Abstract
Influenza viruses pose a significant threat to human health, and vaccination remains the most cost-effective and efficient strategy for controlling outbreaks. This review first introduces the molecular characteristics of influenza A virus (IAV) and examines how conserved epitopes contribute to overcoming its high variability, laying the foundation for broadly protective vaccine design. Different vaccine platforms are then categorized and analyzed through representative examples to highlight their research significance and application potential. The discussion further extends to the role of adjuvants in modulating immune responses, with a focus on how their optimization enhances vaccine efficacy. We explore future directions in vaccine design, highlighting the synergistic potential of conserved epitope targeting and adjuvant improvement in advancing the next generation of influenza vaccines.
1. Introduction
Influenza is a persistent global health threat, with seasonal epidemics causing substantial morbidity and mortality worldwide [1,2,3,4]. Influenza viruses are classified into four types—A (IAV), B (IBV), C (ICV), and D (IDV)—based on antigenic differences in two internal structural proteins: nucleoprotein (NP) and matrix protein (M1). Among them, types A and B pose the greatest threat to human health. IAV, in particular, is responsible for most pandemics and severe seasonal outbreaks, making it the primary focus of universal vaccine development. The influenza virus genome consists of eight segmented RNA strands, which are prone to mutation and reassortment, leading to high genetic diversity. This variation is driven by two major mechanisms: antigenic drift, which refers to the accumulation of point mutations in the genes encoding hemagglutinin (HA) and neuraminidase (NA), causing seasonal strain changes; and antigenic shift, which involves the reassortment of gene segments between different viral subtypes in a shared host, potentially generating novel HA or NA subtypes that can trigger pandemics [5]. The ongoing antigenic variability presents significant challenges for vaccine design and necessitates frequent updates to match circulating strains. While traditional influenza vaccines provide strain-specific protection, their limited cross-protection underscores the urgent need for next-generation vaccines capable of inducing broader and more durable immunity [6,7,8,9].
A promising approach to addressing this challenge is the targeting of conserved epitopes—relatively stable regions of viral proteins that exhibit minimal variation across diverse influenza strains [10,11,12,13]. Vaccines designed to elicit immune responses against these conserved elements hold the potential to circumvent strain-specific limitations and provide broad-spectrum protection against influenza [14,15,16,17,18,19]. However, the effectiveness of such vaccines hinges not only on the strategic selection of epitopes but also on the choice of vaccine platforms and the integration of adjuvants to enhance immunogenicity and durability of the immune response [20,21,22,23,24].
Recent advancements in vaccine platforms, such as recombinant protein-based vaccines, virus-like particles, and nanoparticle-based formulations, offer promising new avenues for enhancing vaccine efficacy [25,26,27,28,29,30]. These platforms provide improved antigen presentation, stability, and scalability, positioning them as strong candidates for next-generation influenza vaccines. Beyond the choice of vaccine platform, adjuvants play a crucial role in modulating immune responses, enhancing antigen processing, and promoting long-lasting immunity [31,32,33,34,35,36]. The synergistic combination of conserved epitope targeting, advanced vaccine platforms, and optimized adjuvants represents a powerful strategy for improving the effectiveness of influenza vaccines [37,38,39].
This review examines the molecular characteristics of IAV and the role of conserved epitopes in addressing antigenic variability. We then discuss various vaccine platforms, evaluating their potential through representative examples, and explore the role of adjuvants in optimizing immune responses. Finally, we conducted an in-depth discussion on the development of universal influenza vaccines and the practical challenges in their clinical translation.
2. Molecular Characterization and Conserved Epitopes of Influenza A Virus
The high variability of influenza viruses poses a significant challenge to the effectiveness of conventional vaccines, prompting the search for universal influenza vaccines targeting conserved antigenic epitopes. Here, we revisit the structural features of influenza viruses and highlight recent advances in recognizing conserved antigenic epitopes, including hemagglutinin (HA), neuraminidase (NA), and the matrix protein 2 extracellular structural domain (M2e). In addition, we discuss the immunological roles of these epitopes and their potential applications in the design of next-generation influenza vaccines.
