Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective
Abstract
1. Introduction
2. Literature Search
3. Current Diagnostic Modalities and Limitations
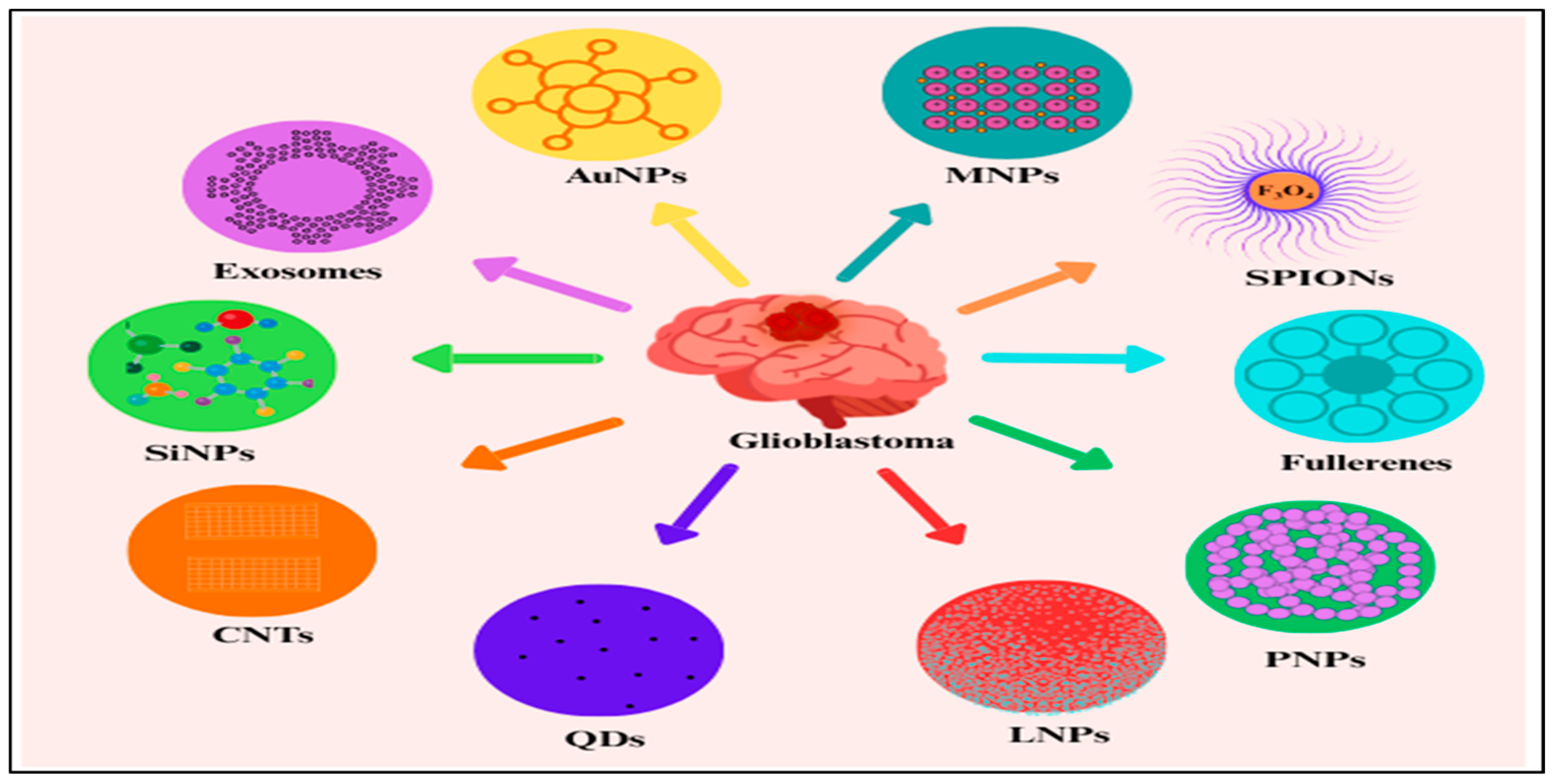
4. Nanotechnology for Glioblastoma Diagnosis
4.1. Nanotechnology for MRI
4.1.1. Computerized Tomography Nanoprobe
4.1.2. Fluorescent Nanoprobe
4.1.3. PA Imaging
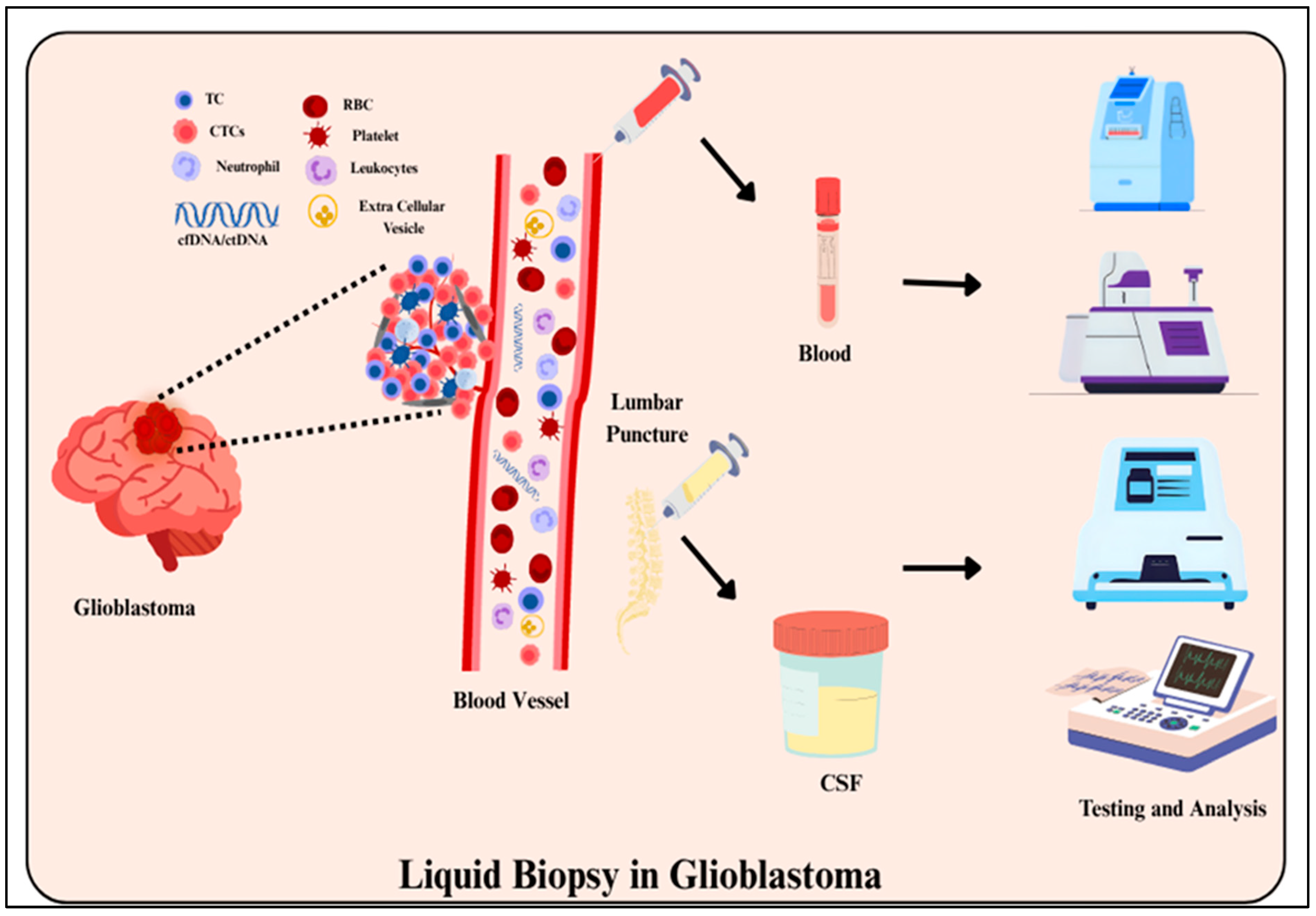
4.2. Liquid Biopsy
4.3. Extracellular Vesicles
4.4. MicroRNAs
4.5. Biosensing for GBM
5. AI for Multi-Omics and Radiomic Integration
Machine Learning Techniques in Multi-Omics Data Integration
6. Bridging Between AI and Nanotechnology
7. Translational Considerations
7.1. Regulatory Landscape for AI in Genomic Medicine
7.2. Challenges in Clinical Implementation and Data Standardization
8. Current Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma Multiform |
| IDH | Isocitrate dehydrogenase |
| 2-HG | 2-hydroxyglutarate |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| EGFR | Epidermal growth factor receptor |
| PTEN | The phosphate and tensin homolog |
| SHH | The Sonic Hedgehog |
| MGMT | 6-methylguanine-DNA methyltransferase |
| TMZ | Temozolomide |
| CCNU | 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea |
| TP53 | Tumor protein p53 |
| MRI | Magnetic resonance imaging |
| ddPCR | Droplet digital PCR |
| FLAIR | Fluid-attenuated inversion recovery |
| PWI | Perfusion-weighted imaging |
| EVs | Extracellular vesicles |
| MNPs | Magnetic nanoparticles |
| IONPs | Iron oxide nanoparticles |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| ECT | Electron Computed Tomography |
| AIE | Aggregation-induced emission |
| LB | Liquid biopsy |
| TiN | Titanium nitride |
| μNMR | Micronuclear magnetic resonance |
| iMER | Immunomagnetic exosome RNA |
| ACE | Alternating current electrokinetic |
| GDPR | General Data Protection Regulation |
| CNNs | Convolutional neural networks |
| SPR | Surface plasmon resonance |
| PCA | principal component analysis |
| t-SNE | t-distributed stochastic neighbor embedding |
| GNNs | Graph neural networks |
| SVM | Support Vector Machine |
| LOOCV | Leave-One-Out Cross-Validation |
| NIH | National Institutes of Health |
| FDA | Food and Drug Administration |
| RNN | Recurrent neural network |
| PIPL | Personal Information Protection Law |
| BBT | Benzobisthiadiazole |
| MoS2 | molybdenum disulfide |
| ICG | Indocyanine green |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.; Dolecek, T.; Horbinski, C.; Ostrom, Q.; Lightner, D.; Barnholtz-Sloan, J.; Villano, J. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Alexopoulos, G.; Zhang, J.; Karampelas, I.; Patel, M.; Kemp, J.; Coppens, J.; Mattei, T.A.; Mercier, P. Long-Term Time Series Forecasting and Updates on Survival Analysis of Glioblastoma Multiforme: A 1975–2018 Population-Based Study. Neuroepidemiology 2022, 56, 75–89. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.; Simjee, S. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
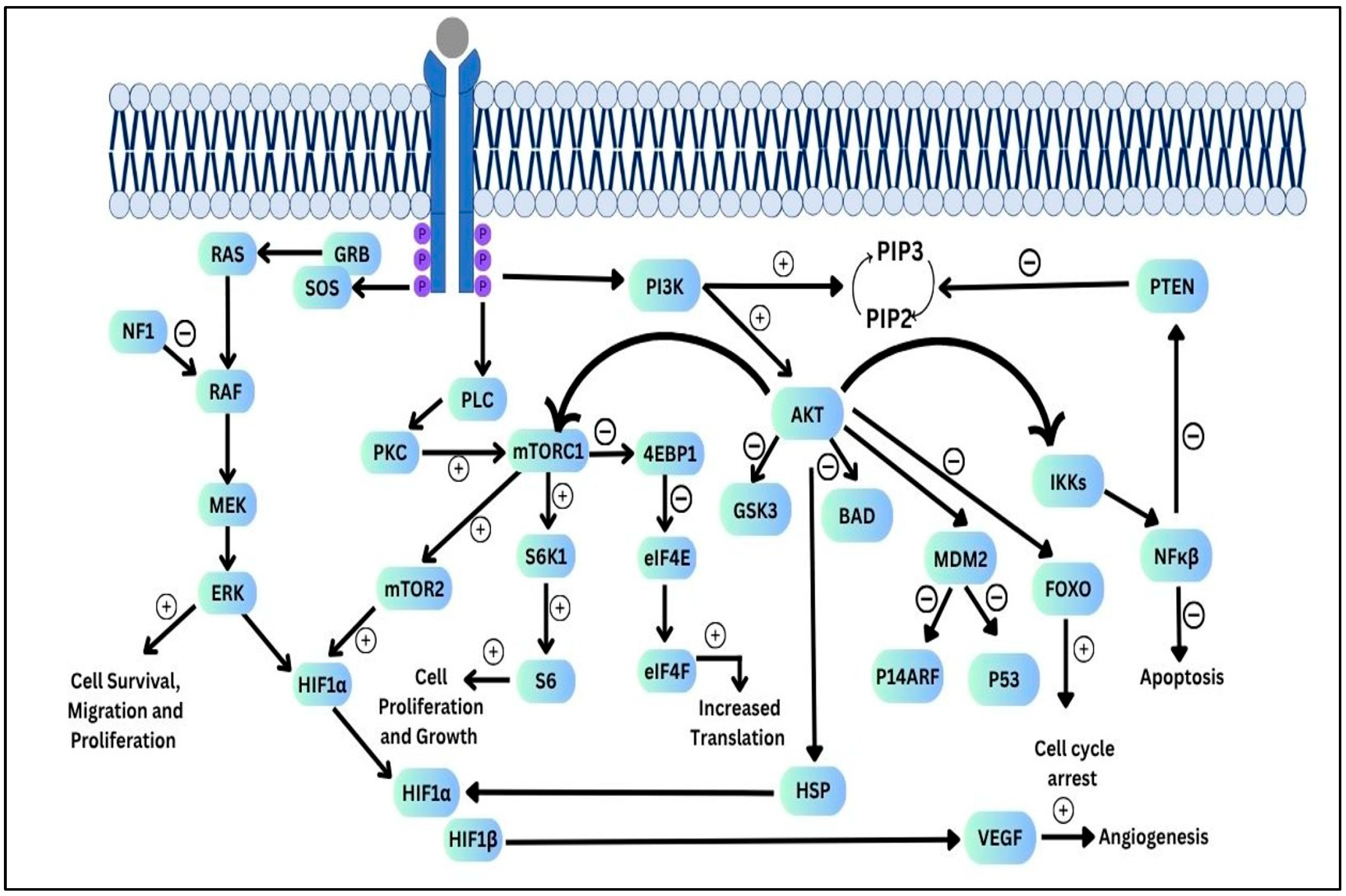
- Khabibov, M.; Garifullin, A.; Boumber, Y.; Khaddour, K.; Fernandez, M.; Khamitov, F.; Khalikova, L.; Kuznetsova, N.; Kit, O.; Kharin, L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review). Int. J. Oncol. 2022, 60, 69. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Nakada, M.; Kita, D.; Watanabe, T.; Hayashi, Y.; Teng, L.; Pyko, I.V.; Hamada, J.-I. Aberrant Signaling Pathways in Glioma. Cancers 2011, 3, 3242–3278. [Google Scholar] [CrossRef]
- Turkalp, Z.; Karamchandani, J.; Das, S. IDH mutation in glioma: New insights and promises for the future. JAMA Neurol. 2014, 71, 1319–1325. [Google Scholar] [CrossRef]
- Du, Y.; Chen, H.; Li, X.-F.; Lu, Y.-C.; Feng, Y.-B.; Zhang, L. Molecular mechanism of Notch signaling with special emphasis on microRNAs: Implications for glioma. J. Cell. Physiol. 2018, 234, 158–170. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Wick, W.; Weller, M.; Weiler, M.; Batchelor, T.; Yung, A.W.; Platten, M. Pathway inhibition: Emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011, 13, 566–579. [Google Scholar] [CrossRef]
- Westermark, B. Platelet-derived growth factor in glioblastoma-driver or biomarker? Ups. J. Med. Sci. 2014, 119, 298–305. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Mantamadiotis, T. Towards Targeting PI3K-Dependent Regulation of Gene Expression in Brain Cancer. Cancers 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Janbazian, L.; Karamchandani, J.; Das, S. Mouse models of glioblastoma: Lessons learned and questions to be answered. J. Neurooncol. 2014, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Chen, L.; Du, W.; Cui, Y.; Piao, X.; Li, Y.; Jiang, C. Targeting glioma stem cells via the Hedgehog signaling pathway. Neuroimmunol. Neuroinflamm. 2014, 1, 9. [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Appin, C.L.; Brat, D.J. Molecular pathways in gliomagenesis and their relevance to neuropathologic diagnosis. Adv. Anat. Pathol. 2015, 22, 50–58. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Kong, X.-T.; Nguyen, N.T.; Choi, Y.J.; Zhang, G.; Nguyen, H.N.; Filka, E.; Green, S.; Yong, W.H.; Liau, L.M.; Green, R.M.; et al. Phase 2 Study of Bortezomib Combined with Temozolomide and Regional Radiation Therapy for Upfront Treatment of Patients with Newly Diagnosed Glioblastoma Multiforme: Safety and Efficacy Assessment. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1195–1203. [Google Scholar] [CrossRef]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.; Loras, A.; González-Bonet, L.; Martínez-Cadenas, C.; Estrela, J.; et al. Glioblastoma Therapy: Past, Present, and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef]
- Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and therapeutic biomarkers in glioblastoma: Current status and future perspectives. BioMed Res. Int. 2017, 2017, 8013575. [Google Scholar] [CrossRef]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Rehman, F.U.; Zheng, M.; Shi, B. Nanomedicine in Brain Diseases: Principles and Application; Xue, X., Ed.; Springer: Singapore, 2019; p. 29. [Google Scholar]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef] [PubMed]
- Kopkova, J.; Sana, P.; Fadrus, O.; Slaby, O. Cerebrospinal fluid microRNAs as diagnostic biomarkers in brain tumors. Clin. Chem. Lab. Med. 2018, 56, 869. [Google Scholar] [CrossRef]
- Hosseini, A.; Ashraf, H.; Rahimi, F.; Alipourfard, I.; Alivirdiloo, V.; Hashemi, B.; Yazdani, Y.; Ghazi, F.; Eslami, M.; Reza, M.A.S.; et al. Recent advances in the detection of glioblastoma, from imaging-based methods to proteomics and biosensors: A narrative review. Cancer Cell Int. 2023, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Kleihues, P.; Ohgaki, H. Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro Oncol. 1999, 1, 44–51. [Google Scholar] [CrossRef]
- Sizoo, E.M.; Braam, L.; Postma, T.J.; Pasman, H.R.; Heimans, J.J.; Klein, M.; Reijneveld, J.C.; Taphoorn, M.J. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010, 12, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Goldberg, T.; Pyka, T.; Bernhofer, M.; Wiestler, B.; Kessel, K.A.; Tafti, P.D.; Nusslin, F.; Braun, A.E.; Zimmer, C.; et al. Combining multimodal imaging and treatment features improves machine learning-based prognostic assessment in patients with glioblastoma multiforme. Cancer Med. 2019, 8, 128–136. [Google Scholar] [CrossRef]
- Price, S.J.; Young, A.M.; Scotton, W.J.; Ching, J.; Mohsen, L.A.; Boonzaier, N.R.; Lupson, V.C.; Griffiths, J.R.; McLean, M.A.; Larkin, T.J. Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J. Magn. Reson. Imaging 2016, 43, 487–494. [Google Scholar] [CrossRef]
- Aoki, T.; Kondo, Y.; Karakida, K.; Naito, H.; Kajiwara, H.; Ota, Y. A mucinous adenocarcinoma of the lip with elevated serum carcinoembryonic antigen levels: A case report. Oral Maxillofac Surg. 2020, 24, 127–132. [Google Scholar] [CrossRef]
- Herman, A.; Gruden, K.; Blejec, A.; Podpečan, V.; Motaln, H.; Rožman, P.; Hren, M.; Zupančič, K.; Veber, M.; Verbovšek, U.; et al. Analysis of Glioblastoma Patients’ Plasma Revealed the Presence of MicroRNAs with a Prognostic Impact on Survival and Those of Viral Origin. PLoS ONE 2015, 10, e0125791. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantages and disadvantage. Clin. Epigenet. 2018, 10, 59. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef]
