Nano-Biofertilizers Synthesis and Applications in Agroecosystems
Abstract
1. Introduction
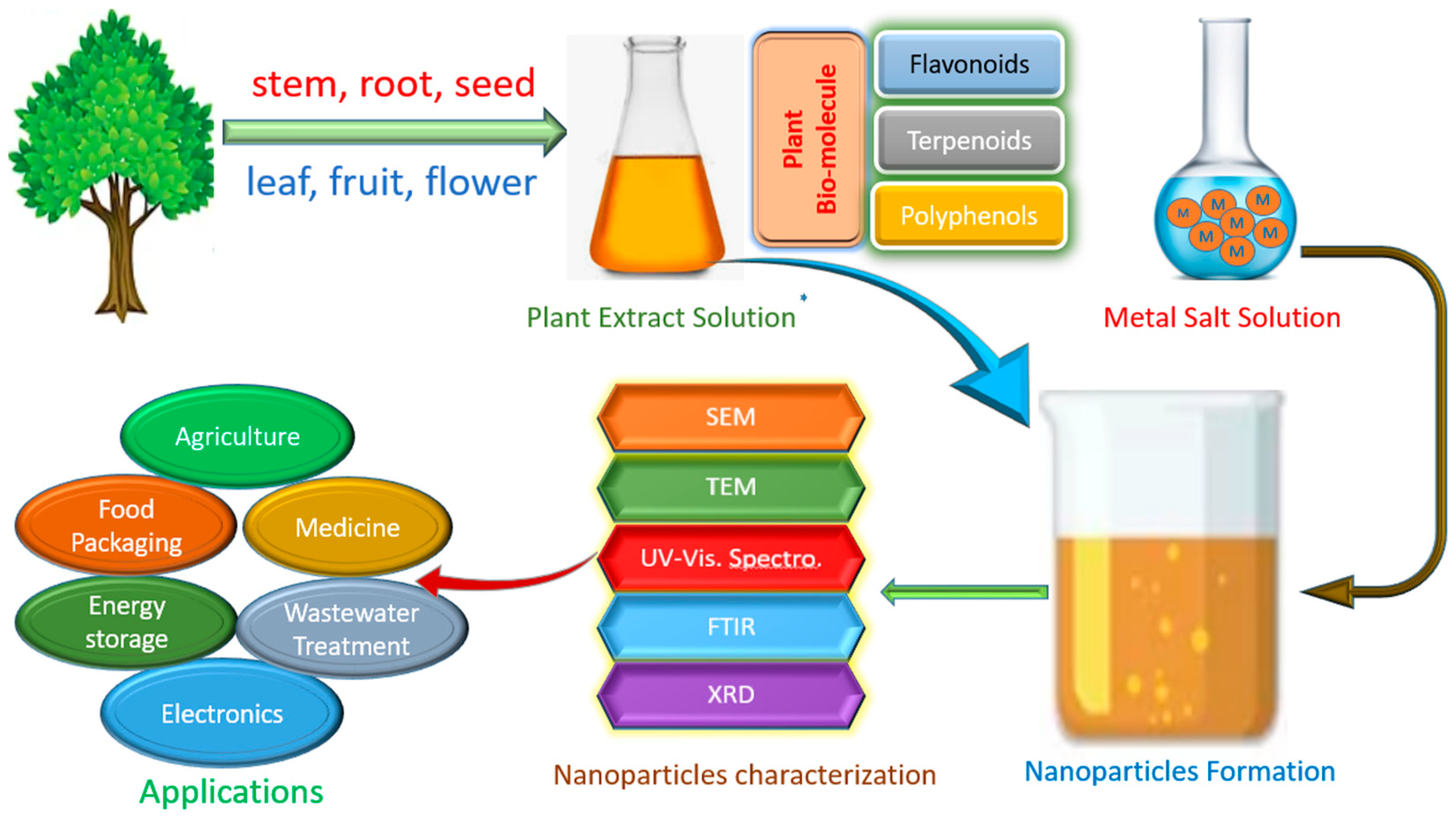
2. Biogenic Synthesis of Nanomaterials as Nanofertilizers
2.1. Microbe-Mediated Synthesis
| S.N. | Bio-Extract | Nanoparticles | Characterization Results | Applications | Ref |
|---|---|---|---|---|---|
| 1 | Twelve bacteria supernatants containing auxin complex (indole-3-acetic, IAA) | Iron and manganese mono- and bimetallic nanoparticles | λmax: 250–300 nm. Spherical shape with size 26.65 nm of FeOx NPs, MnOx NPs at around 22.32 nm, and MnOx/FeOx NPs at around 23.42 nm | Plant growth, especially in germination, root growth, and fresh weight in maize plantlets. Used as micronutrient nanofertilizer. | [40] |
| 2 | Ganoderma lucidum extract | Zinc Oxide nanoparticle | Three hexagonal shapes (discs, rods, and pyramidals) | Nanofertilizers properties on Lepidium sativum | [43] |
| 3 | Calcium phosphate (CaP) biological hard tissues | CaP nanoparticles | Round-shaped with a size of 10–25 nm | Multinutrient nanofertilizers | [44] |
| 4 | Using microalgal algal extract | Iron-oxide nanoparticles | Spherical biofabricated Fe3O4-NPs particle size was 76.5 nm | Plant growth stimulant | [45] |
| 5 | Using Lactobacillus casei Subsp. Casei | Copper-oxide nanoparticles | Spherical in shape with 30 nm to 75 nm size range | Plant micronutrient | [46] |
| 6 | Acidophilus, Lactobacillus casei, and Bifdobacterium sp. | Se nanoparticles | Nanoparticle size ranged from 100–500 nm | Plant disease enhancer and nanofertilizer | [47] |
| 7 | Microalgae | Silver nanoparticles | Nanoparticle size ranged from 13 to 31 nm | Act as nanofertilizer, have antioxidative properties | [48] |
2.2. Plant-Mediated Synthesis
| S.N. | Bio-Extract | Nanoparticles | Characterization Results | Applications | Ref |
|---|---|---|---|---|---|
| 1 | Pomegranate peel (PPE) and coffee ground (CE) extracts | Phosphorous-containing hydroxyapatite nanoparticles (nHAP) | Average diameters were 167.5 nm, 153 nm (nHAPs CE), and 229.6 nm, 120.6 nm (nHAPs PPE), respectively | Investigating improvements in Punica granatum L., metabolites, photosynthetic activity, carbohydrate levels, and biocompatibility. | [64] |
| 2 | Using Bamboo | Silicon nanoparticles | - | Soil application, foliar application | [3] |
| using rice husk | |||||
| using sugar beet bagasse | |||||
| 3 | Fruit extracts of Cornelian cherry | Iron-oxide nanoparticles | Spherical shape with size 20 to 40 nm | Stimulation in both root and shoot biomass | [52] |
| 4 | Leaf extract of Aloe barbadensis Mill | Zinc-oxide nanoparticles | Spherical shape with a size of 35 nm | Nutrient source for plant growth | [59] |
| 5 | Flower extract of Elaeagnus Angustifolia | Zinc-oxide nanoparticles | λmax: 330–340 nm, irregular to nearly spherical shape | Germination and metabolic activities of the plant | [65] |
| 6 | Berberis pachyacantha leaf extract (BPL) | Nickel-oxide nanoparticles | Rhombohedral structure with a size of 22.53 nm | Seed germination | [66] |
| 7 | Coriandrum sativum leaves extract | Zinc-oxide nanoparticles | Rod-shaped and polycrystalline with a size of 100 nm | Growing effects of fertilizer on green grain, Turkish gram, and Bengal gram plants | [57] |