2.1. Structure of the Influenza Virus
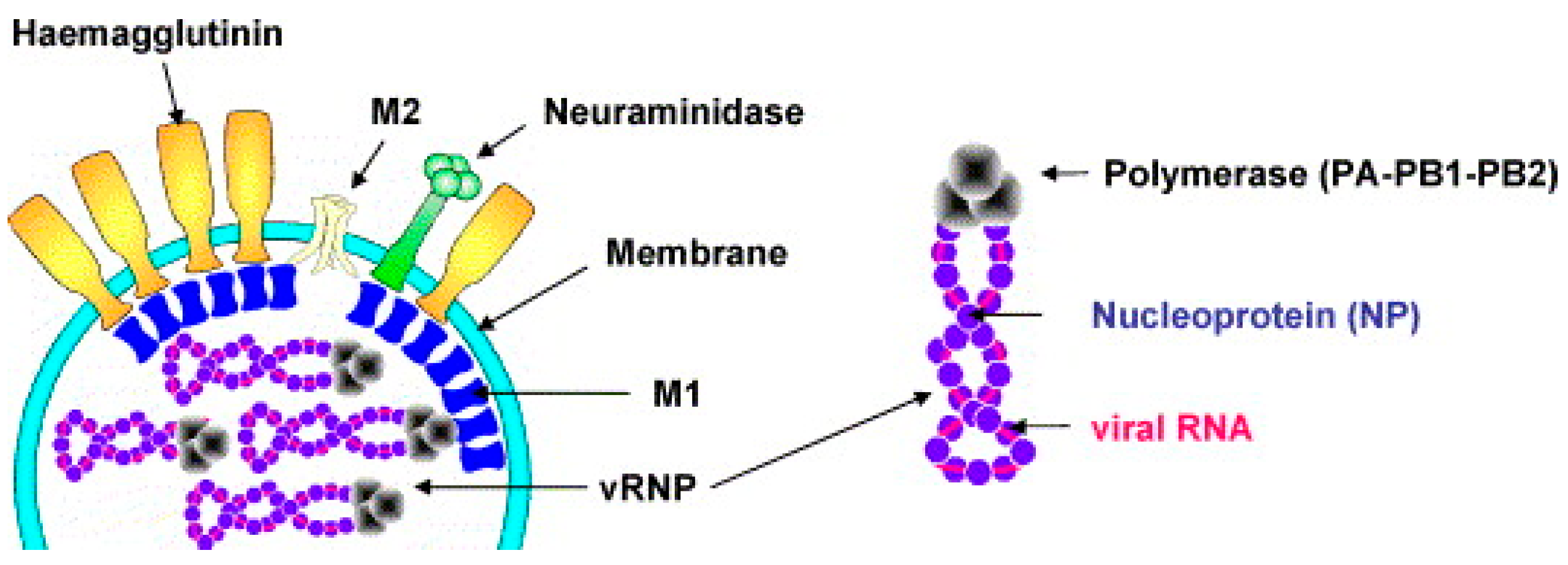
IAV belongs to the Orthomyxoviridae family and is an enveloped, negative-sense, single-stranded RNA virus. IAV exhibits pleomorphism, with the most common form being roughly spherical, approximately 120 nm in diameter (Figure 1) [40,41,42,43]. Its envelope is derived from the host cell membrane and is embedded with three transmembrane proteins: the HA, NA, and M2 proteins [44]. Among them, HA is the most abundant and mediates viral entry into host cells, NA facilitates viral release, and M2 functions as an ion channel involved in viral uncoating [45,46,47,48]. Beneath the envelope, the matrix protein M1 surrounds the viral ribonucleoprotein complex (vRNP), which consists of genomic RNA, nucleoprotein (NP), and an RNA polymerase complex composed of polymerase acidic protein (PA) and polymerase basic proteins (PB1 and PB2) [48]. The IAV genome comprises eight segments encoding 12 proteins, including the aforementioned structural proteins and four non-structural proteins (NS1, NEP/NS2, PB1-F2, and N40), which play crucial roles in viral replication, host immune regulation, and adaptive evolution [49,50,51].
