Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications
Abstract
1. Introduction
2. Method
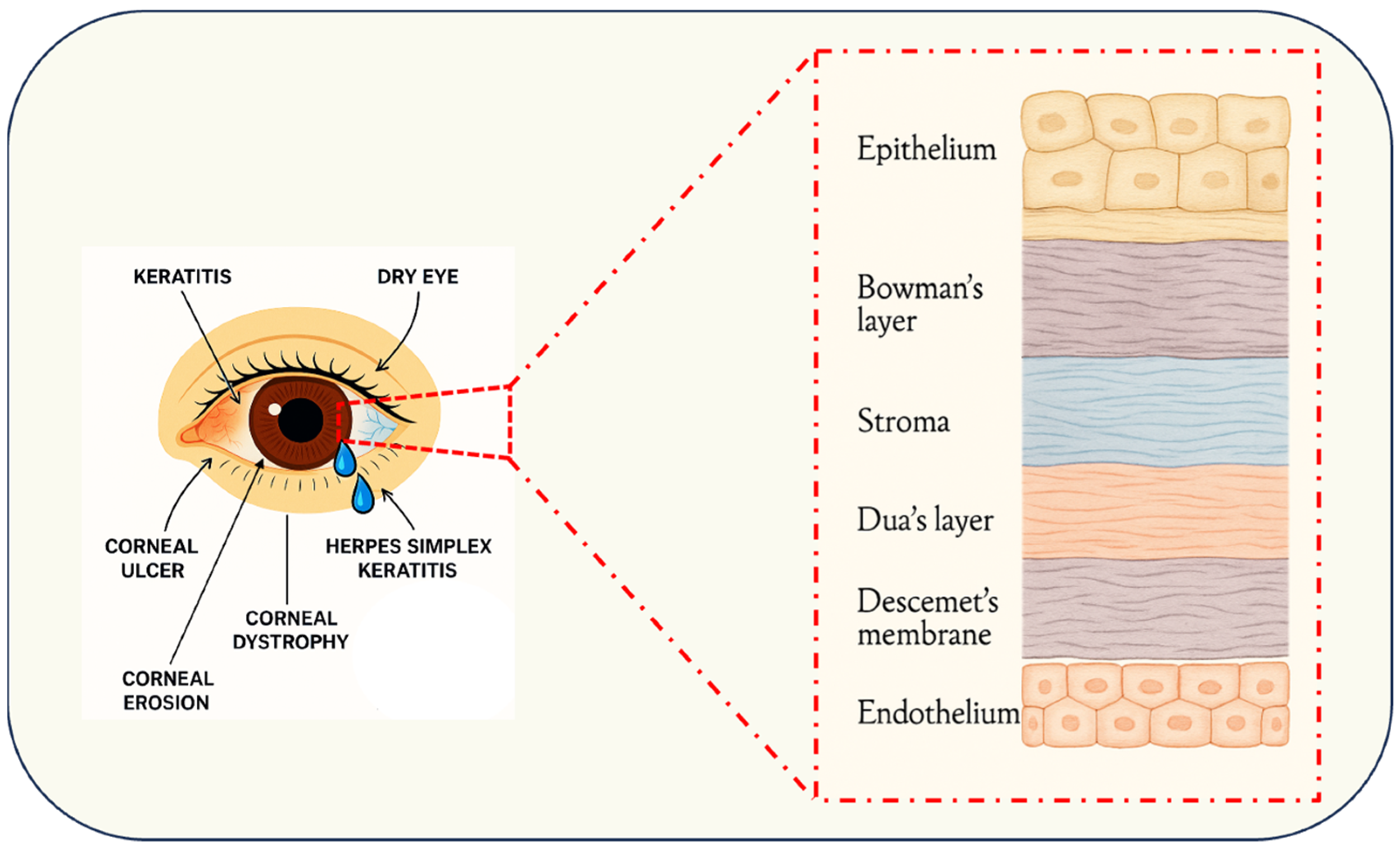
3. Corneal Architecture and Pathological Spectrum
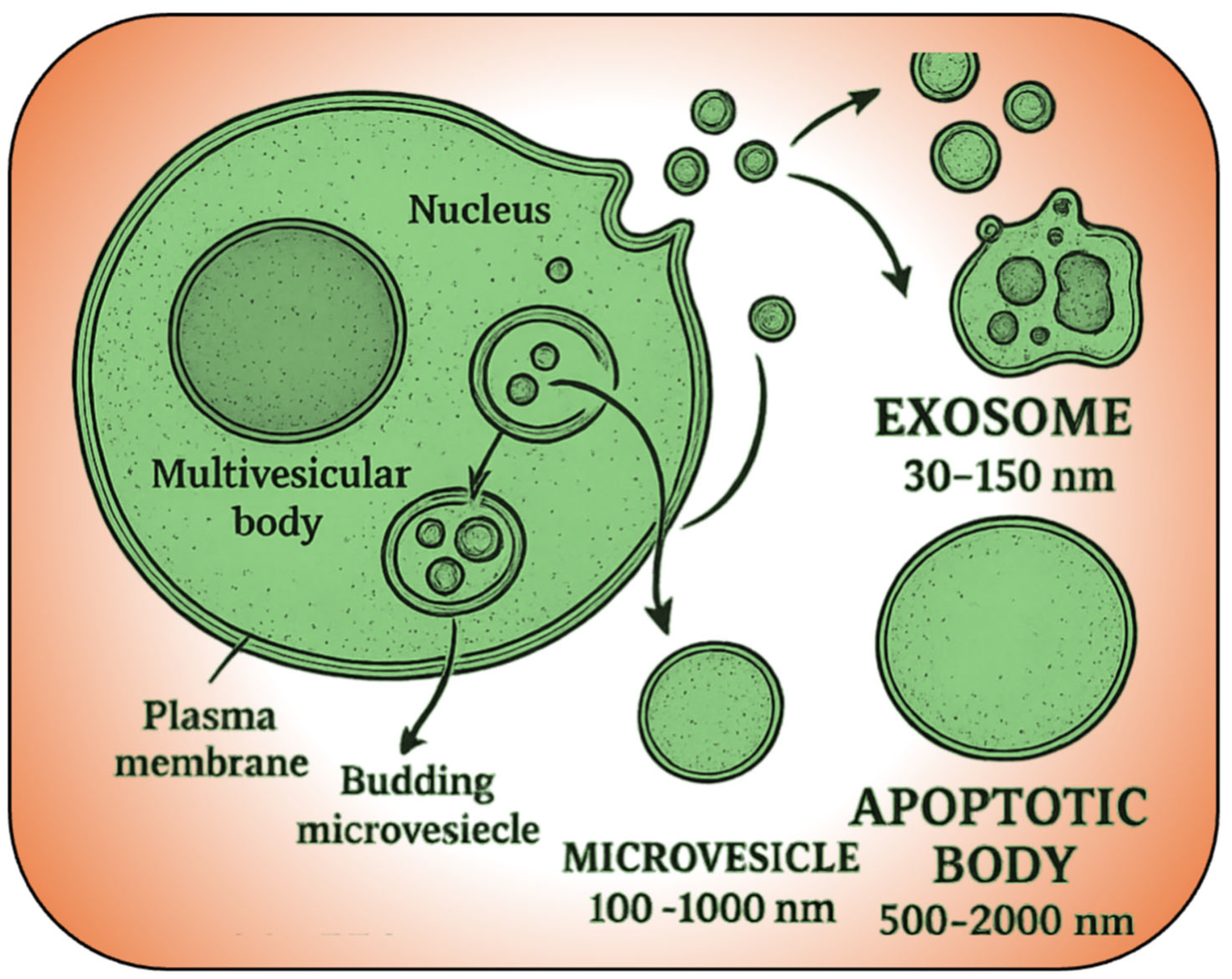
4. EV, Cargo Carriers in Action
5. EV-Associated miRNAs in Corneal Function
| miRNAs | Source Cell | Translational Status | Disease | Target/Pathway | References |
|---|---|---|---|---|---|
| miR-328-3p, miR-532-5p, miR-424-5p. let-7c-5p, miR-665 | Human Corneal stromal cell exosomes | Preclinical | Keratoconus | (unknown) | [118] |
| miR-4466 | Human Corneal stromal exosomes (in vitro); human tears (clinical) | Preclinical | Keratoconus | (unknown; linked to epithelial integrity) | [133] |
| let-7b-5p | Human Corneal Epithelial cell exosomes → macrophages (in vitro) | Preclinical | Fungal keratitis (Aspergillus infection) | Targets SOCS-1; promotes macrophage M1 activation | [134] |
| miR-24-3p | Rabbit ADSC exosomes | Preclinical | Corneal alkali burn/epithelial injury | Upregulates CDC42, EGFR, MMP9; promotes migration | [135] |
| miR-21-5p | Human UMSC exosomes | Preclinical | Corneal epithelial wounding (mechanical) | Downregulates PTEN; activates PI3K/Akt | [102] |
| miR-29b-3p | Mouse BMSC exosomes | Preclinical | General corneal injury (inflammation) | Activates autophagy (↓ PI3K/Akt/mTOR); inhibits NF-κB | [136] |
| miR-19a-3p | Rabbit ADSC exosomes | Preclinical | Corneal stromal fibrosis | Targets HIPK2; anti-fibrotic (↓ collagens, α-SMA) | [137] |
| miR-204-5p | Human (UC-MSC)/Mouse (BMSC) exosomes | Preclinical + Clinical | GVHD-associated dry eye (autoimmune DED) | Targets IL-6/IL-6R/Stat3; anti-inflammatory | [138] |
| miR-223-3p | Mouse adipose MSC exosomes | Preclinical | Dry eye (BAC/scopolamine model) | Targets Fbxw7; anti-inflammatory | [139] |
| miR-127-5p, miR-1273h-3p, miR-1288-5p, miR-130b-5p, miR-139-3p, miR-1910-5p, miR-203b-5p, miR-22-5p, miR-4632-3p | Human tear EVs (clinical study) | Clinical | Non-SS dry eye (tear EVs) | Inflammation-associated (ingenuity analysis) | [107] |
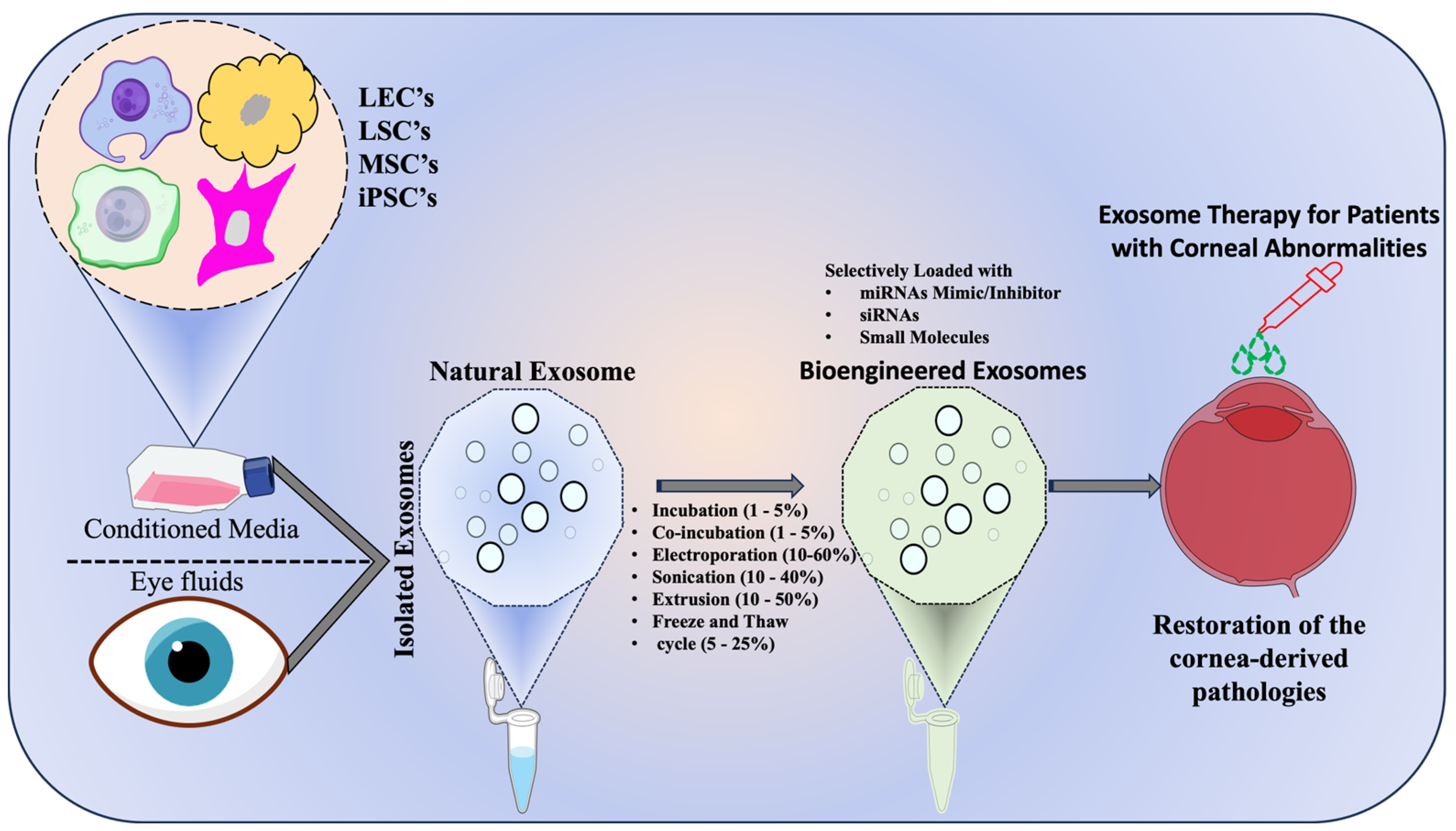
6. Tailored Extracellular Vesicles (TeVs) in Cornea
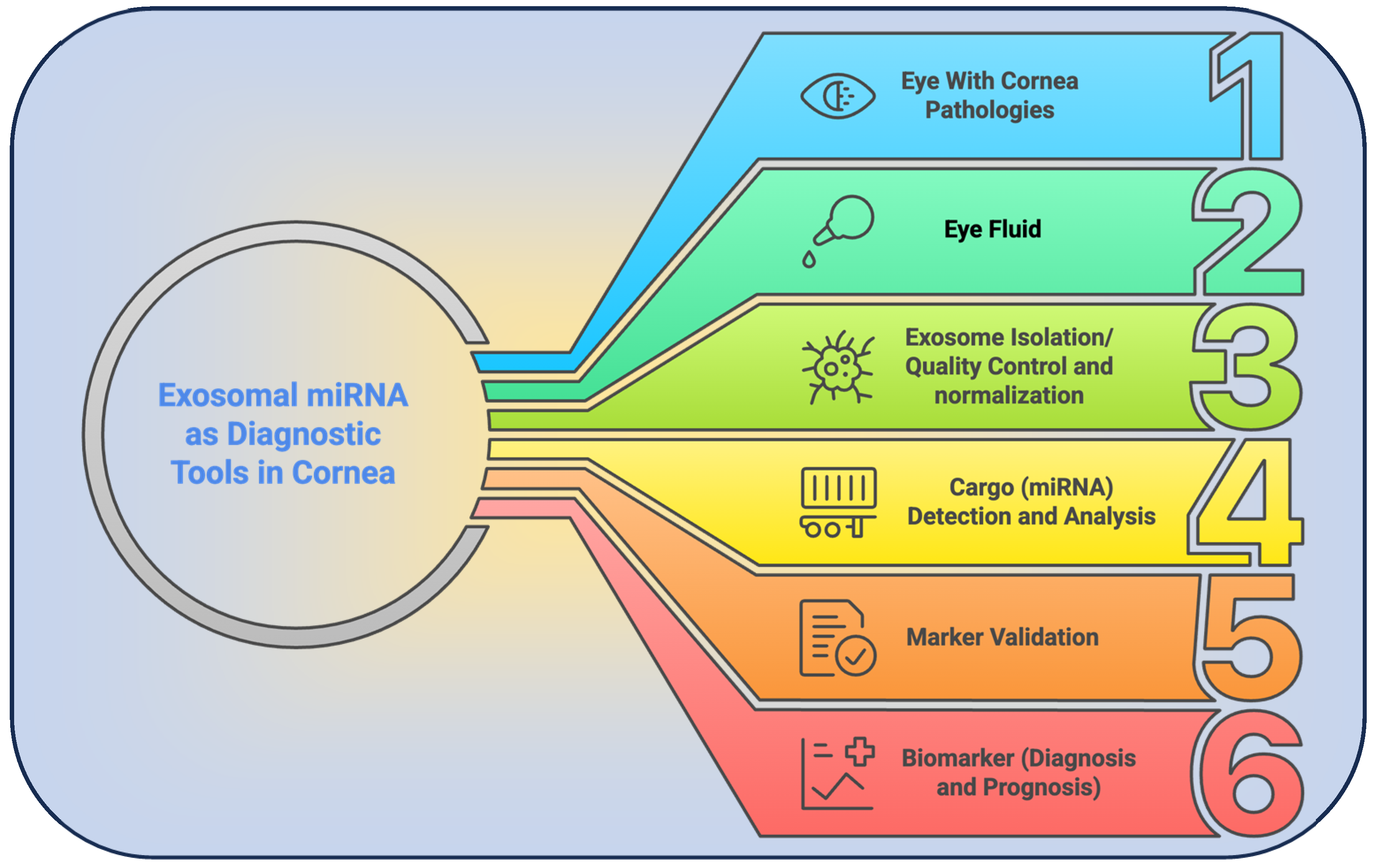
7. Corneal Disease Detection via EV-miRNA Signatures
8. Therapeutic Implications of EV-Associated miRNAs
9. Roadblocks to Breakthroughs
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EV | Extracellular Vesicles |
| FECD | Fuchs endothelial corneal dystrophy |
| LSCD | Limbal Stem Cell Deficiency |
| DLS | Dynamic Light Scattering |
| NTA | Nanoparticle Tracking Analysis |
| LECs | Limbal Epithelial Cells |
| LSCs | Limbal Stromal Cells |
| iPSCs | Induced Pluripotent Stem Cells |
| CSSCs | Corneal Stromal Stem Cells |
| LESCs | Limbal Epithelial Stem Cells |
| LMSCs | Limbal Mesenchymal Stromal Cells |
| MSCs | Mesenchymal Stem Cells |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| OCT | Optical Coherence Tomography |
| UCMSCs | Umbilical Cord-derived Mesenchymal Stem Cells |
| BMSCs | Bone Marrow Stem Cells |
| ADSCs | Adipose-Derived Stem Cells |
References
- Raj, N.; Gupta, N.; Kumar, D.; Vashist, P.; Tandon, R. Population-based study on the prevalence, clinical characteristics and vision-related quality of life in patients with corneal opacity resulting from infectious keratitis: Results from the Corneal Opacity Rural Epidemiological study. Br. J. Ophthalmol. 2023, 107, 476–482. [Google Scholar] [CrossRef]
- Gupta, N.; Vashist, P.; Tandon, R.; Gupta, S.K.; Dwivedi, S.; Mani, K. Prevalence of corneal diseases in the rural Indian population: The Corneal Opacity Rural Epidemiological (CORE) study. Br. J. Ophthalmol. 2015, 99, 147–152. [Google Scholar] [CrossRef]
- Tidke, S.C.; Tidake, P. A Review of Corneal Blindness: Causes and Management. Cureus 2022, 14, e30097. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Z.; Jin, C.; Chen, Q.; Fang, Y.; Jin, J.; Chen, J.; Lu, L.; Tian, H.; Xu, J.; et al. Human amniotic epithelial cell-derived extracellular vesicles provide an extracellular matrix-based microenvironment for corneal injury repair. J. Tissue Eng. 2022, 13, 20417314221122123. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Yeung, V.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles in the Cornea: Insights from Other Tissues. Anal. Cell. Pathol. 2021, 2021, 9983900. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, D.K.; Chirikov, V.; Schmier, J.; Rege, S.; Newton, S. Cost Burden of Endothelial Keratoplasty in Fuchs Endothelial Dystrophy: Real-World Analysis of a Commercially Insured US Population (2014–2019). Clin. Ophthalmol. 2022, 16, 1055–1067. [Google Scholar] [CrossRef]
- Lin, Y.; Anderson, J.D.; Rahnama, L.M.A.; Gu, S.V.; Knowlton, A.A. Exosomes in disease and regeneration: Biological functions, diagnostics, and beneficial effects. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1162–H1180. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Gu, X.; Sheng, X.; Xiao, L.; Wang, X. Exosomes: New regulators of reproductive development. Mater. Today Bio 2023, 19, 100608. [Google Scholar] [CrossRef]
- Sun, B.; Peng, J.; Wang, S.; Liu, X.; Zhang, K.; Zhang, Z.; Wang, C.; Jing, X.; Zhou, C.; Wang, Y. Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev. Neurosci. 2018, 29, 531–546. [Google Scholar] [CrossRef]
- Lasser, C. Exosomes in diagnostic and therapeutic applications: Biomarker, vaccine and RNA interference delivery vehicle. Expert Opin. Biol. Ther. 2015, 15, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Rupp, A.-K.; Altevogt, P. Exosomes as a Potential Tool for a Specific Delivery of Functional Molecules. In Ovarian Cancer: Methods and Protocols; Malek, A., Tchernitsa, O., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 495–511. [Google Scholar]
- Kushch, A.A.; Ivanov, A.V. Exosomes in the life cycle of viruses and the pathogenesis of viral infections. Vopr. Virusol. 2023, 68, 181–197. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Chen, V.T.; Herbst, P.; Zhang, R.; Elfert, A.; Krishan, A.; Azar, D.T.; Chang, J.H.; Hu, W.Y.; Kremsmayer, T.P.; et al. Target specification and therapeutic potential of extracellular vesicles for regulating corneal angiogenesis, lymphangiogenesis, and nerve repair. Ocul. Surf. 2024, 34, 459–476. [Google Scholar] [CrossRef]