| 8 | Clove buds (plant material) | Zinc nanoparticles | - | Growth and yield of Pisum sativum L. | [61] |
| 9 | Leaves of Zataria multiflora Boiss | Zinc nanostructure | - | Foliar application on pomace extract of Punica granatum | [67] |
| 10 | Citrus medica peel extract | Zinc oxide nanoparticles | λmax: 375 nm, the average crystallite size of 20−30 nm | ZnO nanofertilizer improves the growth and yield of Abelmoschus esculentus | [68] |
| 11 | Leaf extract of Parthenium hysterophorus | Zinc oxide nanoparticles | Spherical with a size of 10 nm | Germination of seeds and vegetative growth of Sesamum indicum h | [69] |
| 12 | The seed extract of black seeds (Nigella sativa L.) | Zinc-oxide nanoparticles | Spherical shape with a size of 24 nm | Nano-supplement to improve the production of B. oleracea var. Italicaa | [70] |
| 13 | Vegetable peel extract | Zinc-oxide nanoparticles | - | Boost the value of cluster bean (Cyamopsis tetragonoloba) seeds as well as the higher yield of cluster bean pods | [71] |
| 14 | Cassia occidentalis L. flower extract | Iron-oxide nanoparticles | Irregular surfaces with size 20–50 nm | Enhance germination and overall seedling growth | [72] |
| 15 | Leaf extract of Cassia fistula | Copper-oxide nanoparticles) | λmax: 320 nm, spherical-shaped with size 12–38 nm | Root and foliar application | [73] |
| 16 | Panicum sumatrense grains aqueous extract | Copper-oxide nanoparticles | λmax: 305 nm, crystallite with size 25 nm | Enhance plant growth | [74] |
| 17 | Microalgal algal extract | Iron-oxide nanoparticles | Spherical shape with size 76.5 nm | Plant growth stimulant | [75] |
| 18 | Leaf extracts of Moringa oleifera L | Bimetallic Ag/ZnO nanomaterials | λmax: 366 to 379 nm, spherical shape with sizes from 46 nm to 66 nm | Nitrogen-based fertilizers on biochemical and yield attributes of two wheat varieties | [76] |
| 19 | Peel extract of Citrus reticulate. | Zinc-oxide nanoparticles | Spherical shape with 23–90 nm size Hexagonal structure with 8.89 to 8.62 nm size | Boosting seed germination of Brassica nigra seeds | [77] |
| 20 | Seeds of Juniperus procera | Ag-containing nanoparticles | λmax: 400 and 262 nm, spherical with average size 100 nm | Seed germination | [78] |
3. Agro-Applications of Green Synthesized Nanoparticles
3.1. Silver NPs as Nanofertilizers
3.2. Chitosan Nanoparticles
3.3. Copper NPs as Nanofertilizers
3.4. Iron Nanoparticles as Nanofertilizers
3.5. Zinc Nanoparticles as Nanofertilizers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar] [CrossRef]
- Ball, P. Natural strategies for the molecular engineer. Nanotechnology 2002, 13, R15–R28. [Google Scholar] [CrossRef]
- Snehal, S.; Lohani, P. Silica nanoparticles: Its green synthesis and importance in agriculture. J. Pharmacogn. Phytochem. 2018, 7, 3383–3393. [Google Scholar]
- Carbone, K.; Paliotta, M.; Micheli, L.; Mazzuca, C.; Cacciotti, I.; Nocente, F.; Ciampa, A.; Dell’Abate, M.T. A completely green approach to the synthesis of dendritic silver nanostructures starting from white grape pomace as a potential nanofactory. Arab. J. Chem. 2019, 12, 597–609. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Green nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 145–198. [Google Scholar]
