1. Introduction and Clinical Significance
Echinococcosis is a zoonosis that has two basic forms, alveolar echinococcosis (AE), caused by Echinococcus multilocularis; and cystic echinococcosis (CE), caused by Echinococcus granulosus [
1]. While AE is a rare form and found solely in the northern hemisphere, CE, also known as hydatid disease, is endemic in South America, Eastern and Southern Europe, Russia, the Middle East, Africa, and China [
2].
The liver is the organ most commonly affected, with an infestation rate ranging from 60% to 75%. Among these cases, the right lobe is involved in 80%, while the left lobe is affected in 20% [
3]. Less frequently, other organs such as the lungs (25%), spleen, kidneys, and brain may also be involved [
4]. There are several potential complications, including local manifestations, such as rupture, as well as the risk of hematogenous spread to other organs [
3].
The most commonly used imaging modalities for CE diagnosis are ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) [
5].
Yesilyurt and Esdur reviewed more 500 cases of cystic echinococcosis in their department in their study “Anatomical-Based Imaging of Cystic Echinococcosis and Review of the Current Literature”, giving a detailed discussion of the diagnostic imaging methods for cystic echinococcosis [
6]. Their results stress the significance of imaging in the diagnosis and management of CE, as well as the World Health Organization’s (WHO) classification system for CE staging, which is crucial in making treatment decisions. In addition, they focused on the various complications of CE, such as cyst rupture and organ involvement, which highlights the need for early detection for effective management, furthermore, giving a detailed account of how imaging helps in staging the disease, which, in turn, helps in choosing the treatment.
However, there are some limitations, including their dependence on single-center experience, therefore, not capturing global practices or imaging strategies in different contexts. Also, the study does not present data on the long-term results of different treatment options.
The authors use a combination of literature review and case studies from their own institution to examine the imaging features of CE. Their clinical results indicate the importance of cancer-like stage-based treatment in concordance with WHO classification. Imaging has a major role in diagnosis, stressing the importance of US and MRI prior to CT scanning, as it lacks the ability to specify all of the cystic features, except for calcification. Furthermore, they advised that diffusion-weighted MRI scanning should have an active part in correct diagnosis and staging. Specifically, the peripheral hypointense rim on the T2-weighted images on the MRI scan was named a key feature to guide diagnosis.
This paper aims to expand these results to specific imaging features of the individual stages and combine them with the therapeutic options.
In this paper, we discuss recent implementations in diagnostics and therapy, while presenting a case report of a 23–year-old female patient with a giant cystic echinococcosis, who was followed up for 6 months using MR imaging in a tertiary care hospital in Dortmund, Germany.
This case highlights the possibility of using MRI scans as a preferred method for surveillance, especially in a case where surgery was ruled out. As resources such as MRI scans are not accessible in many countries where CE is endemic, and, in many cases, in an environment with access to more resources, patients often directly undergo surgery, the aim of the paper is to present a different approach of bridging surgery and implementing the principles of surveillance and imaging features.
2. Case Presentation
A 23-year-old female patient presented with nausea, fatigue, and loss of appetite to the emergency department for 6–8 weeks. An abdominal examination did not reveal any significant findings. Laboratory blood tests showed elevated AST 62 (U/L), ALT 74 (U/L), and GGT 107 (U/L). In addition, the eosinophil count was elevated up to 10%. Leucocytes were in a normal range of 7.98 Tsd/μL, as well as C-reactive protein with 1.2 mg/L. The ESR showed no abnormality with a value of 16 mm.
An ultrasound of the abdomen discovered a single hypoechoic lesion with sharp margins in Segment IV (Lobus Quadratus) of the liver and a possible snowstorm sign. The patient’s history involved frequent travels to rural areas of Turkey, a location where CE is endemic. The serology for echinococcosis (IHA and EIA) showed positive values. The patient was admitted to the hospital and further diagnostics were initiated.