- Robbins, B.T.; Montreuil, K.A.; Kundu, N.; Kumar, P.; Agrahari, V. Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue. Pharmaceutics 2024, 16, 1424. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Pan, G. Targeting the Ophthalmic Diseases Using Extracellular Vesicles ‘Exosomes’: Current Insights on Their Clinical Approval and Present Trials. Aging Dis. 2024, 16, 1225–1241. [Google Scholar] [CrossRef]
- Verma, N.; Arora, S.; Singh, A.K.; Ahmed, J. Unlocking the potential of exosomes ‘extracellular vesicles’: Drug delivery advancements and therapeutics in ocular diseases. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, A.; Verma, S.; Stephenson, S.; Bhowmick, T.; Sangwan, V.S. Mini Review: Current Trends and Understanding of Exosome Therapeutic Potential in Corneal Diseases. Front. Pharmacol. 2021, 12, 684712. [Google Scholar] [CrossRef]
- Blanco-Agudín, N.; Ye, S.; González-Fernández, S.; Alcalde, I.; Merayo-Lloves, J.; Quirós, L.M. Exosomes in Ocular Health: Recent Insights into Pathology, Diagnostic Applications and Therapeutic Functions. Biomedicines 2025, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Buono, L.; Scalabrin, S.; De Iuliis, M.; Tanzi, A.; Grange, C.; Tapparo, M.; Nuzzi, R.; Bussolati, B. Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Human Corneal Endothelial Cells from Endoplasmic Reticulum Stress-Mediated Apoptosis. Int. J. Mol. Sci. 2021, 22, 4930. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Aguennouz, M.; Aragona, E.; Gargano, R.; Oliverio, G.W.; Inferrera, L.; Aragona, P. Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact. Genes 2024, 15, 816. [Google Scholar] [CrossRef]
- Sanroque-Muñoz, M.; Garcia, S.G.; Clos-Sansalvador, M.; Font-Morón, M.; Botella-Garcia, J.; Franquesa, M.; Loscos-Arenas, J.; Borràs, F.E. miRNAs from tear fluid-derived extracellular vescicles to identify patients at high risk of fibrotic lesions after glaucoma surgery. Acta Ophthalmol. 2025, 103. [Google Scholar] [CrossRef]
- Arora, S.; Verma, N. Exosomal microRNAs as potential biomarkers and therapeutic targets in corneal diseases. Mol. Vis. 2024, 30, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Luo, S.; Yang, C.; Zhong, G.; Huang, G.; Zhang, X.; Li, B.; Liu, C.; Li, L.; et al. DNA Nanowire Guided-Catalyzed Hairpin Assembly Nanoprobe for In Situ Profiling of Circulating Extracellular Vesicle-Associated MicroRNAs. ACS Sens. 2022, 7, 1075–1085. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Chen, Q.; Wei, Y.; Wei, Z.; Jin, Z.B.; Liang, Q. Extracellular Vesicle MicroRNAs From Corneal Stromal Stem Cell Enhance Stemness of Limbal Epithelial Stem Cells by Targeting the Notch Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 42. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Salem, N.M.; Al-Atabany, W. Impact of Post–Refractive Surgeries on Corneal Biomechanics—A Review. J. Clin. Eng. 2021, 46, 177–188. [Google Scholar] [CrossRef]
- Willoughby, C.E.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function—A review. Clin. Exp. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- McKay, T.B.; Seyed-Razavi, Y.; Ghezzi, C.E.; Dieckmann, G.; Nieland, T.J.F.; Cairns, D.M.; Pollard, R.E.; Hamrah, P.; Kaplan, D.L. Corneal pain and experimental model development. Prog. Retin. Eye Res. 2019, 71, 88–113. [Google Scholar] [CrossRef]
- Guerrero-Moreno, A.; Baudouin, C.; Melik Parsadaniantz, S.; Reaux-Le Goazigo, A. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells-Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef]
- Sprogyte, L.; Park, M.; Girolamo, N.D. Pathogenesis of Alkali Injury-Induced Limbal Stem Cell Deficiency: A Literature Survey of Animal Models. Cells 2023, 12, 1294. [Google Scholar] [CrossRef]
- Loukovitis, E.; Kozeis, N.; Gatzioufas, Z.; Kozei, A.; Tsotridou, E.; Stoila, M.; Koronis, S.; Sfakianakis, K.; Tranos, P.; Balidis, M.; et al. The Proteins of Keratoconus: A Literature Review Exploring Their Contribution to the Pathophysiology of the Disease. Adv. Ther. 2019, 36, 2205–2222. [Google Scholar] [CrossRef]
- Wisse, R.P.; Kuiper, J.J.; Gans, R.; Imhof, S.; Radstake, T.R.; Van der Lelij, A. Cytokine Expression in Keratoconus and its Corneal Microenvironment: A Systematic Review. Ocul. Surf. 2015, 13, 272–283. [Google Scholar] [CrossRef]
- Navel, V.; Malecaze, J.; Belville, C.; Choltus, H.; Henrioux, F.; Dutheil, F.; Malecaze, F.; Chiambaretta, F.; Blanchon, L.; Sapin, V. Dysregulation of Receptor for Advanced Glycation End Products (RAGE) Expression as a Biomarker of Keratoconus. Dis. Markers 2022, 2022, 1543742. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Salvatore, S.; Vingolo, E.M. Corneal sensitivity in keratoconus: A review of the literature. Sci. World J. 2013, 2013, 683090. [Google Scholar] [CrossRef]
- Okumura, N.; Hayashi, R.; Nakano, M.; Yoshii, K.; Tashiro, K.; Sato, T.; Blake, D.J.; Aleff, R.; Butz, M.; Highsmith, E.W.; et al. Effect of Trinucleotide Repeat Expansion on the Expression of TCF4 mRNA in Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 779–786. [Google Scholar] [CrossRef]
- Okumura, N.; Hayashi, R.; Koizumi, N. Perspective of Future Potent Therapies for Fuchs Endothelial Corneal Dystrophy. Open Ophthalmol. J. 2018, 12, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Gattey, D.; Zhu, A.Y.; Stagner, A.; Terry, M.A.; Jun, A.S. Fuchs endothelial corneal dystrophy in patients with myotonic dystrophy: A case series. Cornea 2014, 33, 96–98. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Cai, Y.; Shahipasand, S.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Wilkins, M.R. En face optical coherence tomography angiography for corneal neovascularisation. Br. J. Ophthalmol. 2016, 100, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Barbariga, M.; Vallone, F.; Mosca, E.; Bignami, F.; Magagnotti, C.; Fonteyne, P.; Chiappori, F.; Milanesi, L.; Rama, P.; Andolfo, A.; et al. The role of extracellular matrix in mouse and human corneal neovascularization. Sci. Rep. 2019, 9, 14272. [Google Scholar] [CrossRef]
- Donthula, G.; Daigavane, S. Secondary Glaucoma Following Corneal Transplantation: A Comprehensive Review of Pathophysiology and Therapeutic Approaches. Cureus 2024, 16, e69882. [Google Scholar] [CrossRef]
