprised five compounds (cyclopentene, 1,4−bis(methoxyethoxymethoxy) −, cis−pyrene, acetothioamide, cyclotrisiloxane, tetrasiloxane, and decamethyl−).
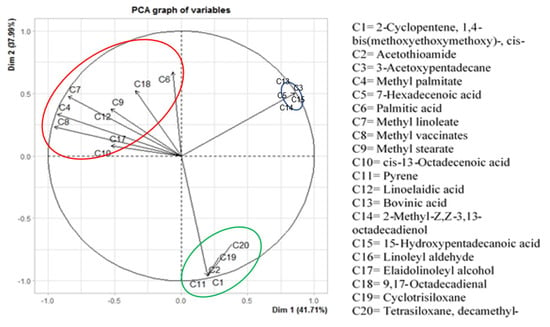
Figure 6.
Score plot of PC1 and PC2 for identified compounds from adlay (C. lacryma-jobi) var. Agrotis extracts.
Figure 7 shows the similarities among the different solvents used to extract C. lacryma-jobi var. Agrotis seeds. It can be observed that chloroform (2) and ethyl acetate (3) extracts were placed into the second group (quadrant II), meaning these solvents possess similarities in terms of the yield of the extracted compounds. Moreover, the hexane extract was arranged into the second group (quadrant II), while the water extract was arranged into the fourth group (quadrant IV), indicating that these solvents produced distinct results.
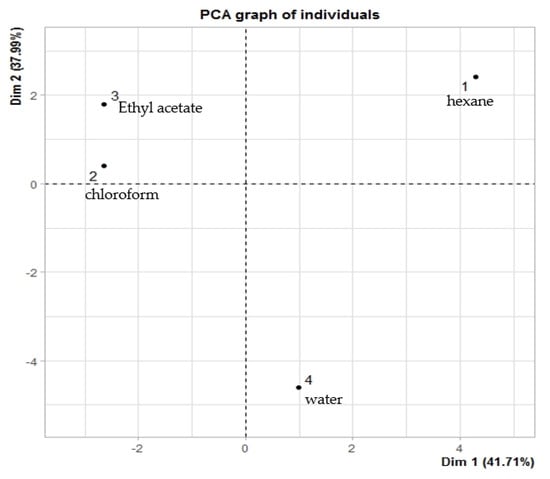
Figure 7.
Score plot of type of different solvents on the concentration of identified compounds: 1 = hexane, 2 = chloroform, 3 = ethyl acetate, and 4 = water.
3.6. Potential Use of Adlay Phytochemicals for Agrochemicals
A potential use of phytochemicals from C. lacrima-joby, based on a literature review, is shown in Table 4. The major constituents of this plant, such as bovinic acid and methyl linoleate in hexane and chloroform extracts, have been reported to possess insecticidal activity against the potato bug (Leptinotarsa decemlineata) [20] and herbicidal activity against wild oat (Avena fatua), sun spurge (Euphorbia helioscopia), goosefoot (Chenopodium album), canary grass (Phalaris minor), and knotweed (Rumex dentatus), respectively [21]. On the other hand, in ethyl acetate extract, some chemical constituents such as palmitic and linoelaidic acids, were found to show larvicidal and insecticidal activities against Aedes aegypti Linn and cotton leafworm (Spodoptera littoralis), respectively [22,23]. Moreover, methyl palmitate presents in the water extract exhibited acaricidal activity against Tetranychus cinnabarinus (Boisduval) [24]. Most of the chemical constituents detected in C. lacryma-jobi have been reported to exhibit potential activity against various plant pests, weeds, fungi, and bacteria. These phytochemicals have the potential to be developed as natural pesticides, but further research is necessary to fully understand their biological activities.

Table 4.
Potential use of chemical constituents from C. lacryma-jobi as crop-protecting agents.