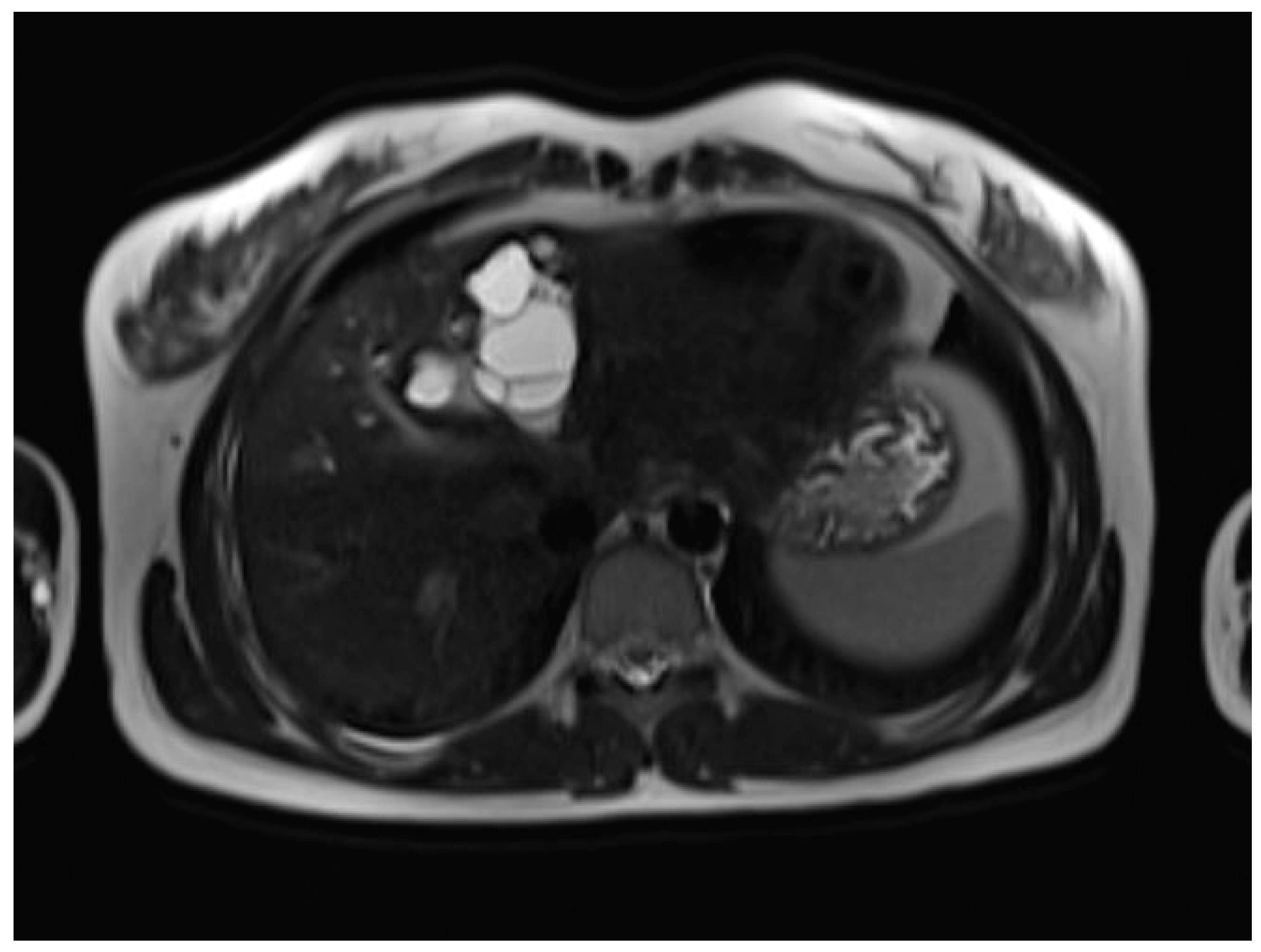
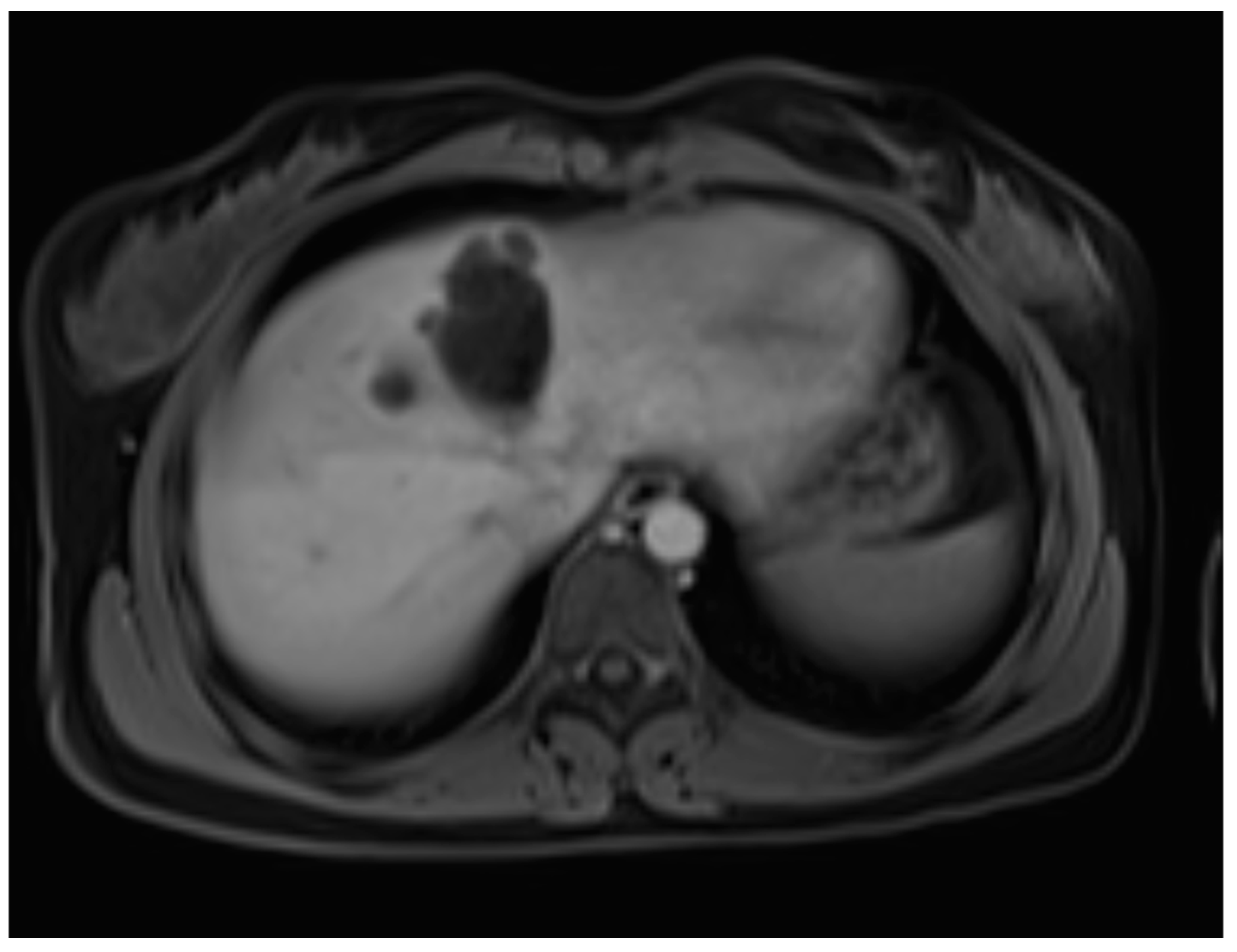
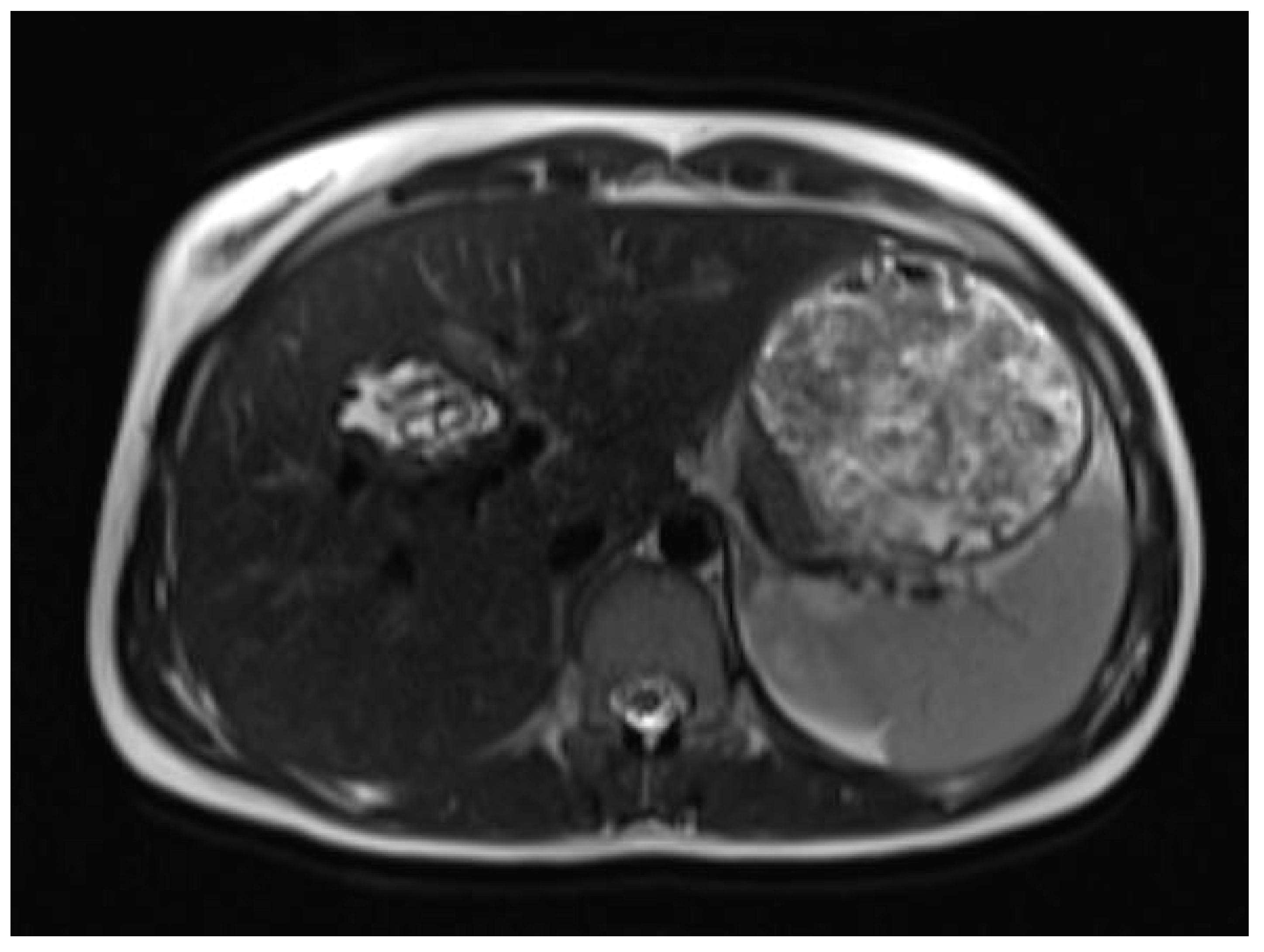
A liver MRI scan confirmed a cystic structure with multiple daughter cysts, hypointense on a T1-weighted image (T1WI), and hyperintense on a T2-weighted image (T2WI), with no solid components, no signs of membrane rupture, and a maximum diameter of 10 cm (craniocaudal) (
Figure 1). Additionally, elevated signals on T2WI in the surrounding liver tissue were found. The T1 sequences did not reveal any contrast uptake after 5 (
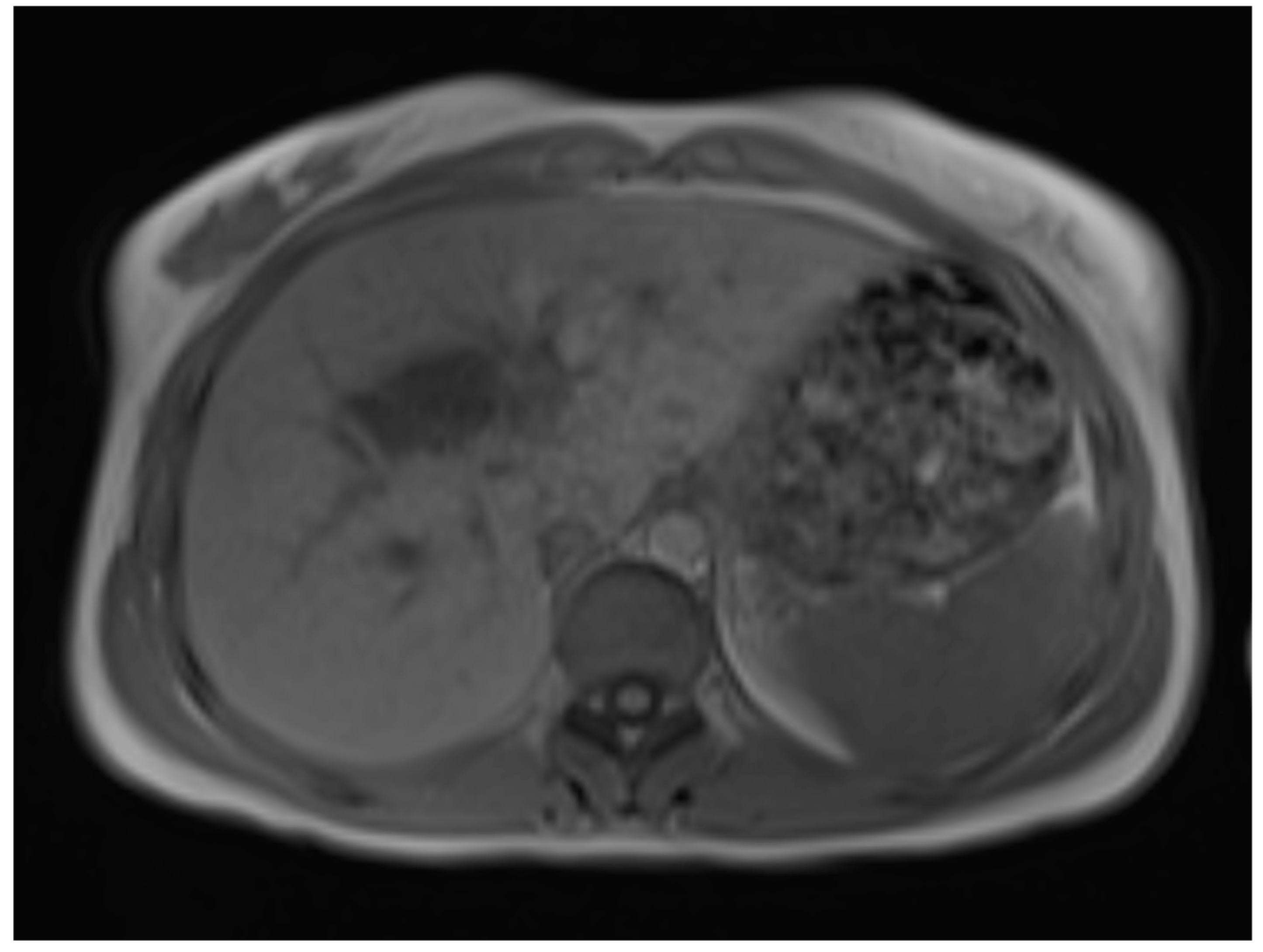
Figure 2), 10, or 20 (
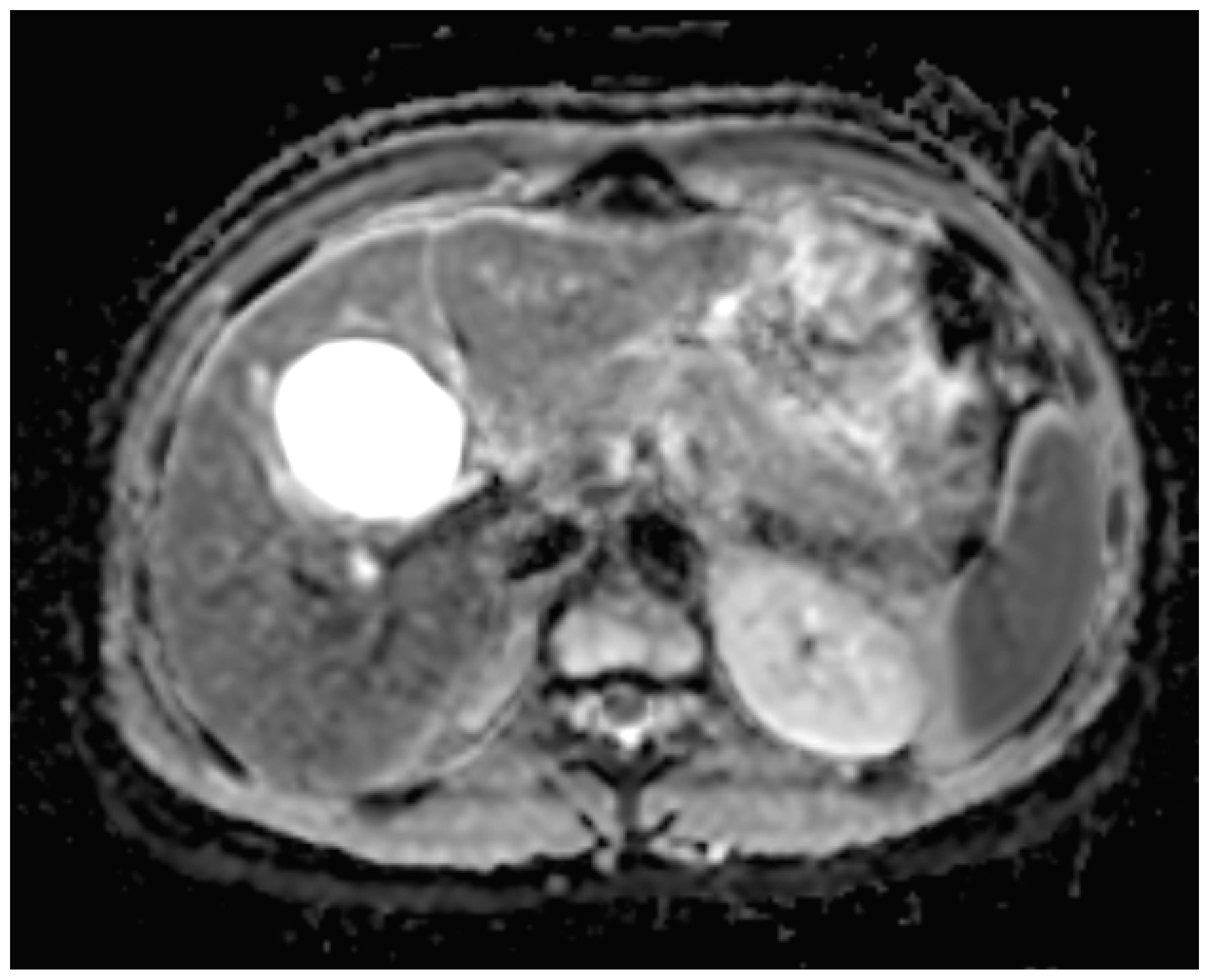
Figure 3) minutes post-injection. Furthermore, DWI/ADC mapping indicated a hydatid cyst. The DWI sequences included a trace DWI (
Figure 4), an ADC map (
Figure 5), and both at b800. Radiomics and AI tools were not involved. The trace DWI showed an area of hypointensity in the region of the cyst that was surrounded by a hypointense layer, depicting the cystic wall. The ADC map revealed hyperintensity within the area, in concordance with a typical depiction of cysts on an MRI scan.