- Kitamoto, K.; Taketani, Y.; Fujii, W.; Inamochi, A.; Toyono, T.; Miyai, T.; Yamagami, S.; Kuroda, M.; Usui, T.; Ouchi, Y. Generation of mouse model of TGFBI-R124C corneal dystrophy using CRISPR/Cas9-mediated homology-directed repair. Sci. Rep. 2020, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Vemuganti, G.K.; Rathi, V.M.; Murthy, S.I. Histological Landmarks in Corneal Dystrophy: Pathology of Corneal Dystrophies. In Corneal Dystrophies; Seitz, B., Lisch, W., Eds.; S.Karger AG: Basel, Switzerland, 2011; Volume 48, pp. 24–50. [Google Scholar]
- Latifi, G. Corneal Dystrophies. In In Vivo Confocal Microscopy in Eye Disease; Latifi, G., Hau, S., Eds.; Springer: London, UK, 2022; pp. 61–89. [Google Scholar] [CrossRef]
- Finis, D.; Stammen, J.; Lisch, W.; Geerling, G. Epithelial Dystrophies of the Cornea. Klin. Monbl. Augenheilkd. 2019, 236, e23–e36. [Google Scholar] [CrossRef]
- Alemi, H.; Dehghani, S.; Forouzanfar, K.; Surico, P.L.; Narimatsu, A.; Musayeva, A.; Sharifi, S.; Wang, S.; Dohlman, T.H.; Yin, J.; et al. Insights into mustard gas keratopathy-characterizing corneal layer-specific changes in mice exposed to nitrogen mustard. Exp. Eye Res. 2023, 236, 109657. [Google Scholar] [CrossRef]
- Dua, S.H.; Darren, S.J.; Mouhamed Al-Aqaba, T.; Said, D.G. Pathophysiology of Keratoconus. In Keratoconus; Izquierdo, L., Henriquez, M., Mannis, M., Eds.; Elsevier: New Delhi, India, 2023; pp. 51–64. [Google Scholar]
- Zhou, H. Deciphering cell fates of the human cornea with a multi-omics meta atlas. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Vincent, A.L.; Rootman, D.; Munier, F.L.; Héon, E. A Molecular Perspective on Corneal Dystrophies. In Genetics in Ophthalmology; Wissinger, B., Kohl, S., Langenbeck, U., Eds.; S.Karger AG: Basel, Switzerland, 2003; Volume 37, pp. 50–66. [Google Scholar]
- Lee, Y.F.; Yong, D.W.W.; Manotosh, R. A Review of Contact Lens-Induced Limbal Stem Cell Deficiency. Biology 2023, 12, 1490. [Google Scholar] [CrossRef]
- Matai, H.; Agarwal, S.; Srinivasan, B.; Iyer, G. Simple Limbal Epithelial Transplantation. In Current Advances in Ocular Surgery; Tsui, E., Fung, S.S.M., Singh, R.B., Eds.; Current Practices in Ophthalmology; Springer Nature: Singapore, 2023; pp. 189–199. [Google Scholar]
- Rossen, J.; Amram, A.; Milani, B.; Park, D.; Harthan, J.; Joslin, C.; McMahon, T.; Djalilian, A. Contact Lens-induced Limbal Stem Cell Deficiency. Ocul. Surf. 2016, 14, 419–434. [Google Scholar] [CrossRef]
- Nureen, L.; Di Girolamo, N. Limbal Epithelial Stem Cells in the Diabetic Cornea. Cells 2023, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liang, Q.F. Research progress on imagine diagnosis of limbal stem cell deficiency. [Zhonghua yan ke za Zhi] Chin. J. Ophthalmol. 2023, 59, 673–676. [Google Scholar] [CrossRef]
- Rama, P. Limbal Stem Cell Deficiency in Inflammatory Disorders. In Complications in Uveitis; Pichi, F., Neri, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 13–21. [Google Scholar]
- Lagali, N. Cellular insights and new therapeutic approaches for limbal stem cell deficiency. Acta Ophthalmol. 2025, 103. [Google Scholar] [CrossRef]
- Lagali, N. The limbal stem cell niche in development, insights from mouse models and children with aniridia. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Hu, J.C.W.; Trief, D. A narrative review of limbal stem cell deficiency & severe ocular surface disease. Ann. Eye Sci. 2023, 8, 13. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Goncalves, D.; Pinto, S.N.; Fernandes, F. Extracellular Vesicles and Infection: From Hijacked Machinery to Therapeutic Tools. Pharmaceutics 2023, 15, 1738. [Google Scholar] [CrossRef]
- Stahl, A.L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Roucourt, B.; Meeussen, S.; Bao, J.; Zimmermann, P.; David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015, 25, 412–428. [Google Scholar] [CrossRef]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Thery, C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, S.; Patel, T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Brief. Funct. Genom. 2016, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Seo, B.; Hussaini, H.M.; Hibma, M.; Rich, A.M. Comparing Two Methods for the Isolation of Exosomes. J. Nucleic Acids 2022, 2022, 8648373. [Google Scholar] [CrossRef]
- Habibian, A.; Soleimanjahi, H.; Hashemi, S.M.; Babashah, S. Characterization and Comparison of Mesenchymal Stem Cell-Derived Exosome Isolation Methods using Culture Supernatant. Arch. Razi Inst. 2022, 77, 1383–1388. [Google Scholar] [CrossRef]
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol. Biotechnol. 2021, 63, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nalabotala, R.; Koo, K.M.; Bose, S.; Nayak, R.; Shiddiky, M.J.A. Separation of distinct exosome subpopulations: Isolation and characterization approaches and their associated challenges. Analyst 2021, 146, 3731–3749. [Google Scholar] [CrossRef]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 2022, 10, 1100892. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Zhang, Z.; Zuo, C.; Wu, H.; Liu, Y. The Advances and Applications of Characterization Technique for Exosomes: From Dynamic Light Scattering to Super-Resolution Imaging Technology. Photonics 2024, 11, 101. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Pariset, E.; Agache, V.; Millet, A. Extracellular Vesicles: Isolation Methods. Adv. Biosyst. 2017, 1, e1700040. [Google Scholar] [CrossRef] [PubMed]
- Shami-Shah, A.; Travis, B.G.; Walt, D.R. Advances in extracellular vesicle isolation methods: A path towards cell-type specific EV isolation. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, J.Y.; Lee, J.H.; Lee, H.H.; Knowles, J.C.; Kim, H.W. Emerging biogenesis technologies of extracellular vesicles for tissue regenerative therapeutics. J. Tissue Eng. 2021, 12, 20417314211019015. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.S.; Ahn, Y.; Im, Y.J.; Kim, S.S.; Lee, K.H.; Kim, J.; Choi, Y.; Lee, D.; Kang, E.; Jin, G.; et al. The characterization of exosomes from fibrosarcoma cell and the useful usage of Dynamic Light Scattering (DLS) for their evaluation. PLoS ONE 2021, 16, e0231994. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 22. [Google Scholar] [CrossRef]
- Nolte-‘t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Vogel, R.; Coumans, F.A.; Maltesen, R.G.; Boing, A.N.; Bonnington, K.E.; Broekman, M.L.; Broom, M.F.; Buzas, E.I.; Christiansen, G.; Hajji, N.; et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles 2016, 5, 31242. [Google Scholar] [CrossRef]