4. Discussion
This study evaluated the phytochemical constituents of Indonesian adlay (C. lacryma-jobi) by screening its three varieties, namely Agrotis, Ma-yuen, and Aquatic, and assessed their potential for use as crop protection agents. Based on DPPH and ABTS assays, the Agrotis variety was chosen for further fractionation due its high antioxidant activity. Total phenolic and flavonoid evaluations in the Agrotis variety using different extraction solvents (Figure 1 and Figure 2) revealed that ethyl acetate extract had the highest content (275.18 ± 16.71 mg GAE/g extract and 37.91 ± 0.68 mg QE/g extract, respectively). This demonstrates that phenolics are not always found in polar extracts, depending on the structure of the phenolic compounds present [40]. According to Woo [41], the darker the color of the extract, the more flavonoids it contains. The yellow color of the hexane fraction is due to the presence of non-polar active chemicals dispersed in the extract [42]. In this study, the total phenol and flavonoid contents and antioxidant activity of adlay extracts were investigated. According to Tuyen et al. [43], phenols and flavonoids are well-known natural antioxidants. Phenolic and flavonoid compounds have been reported to have potential use as natural agrochemicals such as insecticide [44] and herbicide [45].
According to GC-MS analysis, a total of 20 compounds from different chemical groups were identified and quantified (Table 4). The main components, which were mainly volatile compounds, were linoelaidic acid (38.27%), methyl linoleate (14.07%), palmitic acid (9.66%), methyl elaidate (9.51%), and the rest (<9.5%). Based on their similarity and abundance in the extracts, particle component analysis (PCA) clustered them into three different groups (Figure 6). Hexane and water were classified as different groups due to hexane being a nonpolar solvent, while water is a polar one. Additionally, chloroform and ethyl acetate were placed in the same group because they are both are semi-polar solvents, which agrees with the study of Andriana et al. [46], which found ethyl acetate to be an effective solvent for extracting phenolic compounds.
Based on a study of the literature, it has been shown that most of the major chemical constituents from C. lacryma-jobi have potent pesticidal effects against plant pests and weeds, as well as against bacteria and fungi. Generally, certain fatty acids and their derivatives, such as linoleic acid, oleic acid, methyl linoleate, and methyl stearate, have been shown to possess antioxidant and fungicidal properties due to their unsaturated bonds [47]. Furthermore, linoleic acid has demonstrated anti-histaminic, anti-coronary, anti-acne, anti-bacterial, anti-fungal, anti-arthritic, and anti-inflammatory potential [48]. It was also found predominantly in green, black, and white adlay seeds in the range of 66.94–88.29% [49]. Palmitic acid was detected in ethyl acetate extract at 9.66%, and has been reported to possess antioxidant, larvicidal, and insecticidal activities [50]. Mohadjerani [51] also reported that fatty acids and their derivatives have antioxidant activities. Based on the study conducted by Andriana et al. [52], methyl palmitate was also detected in the extract with the highest levels of antioxidants. Therefore, it can be assumed that fatty acids and their derivatives might contribute to antioxidant activity. In addition, there are some saturated fatty acids that show activity as antioxidants as well as antifungal, bactericidal, and pesticidal agents [53]. Table 3 summarizes the pesticidal effects of phytochemical constituents from C. lacryma-jobi. It shows a wide range of inhibitory effects against several weeds, insects, bacteria, and fungi. Further research is required to investigate the phytochemical constituents of this plant in order to explore its potential as a source of natural agrochemicals and to better understand its pesticidal activity.
5. Conclusions
In this study, the antioxidant properties and phytochemical contents of different seed extracts of Indonesian adlay (C. lacryma-jobi) were successfully screened using a GC-MS analysis, with the aim of exploring their potential use as agrochemicals. The Agrotis variety was found to have the highest total phenol and flavonoid content, and its antioxidant capacities were comparable to other studied varieties. Through GC-MS analysis, a total of 20 compounds were identified and quantified, with the majority consisting of fatty acids (FAs) and fatty acid methyl ester (FAMEs) (47.71 and 41.73%, respectively) in all extracts of the Agrotis variety. By PCA analysis, fatty acid methyl esters (FAMEs), including methyl linoleate, methyl stearate, methyl vaccinates, and methyl palmitate, as well as trace components such as acetothioamide and cyclotrisiloxane, were grouped into the second and fourth groups, respectively. Chloroform and ethyl acetate were placed in the same group due to the similarity of the yielded compounds. This study found various concentrations and compositions of the chemical constituents from C. lacryma-jobi seeds, which may have potent pesticidal effects against a range of weeds, insects, fungi, and bacteria. This research suggests that C. lacryma-jobi seeds have potential as natural crop protection agents. However, to detect thermolabile compounds that could not be identified by GC-MS, further research employing liquid chromatography is necessary.