- Mangano, K.; Mazzon, E.; Basile, M.S.; Di Marco, R.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951–17970. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.; Board, R.; Dawson, T.; Jenkinson, M.D.; Baker, M.J. A review of novel analytical diagnostics for liquid biopsies: Spectroscopic and spectrometric serum profiling of primary and secondary brain tumors. Brain Behav. 2016, 6, e00502. [Google Scholar] [CrossRef] [PubMed]
- Krol, I.; Castro-Giner, F.; Maurer, M.; Gkountela, S.; Szczerba, B.M.; Scherrer, R.; Coleman, N.; Carreira, S.; Bachmann, F.; Anderson, S.; et al. Detection of circulating tumour cell clusters in human glioblastoma. Br. J. Cancer 2018, 119, 487–491. [Google Scholar] [CrossRef]
- Thenuwara, G.; Curtin, J.; Tian, F. Advances in Diagnostic Tools and Therapeutic Approaches for Gliomas: A Comprehensive Review. Sensors 2023, 23, 9842. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A. Circulating Liquid Biopsy Biomarkers in Glioblastoma: Advances and Challenges. Int. J. Mol. Sci. 2024, 25, 7974. [Google Scholar] [CrossRef]
- Drake, L.; Hillmer, A.; Cai, Z. Approaches to PET Imaging of Glioblastoma. Molecules 2020, 25, 568. [Google Scholar] [CrossRef] [PubMed]
- Ronvaux, L.; Riva, M.; Coosemans, A.; Herzog, M.; Rommelaere, G.; Donis, N.; D’Hondt, L.; Douxfils, J. Liquid Biopsy in Glioblastoma. Cancers 2022, 14, 3394. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Huang, L.; Yang, Y.; He, J. Enhancing precision medicine: Bispecific antibody-mediated targeted delivery of lipid nanoparticles for potential cancer therapy. Int. J. Pharm. 2024, 654, 123990. [Google Scholar] [CrossRef]
- Choi, Y.; Son, W.; Han, Y.; Chae, J.; Yang, C.S.; Choi, J. Glycan-targeting nanoparticle for photodynamic immunotherapy of melanoma. Acta Pharm. Sin. B 2023, 13, 1903–1918. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [PubMed]
- Chen, Z.; Wu, C.; Yuan, Y.; Xie, Z.; Li, T.; Huang, H.; Li, S.; Deng, J.; Lin, H.; Shi, Z.; et al. CRISPR-Cas13a-powered electrochemical biosensor for the detection of the L452R mutation in clinical samples of SARS-CoV-2 variants. J. Nanobiotechnol. 2023, 21, 141. [Google Scholar]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, Z.; Li, J.; Wu, R.; Zhang, B.; Nie, G.; Xie, Z.; Zhang, H. A Highly Sensitive CRISPR-Empowered Surface Plasmon Resonance Sensor for Diagnosis of Inherited Diseases with Femtomolar-Level Real-Time Quantification. Adv. Sci. 2022, 9, e2105231. [Google Scholar] [CrossRef]
- Gusmão, L.A.; Matsuo, F.S.; Barbosa, H.F.G.; Tedesco, A.C. Advances in nano-based materials for glioblastoma multiforme diagnosis: A mini-review. Front. Nanotechnol. 2022, 4, 836802. [Google Scholar] [CrossRef]
- Shabani, L.; Abbasi, M.; Amini, M.; Amani, A.; Vaez, A. The brilliance of nanoscience over cancer therapy: Novel, promising nanotechnology-based methods for eradicating glioblastoma. J. Neurol. Sci. 2022, 440, 120316. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lakshmi, B. Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications. Int. J. Mol. Sci. 2022, 23, 1641. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rahdar, A.; Kharaba, Z.; Fathi-Karkan, S.; Aboudzadeh, M.; Aliahmad, M.; Ullah, S.; Qindeel, M. Nanotechnology in Glioblastoma Therapy: Advances in Drug Delivery Systems and Diagnostic Approaches. J. Drug Deliv. Sci. Technol. 2024, 102, 106322. [Google Scholar] [CrossRef]
- Qian, H.; Yu, Y.; Song, X. Nanoparticles Mediated the Diagnosis and Therapy of Glioblastoma: Bypass or Cross the Blood-Brain Barrier. Small 2023, 19, e2302613. [Google Scholar] [CrossRef]
- Zottel, A.; Šamec, N.; Jovčevska, I.; Paska, V. Nanomedicine and Immunotherapy: A Step Further towards Precision Medicine for Glioblastoma. Molecules 2020, 25, 490. [Google Scholar] [CrossRef]
- Wu, L.; Han, I.; Zeng, X.; Glaser, T. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Sheervalilou, R.; Sargazi, S.; Shirvalilou, S.; Afzalipour, R.; Khoei, S. New insights into targeted therapy of glioblastoma using smart nanoparticles. Cancer Cell Int. 2024, 24, 160. [Google Scholar] [CrossRef]
- Van Straten, D.; Préat, V.; Broekman, M.; Schiffelers, R.; Zhao, M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Yu, S.; Wei, W.; Jiang, J.; Long, S.; Niu, X.; Chen, L.; Xu, H.; Li, X. Application of nanomaterials in the diagnosis and treatment of glioblastoma. Front. Chem. 2022, 10, 1063152. [Google Scholar] [CrossRef]
- Wang, H.; Liao, C.; Hsu, J.; Wang, Y.; Tsai, M.; Chu, S.; Wang, C.; Huang, H.; Lai, M. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Vallabani, N.V.; Singh, S.; Karakoti, A.S. Magnetic Nanoparticles: Current Trends and Future Aspects in Diagnostics and Nanomedicine. Curr. Drug Metab. 2019, 20, 457–472. [Google Scholar] [CrossRef]
- Tan, J.; Sun, W.; Lu, L.; Xiao, Z.; Wei, H.; Shi, W.; Wang, Y.; Han, S.; Shuai, X. I6P7 peptide modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging detection of low-grade brain gliomas. J. Mater. Chem. B 2019, 7, 6139–6147. [Google Scholar] [CrossRef]
- Lai, J.; Wang, T.; Wang, H.; Shi, F.; Gu, W.; Ye, L. MnO nanoparticles with unique excitation-dependent fluorescence for multicolor cellular imaging and MR imaging of brain glioma. Microchim. Acta 2018, 185, 244. [Google Scholar] [CrossRef]
- Xie, R.; Wu, Z.; Zeng, F.; Cai, H.; Wang, D.; Gu, L.; Zhu, H.; Lui, S.; Guo, G.; Song, B.; et al. Retro-enantio isomer of angiopep-2 assists nanoprobes across the blood-brain barrier for targeted magnetic resonance/fluorescence imaging of glioblastoma. Signal Transduct. Target. Ther. 2021, 6, 309. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for diagnosis and treatment of brain cancer: Recent updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Jackson, P.A.; Rahman, W.N.; Wong, C.J.; Ackerly, T.; Geso, M. Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur. J. Radiol. 2010, 75, 104–109. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhu, J.; Sun, N.; Song, N.; Xing, Y.; Huang, H.; Zhao, J. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Silindir, M.; Erdoğan, S.; Özer, A.Y.; Doğan, A.L.; Tuncel, M.; Uğur, Ö.; Torchilin, V.P. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J. Liposome Res. 2013, 23, 20–27. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Ding, D.; Zhang, G.; Zhu, Z.; Yang, X.; Li, M.; Liang, L.; Shi, X.; Wang, T.; et al. Real-time in vivo imaging reveals specific nanoparticle target binding in a syngeneic glioma mouse model. Nanoscale 2022, 14, 5678–5688. [Google Scholar] [CrossRef]
- An, R.; Liu, L.; Wei, S.; Huang, Z.; Qiu, L.; Lin, J.; Liu, H.; Ye, D. Controlling Disassembly of Paramagnetic Prodrug and Photosensitizer Nanoassemblies for On-Demand Orthotopic Glioma Theranostics. ACS Nano 2022, 16, 20607–20621. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H.; et al. Bright Aggregation-Induced-Emission Dots for Targeted Synergetic NIR-II Fluorescence and NIR-I Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Mater. 2018, 28, e1800766. [Google Scholar] [CrossRef]
- Tang, T.; Chang, B.; Zhang, M.; Sun, T. Nanoprobe-mediated precise imaging and therapy of glioma. Nanoscale Horiz. 2021, 6, 634–650. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, F.; Zhang, H.; Yuan, Q.; Jiang, Z.; Liu, H.; Sun, Q.; Li, Z. Boosting often overlooked long-wavelength emissions of rare-earth nanoparticles for NIR-II fluorescence imaging of orthotopic glioblastoma. Biomaterials 2019, 219, 119364. [Google Scholar] [CrossRef] [PubMed]