- Sina, A.A.; Vaidyanathan, R.; Wuethrich, A.; Carrascosa, L.G.; Trau, M. Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal. Bioanal. Chem. 2019, 411, 1311–1318. [Google Scholar] [CrossRef]
- Guerrini, L.; Garcia-Rico, E.; O’Loghlen, A.; Giannini, V.; Alvarez-Puebla, R.A. Surface-Enhanced Raman Scattering (SERS) Spectroscopy for Sensing and Characterization of Exosomes in Cancer Diagnosis. Cancers 2021, 13, 2179. [Google Scholar] [CrossRef]
- Yurtsever, A.; Yoshida, T.; Badami Behjat, A.; Araki, Y.; Hanayama, R.; Fukuma, T. Structural and mechanical characteristics of exosomes from osteosarcoma cells explored by 3D-atomic force microscopy. Nanoscale 2021, 13, 6661–6677. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell-Cell Communication in the Cornea. Anat. Rec. 2020, 303, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Zhang, Y.; Zhao, S. Expression and function of microRNAs in the cornea. [Zhonghua yan ke za Zhi] Chin. J. Ophthalmol. 2015, 51, 229–235. [Google Scholar]
- Yeung, V.; Zhang, T.C.; Yuan, L.; Parekh, M.; Cortinas, J.; Delavogia, E.; Hutcheon, A.E.K.; Guo, X.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Myofibroblasts Promote Corneal Epithelial Cell Migration. Int. J. Mol. Sci. 2022, 23, 3136. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastrù, W.; Tolosano, E.; Bussolati, B.; et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells 2022, 11, 3892. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver miR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Zou, X.Y.; Yu, Y.; Lin, S.; Zhong, L.; Sun, J.; Zhang, G.; Zhu, Y. Comprehensive miRNA Analysis of Human Umbilical Cord-Derived Mesenchymal Stromal Cells and Extracellular Vesicles. Kidney Blood Press. Res. 2018, 43, 152–161. [Google Scholar] [CrossRef]
- Alli, A.A. Mechanisms of Extracellular Vesicle Biogenesis, Cargo Loading, and Release. In Extracellular Vesicles-Role in Diseases, Pathogenesis and Therapy; Paul, M.K., Ed.; Physiology; IntechOpen: London, UK, 2022; p. 338. [Google Scholar] [CrossRef]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020, 9, 1080. [Google Scholar] [CrossRef]
- Pucker, A.D.; Ngo, W.; Postnikoff, C.K.; Fortinberry, H.; Nichols, J.J. Tear Film miRNAs and Their Association With Human Dry Eye Disease. Curr. Eye Res. 2022, 47, 1479–1487. [Google Scholar] [CrossRef]
- Lee, S.; Ko, J.H.; Kim, S.N. The Extracellular MicroRNAs on Inflammation: A Literature Review of Rodent Studies. Biomedicines 2022, 10, 1601. [Google Scholar] [CrossRef]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef] [PubMed]
- Stunf Pukl, S. Are miRNAs Dynamic Biomarkers in Keratoconus? A Review of the Literature. Genes 2022, 13, 588. [Google Scholar] [CrossRef]
- Dou, S.; Wang, Q.; Zhang, B.; Wei, C.; Wang, H.; Liu, T.; Duan, H.; Jiang, H.; Liu, M.; Qi, X.; et al. Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell Discov. 2022, 8, 66. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ng, T.K.; Choy, K.W.; Wong, H.K.; Chu, W.K.; Pang, C.P.; Jhanji, V. Histological and microRNA Signatures of Corneal Epithelium in Keratoconus. J. Refract. Surg. 2018, 34, 201–211. [Google Scholar] [CrossRef]
- Ouyang, S.; Ma, J.; Sun, Q.; Li, J.; Chen, Y.; Luo, L. Comprehensive Bioinformatics Analysis to Reveal Key RNA Targets and Hub Competitive Endogenous RNA Network of Keratoconus. Front. Genet. 2022, 13, 896780. [Google Scholar] [CrossRef]
- Akoto, T.; Cai, J.; Nicholas, S.; McCord, H.; Estes, A.J.; Xu, H.; Karamichos, D.; Liu, Y. Unravelling the Impact of Cyclic Mechanical Stretch in Keratoconus—A Transcriptomic Profiling Study. Int. J. Mol. Sci. 2023, 24, 7437. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Asada, K.; Ueno, M.; Hiramoto, N.; Fujita, T.; Toda, M.; Sotozono, C.; Kinoshita, S.; Hamuro, J. Cellular Interplay Through Extracellular Vesicle miR-184 Alleviates Corneal Endothelium Degeneration. Ophthalmol. Sci. 2022, 2, 100212. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Q.; Wei, Z.; Xu, X.; Han, D.; Zhang, Y.; Chen, Z.; Liang, Q. MicroRNAs of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the IRAK1/TAB2/NF-kappaB pathway. Ocul. Surf. 2023, 28, 131–140. [Google Scholar] [CrossRef]
- Lozano, V.; Martín, C.; Blanco, N.; Alcalde, I.; Fernandez-Vega Cueto, L.; Merayo-Lloves, J.; Quirós, L.M. Exosomes Released by Corneal Stromal Cells Show Molecular Alterations in Keratoconus Patients and Induce Different Cellular Behavior. Biomedicines 2022, 10, 2348. [Google Scholar] [CrossRef]
- Hadvina, R.; Lotfy Khaled, M.; Akoto, T.; Zhi, W.; Karamichos, D.; Liu, Y. Exosomes and their miRNA/protein profile in keratoconus-derived corneal stromal cells. Exp. Eye Res. 2023, 236, 109642. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Liu, Y.; Wang, J.; Zhu, R.; Jin, X.; Zhang, H. Effect of Tear Derived Exosomes on Corneal Epithelial Cells During Diabetic Keraropathy. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef]
- Tati, V.; Mitra, S.; Basu, S.; Shukla, S. Bone marrow mesenchymal stem cell-derived extracellular vesicles promote corneal epithelial repair and suppress apoptosis via modulation of Caspase-3 in vitro. FEBS Open Bio 2024, 14, 968–982. [Google Scholar] [CrossRef]
- Casado-Santos, A.; Gonzalez-Cubero, E.; Gonzalez-Fernandez, M.L.; Gonzalez-Rodriguez, Y.; Garcia-Rodriguez, M.B.; Villar-Suarez, V. Equine Corneal Wound Healing Using Mesenchymal Stem Cell Secretome: Case Report. Animals 2024, 14, 1842. [Google Scholar] [CrossRef]
- Kumar, A.; Li, Y.; Mallick, S.; Yang, E.; Dhaliwal, D.K.; Price, A.; Xie, T.; Du, Y. Stem Cell Secretome Promotes Scarless Corneal Wound Healing and Rescues Corneal Sensory Nerves. BioRxiv 2022, 2022, 490347. [Google Scholar] [CrossRef]
- Bhujel, B.; Oh, S.H.; Kim, C.M.; Yoon, Y.J.; Kim, Y.J.; Chung, H.S.; Ye, E.A.; Lee, H.; Kim, J.Y. Mesenchymal Stem Cells and Exosomes: A Novel Therapeutic Approach for Corneal Diseases. Int. J. Mol. Sci. 2023, 24, 10917. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Bojana Simovic, M.; Crissy, F.; Aleksandar, A.; Valentin, D.; Nebojsa, A.; Vladislav, V. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Cell Biol. Transl. Med. 2018, 1089, 47–57. [Google Scholar]
- Elyan, M.S.; Nada, H.F.; Hamam, G.G.; El Din Bayomi, N.S. Effect of Mesenchymal Stem Cells-Derived Exosomes on Healing of a Rabbit Model of Corneal Alkali Burn. Histological and Immunohistochemical Study. QJM Int. J. Med. 2024, 117, hcae070. [Google Scholar] [CrossRef]