The previous findings were highly suspicious of echinococcosis, and, according to the World Health Organization Informal Working Groups on Echinococcosis (WHO-IWGE), were classified as CE2.
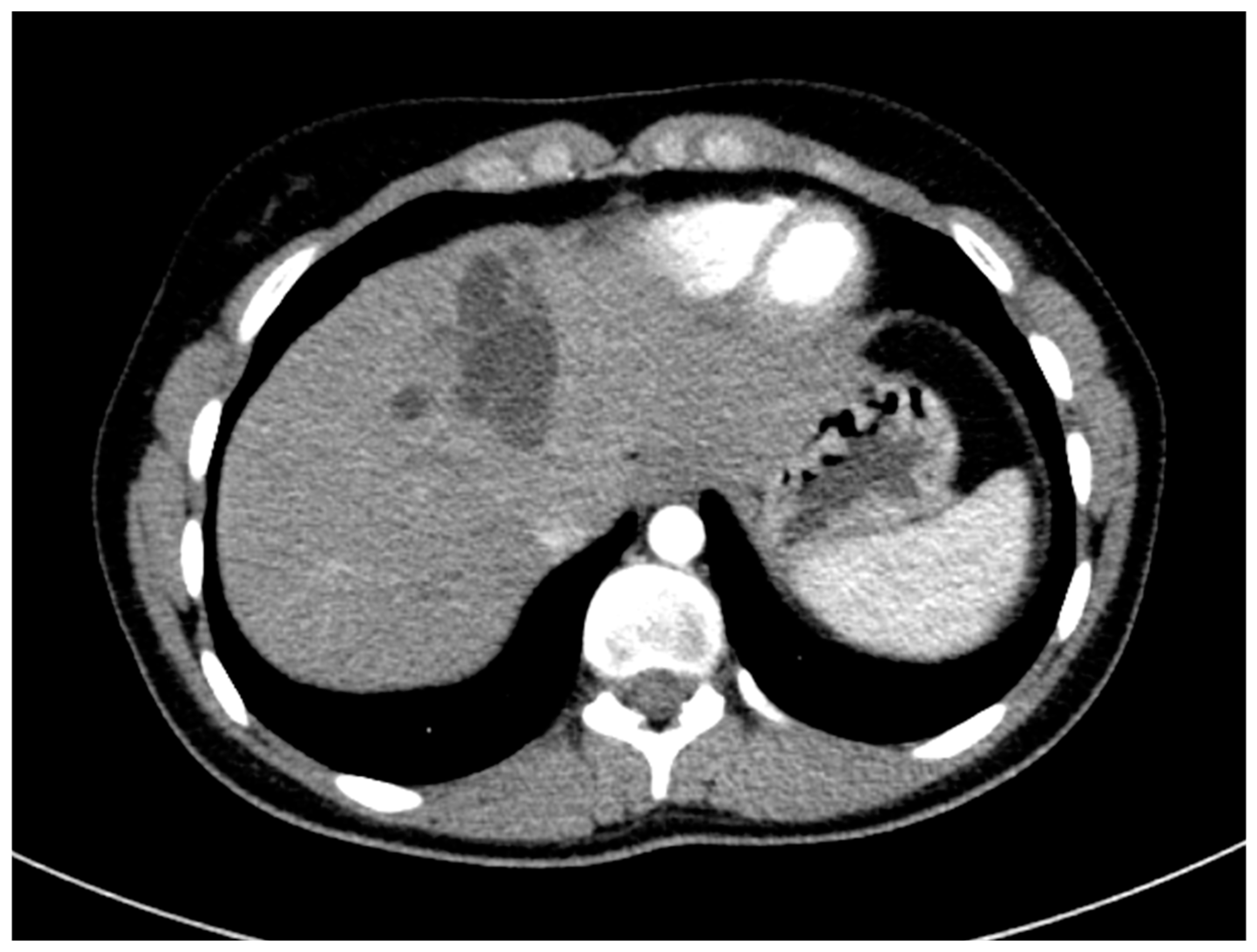
The surgical department decided not to opt for a surgical resection due to difficult access to the cyst, and therapy with the antihelminthic medication albendazole for four weeks was initiated. Additional diagnostics, including chest CT and an MRI scan of the brain, did not find any further extrahepatic manifestations of the hydatid disease. The chest CT scan included parts of the abdomen and also depicted the cyst and lower-attenuated daughter cysts (
Figure 6).
Six months later, a second ultrasound scan and a consecutive MRI scan to follow-up and control the success of the therapy were performed. The ultrasound revealed a decrease in the size of the cyst from 10 cm in maximal diameter to 9 cm and showed multiple circular septations, indicating an involuting cyst (
Figure 7). In contrast to the preceding MRI scan, typical T2 hypointense membrane-like structures were observed within the lesion in T2WI. These represented most probably the floating membranes, the typical water-lily sign (
Figure 8). T1WI (