Author Contributions
Conceptualization, Y.A. and T.D.X.; methodology, Y.A. and A.C.I.; data curation, N.A.F.; writing—original draft preparation, Y.A.; writing—review and editing, Y.A. and T.D.X.; supervision, T.D.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Research Center for Appropriate Technology, National Research and Innovation Agency (BRIN) for providing facilities for this research. The authors also thank Yusep Ikrawan from Pasundan University for his suggestions to improve this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loiseleur, O. Natural products in the discovery of agrochemicals. CHIMIA Int. J. Chem. 2017, 71, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Maisuthisakul, P.; Pasuk, S.; Ritthiruangdej, P. Relationship between antioxidant properties and chemical composition of some Thai plants. J. Food Compos. Anal. 2008, 21, 229–240. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Phytochemical Constituent Grass, Herbs and Other Economic Plants: Herbal Reference Library; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Bon, S.G.; Enicola, E.E.; Padasas, G.Y.; Galvez, H.F. Diversity conservation of adlay germplasm in the Philippines. Philipp. J. Crop. Sci. 2018, 42, 98–140. [Google Scholar]
- Diningrat, D.S.; Risfandi, M.; Harahap, N.S.; Sari, A.N.; Siregar, H.K. Phytochemical screening and antibacterial activity Coix lacryma-jobi oil. J. Plant Biotechnol. 2020, 47, 100–106. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Ali, B.; Yang, N.; Chen, Y.S.; Wu, F.F.; Xu, X.M. Changes of the phenolic compounds and antioxidant activities in germinated adlay seeds. J. Sci. Food Agric. 2017, 97, 4227–4234. [Google Scholar] [CrossRef]
- Wang, L.F.; Chen, C.; Su, A.X.; Zhang, Y.Y.; Yuan, J.; Ju, X.R. Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay (Coix lachryma-jobi L. var. Ma-yuen Stapf) seed meal. Food Chem. 2016, 196, 509–517. [Google Scholar]
- Yu, Q.; Ye, G.; Lei, Z.; Yang, R.; Chen, R.; He, T.; Huang, S. An isolated compound from stems and leaves of Coix lacryma-jobi L. and its anticancer effect. Food Biosci. 2021, 42, 101047. [Google Scholar] [CrossRef]
- Son, E.S.; Kim, S.H.; Kim, Y.O.; Lee, Y.E.; Kyung, S.Y.; Jeong, S.H.; Kim, Y.J.; Park, J.W. Coix lacryma-jobi var. Ma-yuen Stapf sprout extract induces cell cycle arrest and apoptosis in human cervical carcinoma cells. BMC Complement. Altern. Med. 2019, 19, 312–327. [Google Scholar]
- Rahman, M.; Rahaman, M.; Islam, M.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Das, S.; Akhter, R.; Khandaker, S.; Huque, S.; Das, P.; Anwar, M.R.; Tanni, K.A.; Shabnaz, S.; Shahriar, M. Phytochemical screening, antibacterial and anthelmintic activities of leaf and seed extracts of Coix lacryma-jobi L. J. Coast Life Med. 2017, 5, 360–364. [Google Scholar] [CrossRef]
- Breitenbach, G.L.; Rosenberger, M.G.; Rosenberger, A.G.; Caetano, J.; Pellá, M.C.G.; Scheidt, D.T.; Martins, C.V.B.; Munizc, E.C.; Dragunski, D.C. Antimicrobial activity of polymeric microfibers containing Coix lacryma-jobi extract. Macromol. Res. 2020, 28, 869–876. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Minh, T.N.; Van, T.M.; Viet, T.D. Antihyperuricemia, antioxidant, and antibacterial activities of Tridax procumbens L. Foods 2019, 8, 21. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.D.; Le, Q.T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef]
- Iwansyah, A.C.; Desnilasari, D.; Agustina, W.; Pramesti, D.; Indriati, A.; Mayasti, N.K.I.; Andriana, Y.; Kormin, F.B. Evaluation on the physicochemical properties and mineral contents of Averrhoa bilimbi L. leaves dried extract and its antioxidant and antibacterial capacities. Food Sci. Technol. 2021, 41, 987–992. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Tran, H.D.; Van, T.M.; Andriana, Y.; Khanh, T.D.; Quan, N.V.; Ahmad, A. Isolation and purification of bioactive compounds from the stem bark of Jatropha podagrica. Molecules 2019, 24, 88. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 23 June 2022).