- Khan, S.H.; Alaie, S.A. Green nanomaterials for environmental applications. In Green Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 365–396. [Google Scholar] [CrossRef]
- Aithal, S.; Aithal, P.S. Green Nanotechnology Innovations to Realize UN Sustainable Development Goals 2030. Int. J. Appl. Eng. Manag. Lett. 2021, 5, 96–105. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Khan, S.H. Green Nanotechnology for the Environment and Sustainable Development. In Green Materials for Wastewater Treatment. Environmental Chemistry for a Sustainable World, 2020, 38; Naushad, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. Monit. Manag. 2020, 15, 100418. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. The Role of Beneficial Elements in Triggering Adaptive Responses to Environmental Stressors and Improving Plant Performance. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 137–172. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A. Plant Science Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent Developments on Nanotechnology in Agriculture: Plant Mineral Nutrition, Health, and Interactions with Soil Microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Rameshaiah, G.N.; Pallavi, J.; Shabnam, S. Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015, 3, 314–320. [Google Scholar]
- Mastronardi, E.; Tsae, P.; Zhang, X.; Monreal, C.; DeRosa, M.C. Strategic Role of Nanotechnology in Fertilizers: Potential and Limitations. In Anotechnologies in Food and Agriculture; Springer International Publishing: Cham, Switzerland, 2015; pp. 25–67. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of na-noparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages. In 5th National Conference on Thermophysical Properties: (NCTP-09); AIP Publishing LLC.: Melville, NY, USA, 2016; Volume 1724, p. 020048. [Google Scholar]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-Inspired Green Nanoparticles: Synthesis, Mechanism, and Antibacterial Application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef]
- Abbasifar, A.; ValizadehkKaji, B.; Iravani, M.A. Effect of green synthesized molybdenum nanoparticles on nitrate accumulation and nitrate reductase activity in spinach. J. Plant Nutr. 2019, 43, 13–27. [Google Scholar] [CrossRef]
- Abbasifar, A.; Shahrabadi, F.; ValizadehkKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2017, 25, 10164–10183. [Google Scholar] [CrossRef]
- Solgi, M.; Taghizadeh, M. Biogenic synthesis of metal nanoparticles by plants. In Biogenic Nano-Particles and their Use in Agro-Ecosystems; Springer: Berlin/Heidelberg, Germany, 2020; pp. 593–606. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Aslam, A.A.; Aslam, A.A.; Aslam, M.S.; Quazi, S. An Overview on Green Synthesis of Nanomaterials and Their Advanced Applications in Sustainable Agriculture. Preprints 2022, 2022020315. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2005, 69, 485–492. [Google Scholar] [CrossRef]