- Kurbegovic, S.; Juhl, K.; Chen, H.; Qu, C.; Ding, B.; Leth, J.M.; Drzewiecki, K.T.; Kjaer, A.; Cheng, Z. Molecular-targeted NIR-II probes for image-guided brain tumor surgery. Bioconjug. Chem. 2018, 29, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Kirbas Cilingir, E.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin-derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, e1805875. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X. Perylene-Diimide-Based Nanoparticles as Highly Efficient Photoacoustic Agents for Deep Brain Tumor Imaging in Living Mice. Adv. Mater. 2015, 27, 843. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Zhu, Y.; Gong, X.; Zheng, R.; Chen, N.; Chen, D.; Yan, H.; Zhang, P.; Zheng, H.; et al. Highly Sensitive MoS2–Indocyanine Green Hybrid for Photoacoustic Imaging of Orthotopic Brain Glioma at Deep Site. Nano-Micro Lett. 2018, 10, 48. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Lin, X.; Xu, S.; Liu, C.; Zheng, H.; Liu, B. Molecular engineering of conjugated polymers for biophotonic imaging and therapy. Mater. Hor. 2017, 4, 1151. [Google Scholar]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic: Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Antoñanzas, A.; Auzmendi-Iriarte, J.; Carrasco-Garcia, E.; Moreno-Cugnon, L.; Ruiz, I.; Villanua, J.; Egaña, L.; Otaegui, D.; Samprón, N.; Matheu, A. Liquid biopsy in glioblastoma: Opportunities, applications, and challenges. Cancers 2019, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qiao, Y.; Tu, J. Microfluidic technologies for cfDNA isolation and analysis. Micromachines 2019, 10, 672. [Google Scholar] [CrossRef]
- Su, X.; Shan, Z.; Duan, S. Harnessing extracellular vesicles using liquid for cancer diagnosis and monitoring: Highlights from AACR Annual Meeting 2024. J. Hematol. Oncol. 2024, 17, 55. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, M.; Zhang, Y.; Zhang, X.; Zhang, X.; Zhang, B. Liquid biopsies in cancer. Mol. Biomed. 2025, 6, 18. [Google Scholar] [CrossRef]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef]
- Jamshidi, A.; Liu, M.C.; Klein, E.A.; Venn, O.; Hubbell, E.; Beausang, J.F.; Gross, S.; Melton, C.; Fields, A.P.; Liu, Q.; et al. Evaluation of cell-free DNA approaches for multi-cancer early detection. Cancer Cell 2022, 40, 1537–1549.e12. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef]
- Zottel, A.; Paska, A.V.; Jovcevska, I. Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis, and therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef]
- Schuurmans, C.; Balakrishnan, A.; Roy, S.; Fleming, T.; Leong, H.S. The emerging role of extracellular vesicles in the glioma microenvironment: Biogenesis and clinical relevance. Cancers 2020, 12, 1964. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Carter, B.S. Detection of glioblastoma in biofluids. J. Neurosurg. 2018, 129, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Nimgaonkar, V.; Issadore, D.; Carpenter, E. Extracellular Vesicle-Based Multianalyte Liquid Biopsy as a Diagnostic for Cancer. Annu. Rev. Biomed. Data Sci. 2022, 5, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Cellot, G.; Franceschi Biagioni, A.; Ballerini, L. Nanomedicine and graphene-based materials: Advanced technologies for potential treatments of diseases in the developing nervous system. Pediatr. Res. 2021, 92, 71–79. [Google Scholar] [CrossRef]
- Kutwin, M.; Sosnowska, M.E.; Strojny-Cieślak, B.; Jaworski, S.; Trzaskowski, M.; Wierzbicki, M.; Chwalibog, A.; Sawosz, E. MicroRNA delivery by graphene-based complexes into glioblastoma cells. Molecules 2021, 26, 5804. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Verduin, M.; Compter, I.; Steijvers, D.; Postma, A.A.; Eekers, D.B.P.; Anten, M.M.; Ackermans, L.; Ter Laan, M.; Leijenaar, R.T.H.; van de Weijer, T.; et al. Noninvasive Glioblastoma Testing: Multimodal Approach to Monitoring and Predicting Treatment Response. Dis. Markers 2018, 2018, 2908609. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Ibsen, D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.-Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 2017, 11, 6641. [Google Scholar] [CrossRef]
- Qiu, G.; Thakur, A.; Xu, C.; Ng, S.P.; Lee, Y.; Wu, C.M.L. Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 2019, 29, 1806761. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Skog, J.; Akers, J.; Li, H.; Komotar, R.; Jensen, R.; Ringel, F.; Yang, I.; Kalkanis, S.; Thompson, R. Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol. 2017, 19, 1494. [Google Scholar] [CrossRef] [PubMed]
- Drusco, A.; Bottoni, A.; Lagana, M.; Acunzo, M.; Fassan, L.; Cascione, A.; Antenucci, P.; Kumchala, C.; Vicentini, M.P.; Gardiman, M.P. A differentially expressed set of microRNAs in cerebro-spinal fluid (CSF) can diagnose CNS malignancies. Oncotarget 2015, 6, 20829. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, S.; Zhu, Z. What can AI-TENG do for Low Abundance Biosensing? Front. Bioeng. Biotechnol. 2022, 10, 899858. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Kasture, K.; Shende, P. Amalgamation of Artificial Intelligence with Nanoscience for Biomedical Applications. Arch. Comput. Methods Eng. 2023, 30, 4667–4685. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Niu, Z.; Ismail, M.; Morsch, M.; Zhang, X.; Li, M.; Shi, B. Nanotechnology-based strategies for early diagnosis of central nervous system disorders. Adv. NanoBiomed Res. 2021, 1, 2100008. [Google Scholar] [CrossRef]
- Ozaki, Y.; Broughton, P.; Abdollahi, H.; Valafar, H.; Blenda, A.V. Integrating omics data and AI for cancer diagnosis and prognosis. Cancers 2024, 16, 2448. [Google Scholar] [CrossRef] [PubMed]
- Shiri, F.M.; Perumal, T.; Mustapha, N.; Mohamed, R. A comprehensive overview and comparative analysis on deep learning models: CNN, RNN, LSTM, GRU. arXiv 2023, arXiv:2305.17473. [Google Scholar] [CrossRef]
- Tan, K.; Huang, W.; Hu, J.; Dong, S. A multi-omics supervised autoencoder for pan-cancer clinical outcome endpoints prediction. BMC Med. Inform. Decis. Mak. 2020, 20, 129. [Google Scholar] [CrossRef]
- Ali, M.; Aittokallio, T. Machine learning and feature selection for drug response prediction in precision oncology applications. Biophys Rev. 2019, 11, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Scott-Boyer, M.P.; Bodein, A.; Périn, O.; Droit, A. Integration strategies of multi-omics data for machine learning analysis. Comput. Struct. Biotechnol. J. 2021, 19, 3735–3746. [Google Scholar] [CrossRef]
- Bian, Y.; Xie, X.Q. Generative chemistry: Drug discovery with deep learning generative models. J. Mol. Model. 2021, 27, 1–18. [Google Scholar] [CrossRef]
- Rhee, S.; Seo, S.; Kim, S. Hybrid Approach of Relation Network and Localized Graph Convolutional Filtering for Breast Cancer Subtype Classification. arXiv 2018, arXiv:1711.05859. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- McCague, C.; Ramlee, S.; Reinius, M.; Selby, I.; Hulse, D.; Piyatissa, P.; Bura, V.; Crispin-Ortuzar, M.; Sala, E.; Woitek, R. Introduction to radiomics for a clinical audience. Clin. Radiol. 2023, 78, 83–98. [Google Scholar] [CrossRef]
- Fawaz, A.; Ferraresi, A.; Isidoro, C. Systems biology in cancer diagnosis: Integrating omics technologies and artificial intelligence to support physician decision making. J. Pers. Med. 2023, 13, 1590. [Google Scholar] [CrossRef]
- Sharifi-Noghabi, H.; Zolotareva, O.; Collins, C.C.; Ester, M. MOLI: Multi-Omics Late Integration with Deep Neural Networks for Drug Response Prediction. Bioinformatics 2019, 35, i501–i509. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Kim, Y.; Lee, S.; Namkung, J.; Yun, T.; Yi, S.G.; Han, S.; Kang, M.; Kim, S.W.; Jang, J.-Y.; et al. Integrative Analysis of Multi-Omics Data for Identifying Multi-Markers for Diagnosing Pancreatic Cancer. BMC Genom. 2015, 16, S4. [Google Scholar] [CrossRef]
- Ali, H. Artificial intelligence in multi-omics data integration: Advancing precision medicine, biomarker discovery, and genomic-driven disease interventions. Int. J. Sci. Res. Arch. 2023, 8, 1012–1030. [Google Scholar] [CrossRef]
- Henderson, S.; Perez-Gil, D.; Caulfield, M.; Rendon, A.; Chubb, D.; Hing, S.; Bentley, D.; Chan, G.; Fowler, T.; Siddiq, A.; et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 2024, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A.; Lacroix, L.; Mège, A.; Isambert, N.; Mosele, F.; André, F.; Filleron, T.; Hajjaji, N.; Jacot, W.; Rouleau, E.; et al. Genomics to select treatment for patients with metastatic breast cancer. Nature 2022, 610, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, S.; Zhao, Q.; Shia, B.; Huang, J.; Shi, X. Combining multidimensional genomic measurements for predicting cancer prognosis: Observations from TCGA. Brief. Bioinform. 2015, 16, 291–303. [Google Scholar] [CrossRef]
- Rao, J.; Zhao, H.; Zhou, X.; Zhang, Z.; Yang, Y.; Chai, H. Integrating multi-omics data through deep learning for accurate cancer prognosis prediction. Comput. Biol. Med. 2021, 134, 104481. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, D.; Lindeman, N.; Lu, M.; Mahmood, F.; Chen, R.; Rodig, S. Pathomic Fusion: An Integrated Framework for Fusing Histopathology and Genomic Features for Cancer Diagnosis and Prognosis. IEEE Trans. Med. Imaging 2022, 41, 757–770. [Google Scholar] [CrossRef]