Figure 9) showed the cyst as a hypointense lesion, but the membranes could only be clearly observed in T2WI. Consecutively, the cyst was classified as WHO-CE3A.
The further options of surgery after the cyst reduction were explained to the patient, but they left with a further scheduled check-up.
The patient returned a month later with recurrent upper abdominal pain. Another therapy with albendazole for four weeks was initiated, and the patient was referred to a more specialized hospital for liver surgeries.
The patient underwent a diagnostic laparoscopy after four weeks, as the albendazole cycle was completed. The surgical team took the decision of conversion to an open mesohepatectomy because of the central location of the cyst. A total cystectomy was performed. The operation resulted in an insufficiency of the bile ducts, which was successfully treated with ERCP and a bilary stent.
The patient received a third cycle of a four-week antihelminthic treatment of albendazole after the operation. The patient was discharged without further complaints.
3. Discussion
The life cycle of Echinococcus granulosus involves two hosts, as follows: a definitive host, typically a dog (or other carnivores) and an intermediate host, usually a sheep. The adult worm lives in the small intestine of the definitive host, releasing eggs that are excreted in the feces. Sheep ingest the eggs while grazing, and the larvae (oncospheres) penetrate the intestinal wall, entering portal circulation and forming cysts in the liver. The cycle is completed when the definitive host consumes the viscera of the infected intermediate host. Humans can become accidental intermediate hosts through contact with infected dogs or the consumption of contaminated water or food. In the human liver, cysts grow up to 1 cm in the first 6 months, and 2–3 cm annually, thereafter, depending on the host’s immune response [
7]. Due to their slow growth, they are usually asymptomatic or have unspecific clinical presentation [
8].
The natural progression of hepatic hydatid cysts predisposes rupture as a complication in 50% to 90% of cases, potentially leading to an anaphylactic reaction. Besides rupturing in the blood stream, more rarely, 0.5% of cysts rupture in the hollow organs and present with hydatidemesis or hydatidorrhea. In 5% to 15% of cases, they can rupture into the biliary tree and lead to symptoms of biliary obstruction. Furthermore, a cyst can become infected or undergo exophythic growth or transdiaphragmatic involvement, with the bare area of the liver being one of the most common pathways. Peritoneal echinococcosis occurs in 13% of cases, mostly after surgeries for hepatic diseases [
7]. Another complication is compression or thrombosis of the portal vein [
9]. Echinococcosis can act like a tumor and spread hematogenously to every organ, found even in the pericard, spinal cord, adrenal glands, and muscle [
10]. Therefore, a thorough assessment of the patient is necessary, including ruling out pulmonary and cerebral lesions, as was performed in our presented case.
Our case did not give any indication for a cardiac mass, but here the approach of multimodality imaging for patients with CE is of importance. If there are any indications of a possible cardiac mass, patients require a distinguished approach to detect any possible cardiac lesions, with cardiac MRI scanning being the gold standard [
11]. Imaging modalities used for the diagnosis of CE are US, CT, and MRI scans [
5]. Unlike in AE, where FDG-PET/CT imaging can be used to examine the biological activity, recent studies of Salvador et al. did not find a correlation between biological activity and FDG-PET/CT [
12]. Serology has only a confirmational role after imaging [
10].