- Ong, H.S.; Riau, A.K.; Yam, G.H.-F.; Yusoff, N.Z.B.M.; Han, E.J.Y.; Goh, T.-W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Verma, N.; Khare, D.; Poe, A.J.; Amador, C.; Ghiam, S.; Fealy, A.; Ebrahimi, S.; Shadrokh, O.; Song, X.Y.; Santiskulvong, C.; et al. MicroRNA and Protein Cargos of Human Limbal Epithelial Cell-Derived Exosomes and Their Regulatory Roles in Limbal Stromal Cells of Diabetic and Non-Diabetic Corneas. Cells 2023, 12, 2524. [Google Scholar] [CrossRef]
- Kistenmacher, S.; Schwammle, M.; Martin, G.; Ulrich, E.; Tholen, S.; Schilling, O.; Giessl, A.; Schlotzer-Schrehardt, U.; Bucher, F.; Schlunck, G.; et al. Enrichment, Characterization, and Proteomic Profiling of Small Extracellular Vesicles Derived from Human Limbal Mesenchymal Stromal Cells and Melanocytes. Cells 2024, 13, 623. [Google Scholar] [CrossRef]
- Kobal, N.; Marzidovsek, M.; Schollmayer, P.; Malicev, E.; Hawlina, M.; Marzidovsek, Z.L. Molecular and Cellular Mechanisms of the Therapeutic Effect of Mesenchymal Stem Cells and Extracellular Vesicles in Corneal Regeneration. Int. J. Mol. Sci. 2024, 25, 11121. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef]
- Tavakkoli, F.; Eleiwa, T.K.; Elhusseiny, A.M.; Damala, M.; Rai, A.K.; Cheraqpour, K.; Ansari, M.H.; Doroudian, M.; Keshel, S.H.; Soleimani, M.; et al. Corneal stem cells niche and homeostasis impacts in regenerative medicine; concise review. Eur. J. Ophthalmol. 2023, 33, 1536–1552. [Google Scholar] [CrossRef]
- Altman, J.; Jones, G.; Ahmed, S.; Sharma, S.; Sharma, A. Tear Film MicroRNAs as Potential Biomarkers: A Review. Int. J. Mol. Sci. 2023, 24, 3694. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X. Exosomal let-7b-5p derived from Aspergillus fumigatus-treated human corneal epithelial cells promotes M1 macrophage activation via targeting SOCS-1. Front. Immunol. 2025, 16, 1548802. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Lu, P.; Wang, Y.; Xing, S.; Yan, Y.; Han, R.; Hao, P.; Li, X. Mesenchymal Stem Cell-Derived Exosomes as Drug Carriers for Delivering miRNA-29b to Ameliorate Inflammation in Corneal Injury Via Activating Autophagy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 16. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging 2020, 12, 4093–4110. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Liu, Y.; Yang, M.; Zeng, L. Mesenchymal Stem Cells-Derived Exosomal miR-223-3p Alleviates Ocular Surface Damage and Inflammation by Downregulating Fbxw7 in Dry Eye Models. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Xia, J.; Xiao, Y.; Khan, N.; Jiang, B.; Liang, Y.; Duan, L. Micro Trojan horses: Engineering extracellular vesicles crossing biological barriers for drug delivery. Bioeng. Transl. Med. 2024, 9, e10623. [Google Scholar] [CrossRef]
- Liu, G.-S.; Chen, H.-A.; Chang, C.-Y.; Chen, Y.-J.; Wu, Y.-Y.; Widhibrata, A.; Yang, Y.-H.; Hsieh, E.-H.; Delila, L.; Lin, I.C.; et al. Innovative platelet-derived extracellular vesicle drug delivery system for the treatment of corneal neovascularization. bioRxiv 2024, 2024, 614855. [Google Scholar] [CrossRef]
- Fan, Z.; Jiang, C.; Wang, Y.; Wang, K.; Marsh, J.; Zhang, D.; Chen, X.; Nie, L. Engineered extracellular vesicles as intelligent nanosystems for next-generation nanomedicine. Nanoscale Horiz. 2022, 7, 682–714. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.N.; Tian, C. Engineered Extracellular Vesicles: Emerging Therapeutic Strategies for Translational Applications. Int. J. Mol. Sci. 2023, 24, 15206. [Google Scholar] [CrossRef]
- Su, Y.; Chen, M.; Xu, W.; Gu, P.; Fan, X. Advances in Extracellular-Vesicles-Based Diagnostic and Therapeutic Approaches for Ocular Diseases. ACS Nano 2024, 18, 22793–22828. [Google Scholar] [CrossRef]
- Durmaz, E.; Dribika, L.; Kutnyanszky, M.; Mead, B. Utilizing extracellular vesicles as a drug delivery system in glaucoma and RGC degeneration. J. Control. Release 2024, 372, 209–220. [Google Scholar] [CrossRef]
- Lybecker, J.R.; Van de Ven, A.; Braesch-Andersen, K.; Juriga, D.; Norein, N.; Hansson, P.; Samanta, A. Comprehensive Characterization and Sustained, on-Demand Delivery of Corneal Epithelial Extracellular Vesicles for Healing Corneal Epithelium. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Puistola, P.; Kethiri, A.; Nurminen, A.; Turkki, J.; Hopia, K.; Miettinen, S.; Moro, A.; Skottman, H. Cornea-Specific Human Adipose Stem Cell-Derived Extracellular Matrix for Corneal Stroma Tissue Engineering. ACS Appl. Mater. Interfaces 2024, 16, 15761–15772. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Dhrisya, C.; Lin, F.H.; Sadhasivam, S. The Design and Developments of Protein-Polysaccharide Biomaterials for Corneal Tissue Engineering. Adv. Mater. Technol. 2023, 8, 2300171. [Google Scholar] [CrossRef]
- Ma, F.; Feng, J.; Liu, X.; Tian, Y.; Wang, W.J.; Luan, F.X.; Wang, Y.J.; Yang, W.Q.; Bai, J.Y.; Zhang, Y.Q.; et al. A synergistic therapeutic nano-eyedrop for dry eye disease based on ascorbic acid-coupled exosomes. Nanoscale 2023, 15, 1890–1899. [Google Scholar] [CrossRef]
- Zhao, W.; He, X.; Liu, R.; Ruan, Q. Accelerating corneal wound healing using exosome-mediated targeting of NF-kappaB c-Rel. Inflamm. Regen. 2023, 43, 6. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, X.; Wang, Q.; Fang, P.H.; Liu, L.; Du, X.; Li, W.; He, D.; Zhang, T.; Bai, Y.; et al. Engineered Mesenchymal Stromal Cell Exosomes-Loaded Microneedles Improve Corneal Healing after Chemical Injury. ACS Nano 2024, 18, 20065–20082. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Q.; Zhang, J.; Li, C.; Miao, Y.R.; Lei, Q.; Li, Q.; Guo, A.Y. EVmiRNA: A database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019, 47, D89–D93. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Dudzińska, D.; Kosior-Jarecka, E.; Ćwikińska-Haszcz, A.; Czop, M.; Kocki, J.; Żarnowski, T. miRNA profiling in the aqueous humour in patients with Fuch’s endothelial corneal dystrophy. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Al Bdour, M.; Sabbagh, H.M.; Jammal, H.M. Multi-modal imaging for the detection of early keratoconus: A narrative review. Eye Vis. 2024, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Torimura, A.; Kanei, S.; Shimizu, Y.; Baba, T.; Uotani, R.; Sasaki, S.I.; Nagase, D.; Inoue, Y.; Ochiya, T.; Miyazaki, D. Profiling miRNAs in tear extracellular vesicles: A pilot study with implications for diagnosis of ocular diseases. Jpn. J. Ophthalmol. 2024, 68, 70–81. [Google Scholar] [CrossRef]