- Shady, M.; Joo, B.; Shaban, M.; Williamson, D.; Lipková, J.; Lu, M.; Williams, M.; Mahmood, F.; Chen, R.; Chen, T.; et al. Pan-cancer integrative histology-genomic analysis via multimodal deep learning. Cancer Cell 2022, 40, 865–878.e6. [Google Scholar] [CrossRef]
- Durand, S.; Corcos, L.; Jossic-Corcos, C.; Badic, B.; Visvikis, D.; Hatt, M.; Simon, B. Radiogenomics-based cancer prognosis in colorectal cancer. Sci. Rep. 2019, 9, 9743. [Google Scholar] [CrossRef]
- Tabernero, J.; Mouillet-Richard, S.; Agueeff, N.; Laurent-Puig, P.; De Reyniès, A.; Sroussi, M.; Dourthe, L.; Lécaille, C.; Bachet, J.; Coutzac, C.; et al. Prognostic Models from Transcriptomic Signatures of the Tumor Microenvironment and Cell Cycle in Stage III Colon Cancer From PETACC-8 and IDEA-France Trials. J. Clin. Oncol. 2025, 43, 1765–1776. [Google Scholar] [CrossRef]
- Tsimberidou, A.; Kurzrock, R.; Fountzilas, E.; Bleris, L. Transcriptomics and Solid Tumors: The Next Frontier in Precision Cancer Medicine. Semin. Cancer Biol. 2020, 84, 50–59. [Google Scholar] [CrossRef]
- Chapman, L.; Rubin, E.; Srinivasan, A.; Dinstag, G.; Wang, K.; Cha, H.; Schperberg, A.; Kim, D.; Berger, R.; Chung, Y.; et al. Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell 2021, 184, 2487–2502.e13. [Google Scholar] [CrossRef]
- Pellegrini, B.; Yip, S.; Taylor, S.; Reisle, C.; Nelson, J.; Jones, M.; Pleasance, E.; Feng, X.; Tinker, A.; Sadeghi, S.; et al. Whole genome and transcriptome analysis enhances precision cancer treatment options. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 939–949. [Google Scholar] [CrossRef]
- Moore, R.; Wee, K.; Mungall, K.; Titmuss, E.; Zhao, Y.; Rassekh, S.; Marra, M.; Jones, S.; Nelson, J.; Ch’ng, C.; et al. Whole-genome and transcriptome integrated analyses guide clinical care of pediatric poor-prognosis cancers. Nat. Commun. 2024, 15, 4165. [Google Scholar] [CrossRef]
- Blakely, C.; Benes, C.; Schäffer, A.; Bivona, T.; Sinha, S.; Yosef, N.; Kammula, A.; Jiang, P.; Dhruba, S.; Ruppin, E.; et al. PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors. Nat. Cancer 2024, 5, 938–952. [Google Scholar] [CrossRef]
- Zhang, C.; Pontén, F.; Micke, P.; Uhlén, M.; Lundberg, E.; Lindskog, C.; Sjöblom, T.; Edfors, F.; Bidkhori, G.; Arif, M.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, N.; Zhuang, L.; Li, J.; Li, J. Development and validation of epigenetic modification-related signals for the diagnosis and prognosis of colorectal cancer. BMC Genom. 2024, 25, 51. [Google Scholar] [CrossRef]
- Jin, S.; Dai, R.; Xu, Q.; Zhang, R. Exploring the role of epigenetic regulation in cancer prognosis with the epigenetic score. Front. Pharmacol. 2025, 16, 1538205. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, K.; Wang, W.; Wu, Z.; Lin, R. Epigenetic and Tumor Microenvironment for Prognosis of Patients with Gastric Cancer. Biomolecules 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Alam, A.; Tan, J.; Lim, I.; Della Coletta, R.; Idrees, M.; Brenan, P. Epigenetics in the diagnosis and prognosis of head and neck cancer: A systematic review. J. Oral Pathol. Med. 2024, 53, 90–106. [Google Scholar] [CrossRef]
- Bolt, I.; Dillner, J.; Beaufort, I.; Rebitschek, F.; Joly, Y.; Tassé, A.; Colombo, N.; Knoppers, B.; Jones, A.; Sroczynski, G.; et al. Epigenome-based cancer risk prediction: Rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 292–309. [Google Scholar] [CrossRef]
- Grady, W.; Markowitz, S.; Yu, M. Epigenetic alterations in the gastrointestinal tract: Current and emerging use for biomarkers of cancer. Gastroenterology 2020, 160, 690–709. [Google Scholar] [CrossRef]
- Eritja, N.; Matias-Guiu, X.; Gatius, S.; Megino-Luque, C.; Albertí-Valls, M.; Macià, A. Metabolomic-Based Approaches for Endometrial Cancer Diagnosis and Prognosis: A Review. Cancers 2023, 16, 185. [Google Scholar] [CrossRef]
- Werner, H.; Rižner, T.; Schröder, C.; Lowy, C.; Fishman, D.; Semczuk, A.; Griesbeck, A.; Tokarz, J.; Romano, A.; Adamski, J. Endometrial cancer diagnostic and prognostic algorithms based on proteomics, metabolomics, and clinical data: A systematic review. Front. Oncol. 2023, 13, 1120178. [Google Scholar] [CrossRef]
- Srivastava, A.; Creek, D. Discovery and Validation of Clinical Biomarkers of Cancer: A Review Combining Metabolomics and Proteomics. Proteomics 2018, 19, e1700448. [Google Scholar] [CrossRef]
- Shi, H.; Weng, W.; Huang, W.; Shan, Y.; Chen, J.; Xiang, Y. Development of cancer prognostic signature based on pan-cancer proteomics. Bioengineered 2020, 11, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, Z.; Feng, Y.; Ye, H.; Lou, H.; Zhang, H.; Tian, J.; Wang, L.; Wang, Z.; Ni, J.; et al. Plasma-based proteomic and metabolomic characterization of lung and lymph node metastases in cervical cancer patients. J. Pharm. Biomed. Anal. 2024, 253, 116521. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Yang, L.; Song, H.; Jin, P.; Tian, Y.; Yang, T.; Wang, B.; Lou, S.; Wang, M.; Chen, Y.; et al. Metabolomic machine learning predictor for diagnosis and prognosis of gastric cancer. Nat. Commun. 2024, 15, 1657. [Google Scholar] [CrossRef]
- Haider, M.; Khalvati, F.; Zhang, Y.; Wong, A.; Oikonomou, A. Radiomics-based Prognosis Analysis for Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, srep46349. [Google Scholar] [CrossRef]
- Vallières, M.; Naqa, I.; Kay-Rivest, E.; Perrin, L.; Liem, X.; Khaouam, N.; Aerts, H.; Sultanem, K.; Seuntjens, J.; Wang, C.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Xia, Y.; Zhang, Y.; Zhu, F.; Zhang, Y.; Jiang, W.; Wang, X.; Zhang, H.; Ji, G. Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes. Radiology 2019, 290, 90–98. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kha, Q.H.; Le, V.H.; Le, N.Q.K.; Le, V.L.; Minh, T.N.T. Development and Validation of CT-Based Radiomics Signature for Overall Survival Prediction in Multi-organ Cancer. J. Digit. Imaging 2023, 36, 911–922. [Google Scholar] [CrossRef]
- Wang, W.; Cai, D.; Zhong, M.; Wu, X.; Kou, W.; Gao, F.; Li, C.; Zhu, Q.; Huang, Z.; Duan, X.; et al. A Metabolism-Related Radiomics Signature for Predicting the Prognosis of Colorectal Cancer. Front. Mol. Biosci. 2021, 7, 613918. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Sun, X.; Chen, S.; Zhong, Y.; Chen, C.; Xie, D.; Zhao, M.; Wang, T.; Yang, Y.; She, Y.; et al. Radiomics for Survival Risk Stratification of Clinical and Pathologic Stage IA Pure-Solid Non-Small Cell Lung Cancer. Radiology 2021, 302, 425–434. [Google Scholar] [CrossRef]
- Gomez, D.; Liao, Z.; Followill, D.; Balter, P.; Yang, J.; Fave, X.; Court, L.; Zhang, L.; Stingo, F.; Mackin, D.; et al. Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer. Sci. Rep. 2017, 7, 588. [Google Scholar] [CrossRef]
- Lehrer, E.; Kothari, G.; Korte, J.; Hardcastle, N.; Kron, T.; Zaorsky, N.; Lazarakis, S.; Siva, S. A systematic review and meta-analysis of the prognostic value of radiomics-based models in non-small cell lung cancer treated with curative radiotherapy. Radiother. Oncol. 2020, 155, 188–203. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, J.; Qiu, X.; Luo, J.; Luo, Y.; Kang, W.; Yang, Z.; Li, X. Application of radiomics-based multiomics combinations in the tumor microenvironment and cancer prognosis. J. Transl. Med. 2023, 21, 598. [Google Scholar] [CrossRef]
- Nandipati, M.; Fatoki, O.; Desai, S. Bridging nanomanufacturing and artificial intelligence—A comprehensive review. Materials 2024, 17, 1621. [Google Scholar] [CrossRef]
- Badini, S.; Regondi, S.; Pugliese, R. Unleashing the power of Artificial Intelligence in materials design. Materials 2023, 16, 5927. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Integrating Artificial Intelligence for drug discovery in the context of revolutionizing drug delivery. Life 2024, 14, 233. [Google Scholar] [CrossRef]
- Nayak, M.R.; Bayannavar, P.K.; Kamble, R.R. Design of Medicinally Pertinent Multifunctional Inorganic Nanomaterials Using Artificial Intelligence. In Multifunctional Inorganic Nanomaterials for Energy Applications; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Wang, Z.; Zhu, X. Enhancing nanocrystal synthesis: A comparative study of online artificial intelligence optimization and offline high-throughput experimentation in chemical material discovery. ACS Appl. Nano Mater. 2024, 7, 6499–6505. [Google Scholar] [CrossRef]