- Clements, J.; Groves, R.L.; Cava, J.; Barry, C.C.; Chapman, S.; Olson, J.M. Conjugated linoleic acid as a novel insecticide targeting the agricultural pest Leptinotarsa decemlineata. PLoS ONE 2019, 14, 0220830. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Mahnashi, M.H.; Kabir, F.; Parveen, N.; Ahmed, D.; Afzal, U.; Batool, S.; Awais, M.; Ahmed, A.S.; et al. Bioherbicidal ability and weed management of allelopathic methyl esters from Lantana camara. Saudi J. Biol. Sci. 2021, 28, 4365–4374. [Google Scholar] [CrossRef]
- Bharathithasan, M.; Ravindran, D.R.; Rajendran, D.; Chun, S.K.; Abbas, S.A.; Sugathan, S. Analysis of chemical compositions and larvicidal activity of nut extracts from Areca catechu Linn against Aedes (Diptera: Culicidae). PLoS ONE 2021, 16, e0260281. [Google Scholar] [CrossRef]
- Yousef, H.; El-Lakwah, S.F.; El-Sayed, Y.A. Insecticidal activity of linoleic acid against Spodoptera littoralis (Boisd.). Egypt. J. Agric. Res. 2013, 91, 573–580. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, H.X.; Shen, Z.J.; Zhao, L.L.; Clarke, S.R.; Sun, J.H.; Du, Y.Y.; Shi, G.L. Methyl palmitate, an acaricidal compound occurring in green walnut husks. J. Econ. Entomol. 2009, 102, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.N.; Yahayu, M.; Sarip, S.H.M.; Rizan, N.H.; Min, C.B.; Mustafa, N.F.; Ngadiran, S.; Ujang, S.; Zakaria, Z.A. Evaluation on efficiency of pyroligneous acid from palm kernel shell as antifungal and solid pineapple biomass as antibacterial and plant growth promoter. Sains Malays. 2016, 45, 1423–1434. [Google Scholar]
- Huang, G.; Huang, H. Synthesis, antiasthmatic, and insecticidal/antifungal activities of allosamidins. J. Enzym. Inhib. Med. Chem. 2019, 34, 1226–1232. [Google Scholar] [CrossRef]
- Matsumura, F.; Tai, A.; Coppel, H.C.; Imaida, M. Chiral specificity of the sex pheromone of the red-headed pine sawfly, Neodiprion lecontei. J. Chem. Ecol. 1979, 5, 237–249. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.)(Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. Nematicidal effect of methyl palmitate and methyl stearate against Meloidogyne incognita in bananas. J. Agric. Food Chem. 2020, 68, 6502–6510. [Google Scholar] [CrossRef]
- Enenebeaku, U.E.; Duru, C.E.; Mgbemena, I.C.; Ukwandu, N.C.D.; Nwigwe, H.C.; Enenebeaku, C.K.; Okotcha, E.N. Phytochemical evaluation and molecular docking of bioactive compounds from the roots of Dictyandra arborescens (Welw.) against Plasmodium bergheiprotein targets. Trop. J. Nat. Prod. Res. 2021, 5, 370–381. [Google Scholar]
- Wang, X.; Zhu, X.; Chen, X.; Lv, B.; Wang, X.; Wang, D. Phenanthrene and pyrene disturbed the growth of Microcystis aeruginosa as co-cultured with Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2020, 27, 45957–45964. [Google Scholar] [CrossRef]
- Fang, L.; Wang, X.; Guo, L.; Liu, Q. Antioxidant, anti-microbial properties and chemical composition of cumin essential oils extracted by three methods. Open Chem. 2018, 16, 291–297. [Google Scholar] [CrossRef]
- Yang, A.; Zeng, S.; Yu, L.; He, M.; Yang, Y.; Zhao, X.; Jiang, C.; Hu, D.; Song, B. Characterization and antifungal activity against Pestalotiopsis of a fusaricidin-type compound produced by Paenibacillus polymyxa Y-1. Pestic. Biochem. Physiol. 2018, 147, 67–74. [Google Scholar] [CrossRef]
- Tomčala, A.; Jirošová, A.; Žáček, P.; Kaušková, M.; Hovorka, O.; Koutek, B. Species specificity of aldehyde and fatty acid profiles of four family group representatives within the insect Infraorder pentatomomorpha (Hemiptera: Heteroptera). Chem. Biodivers. 2017, 14, e1600420. [Google Scholar] [CrossRef]