- Al-Sharify, N.T.; Yussof, S.; Ghaeb, N.H.; Al-Sharify, Z.T.; Naser, H.Y.; Ahmed, S.M.; See, O.H.; Weng, L.Y. Advances in Corneal Diagnostics Using Machine Learning. Bioengineering 2024, 11, 1198. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Cheng, H.L.; Fu, C.Y.; Kuo, W.C.; Chen, Y.W.; Chen, Y.S.; Lee, Y.M.; Li, K.H.; Chen, C.; Ma, H.P.; Huang, P.C.; et al. Detecting miRNA biomarkers from extracellular vesicles for cardiovascular disease with a microfluidic system. Lab Chip 2018, 18, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Ramshani, Z.; Zhang, C.; Richards, K.; Chen, L.; Xu, G.; Stiles, B.L.; Hill, R.; Senapati, S.; Go, D.B.; Chang, H.C. Extracellular vesicle microRNA quantification from plasma using an integrated microfluidic device. Commun. Biol. 2019, 2, 189. [Google Scholar] [CrossRef]
- Paolini, A.; Baldassarre, A.; Bruno, S.P.; Felli, C.; Muzi, C.; Ahmadi Badi, S.; Siadat, S.D.; Sarshar, M.; Masotti, A. Improving the Diagnostic Potential of Extracellular miRNAs Coupled to Multiomics Data by Exploiting the Power of Artificial Intelligence. Front. Microbiol. 2022, 13, 888414. [Google Scholar] [CrossRef] [PubMed]
- Muthamilselvan, S.; Ramasami Sundhar Baabu, P.; Palaniappan, A. Microfluidics for Profiling miRNA Biomarker Panels in AI-Assisted Cancer Diagnosis and Prognosis. Technol. Cancer Res. Treat. 2023, 22, 15330338231185284. [Google Scholar] [CrossRef]
- Jeffet, J.; Mondal, S.; Federbush, A.; Tenenboim, N.; Neaman, M.; Deek, J.; Ebenstein, Y.; Bar-Sinai, Y. Machine-Learning-Based Single-Molecule Quantification of Circulating MicroRNA Mixtures. ACS Sens. 2023, 8, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Huang, C.-H.; Hsu, P.-W.; Chen, Y.-C.; Liu, Y.-H.; Wei, Z.-Y.; Chen, C.-H.; Ho, L.-H.; Yao, H.-W.; Lin, T.-Y.; et al. (Invited) liquid Biopsy Tool: Integrated Aiot Digital Bead-Based Sensor for Molecular-Fingerprint Profiling of Extracellular Vesicles. ECS Meet. Abstr. 2023, 243, 1914. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Juthani, N.; Doyle, P.S. Quantitative and Multiplex Detection of Extracellular Vesicle-Derived MicroRNA via Rolling Circle Amplification within Encoded Hydrogel Microparticles. Adv. Healthc. Mater. 2022, 11, e2102332. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, J.; Hou, H.; Huang, L.; Li, J. High-throughput microfluidic systems accelerated by artificial intelligence for biomedical applications. Lab Chip 2024, 24, 1307–1326. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K.; Lalgudi, V.G.; Kundu, G.; Mimouni, M.; Liu, H.; Jhanji, V.; Prakash, G.; Roy, A.S.; Shetty, R.; et al. Role of artificial intelligence, machine learning and deep learning models in corneal disorders—A narrative review. J. Fr. Ophtalmol. 2024, 47, 104242. [Google Scholar] [CrossRef]
- Nguyen, T.; Ong, J.; Masalkhi, M.; Waisberg, E.; Zaman, N.; Sarker, P.; Aman, S.; Lin, H.; Luo, M.; Ambrosio, R.; et al. Artificial intelligence in corneal diseases: A narrative review. Cont. Lens Anterior Eye 2024, 47, 102284. [Google Scholar] [CrossRef]
- Adampourezare, M.; Hasanzadeh, M.; Seidi, F. Microfluidic assisted recognition of miRNAs towards point-of-care diagnosis: Technical and analytical overview towards biosensing of short stranded single non-coding oligonucleotides. Biomed. Pharmacother. 2022, 153, 113365. [Google Scholar] [CrossRef]
- Qu, J.-h.; Qin, X.-r.; Xie, Z.-j.; Qian, J.-h.; Zhang, Y.; Sun, X.-n.; Sun, Y.-z.; Peng, R.-m.; Xiao, G.-g.; Lin, J.; et al. Establishment of an automatic diagnosis system for corneal endothelium diseases using artificial intelligence. J. Big Data 2024, 11, 67. [Google Scholar] [CrossRef]
- Kryszan, K.; Wylegala, A.; Kijonka, M.; Potrawa, P.; Walasz, M.; Wylegala, E.; Orzechowska-Wylegala, B. Artificial-Intelligence-Enhanced Analysis of In Vivo Confocal Microscopy in Corneal Diseases: A Review. Diagnostics 2024, 14, 694. [Google Scholar] [CrossRef]
- Kosaka, N.; Yoshioka, Y.; Hagiwara, K.; Tominaga, N.; Katsuda, T.; Ochiya, T. Trash or Treasure: Extracellular microRNAs and cell-to-cell communication. Front. Genet. 2013, 4, 173. [Google Scholar] [CrossRef]
- Caporali, A.; Miscianinov, V.; Saif, J.; Emanueli, C. MicroRNA transport in cardiovascular complication of diabetes. Biochim. Biophys. Acta 2016, 1861, 2111–2120. [Google Scholar] [CrossRef]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Quarato, C.M.I.; Foschino Barbaro, M.P.; Scioscia, G. EVs-miRNA: The New Molecular Markers for Chronic Respiratory Diseases. Life 2022, 12, 1544. [Google Scholar] [CrossRef]
- Rautavaara, Y. MSC-EV mechanisms in corneal wound healing and inflammation. Acta Ophthalmol. 2025, 103. [Google Scholar] [CrossRef]
- Deng, S.X.; Dos Santos, A.; Gee, S. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Ratuszny, D.; Gras, C.; Bajor, A.; Borger, A.K.; Pielen, A.; Borgel, M.; Framme, C.; Blasczyk, R.; Figueiredo, C. miR-145 Is a Promising Therapeutic Target to Prevent Cornea Scarring. Hum. Gene Ther. 2015, 26, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, S.S.; Shah, B.; Kapre, S.; Charbe, N.; Immanuel, S.; Pasham, S.; Thalla, M.; Jain, A.; Palakurthi, S. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Dave, K.M.; Pinky, P.P.; S Manickam, D. Molecular engineering of extracellular vesicles for drug delivery: Strategies, challenges, and perspectives. J. Control. Release 2025, 386, 114068. [Google Scholar] [CrossRef] [PubMed]
- Dowaidar, M. Gene therapy using extracellular vesicles loaded with miRNA derived from Bone Marrow Mesenchymal Stem Cells is a cell-free medication delivery method used in a variety of diseases. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar]
- Hernandez, M.J.; Gaetani, R.; Pieters, V.M.; Ng, N.W.; Chang, A.E.; Martin, T.R.; Ingen, E.v.; Mol, E.A.; Dzieciatkowska, M.; Hansen, K.C.; et al. Decellularized Extracellular Matrix Hydrogels as a Delivery Platform for MicroRNA and Extracellular Vesicle Therapeutics. Adv. Ther. 2018, 1, 1800032. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.M.; Wang, J.; Liu, B.J.; Gao, Q.; Zhang, J.; Qian, H.D.; Pan, J.Y.; Liu, M.; Huang, Q.; et al. Circulating Small Extracellular Vesicles Involved in Systemic Regulation Respond to RGC Degeneration in Glaucoma. Adv. Sci. 2024, 11, e2309307. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Reclusa Asiain, P.; Durendez Saez, E.; Jantus-Lewintre, E.; Malarani, M.; Khan, S.; Fontana, S.; Naing, A.; Passiglia, F.; Raez, L.E.; et al. Extracellular Vesicles As miRNA Nano-Shuttles: Dual Role in Tumor Progression. Target. Oncol. 2018, 13, 175–187. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kumar, U.S.; Sadeghipour, N.; Massoud, T.F.; Paulmurugan, R. A Microfluidics-Based Scalable Approach to Generate Extracellular Vesicles with Enhanced Therapeutic MicroRNA Loading for Intranasal Delivery to Mouse Glioblastomas. ACS Nano 2021, 15, 18327–18346. [Google Scholar] [CrossRef]