- Khan, T.; Abbas, S.; Fariq, A.; Yasmin, A. Microbes: Nature’s cell factories of nanoparticles synthesis. In Exploring the Realms of Nature for Nanosynthesis; Prasad, R., Jha, A.K., Prasad, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 25–50. [Google Scholar]
- Kato, Y.; Suzuki, M. Synthesis of Metal Nanoparticles by Microorganisms. Crystals 2020, 10, 589. [Google Scholar] [CrossRef]
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929. [Google Scholar] [CrossRef]
- Chhipa, H. Mycosynthesis of nanoparticles for smart agricultural practice: A green and eco-friendly approach. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–109. [Google Scholar]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surfaces B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef]
- Bettencourt, G.M.D.F.; Degenhardt, J.; Torres, L.A.Z.; de Andrade Tanobe, V.O.; Soccol, C.R. Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal. Agric. Biotechnol. 2020, 30, 101822. [Google Scholar] [CrossRef]
- Delilah, D.; Mathew, N.M.; Joseph, A.; Jose, S.; Kumar, S. Plant Micronutrient Nanoparticles from Microalgae-Biosynthesis and their Applications–A Review. Int. J. Bot. Stud. 2022, 7, 135–139. [Google Scholar]
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.; Shende, S.; Gaikwad, S.; Ingle, A. Fusarium as a Novel Fungus for the Synthesis of Nanoparticles: Mechanism and Applications. J. Fungi 2021, 7, 139. [Google Scholar] [CrossRef]
- Sedefoglu, N.; Zalaoglu, Y.; Bozok, F. Green synthesized ZnO nanoparticles using Ganoderma lucidum: Characterization and In Vitro Nanofertilizer effects. J. Alloys Compd. 2022, 918, 165695. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-De-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.A.; Allahyari-Devin, M. Biosynthesis of Copper Oxide Nanoparticles Using Lactobacillus casei Subsp. Casei and its Anticancer and Antibacterial Activities. Curr. Nanosci. 2020, 16, 101–111. [Google Scholar] [CrossRef]
- Eszenyi, P.; Sztrik, A.; Babka, B.; Prokisch, J. Elemental, Nano-Sized (100–500 nm) Selenium Production by Probiotic Lactic Acid Bacteria. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 148–152. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; De Morais, M.G. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles–a review on biomolecules involved, characterisation and antibacterial activity. Chem. -Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Rostamizadeh, E.; Iranbakhsh, A.; Majd, A.; Arbabian, S.; Mehregan, I. Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J. Nanostructure Chem. 2020, 10, 125–130. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; El-Aziz, M.E.A.; Elwahed, M.S.A.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019, 3476347. [Google Scholar] [CrossRef]
- Singh, M.; Singh, J.; Sharma, D.; Kaur, B.; Rawat, M. Plant leaves mediated synthesis of semiconductor ZnO nanoparticles and its application for seed germination. In Proceedings of the Recent Advances in Experimental and Theoretical Physics (RAETP-201), Jammu, India, 17–18 April 2018; AIP Publishing LLC.: Melville, NY, USA, 2018; Volume 2006, p. 030031. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Ukidave, V.V.; Ingale, L.T. Green Synthesis of Zinc Oxide Nanoparticles from Coriandrum sativum and Their Use as Fertilizer on Bengal Gram, Turkish Gram, and Green Gram Plant Growth. Int. J. Agron. 2022, 2022, 8310038. [Google Scholar] [CrossRef]