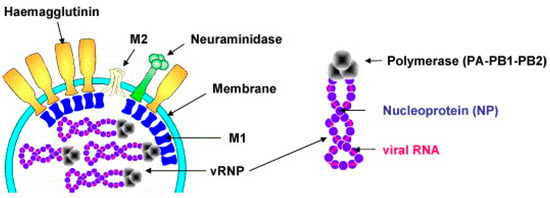
Figure 1.
Influenza virus particles contain HA, NA, M1, and M2. Schematic diagram of viral RNA, nucleoprotein, and RNA polymerase complex. Reproduced with permission from ref. [40], copyright (2006) Elsevier B.V. All rights reserved.
2.2. Conserved Epitopes
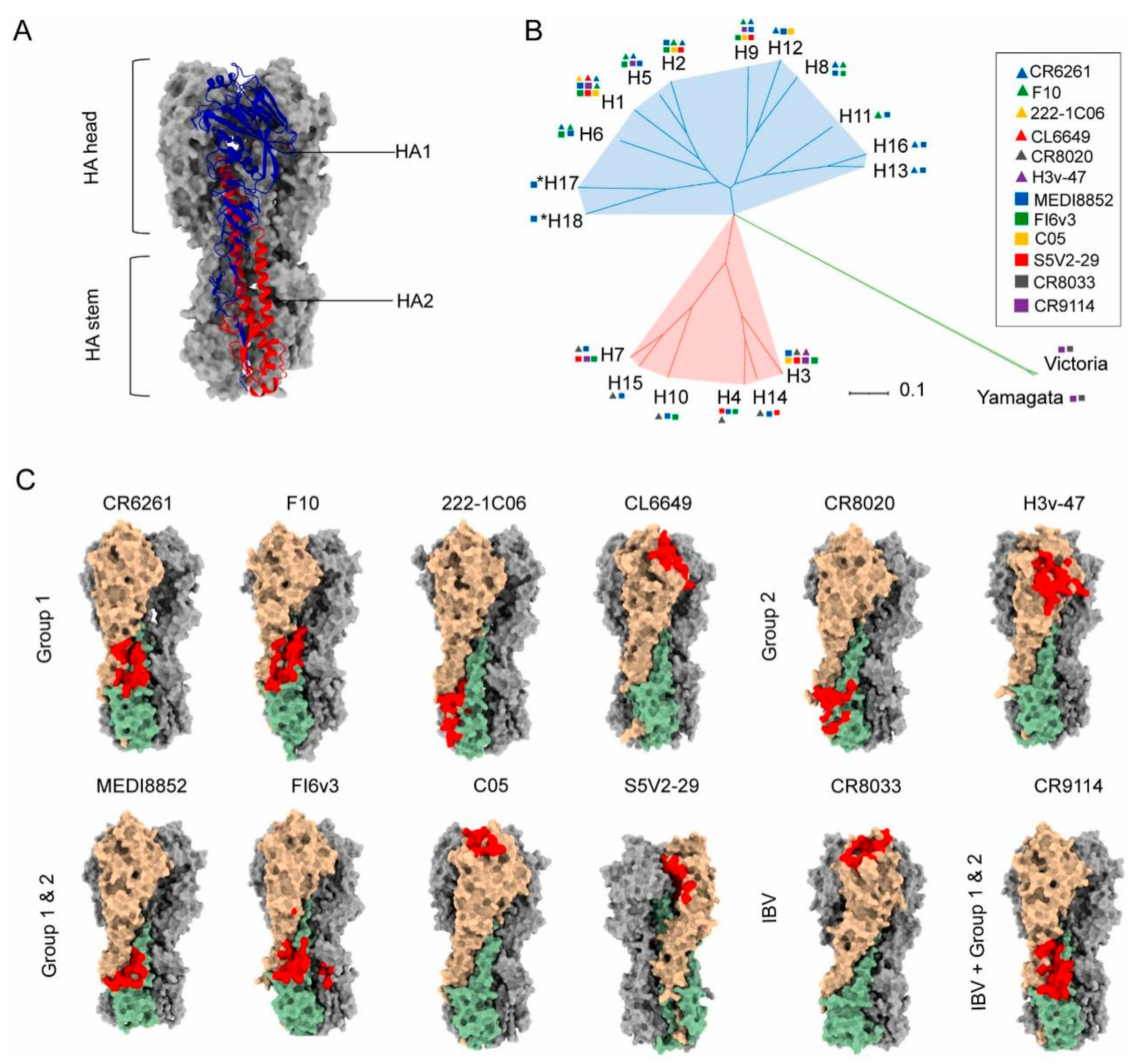
HA, a transmembrane glycoprotein on the influenza viral envelope, comprises two distinct structural domains: the globular head and the helical stalk regions [52,53,54]. The HA head domain demonstrates potent immunogenicity capable of eliciting antibody responses [55,56,57]. However, frequent genetic mutations and reassortment in influenza viruses induce antigenic drift in the HA head, thereby compromising the broad protective efficacy of conventional vaccines [58]. In contrast, the HA stalk region exhibits relative structural conservation with cross-subtype similarity, establishing it as a promising target for universal influenza vaccine development [53,54,59,60,61]. The principal challenge lies in overcoming its weak immunodominance in natural immune responses. Recent advancements involve engineered headless HA immunogens and recombinant stalk-based vaccines that significantly enhance stalk-directed immunity (Figure 2) [8,59,62,63,64]. Notably, Yassine et al. implemented six rounds of iterative optimization (Gen1–Gen6) using HA from H1N1 A/New Caledonia/20/1999 as a template [65]. Through the sequential removal of