- Verma, N.; Arora, S. Navigating the Global Regulatory Landscape for Exosome-Based Therapeutics: Challenges, Strategies, and Future Directions. Pharmaceutics 2025, 17, 990. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Peh, G.S.L.; Adnan, K.B.; Mehta, J.S. Translational and Regulatory Challenges of Corneal Endothelial Cell Therapy: A Global Perspective. Tissue Eng. Part B Rev. 2020, 28, 52–62. [Google Scholar]
- Yadav, A.; Xuan, Y.; Sen, C.K.; Ghatak, S. Standardized Reporting of Research on Exosomes to Ensure Rigor and Reproducibility. Adv. Wound Care 2024, 13, 584–599. [Google Scholar] [CrossRef]
- Nelson, B.C.; Samantha, M.; Ghiran, G.I.; Jones, C.J.; DeRose, P.C.; Elzafir, E.; Vreeland, W.N.; Wang, L. Measurement and Standardization Challenges for Extracellular Vesicle Therapeutic Delivery Vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019, 26, 933–944.e4. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Cheng, L.; Vella, L.J.; Barnham, K.J.; McLean, C.; Masters, C.L.; Hill, A.F. Small RNA fingerprinting of Alzheimer’s disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1766822. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Min, L.; Zhu, S.; Chen, L.; Liu, X.; Wei, R.; Zhao, L.; Yang, Y.; Zhang, Z.; Kong, G.; Li, P.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.-W.; Zhang, Y.; Liu, J.; Kim, Y.; Dong, H.; Lucien, F.; Liu, Y. Abstract A030: Rapid extraction and detection of extracellular vesicle-derived PD-L1 in a microfluidic platform. Clin. Cancer Res. 2024, 30, A30. [Google Scholar]
- Bracht, J.W.P.; Los, M.; van der Pol, E.; Verkuijlen, S.; van Eijndhoven, M.A.J.; Pegtel, D.M.; Nieuwland, R. Choice of size-exclusion chromatography column affects recovery, purity, and miRNA cargo analysis of extracellular vesicles from human plasma. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 497–508. [Google Scholar] [CrossRef]
- Miceli, R.T.; Chen, T.Y.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef]
- Srinivasan, S.; Duval, M.X.; Kaimal, V.; Cuff, C.; Clarke, S.H. Assessment of methods for serum extracellular vesicle small RNA sequencing to support biomarker development. J. Extracell. Vesicles 2019, 8, 1684425. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, R.; Falcon-Perez, J.M.; Thery, C.; Witwer, K.W. Rigor and standardization of extracellular vesicle research: Paving the road towards robustness. J. Extracell. Vesicles 2020, 10, e12037. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Culot, M. Minimum information for studies of extracellular vesicles (MISEV) as toolbox for rigorous, reproducible and homogeneous studies on extracellular vesicles. Toxicol. In Vitro 2025, 106, 106049. [Google Scholar] [CrossRef]
- Li, D.; Yao, X.; Yue, J.; Fang, Y.; Cao, G.; Midgley, A.C.; Nishinari, K.; Yang, Y. Advances in Bioactivity of MicroRNAs of Plant-Derived Exosome-Like Nanoparticles and Milk-Derived Extracellular Vesicles. J. Agric. Food Chem. 2022, 70, 6285–6299. [Google Scholar] [CrossRef]
- Zhong, H.; Guan, G.; Jin, Y. Roles of helminth extracellular vesicle-derived let-7 in host-parasite crosstalk. Front. Immunol. 2024, 15, 1449495. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rao, J.; Liang, Z.; Xu, X.; Lin, F.; Lin, Y.; Wang, C.; Chen, C. Efficacy of miRNA-modified mesenchymal stem cell extracellular vesicles in spinal cord injury: A systematic review of the literature and network meta-analysis. Front. Neurosci. 2022, 16, 989295. [Google Scholar] [CrossRef]
- Takakura, Y.; Hanayama, R.; Akiyoshi, K.; Futaki, S.; Hida, K.; Ichiki, T.; Ishii-Watabe, A.; Kuroda, M.; Maki, K.; Miura, Y.; et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm. Res. 2024, 41, 1573–1594. [Google Scholar] [CrossRef]
- Lim, Y.J.; Seo, M.S.; Park, W.T.; Park, S.; Lee, G.W. Extracellular vesicle-derived MicroRNAs as potential therapies for spinal cord and peripheral nerve injuries. RNA Biol. 2025, 22, 1–9. [Google Scholar] [CrossRef]
- DaCunza, J.T.; Wickman, J.R.; Ajit, S.K. miRNA packaging into small extracellular vesicles and implications in pain. Pain Rep. 2024, 9, e1198. [Google Scholar] [CrossRef]
- Leavitt, R.J.; Limoli, C.L.; Baulch, J.E. miRNA-based therapeutic potential of stem cell-derived extracellular vesicles: A safe cell-free treatment to ameliorate radiation-induced brain injury. Int. J. Radiat. Biol. 2019, 95, 427–435. [Google Scholar] [CrossRef]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Immunological Off-Target Effects of microRNA Control Sequences. Res. Sq. 2020. [Google Scholar] [CrossRef]
- O’Brien, K.P.; Khan, S.; Gilligan, K.E.; Zafar, H.; Lalor, P.; Glynn, C.; O’Flatharta, C.; Ingoldsby, H.; Dockery, P.; De Bhulbh, A.; et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 2018, 37, 2137–2149. [Google Scholar] [CrossRef]
- Albanese, M.; Chen, Y.A.; Huls, C.; Gartner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Gobel, C.; Zeidler, R.; Shein, M.; et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021, 17, e1009951. [Google Scholar] [CrossRef]
- Jiang, L.; Vader, P.; Schiffelers, R.M. Extracellular vesicles for nucleic acid delivery: Progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017, 24, 157–166. [Google Scholar] [CrossRef]