- Prakashraj, R.; Vijayakumar, S.; Punitha, V.N.; Vidhya, E.; Nilavukkarasi, M.; Praseetha, P.K. Fabricated TiO2 Nanofertilizers for Foliar Assimilation to Enhance Yield and Disease Resistance in Capsicum annuum L. J. Plant Growth Regul. 2021, 41, 3387–3394. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.; Bolan, N.; Kim, K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12, 1921. [Google Scholar] [CrossRef]
- Ahmed, S.; Qasim, S.; Ansari, M.; Shah, A.A.; Rehman, H.U.; Shah, M.N.; Ghafoor, U.; Naqvi, S.A.H.; Hassan, M.Z.; Rehman, S.U.; et al. Green synthesis of zinc nanoparticles and their effects on growth and yield of Pisum sativum. J. King Saud Univ. Sci. 2022, 34, 102132. [Google Scholar] [CrossRef]
- Reshma, Z.; Meenal, K. Foliar application of biosynthesised zinc nanoparticles as a strategy for ferti-fortification by improving yield, zinc content and zinc use efficiency in amaranth. Heliyon 2022, 8, e10912. [Google Scholar] [CrossRef]
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green synthesis of anatase titanium dioxide nanoparticles using Cuminum cyminum seed extract; effect on Mung bean (Vigna radiata) seed germination. Inorg. Chem. Commun. 2021, 126, 108485. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef]
- Uddin, S.; Iqbal, J.; Safdar, L.B.; Ahmad, S.; Abbasi, B.A.; Capasso, R.; Kazi, M.; Quraihi, U.M. Green synthesis of BPL-NiONPs using leaf extract of Berberis pachyacantha: Characterization and multiple in vitro biological applications. Molecules 2022, 27, 2064. [Google Scholar] [CrossRef]
- Bahmanzadegan, A.; Tavallali, H.; Tavallali, V.; Karimi, M.A. Variations in biochemical characteristics of Zataria multiflora in response to foliar application of zinc nano complex formed on pomace extract of Punica granatum. Ind. Crop. Prod. 2022, 187, 115369. [Google Scholar] [CrossRef]
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.N.; Nilavukkarasi, M.; Praseetha, P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762. [Google Scholar]
- Sharma, P.; Urfan, M.; Anand, R.; Sangral, M.; Hakla, H.R.; Sharma, S.; Das, R.; Pal, S.; Bhagat, M. Green synthesis of zinc oxide nanoparticles using Eucalyptus lanceolata leaf litter: Characterization, antimicrobial and agricultural efficacy in maize. Physiol. Mol. Biol. Plants 2022, 28, 363–381. [Google Scholar] [CrossRef]
- Awan, S.; Shahzadi, K.; Javad, S.; Tariq, A.; Ahmad, A.; Ilyas, S. A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J. Saudi Soc. Agric. Sci. 2020, 20, 18–24. [Google Scholar] [CrossRef]
- Rexlin, J.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Alharthi, N.S.; Sajjad, M.; Punitha, V.N.; Praseetha, P.K. Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L).Taub. to improve yield and disease resistance. Appl. Nanosci. 2022, 1–9. [Google Scholar] [CrossRef]
- Afzal, S.; Sharma, D.; Singh, N.K. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 40275–40287. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Ahmad, I.S.; Irudayaraj, J.; Javed, S.; Qaiser, U.; Naseem, S. Inhibition mechanism of green-synthesized copper oxide nanoparticles from Cassia fistula towards Fusarium oxysporum by boosting growth and defense response in tomatoes. Environ. Sci. Nano 2021, 8, 1729–1748. [Google Scholar] [CrossRef]