- Putra, R.V.W.; Marchisio, A.; Zayer, F.; Dias, J.; Shafique, M. Embodied Neuromorphic Artificial Intelligence for Robotics: Perspectives, Challenges, and Research Development Stack. In Proceedings of the 2024 18th International Conference on Control, Automation, Robotics and Vision (ICARCV), Dubai, United Arab Emirates, 12–15 December 2024; pp. 612–619. [Google Scholar]
- Kumar, A.; Panda, D.; Gangawane, K. Computational modeling on the design of the morphology of aerogels. In Hybrid Aerogels: Energy and Environmental Applications; De Gruyter: Berlin, Germany, 2024; pp. 269–290. [Google Scholar]
- Vijaya, R.; Lincy, B.C.; RamanG, G.; Kirubakaran, N.; Chacko, L.; Bhupathyraaj, M.; Kiruba, M.; Alharbi, H.F. Applications of Artificial Intelligence in Drug Delivery Systems. In Artificial intelligence in Pharmaceutical Sciences; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Deori, C.; Hujuri, L.; Sarma, G. Artificial Intelligence (AI): It’s Role in Drug Discovery and Novel Drug Delivery System. Int. J. Sci. Res. 2024, 13, 1434–1439. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.L.A.; Govan, J.E. Machine learning techniques for improving nanosensors in agroenvironmental applications. Agronomy 2024, 14, 341. [Google Scholar] [CrossRef]
- Das, K.; J, C. Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: Current progress and challenges. Front. Med. Technol. 2023, 4, 1067144. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, J.; Gandhi, S.; Prajapati, B.; Thanawuth, K.; Limmatvapirat, S.; Sriamornsak, P. AI-driven design and optimization of nanoparticle-based drug delivery systems. Science. Eng. Health Stud. 2024, 18, 24010003. [Google Scholar] [CrossRef]
- Akhtar, M.; Nehal, N.; Gull, A.; Parveen, R.; Khan, S.; Khan, S.; Ali, J.; Hamdard, J. Explicating the transformative role of artificial intelligence in designing targeted nanomedicine. Expert Opin. Drug Deliv. 2025, 22, 971–991. [Google Scholar] [CrossRef] [PubMed]
- Alafeef, M.; Srivastava, I.; Pan, D. Machine-learning for Precision Breast Cancer Diagnosis and Prediction of the Nanoparticles Cellular internalization. ACS Sens. 2020, 5, 1689–1698. [Google Scholar] [CrossRef]
- Esmaeilpour, D.; Ghomi, M.; Zare, E.; Sillanpää, M. Nanotechnology-Enhanced siRNA Delivery: Revolutionizing Cancer Therapy. ACS Appl. Bio. Mater. 2025, 8, 4549–4579. [Google Scholar] [CrossRef]
- Chou, W.; Chen, Q.; Yuan, L.; Cheng, Y.; He, C.; Monteiro-Riviere, N.; Riviere, J.; Lin, Z. An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef]
- Moore, J.; Chow, J. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 22001. [Google Scholar] [CrossRef]
- Ho, D.; Wang, P.; Kee, T. Artificial intelligence in nanomedicine. Nanoscale Horiz. 2019, 4, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2019, 32, e1901989. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Pan, M.; Mo, K.; Mao, Y.; Zou, D. Emerging Role of Artificial Intelligence in Diagnosis, Classification, and Clinical Management of Glioma. Semin. Cancer Biol. 2023, 91, 110–123. [Google Scholar] [CrossRef]
- Rudie, M.; Rauschecker, M.; Bryan, M.; Davatzikos, P.; Mohan, M. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology 2019, 290, 607–618. [Google Scholar] [CrossRef]
- Khalighi, S.; Reddy, K.; Midya, A.; Pandav, K.; Madabhushi, A.; Abedalthagafi, M. Artificial intelligence in neuro-oncology: Advances and challenges in brain tumor diagnosis, prognosis, and precision treatment. NPJ Precis. Oncol. 2024, 8, 80. [Google Scholar] [CrossRef]
- Razek, A.; Alksas, A.; Shehata, M.; Abdelkhalek, A.; Baky, K.; El-Baz, A.; Helmy, E. Clinical applications of artificial intelligence and radiomics in neuro-oncology imaging. Insights Into Imaging 2021, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shi, F.; Chun, Q.; Chen, H.; Ma, Y.; Wu, S.; Hameed, N.; Mei, C.; Lu, J.; Zhang, J.; et al. Artificial Intelligence Neuropathologist for Glioma Classification using Deep Learning on Hematoxylin and Eosin-Stained Slide images and Molecular Markers. Neuro Oncol. 2020, 23, 44–52. [Google Scholar] [CrossRef]
- Zhou, K.; Xiao, Z.; Liu, Q.; Wang, X.; Huo, J.; Wu, X.; Zhao, X.; Feng, X.; Fu, B.; Xu, P.; et al. Comprehensive application of AI algorithms with TCR NGS data for glioma diagnosis. Sci. Rep. 2024, 14, 15361. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Chen, Z.; Zhang, S.; Li, S.; Wageh, S.; Al-Hartomy, O.; Al-Sehemi, A.; Xie, Z.; Kankala, R.; et al. Glioma diagnosis and therapy: Current challenges and nanomaterial-based solutions. J. Control. Release 2022, 352, 338–370. [Google Scholar] [CrossRef]
- Palkar, A.; Dias, C.; Chadaga, K.; Sampathila, N. Empowering Glioma Prognosis with Transparent Machine Learning and Interpretative Insights Using Explainable AI. IEEE Access 2024, 12, 31697–31718. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial Intelligence Aids in the Development of Nanomedicines for Cancer Management. Semin. Cancer Biol. 2023, 89, 61–75. [Google Scholar] [CrossRef]
- Ameh, B. Advancing national security and economic prosperity through resilient and technology-driven supply chains. World J. Adv. Res. Rev. 2024, 24, 483–500. [Google Scholar] [CrossRef]
- Marima, R.; Hull, R.; Mbeje, M.; Molefi, T.; Mathabe, K.; Elbagory, A.M.; Demetriou, D.; Dlamini, Z. Role of precision oncology in type II endometrial and prostate cancers in the African population: Global cancer genomics disparities. Int. J. Mol. Sci. 2022, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Pauly, R.; Schwartz, C.E. The future of clinical diagnosis: Moving functional genomics approaches to the bedside. Adv. Mol. Pathol. 2019, 40, 221–230. [Google Scholar]
- Pauly, R.; Ziats, C.A.; Abenavoli, L.; Schwartz, C.E.; Boccuto, L. New strategies for clinical trials in autism spectrum disorder. Rev. Recent Clin. Trials 2021, 16, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Krentel, F.; Singer, F.; Rosano-Gonzalez, M.L.; Gibb, E.A.; Liu, Y.; Davicioni, E.; Keller, N.; Stekhoven, D.J.; Kruithof-de Julio, M.; Seiler, R. A showcase study on personalized in silico drug response prediction based on the genetic landscape of muscle invasive bladder cancer. Sci. Rep. 2021, 11, 5849. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Hu, Y.; Pan, B.; Huang, Q.; Dong, Q.; Xue, X.; Shen, X.; Chen, X. Utilizing machine learning to integrate single-cell and bulk RNA sequencing data for constructing and validating a novel cell adhesion molecules-related prognostic model in gastric cancer. Comput. Biol. Med. 2024, 180, 108998. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Zeh, H.J.; Kang, R.; Bai, L.; Tang, D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 2020, 11, 6339. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Sign. Transduct. Target Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Lian, J.X.; Lan, Z.; Zou, K.L.; Wang, W.M.; Yu, G.T. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2023, 29, 933–941. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M. Pharmacological inhibition of cystine-glutamate exchange induces endo plasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Pitini, E.; Baccolini, V.; Isonne, C.; Maran, P.; Marzuillo, C.; Villari, P.; Galeone, D.; Vaia, F. Public health genomics research in Italy: An overview of ongoing projects. Front. Public Health 2024, 12, 1343509. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Zhao, Y.; Li, M.C.; Polley, E.C.; Simon, R.M. OpenGeneMed: A portable, flexible, and customizable informatics hub for the coordination of next-generation sequencing studies in support of precision medicine trials. Brief. Bioinform. 2017, 18, 723–734. [Google Scholar] [CrossRef]
- Majumder, M.M. Improving Precision in Therapies for Hematological Malignancies. Diss. Sch. Dr. Ad Sanit. Investig. Univ. Hels. 2018, 66, 2018. [Google Scholar]
- De Mattos-Arruda, L.; Siravegna, G. How to use liquid biopsies to treat patients with cancer. ESMO Open 2021, 6, 100060. [Google Scholar] [CrossRef] [PubMed]