Our case proved an entity with specific features in all three modalities, although the diagnosis-revealing modality was eventually MRI scanning. The confirmational element of serology was also proven in our case.
Because of the difference in growing patterns, AE and CE have different imaging findings but have a similar therapy approach. CE has a concentric expansion, and AE has vesicle-to-vesicle growth, forming grapes-like clusters with an infiltrative nature [
2]. CE is the more common type of echinococcosis [
5]. In this paper, we will focus on the typical imaging findings and therapy plan for CE.
The most important differential diagnosis for CE is a cyst with calcification [
2]. Ultrasound depicts calcification and is often used as a screening tool because it is not only highly sensitive and specific, but also a non-invasive, widely available, and cost-efficient mode of investigation [
2,
13]. The cyst wall typically appears as two echogenic lines with a hypoechogenic fluid layer in between. Simple cysts lack internal structures, but a hydatid cyst may show echogenic foci, known as hydatid sand, that shift to the lowest part of the cyst when the patient is repositioned. This movement creates a snowstorm sign, where the foci appear scattered, like a snowstorm, without forming distinct layers [
7], as observed in our case. A water-lily sign on an ultrasound, CT, and MRI scan is used for describing the detachment of the membrane inside of the cyst. Depending on the membrane configuration, it can also be observed as a snake sign (also called serpent sign) [
14].
CT scanning, with its high sensitivity and specificity, is an effective method for describing rupture, infection, calcification, and extrahepatic spread [
2,
5,
7]. On the other hand, MRI scanning is a modality of choice for biliary complications [
2]. CT scans depict air–fluid levels in cases of infection or perforation into the hollow viscera. It is a modality of choice in cases of peritoneal seeding, because it enables the imaging of the whole abdomen [
7]. CT scanning is not suitable for the staging of hydatid disease. Furthermore, a finding from CT and MRI scan, that helps to rule out CE, is internal enhanced septa [
2]. On the contrary, specific signs of the disease are daughter cysts and an internal detached membrane [
15].
In diagnosing CE, MRI scanning is of more value than CT [
16]. On an MRI scan, hepatic cysts show homogeneous very-low signal intensity on T1WI and homogeneous very-high signal intensity on T2WI. Due to its fluid content, there is a notable increase in signal intensity on heavily T2WI, which helps to differentiate these cysts from metastatic lesions [
17]. No enhancement is observed after the administration of gadolinium contrast. In rare cases of intracystic hemorrhage, when blood products are mixed within the cyst, the signal intensity becomes high, and a fluid–fluid level is seen both on T1WI and T2WI [
15]. These features are observed within hydatid cysts as well, although there are differences regarding their histopathological features. A hydatid cyst has three distinct layers, as follows: the outer adventitial layer formed by the host’s liver tissue (pericyst), the middle laminated layer derived from the parasite, and the inner germinal layer derived from the parasite [
7,
16].
MR imaging clearly visualizes pericyst, matrix, and daughter cysts. The pericyst appears as a hypointense rim on both T1WI and T2WI due to its fibrous structure and the presence of calcifications. This is a specific feature of hydatid cysts, called the rim sign, mostly better visualized on T2WI [
5]. The matrix represents hydatid-fluid-containing membranes of broken daughter vesicles, scolices, and hydatid sand [
7]. The hydatid matrix appears hypointense on T1WI and significantly hyperintense on T2WI. However, the limitations of MRI scans are especially observed in stages CE4–CE5, as bigger calcifications are better observed on a CT scan. When daughter cysts are present, they are typically more hypointense than the matrix on T2WI, as observed in our case report [
17] (
Figure 1). If the membrane is separated, it can shift with movement, resembling a water lily floating on the surface of a pond, previously described as water-lily sign, which was seen in our case report as well [
18] (
Figure 8).
Diffusion-weighted imaging (DWI) is an MRI technique that allows the quantitative and non-invasive measurement of water molecule diffusion in biological tissues. DWI enables us to calculate apparent diffusion coefficients using different b-values. The b-value measures the degree of diffusion weighting applied [
17]. Inan et al. and Yalcinoz et al. found a significant difference between hydatid cysts type CE 1 and CE 2 and simple cysts based on the significantly lower mean ADCs found in hydatid cysts [
19,
20]. On the contrary, Konukoglu et al. did not find any significant difference between these two entities in ADC when comparing all stages of hydatid cyst and simple cysts [
21]. The distinction between simple cysts and hydatid cysts (CE 1 and CE 2) can be identified using ADC measurements at b600 and b1000 values. Both simple and hydatid cysts (CE 1 and CE 2) appear isointense on DWI with b1000, but simple cysts exhibit a peripheral moderate hyperintensity when compared to hydatid cysts [
21]. Although diffusion coefficients of b600 and b1000 were not available in our case, the ADC map on b800 clearly depicted a hyperintense signal of the cyst, which is typical for cysts. However, the hypointense surrounding rim of the cyst on trace DWI led to a clear depiction of the cyst, helping in correctly identifying the activity. The results of Inan et al. were not applicable, due to the use of a b800 value [
19,
20]. However, since the use of DWI and a diffusion coefficient has yet to be discussed, the MRI standard protocols allowed us to safely diagnose the disease with the described imaging features in T1WI, T2WI, and the use of a contrast agent. Therefore, the specific morphology of the lesion plays a crucial role in correctly identifying the activity of the disease.