- Velsankar, K.; Parvathy, G.; Mohandoss, S.; Kumar, R.M.; Sudhahar, S. Green synthesis and characterization of CuO nanoparticles using Panicum sumatrense grains extract for biological applications. Appl. Nanosci. 2022, 12, 1993–2021. [Google Scholar] [CrossRef]
- Ehsan, M.; Raja, N.I.; Mashwani, Z.U.R.; Zohra, E.; Abasi, F.; Ikram, M.; Mustafa, N.; Wattoo, F.H.; Proćków, J.; de la Lastra, J.M.P. Effects of Phytogenically Synthesized Bimetallic Ag/ZnO Nanomaterials and Nitrogen-Based Fertilizers on Biochemical and Yield Attributes of Two Wheat Varieties. Nanomaterials 2022, 12, 2894. [Google Scholar] [CrossRef]
- Rafique, M.; Sohaib, M.; Tahir, R.; Tahir, M.B.; Rizwan, M. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Peel Extract of Citrus reticulate for Boosting Seed Germination of Brassica nigra Seeds. J. Nanosci. Nanotechnol. 2021, 21, 3573–3579. [Google Scholar] [CrossRef]
- Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Prakash, O. Chapter-5 the Impact of Chemical Fertilizers on Our Environment and Ecosystem. In Research Trends in Environmental Science, 2nd ed.; AkiNik: Delhi, India, 2019; Volume 35, p. 69. [Google Scholar]
- Itelima, J.U.; Bang, W.J.; Onyimba, I.A.; Sila, M.D.; Egbere, O.J. Bio-fertilizers as key player in enhancing soil fertility and crop productivity: A review. Direct Res. J. Agric. Food Sci. 2018, 6, 73–83. [Google Scholar]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Katya, C.; De Angelis, A.; Claudia, M.; Enrico, S. Microwave-assisted synthesis of catalytic silver nanoparticles by hyperpigmented tomato skins: A green approach. LWT 2020, 133, 110088. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Fouda, M.M.; Abdelsalam, N.R.; El-Naggar, M.E.; Zaitoun, A.F.; Salim, B.M.; Bin-Jumah, M.; Allam, A.; Abo-Marzoka, S.A.; Kandil, E.E. Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int. J. Biol. Macromol. 2020, 149, 1304–1317. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of Metal Nanoparticles on the Soil Microbial Community and Germination of Lettuce Seeds. Water Air, Soil Pollut. 2008, 197, 143–148. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Jhanzab, H.M.; Razzaq, A.; Jilani, G.; Rehman, A.; Hafeez, A.; Yasmeen, F. Silver nano-particles enhance the growth, yield and nutrient use efficiency of wheat. Int. J. Agron. Agri. Res. 2015, 7, 15–22. [Google Scholar]
- Ding, H.; Zhang, Y.; Zhignuol, Z.; Mingjhan, Y. Effect of controlled release fertilizer on the yield and quality of Chinese cabbage and nutrient use efficiency. J. Chang. Veg. 2009, 12, 1–6. [Google Scholar]
- Abbas, M.M. Enhancement of the Nutrients efficiency and Prpductivity of tomato (Lycopersicum esculentum Mill.) plants by using Noano silver. Plant Arch. 2020, 20, 4242–4244. [Google Scholar]
- Sharma, G.; Kumar, A.; Devi, K.A.; Prajapati, D.; Bhagat, D.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Chitosan nanofertilizer to foster source activity in maize. Int. J. Biol. Macromol. 2020, 145, 226–234. [Google Scholar] [CrossRef]