- Klocker, E.V.; Hasenleithner, S.; Bartsch, R.; Gampenrieder, S.P.; Egle, D.; Singer, C.F.; Rinnerthaler, G.; Hubalek, M.; Schmitz, K.; Bago-Horvath, Z.; et al. Clinical applications of next-generation sequencing-based ctDNA analyses in breast cancer: Defining treatment targets and dynamic changes during disease progression. Mol. Oncol. 2024, 19, 1897–1917. [Google Scholar] [CrossRef]
- Kawauchi, D.; Ohno, M.; Miyakita, Y.; Takahashi, M.; Yanagisawa, S.; Omura, T.; Yoshida, A.; Kubo, Y.; Igaki, H.; Ichimura, K.; et al. Early Diagnosis and Surgical Intervention Within 3 Weeks from Symptom Onset Are Associated with Prolonged Survival of Patients with Glioblastoma. Neurosurgery 2022, 91, 741–748. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Goldberg Allela, O.; Pecho, R.; Jayasankar, N.; Rao, D.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; Saadh, M.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Khan, M.; Alshahrani, M.; Wahab, S.; Gupta, G.; Kesharwani, P. Evolution of artificial intelligence as a modern technology in advanced cancer therapy. J. Drug Deliv. Sci. Technol. 2024, 98, 105892. [Google Scholar] [CrossRef]
- Pandurangan, P.; Dinesh, R.; MohanaSundaram, A.; Samrat, A.; Meenambika, S.; Vedanarayanan, V.; Meena, R.; Namasivayam, S.; Moovendhan, M. Integrating cutting-edge technologies: AI, IoT, blockchain and nanotechnology for enhanced diagnosis and treatment of colorectal cancer—A review. J. Drug Deliv. Sci. Technol. 2023, 91, 105197. [Google Scholar] [CrossRef]
- Goldberg, M. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Song, W.; Anselmo, A.; Huang, L. Nanotechnology intervention of the microbiome for cancer therapy. Nat. Nanotechnol. 2019, 14, 1093–1103. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, H.; Huang, R.; Zhou, J.; Zhang, J.; Yang, X.; Zhou, W.; Jiang, W.; Chen, S. Harnessing nanotechnology for cancer treatment. Front. Bioeng. Biotechnol. 2025, 12, 1514890. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Xing, Y.; Kim, G.; Simons, J. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Kawauchi, D.; Ohno, M.; Takahashi, M.; Yanagisawa, S.; Omura, T.; Miyakita, Y. AB023. Early diagnosis and treatment lead to improved survival of glioblastoma patients. Chin. Clin. Oncol. 2024, 13 (Suppl. S1), AB023. [Google Scholar] [CrossRef]
- Zhang, Y.; Lucas, C.; Young, J.; Morshed, R.; McCoy, L.; Bush, O.; Taylor, J.; Daras, M.; Butowski, N.; Villanueva-Meyer, J.; et al. Prospective genomically guided identification of “early/evolving” and “undersampled” IDH-wildtype glioblastoma leads to improved clinical outcomes. Neuro Oncol. 2022, 24, 1749–1762. [Google Scholar] [CrossRef]
- Qi, D.; Li, J.; Quarles, C.; Fonkem, E.; Wu, E. Assessment and prediction of glioblastoma therapy response: Challenges and opportunities. Brain 2022, 146, 1281–1298. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Xu, Y.; Sang, X.; Lu, X. Research on liquid biopsy for cancer: A bibliometric analysis. Heliyon 2023, 9, e14145. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.; Gulyás, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef]
- Crist, R.; Dasa, S.; Liu, C.; Clogston, J.; Dobrovolskaia, M.; Stern, S. Challenges in the development of nanoparticle-based imaging agents: Characterization and biology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1665. [Google Scholar] [CrossRef]
- Appenzeller, B.M.; Chadeau-Hyam, M.; Aguilar, L. Skin exposome science in practice: Current evidence on hair biomonitoring and future perspectives. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 26–30. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Papageorgiou, L.G.; Theofilatos, K.; Tsoka, S. Optimisation models for pathway activity inference in cancer. Cancers 2023, 15, 1787. [Google Scholar] [CrossRef]
- Park, I.; Kim, N.; Lee, S.; Park, K.; Son, M.Y.; Cho, H.S.; Kim, D.S. Characterization of signature trends across the spectrum of non-alcoholic fatty liver disease using a deep learning method. Life Sci. 2023, 314, 121195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, J.; Zhang, S.; Jiao, J. Applications of artificial intelligence (AI) in research on non-alcoholic fatty liver disease (NAFLD): A systematic review. Rev. Endocr. Metab. Disord. 2022, 23, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Zhu, Z.; Zhao, L.; Wang, H.; Song, C.; Chen, Y.; Zhao, Q.; Yang, J.; Pei, Y. A comprehensive review on the synergy of multi-modal data and ai technologies in medical diagnosis. Bioengineering 2024, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Yala, A.; Mikhael, P.; Strand, F.; Lin, G.; Satuluru, S.; Kim, T.; Banerjee, I.; Gichoya, J.; Trivedi, H.; Lehman, C.; et al. Multi-Institutional Validation of a Mammography-Based Breast Cancer Risk Model. J. Clin. Oncol. 2021, 40, 1732–1740. [Google Scholar] [CrossRef]
- Lipková, J.; Chen, R.; Chen, B.; Lu, M.; Barbieri, M.; Shao, D.; Vaidya, A.; Chen, C.; Zhuang, L.; Williamson, D.; et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 2022, 40, 1095–1110. [Google Scholar] [CrossRef]
- Arya, S.; Dias, S.; Jelinek, H.; Hadjileontiadis, L.; Pappa, A. The convergence of traditional and digital biomarkers through AI-assisted biosensing: A new era in translational diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef] [PubMed]
| Nanotechnology Approach | Diagnostic Application | Advantages | Challenges/Limitations | References |
|---|---|---|---|---|
| Nanoparticle-based MRI contrast agents | Enhanced tumor imaging, intraoperative guidance | Improved sensitivity, real-time tracking | BBB penetration, potential toxicity | [55,56,57,58] |
| Fluorescent nanoparticles | Tumor visualization during surgery | Increased tumor cell visibility, precision | Limited clinical translation | [55,56,58] |
| Gold and iron oxide nanoparticles | MRI, PET, photoacoustic imaging | Multifunctional, high contrast, targeting | Biocompatibility, clearance | [55,56,59,60] |
| Liposomes, dendrimers, micelles | Drug delivery + imaging (theranostics) | Dual function (diagnosis + therapy), tunability | Stability, manufacturing complexity | [59,61,62,63] |
| Smart/inorganic nanoparticles | Targeted molecular imaging | Specificity, surface modification | Off-target effects, immune response | [55,56,60] |
| Nanocarriers for liquid biopsy | Detection of circulating tumor DNA/cells | Non-invasive, early detection | Sensitivity, standardization | [56,58,62] |
| Nanomedicine-enabled PET/MRI agents | Multimodal imaging | Comprehensive tumor characterization | Cost, regulatory hurdles | [55,56,57] |
| Extracellular vesicle analysis | Biomarker discovery, monitoring | Personalized diagnosis, prognosis | Isolation techniques, reproducibility | [56,58,62] |
| Polymer-based nanoparticles | Targeted imaging and drug delivery | BBB penetration, controlled release | Long-term safety, scalability | [56,59,61,63] |
| CRISPR/Cas9 delivery via nanoparticles | Molecular diagnostics, gene editing | Precision, potential for personalized medicine | Delivery efficiency, ethical concerns | [56,59] |
| Genomics-Based Prediction of Cancer Prognosis | ||||
|---|---|---|---|---|
| Study Title | Cancer Type(s) | Approach | Key Findings | Reference |
| Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme | 33 solid tumor types | Whole-genome sequencing (WGS) integrated with clinical data | Linking WGS and clinical outcomes enables survival analysis and identification of prognostic cancer genes, supporting precision oncology | [122] |
| Genomics to select treatment for patients with metastatic breast cancer (SAFIR02-BREAST trial) | Metastatic breast cancer | Genomic profiling for therapy selection | Targeted therapies matched to actionable genomic alterations improve progression-free survival; benefit depends on actionability level | [123] |
| Combining multidimensional genomic measurements for predicting cancer prognosis: observations from TCGA | Breast, glioblastoma, AML, lung SCC | mRNA, DNA methylation, miRNA, copy number | Multidimensional genomic data can improve prognosis prediction, but gene expression and clinical data are most predictive | [124] |
| Integrating multi-omics data through deep learning for accurate cancer prognosis prediction | 15 cancer types (TCGA) | Multi-omics (genomics, transcriptomics, etc.) with deep learning | Denoising autoencoder improves prognostic accuracy; robust integration of multi-omics data identifies prognostic markers | [125] |