- Drees, E.E.E.; Groenewegen, N.J.; Verkuijlen, S.; van Eijndhoven, M.A.J.; Ramaker, J.; Veenstra, P.; Hussain, M.; Groothuis-Oudshoorn, C.G.M.; de Jong, D.; Zijlstra, J.M.; et al. Towards IVDR-compliance by implementing quality control steps in a quantitative extracellular vesicle-miRNA liquid biopsy assay for response monitoring in patients with classic Hodgkin lymphoma. J. Extracell. Biol. 2024, 3, e164. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, P.; Turner, N.; Mitchell, M.D. A comparative analysis of small extracellular vesicle (sEV) micro-RNA (miRNA) isolation and sequencing procedures in blood plasma samples. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Gouin, K.; Peck, K.; Antes, T.; Johnson, J.L.; Li, C.; Vaturi, S.D.; Middleton, R.; de Couto, G.; Walravens, A.S.; Rodriguez-Borlado, L.; et al. A comprehensive method for identification of suitable reference genes in extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1347019. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Vigano, M.; Colombini, A.; Lugano, G.; Bollati, V.; de Girolamo, L. miR-22-5p and miR-29a-5p Are Reliable Reference Genes for Analyzing Extracellular Vesicle-Associated miRNAs in Adipose-Derived Mesenchymal Stem Cells and Are Stable under Inflammatory Priming Mimicking Osteoarthritis Condition. Stem Cell Rev. Rep. 2019, 15, 743–754. [Google Scholar] [CrossRef]
- Buschmann, D.; Kirchner, B.; Hermann, S.; Marte, M.; Wurmser, C.; Brandes, F.; Kotschote, S.; Bonin, M.; Steinlein, O.K.; Pfaffl, M.W.; et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J. Extracell. Vesicles 2018, 7, 1481321. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, J.J.; Giovanazzi, A.; Rocha, A.M.; Rocha, S.; Silva, I.; Saraiva, N.; Bonito, N.; Carvalho, J.; Maia, L.; Wauben, M.H.M.; et al. Adapter dimer contamination in sRNA-sequencing datasets predicts sequencing failure and batch effects and hampers extracellular vesicle-sRNA analysis. J. Extracell. Biol. 2023, 2, e91. [Google Scholar] [CrossRef] [PubMed]
- Shukuya, T.; Ghai, V.; Amann, J.M.; Okimoto, T.; Shilo, K.; Kim, T.K.; Wang, K.; Carbone, D.P. Circulating MicroRNAs and Extracellular Vesicle-Containing MicroRNAs as Response Biomarkers of Anti-programmed Cell Death Protein 1 or Programmed Death-Ligand 1 Therapy in NSCLC. J. Thorac. Oncol. 2020, 15, 1773–1781. [Google Scholar] [CrossRef]
- Chen, X.; Jin, Y.; Feng, Y. Evaluation of Plasma Extracellular Vesicle MicroRNA Signatures for Lung Adenocarcinoma and Granuloma With Monte-Carlo Feature Selection Method. Front. Genet. 2019, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Cheng, L.; Wang, Y.; Li, M.; Yu, J.; Ma, Z.; Ho, P.C.; Sethi, G.; Chen, X.; et al. Extracellular vesicle-derived biomarkers in prostate cancer care: Opportunities and challenges. Cancer Lett. 2024, 601, 217184. [Google Scholar] [CrossRef] [PubMed]
| Method | Yield | Purity | Throughput | Advantages | Limitations | Reference(s) |
|---|---|---|---|---|---|---|
| Ultracentrifugation (UC) | Moderate | Moderate–Low | Low | Widely used; no special reagents | Labor-intensive; vesicle damage; variable recovery | [83] |
| Ultrafiltration (UF) | Moderate | Moderate | Moderate | Simple; faster than UC | Membrane clogging; non-specific binding | [84] |
| Polymer-Based Precipitation | High | Low | High | Simple; no ultracentrifuge needed | Co-precipitates proteins/polymers; interferes with downstream assays | [83,85] |
| Size-Exclusion Chromatography | Moderate | High | Moderate | Preserves vesicle integrity; high purity | Sample dilution; needs reconcentration | [63,86,87,88] |
| Tangential Flow Filtration | High | Moderate | High | Scalable; gentle shear | Equipment complexity; potential shear stress on EVs | [63,86,87] |
| Immunoaffinity Capture | Low | High | Low | Selective isolation of EV subpopulations | High cost; low capacity; antibody carry-over | [83,85] |
| Microfluidic Immunocapture | Low–Moderate | High | High | Rapid; integrates with on-chip analysis; minimal sample usage | Difficult to scale for large volumes; potential channel clogging | [86,87] |
| Method | Principle | Advantages | Limitations | Applications | Reference(s) |
|---|---|---|---|---|---|
| Nanoparticle Tracking Analysis (NTA) | Tracks Brownian motion of individual vesicles under a laser beam to calculate size and concentration |
|
| Size/concentration profiling, QC of isolation protocols | [89] |
| Dynamic Light Scattering (DLS) | Measures intensity fluctuations of scattered light from particles to derive hydrodynamic diameter |
|
| Preliminary size estimation, comparing batch consistency | [90] |
| Transmission Electron Microscopy (TEM) | Electron beam imaging for ultrastructural morphology |
| Laborious sample prep (fixation, staining)
| Morphological validation, purity checks, size confirmation | [91] |
| Western Blotting (WB) | Immunodetection of exosome-enriched marker proteins |
|
| Verification of marker expression, confirmation of exosomal identity | [69] |
| High-Resolution Flow Cytometry | Light scatter and fluorescence detection of antibody-labeled vesicles |
| Detection limit ~200 nm
| Surface marker profiling, subpopulation analysis | [92] |
| Tunable Resistive Pulse Sensing (TRPS) | Electrical pulse blockade as vesicles traverse a nanopore |
|
| Accurate size distribution, concentration in heterogeneous samples | [93] |
| Surface Plasmon Resonance (SPR) | Monitors refractive index changes upon exosome binding to a functionalized sensor surface |
|
| Quantification of surface proteins, binding affinity studies | [94] |
| Raman Spectroscopy (including SERS) | Detects molecular vibrational fingerprints of vesicle biomolecules |
|
| Biochemical fingerprinting, disease biomarker discovery | [95] |
| Atomic Force Microscopy (AFM) | Nanoscale probe scans to map surface topology and measure mechanical properties |
|
| Surface morphology, vesicle elasticity, interaction forces | [96] |
| Single-Particle Interferometric Reflectance Imaging (SP-IRIS) | Interferometric detection of exosomes captured on antibody microarrays, enabling counts and sizing |
|
| Phenotyping of exosome subpopulations, clinical biomarker validation | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, N.; Arora, S.; Singh, A.K.; Kumar, A. Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications. Targets 2025, 3, 32. https://doi.org/10.3390/targets3040032
Verma N, Arora S, Singh AK, Kumar A. Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications. Targets. 2025; 3(4):32. https://doi.org/10.3390/targets3040032
Chicago/Turabian StyleVerma, Nagendra, Swati Arora, Anurag Kumar Singh, and Amrendra Kumar. 2025. "Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications" Targets 3, no. 4: 32. https://doi.org/10.3390/targets3040032
APA StyleVerma, N., Arora, S., Singh, A. K., & Kumar, A. (2025). Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications. Targets, 3(4), 32. https://doi.org/10.3390/targets3040032