Due to the cancer-like manifestation and staging, the application of AI-based tools and radiomics is an important consideration. In many other entities, such as the grading of bladders tumors, radiomics already plays an important role, as proven by Benfante et al. with prognostic and predictive models about grading bladder tumors into low-grade and high-grade tumors by the use of a total of 120 radiomics features. These results should be taken into consideration, as the grading process of a hydatid cyst into the correct WHO stage has similar requirements [
22].
Radiomics generates quantitative characteristics by extracting features of shape, texture, intensity, and filtering. However, the limitations of these features are in their design, which is entirely based on prior knowledge of humans. Radiomics may fail to capture important features that have not been discovered yet. This is where deep learning, with its capability of self-learning, plays a key role. Deep learning networks can identify hidden features that are predictive of outcomes, revealing higher-level characteristics that humans may not recognize. The DLR method, which combines deep learning features with radiomics features, enhances the feature set of the diagnostic models and, thus, improves their reliability by increasing the data input dimensions.
Nijati et al. applied a deep learning radiomics model to the activity of CE on CT imaging. The calibration curve showed a strong agreement between the nomogram’s predicted results and the actual outcomes [
23]. However, the application of deep learning models to MR imaging of CE is not yet described, but will be of importance for a better therapeutic approach.
Hydatid cysts can be staged with six types according to the WHO-IWGE classification, a fusion of the former WHO classification and the Gharbi classification. Originally based on ultrasound imaging, it is commonly applied to MRI and CT modalities [
14] (
Table 1).
The imaging and appropriate classification have a key role in the therapeutic approach for hydatid disease [
2]. The cancer-like nature of the disease has conditioned the management to become multidisciplinary, to combine drug therapy and surgery along with a long-term follow up [
16].
The therapeutic options are largely influenced by the environmental context in which the patient resides [
14]. The individual therapeutic decision is not only based on the definite classification of the disease, but also influenced by the symptoms of the patient, the interdisciplinary approach, and the size of the lesion.
MRI scanning has a huge influence regarding the possibility of exacter measurements and gives a clearer indication of the activity of the disease, as seen in our case. The use of only albendazole is reserved for early stages where the cysts are smaller than 5 cm. In the later stages, as the cysts grow, the implementation of Puncture–Aspiration–Injection–Reapiration, modified catheterization technique, and surgery becomes necessary. In contrast, for inactive cysts (CE4 and CE5), the watch-and-wait approach is advised [
2,
14,
16].
The only curative treatment is total cystectomy [
2]. Other invasive methods include sub-total and partial cystectomy, Puncture–Aspiration–Injection–Reaspiration (PAIR), and the modified catheterization technique (MoCAT) [
2,
16].
When the cyst is adjacent to major vessels, sub-total cystectomy, which avoids dissection of these vessels, is encouraged. Sub-total cystectomy includes the partial resection of the adventitial layer while completely resecting the other layers. Partial cystectomy includes the opening of the cyst and may leave all parts of middle and inner layers [
16]. It depends on the use of protoscolecidal agents, but still has a high risk of recurrence and, therefore, it is not recommended [
24]. In our case, surgery was not considered due to the cyst’s inaccessible location.
MoCAT represents a procedure suitable for cysts of up to 10 cm in diameter and involves aspirating both the cyst content and the parasitic membranes, with a catheter being left in place during the post-intervention period [
25].
The application of protoscolecidal agents into the cyst is important for reducing the risk of recurrence; moreover, WHO-IWGE recommends 20% hypertonic saline as the preferred protoscolecide agent in surgery and 20% hypertonic saline or 95% alcohol in PAIR [
16]. Furthermore, a combination of interventional techniques with albendazole is recommended [
9]. As a last option in selected cases, liver transplantation may be considered [
16].
Because of the risk of recurrence after surgery, biological and imaging follow-up is necessary for 5 years [
2].
4. Conclusions
The main focus of this paper is to highlight the crucial role of imaging modalities in the classification of echinococcosis, which is essential for making informed therapeutic decisions, taking into consideration the limited predictive value of serology for CE.
Our case report gives important insight into the better predictive value of MRI scans, when available. In our case, it could specifically facilitate surveillance and could be used to bridge the time until the operation and cyst reduction, within the possibilities of anthelmintic therapy.
Due to globalization, echinococcosis is no longer confined only to tropical regions, stretching the need for heightened awareness and the inclusion of this disease in differential diagnoses.
MRI scanning with DWI/ADC mapping is a specific tool not only for diagnosing a hydatid cyst in its earliest stage, but also for establishing and surveilling a therapy plan, as it can be proven to be a useful indicator. Diffusion coefficients are not necessarily needed to do so, but they can be useful, as observed in our case, where DWI also proved to have a stake in further depicting the cystic wall and, therefore, giving guidance on the activity.
The future of MRI-guided surveillance and therapy still needs more scientific engagement, especially regarding the use of deep learning models.
On the contrary, in a low-setting environment, where the disease is endemic, not all diagnostic tools are available, which makes the diagnosis more challenging.
The application and continuous development of clear guidelines, an accessible tailored therapeutical approach, and the establishment of a multidisciplinary team, where possible, are essential for effectively managing this disease and preventing its spread.