| Pathomic Fusion: An Integrated Framework for Fusing Histopathology and Genomic Features for Cancer Diagnosis and Prognosis | Glioma, renal cell carcinoma | Histology + genomics (mutations, CNV, RNA-Seq) | Multimodal deep learning fusion improves survival prediction over unimodal models | [126] |
| Pan-cancer integrative histology-genomic analysis via multimodal deep learning | 14 cancer types | Histology + genomics | Multimodal deep learning predicts outcomes and discovers prognostic features across cancers | [127] |
| Radiogenomics-based cancer prognosis in colorectal cancer | Colorectal cancer | Radiomics + gene expression | Combining imaging and gene expression enhances prognostic stratification | [128] |
| Transcriptomic-Based Prediction of Cancer Prognosis. | ||||
| PETACC-8 & IDEA-France (2025) | Stage III colon cancer | 3′RNA sequencing, TME, and cell cycle signatures | Developed prognostic models integrating transcriptomic signatures, improving risk stratification for recurrence | [129] |
| WINTHER Trial (2020) | Diverse solid tumors | RNA expression profiling | Combined genomics and transcriptomics increased actionable targets; improved patient matching to therapies | [130,131] |
| POG Program (2022) | Advanced/metastatic cancers | Whole genome and transcriptome analysis (WGTA) | RNA data informed 67% of treatments; 46% of WGTA-informed treatments led to clinical benefit | [132] |
| Pediatric Poor Prognosis Study (2024) | Pediatric cancers | WGTA | Integrating transcriptome data identified actionable variants in 96% of cases, guiding therapy | [133] |
| PERCEPTION (2024) | Multiple myeloma, breast, and lung cancer | Single-cell transcriptomics | scRNA-seq-based models outperformed bulk predictors in clinical response prediction | [134] |
| SELECT (2021) | 10 cancer types | Synthetic lethality via transcriptome | Predicted therapy response in 80% of 35 clinical trials, including WINTHER | [131] |
| Pathology Atlas (2017) | 17 cancer types | Genome-wide transcriptomics | Identified prognostic genes and created an open-access atlas for survival prediction | [135] |
| Epigenomics-Based Prediction of Cancer Prognosis. | ||||
| Development and validation of epigenetic modification-related signals for the diagnosis and prognosis of colorectal cancer | Colorectal cancer | Predictive model using gene expression and epigenetic-related genes, validated on patient cohorts | An 8-gene epigenetic signature effectively predicts prognosis and may guide targeted therapies | [136] |
| Exploring the role of epigenetic regulation in cancer prognosis with the epigenetic score | Pan-cancer (TCGA datasets) | LASSO Cox model to create an epigenetic score, a nomogram integrating clinical features | Epigenetic score correlates with cancer hallmarks and predicts survival across cancer types | [137] |
| Epigenetic and Tumor Microenvironment for Prognosis of Patients with Gastric Cancer | Gastric cancer | Machine learning (NMF, LASSO, SVM) to identify prognostic gene signatures | Identified hub genes for prognosis; signatures performed well in survival prediction and immunotherapy response | [138] |
| Epigenetics in the diagnosis and prognosis of head and neck cancer: A systematic review | Head and neck squamous cell carcinoma | Systematic review of 25 studies on DNA methylation and histone modifications | Several biomarkers (e.g., DAPK, TIMP3) show promise for early detection, but more robust trials are needed | [139] |
| Epigenome-based cancer risk prediction: rationale, opportunities and challenges | General/Multiple cancers | Review of DNA methylation-based risk prediction tests | DNA methylation tests are promising for risk prediction, but challenges include cell-type specificity and implementation | [140] |
| Epigenetic alterations in the gastrointestinal tract: Current and emerging use for biomarkers of cancer | GI cancers (colorectal, liver, etc.) | Review of clinical and emerging epigenetic biomarkers | Epigenetic alterations are robust biomarkers for prognosis and are being integrated into clinical tests | [141] |
| Proteomics and Metabolomics-Based Prediction of Cancer Prognosis. | ||||
| Metabolomic-Based Approaches for Endometrial Cancer Diagnosis and Prognosis: A Review | Endometrial | Metabolomics | Identifies metabolite biomarkers for improved diagnosis, prognosis, and monitoring | [142] |
| Endometrial cancer diagnostic and prognostic algorithms based on proteomics, metabolomics, and clinical data: a systematic review | Endometrial | Proteomics and Metabolomics | Reviews diagnostic/prognostic biomarker discovery using omics and clinical data | [143] |
| Discovery and Validation of Clinical Biomarkers of Cancer: A Review Combining Metabolomics and Proteomics | Various | Proteomics and Metabolomics | Highlights advance in biomarker discovery and clinical translation | [144] |
| Development of a cancer prognostic signature based on pan-cancer proteomics | Multiple (pan-cancer) | Proteomics | Proteomics-based model accurately predicts survival across cancers | [145] |
| Plasma-based proteomic and metabolomic characterization of lung and lymph node metastases in cervical cancer patients | Cervical | Proteomics and Metabolomics | Identifies biomarker panels for predicting lung and lymph node metastasis | [146] |
| Metabolomic machine learning predictor for diagnosis and prognosis of gastric cancer | Gastric | Metabolomics and Machine Learning | Machine learning model outperforms traditional markers for diagnosis and prognosis | [147] |
| Radiomic-Based Prediction of Cancer Prognosis. | ||||
| Radiomics-based Prognosis Analysis for Non-Small Cell Lung Cancer | NSCLC | 112 patients, CT-based radiomics, various modeling strategies | Random Forest and PCA improved the prediction of recurrence and survival; addressing data imbalance increased accuracy | [148] |
| Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer | Head and Neck | 300 patients, multi-cohort, PET/CT radiomics | Radiomics plus clinical data predicted recurrence/metastasis risk; validated across cohorts | [149] |
| Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes | Biliary Tract | 247 patients, CT-based, retrospective | Radiomics model predicted lymph node metastasis and survival; the high-risk group had worse outcomes | [150] |
| Development and Validation of CT-Based Radiomics Signature for Overall Survival Prediction in Multi-organ Cancer | Lung, Kidney, Head and Neck | 905 patients, multi-organ, CT-based | Radiomics signature predicted survival across cancer types; the combined model improved accuracy | [151] |
| A Metabolism-Related Radiomics Signature for Predicting the Prognosis of Colorectal Cancer | Colorectal | 381 patients, CT-based, LASSO regression | Radiomics score independently predicted disease-free survival; the nomogram outperformed the TNM stage | [152] |
| Radiomics for Survival Risk Stratification of Clinical and Pathologic Stage IA Pure-Solid Non-Small Cell Lung Cancer | NSCLC (Stage IA) | 592 patients, multi-region radiomics | Multiregional radiomics signature stratified survival risk, improved over clinical predictors | [153] |
| Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer | NSCLC | 107 patients, longitudinal CT | Radiomics features changed during therapy; end-of-treatment features predicted response | [154] |
| Radiogenomics-based cancer prognosis in colorectal cancer | Colorectal | 64 patients, CT radiomics + gene expression | Combined radiomics and genomics improved prognostic stratification | [128] |
| Systematic review and meta-analysis of radiomics-based models in NSCLC | NSCLC | 40 studies, 6223 patients | Radiomics models showed modest prognostic value; need for standardization | [155] |
| Application of radiomics-based multiomics combinations in the tumor microenvironment and cancer prognosis | Various | Review | Multiomics (radiomics, pathomics, genomics) enhances TME assessment and prognosis prediction | [156] |
| Nanoparticle Type | AI Algorithm/Approach | Clinical Outcome/Use Case | Citations |
|---|---|---|---|
| Polymeric nanoparticles | Machine learning | Enhanced targeted drug delivery in cancer | [168,169,170] |
| Dendrimers | Neural networks | Improved drug loading and release profiles | [169,170] |
| Micelles | Optimization algorithms | Controlled drug release, reduced toxicity | [169,170] |