- Mitchell, E.R.; McLaughlin, J.R. Suppression of mating and oviposition by fall armyworm and mating by corn earworm in corn, using the air permeation technique. J. Econ. Entomol. 1982, 75, 270–274. [Google Scholar] [CrossRef]
- Farag, S.M.; Essa, E.E.; Alharbi, S.A.; Alfarraj, S.; El-Hassan, G.A. Agro-waste derived compounds (flax and black seed peels): Toxicological effect against the West Nile virus vector, Culex pipiens L. with special reference to GC–MS analysis. Saudi J. Biol. Sci. 2021, 28, 5261–5267. [Google Scholar] [CrossRef]
- Shilaluke, K.C.; Moteetee, A.N. Insecticidal activities and GC-MS analysis of the selected family members of Meliaceae used traditionally as insecticides. Plants 2022, 11, 3046. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A. Biological control and plant growth promotion properties of volatile organic compound producing antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. An introduction to natural products isolation. Nat. Prod. Isol. 2012, 864, 1–25. [Google Scholar]
- Woo, K.S. Use of bee venom and propolis for apitherapy in Korea. In Proceedings of the Seventh Asian Apicultural Association Conference and Tenth BEENET Symposium and Technofora, College, Laguna, Philippines, 23–27 February 2004. [Google Scholar]
- Tensiska, B.N.; Endah, W.; Yushini, A.L.R. Antioxidant activity of Adlay (Coix lachryma-jobi L.) extracts with different extracting solvents. J. Agroindustri 2020, 10, 1–11. (In Indonesian) [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Anh, T.T.T.; Anh, L.H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Andriana, Y.; Ade, I.C.; Nguyen, Q.T.; Bui, Q.M.; Nguyen, M.D.; Phung, T.T.; Le, V.A.; Nguyen, H.K.; Truong, N.M. Efficacy of different solvents on the extraction process of antioxidant and potential compounds in Alpinia galanga L. from Indonesia. J. Mod. Agric. Biotechnol. 2022, 1, 5. [Google Scholar]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharm. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.E.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Ciênc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Azzahra, G. Phytochemicals Analysis of Seeds and Seed Stalk of Wild Adlay (Coix lacryma-jobi L.) and Its Culture. Undergraduate Thesis, Indonesian University of Education, Kota Bandung, Indonesia, 2019. Available online: http://repository.upi.edu/ (accessed on 23 January 2021).
- Jin, X.L.; Wang, K.; Li, Q.Q.; Tian, W.L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Antioxidant and anti-inflammatory effects of Chinese propolis during palmitic acid-induced lipotoxicity in cultured hepatocytes. J. Funct. Foods 2017, 34, 216–223. [Google Scholar] [CrossRef]
- Mohadjerani, M.; Tavakoli, R.; Hosseinzadeh, R. Fatty acid composition, antioxidant and antibacterial activities of Adonis wolgensis L. extract. Avicenna J. Phytomed. 2013, 4, 24–30. [Google Scholar]
- Andriana, Y.; Xuan, T.D.; Quan, N.V.; Quy, T.N. Allelopathic potential of Tridax procumbens L. on radish and identification of allelochemicals. Allelopath. J. 2018, 43, 223–238. [Google Scholar] [CrossRef]
- Jadeeswari, P.; Nishanthini, A.; Muthukumarasamy, S.M.V.R.; Mohan, V.R. GC-MS analysis of bioactive components of Aristolochia krysagathra (Aristolochiaceae). J. Curr. Chem. Pharm. Sci. 2012, 2, 226–232. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).