- Hartoyo, A.P.P.; Octaviani, E.A.; Syamani, F.A.; Mulsanti, I.W.; Solikhin, A. Potential of chitosan/carbon nanoparticles and chitosan/lignocellulose nanofiber composite as growth media for peatland paddy seeds. Environ. Res. 2022, 212, 113235. [Google Scholar] [CrossRef]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.-L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2018, 45, 51–63. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; da Silva, S.S. Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 2018, 213, 44–51. [Google Scholar] [CrossRef]
- Bhagat, M.; Anand, R.; Sharma, P.; Rajput, P.; Sharma, N.; Singh, K. Review—Multifunctional Copper Nanoparticles: Synthesis and Applications. ECS J. Solid State Sci. Technol. 2021, 10, 063011. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2013, 77, 367–379. [Google Scholar] [CrossRef]
- Shende, S.; Rathod, D.; Gade, A.; Rai, M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotechnol. 2017, 11, 773–781. [Google Scholar] [CrossRef]
- Saranyaadevi, K.; Subha, V.; Ravindran, R.E.; Renganathan, S. Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int. J. Chemtech Res. 2014, 6, 4533–4541. [Google Scholar]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Lam, P.-L.; Wong, R.S.-M.; Lam, K.-H.; Hung, L.-K.; Wong, M.-M.; Yung, L.-H.; Ho, Y.-W.; Wong, W.-Y.; Hau, D.K.-P.; Gambari, R.; et al. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem. Interactions 2020, 320, 109023. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Devra, V.; Rathore, A. Single-Step Green Synthesis of Iron Nanoparticles in the Aqueous Phase for Catalytic Application in Degradation of Malachite Green. Adv. Energy Convers. Mater. 2021, 3, 16–29. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2020, 28, 1292–1303. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation. Crit. Rev. Environ. Sci. Technol. 2015, 46, 443–466. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2017, 53, 185–201. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Singh, N.B.; Amist, N.; Yadav, K.; Singh, D.; Pandey, J.K.; Singh, S.C. Zinc Oxide Nanoparticles as Fertilizer for the Germination, Growth and Metabolism of Vegetable Crops. J. Nanoeng. Nanomanufacturing 2013, 3, 353–364. [Google Scholar] [CrossRef]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bairwa, P.; Kumar, N.; Devra, V.; Abd-Elsalam, K.A. Nano-Biofertilizers Synthesis and Applications in Agroecosystems. Agrochemicals 2023, 2, 118-134. https://doi.org/10.3390/agrochemicals2010009
Bairwa P, Kumar N, Devra V, Abd-Elsalam KA. Nano-Biofertilizers Synthesis and Applications in Agroecosystems. Agrochemicals. 2023; 2(1):118-134. https://doi.org/10.3390/agrochemicals2010009
Chicago/Turabian StyleBairwa, Preeti, Nimish Kumar, Vijay Devra, and Kamel A. Abd-Elsalam. 2023. "Nano-Biofertilizers Synthesis and Applications in Agroecosystems" Agrochemicals 2, no. 1: 118-134. https://doi.org/10.3390/agrochemicals2010009
APA StyleBairwa, P., Kumar, N., Devra, V., & Abd-Elsalam, K. A. (2023). Nano-Biofertilizers Synthesis and Applications in Agroecosystems. Agrochemicals, 2(1), 118-134. https://doi.org/10.3390/agrochemicals2010009