| Liposomes | Deep learning | Personalized dosing, improved therapeutic index | [169,170,171] |
| Protein nanoparticles | Pattern recognition | Tumor-specific targeting, better diagnostics | [169,172] |
| Cell membrane nanoparticles | AI-driven design | Enhanced immune evasion, longer circulation | [169,173] |
| Mesoporous silica nanoparticles | Predictive modeling | Increased delivery efficiency to tumors | [169,174] |
| Gold nanoparticles | Mathematical modeling, ML | Optimized photothermal therapy, improved imaging | [169,175] |
| Iron oxide nanoparticles | AI-assisted PBPK modeling | Accurate prediction of biodistribution | [169,174] |
| Quantum dots | Classification algorithms | Improved cancer cell identification | [169,172] |
| Carbon nanotubes | ANN, ML | High-accuracy cancer cell classification | [169,172] |
| Black phosphorus | AI-based optimization | Enhanced drug delivery, reduced side effects | [169,170] |
| MOF nanoparticles | QSAR, ML | Optimized structure for drug delivery | [169,171] |
| Exosome-mimicking nanoparticles | AI-driven nanocarrier design | Improved biocompatibility, targeted siRNA delivery | [169,173] |
| Multifunctional nanoparticles | ML for synergy prediction | Combined chemo/immunotherapy, reduced resistance | [173,176] |
| Biomimetic nanocarriers | ML, optimization | Enhanced tumor targeting, immune evasion | [169,173] |
| Smart nanoparticles (stimuli-responsive) | AI-powered design | Precision therapy, individualized treatment | [168,169] |
| Carbon nanoparticles (CNPs) | ANN, ML | Subclassification of breast cancer, >98% diagnostic accuracy | [172] |
| Nanoparticle-modified drugs | AI for dose optimization | Improved combination therapy outcomes | [176] |
| Nanoparticle-based imaging agents | AI for image analysis | Enhanced diagnostic accuracy, better treatment planning | [169,177] |
| Technology/ Model | Nanotechnology Type and Example | Application (What It Does) | Example (Specific Implementation) | Clinical Status | References |
|---|---|---|---|---|---|
| Deep learning on MRI/Pathology | Metal nanoparticles (e.g., gold, iron oxide) for enhanced imaging contrast and targeted delivery | Tumor segmentation, grading, and molecular subtyping | CNNs and transformer-based models for classifying glioma subtypes from histopathology and MRI | Clinically validated | [178,179,180,181,182] |
| AI on TCR NGS data | Persistent luminescence nanoparticles (e.g., TRZD: ZnGa2O4:Cr3+, Sn4+) for long-term NIR imaging and therapy | Immune repertoire-based glioma diagnosis | AI models using T-cell receptor sequencing to classify glioma with an AUC of up to 96.7% | Early clinical | [183,184] |
| AI model optimization | Chlorotoxin peptide-functionalized gold nanoparticles (CTX-PEI-AuNPs) for targeted SPECT/CT imaging/therapy | Deploying AI in low-resource clinical settings | Post-training optimization of ResUNet for tumor delineation, reducing latency and memory usage | Early clinical | [177,184] |
| Explainable AI (XAI) for prognosis | AI-guided nanomedicine design for optimizing nanoparticle properties for diagnosis and therapy | Transparent, interpretable prediction of glioma outcomes | XGBoost, SHAP, LIME, and other XAI tools for feature importance and model explanation | Preclinical | [177,185,186] |
| Deep learning for digital pathology | Albumin-based nanotheranostic probes (e.g., ICG/AuNR@BCNP) for multimodal imaging and phototherapy | Automated histopathological subtype classification | SD-Net_WCE (DenseNet variant) for classifying five glioma subtypes from H&E slides | Clinically validated | [179,182] |
| Radiomics and radiogenomics | Multifunctional metal nanoparticles for targeted drug delivery, imaging, and therapy | Linking imaging features to molecular/ genomic profiles | AI models predicting IDH mutation and 1p/19q codeletion from MRI features | Clinically validated | [179,180,184] |
| AI for treatment response prediction | Surface-modified nanoparticles (e.g., PEGylated, ligand-targeted) for BBB penetration and targeted delivery | Predicting therapy outcomes and recurrence | Machine learning models integrating imaging, genomics, and clinical data | Preclinical | [179,184,186] |
| AI-enabled nanomedicine design | AI-enabled design of nanomedicines for personalized dosing and reduced nanotoxicity | Optimizing nanomaterial properties for diagnosis/therapy | AI algorithms predicting nanomaterial interactions for improved efficacy and safety | Preclinical | [177,186] |
| Nanoparticle-based imaging agents | Iron oxide nanoparticles (IONPs) for MRI and therapy; gold nanoparticles for SPECT/CT imaging and therapy | Enhanced Imaging for glioma detection | Iron oxide nanoparticles (IONPs) for MRI contrast, cell tracking, and magnetic hyperthermia | Mostly preclinical | [177,186] |
| Region | Key Regulatory Frameworks | Focus Areas |
|---|---|---|
| United States | FDA, NIH, GINA | AI software validation, genetic privacy laws |
| European Union | GDPR, AI Act | Data protection, high-risk AI regulation |
| China | PIPL, AI Governance Initiatives | National AI strategies, genomic data security |
| Japan | Ethical AI Guidelines, Genome Research Laws | AI in genomics, patient data ethics |
| Translation Gap | Nanotechnology Example(s) | AI Example(s) | Citations |
|---|---|---|---|
| Limited Clinical Approvals | Liposomal doxorubicin (Doxil), albumin-bound paclitaxel (Abraxane); most nanomedicines remain preclinical | AI-driven design of nanocarriers for siRNA delivery, but few AI-optimized nanomedicines have clinical approval | [173,177,203,204] |
| Biological Barriers | Nanoparticles for siRNA/chemotherapy delivery face rapid degradation, poor tumor accumulation, and immune clearance | AI models predict nanoparticle–biological interactions to optimize delivery, but translation to humans is limited | [173,177,204,205] |
| Tumor Heterogeneity | Multifunctional nanoparticles for combined therapy (e.g., chemo-immuno- or photothermal therapy) to address tumor diversity | AI analyzes patient omics/imaging data to tailor nanomedicine, but heterogeneity complicates universal solutions | [177,206,207,208] |
| Safety and Toxicity Concerns | Biomimetic nanocarriers (e.g., exosome-mimicking) improve biocompatibility, but long-term human safety data lacking | AI used to predict and minimize nanotoxicity, but real-world validation is limited | [173,186,206,209] |
| Regulatory and Manufacturing Challenges | Few standardized protocols for nanomedicine production; scalability and reproducibility issues | AI can optimize manufacturing processes, but regulatory pathways for AI-designed nanomedicines are unclear | [177,205,210,211] |
| Data Integration and Patient Stratification | Nanodiagnostics and nano-imaging generate large datasets for patient profiling | AI integrates multi-omics, imaging, and clinical data for personalized therapy, but clinical implementation is rare | [177,207,208] |
| Gap Between Preclinical and Clinical | Most nanoformulations (e.g., targeted nanoparticles, nano-theranostics) show efficacy in animals but not in humans | AI-optimized nanomedicines are often validated only in silico or in animals, not in clinical trials | [173,177,203,206] |
| Incomplete Understanding of Cancer Biology | Nanoparticles are designed for targeted delivery, but incomplete knowledge of the tumor microenvironment limits success | AI helps uncover new biomarkers and drug targets, but translation to effective therapies is ongoing | [177,206,210,211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryawanshi, M.V.; Bagban, I.; Patne, A.Y. Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective. Targets 2025, 3, 31. https://doi.org/10.3390/targets3040031
Suryawanshi MV, Bagban I, Patne AY. Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective. Targets. 2025; 3(4):31. https://doi.org/10.3390/targets3040031
Chicago/Turabian StyleSuryawanshi, Meghraj Vivekanand, Imtiyaz Bagban, and Akshata Yashwant Patne. 2025. "Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective" Targets 3, no. 4: 31. https://doi.org/10.3390/targets3040031
APA StyleSuryawanshi, M. V., Bagban, I., & Patne, A. Y. (2025). Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective. Targets, 3(4), 31. https://doi.org/10.3390/targets3040031