immunodominant head domains and incorporation of the HIV-1 gp41 trimerization domain with ferritin self-assembling nanoparticle platform (H1-SS-np), they achieved enhanced conformational stability of stalk epitopes. This vaccine induced cross-reactive antibodies conferring complete protection in murine models and partial resistance in ferrets against H5N1 challenge, validating the protective mechanism mediated by Fc effector functions of non-neutralizing antibodies. These findings underscore the translational potential of stalk-focused strategies despite the continued dominance of head-targeting approaches in current vaccine design.
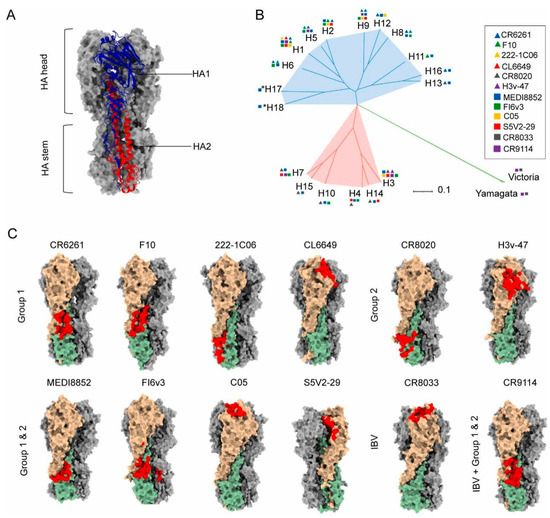
Figure 2.
Structural basis for the induction of broadly neutralizing antibodies against HA: (A) Structure of Influenza hemagglutinin (HA).). Ha1 subunit is blue, H2 subunit is red (B) Phylogenetic tree of influenza A and influenza B HA subtypes. (C) The epitopes of anti-HA head and stem antibodies, including IAV group 1 neutralizing mAbs CR6261 (PDB 3GBN), F10 (PDB 3FKU), 222-1C06 (PDB 7T3D), CL6649 (PDB 5W6G), group 2 neutralizing mAbs CR8020 (PDB 3SDY), H3v-47 (PDB 5W42), IAV cross-group neutralizing mAbs FI6v3 (PDB 3ZTJ), MEDI8852 (PDB 5JW4), C05 (PDB 4FQR), S5V2-29 (PDB 6E4X), IBV neutralizing mAbs CR8033 (PDB 4FQM), and both IAV and IBV reactive mAb CR9114 (PDB 4FQI) on their cognate HA ligands. HA1 and HA2 monomer molecules are shown in light brown and dark sea green, respectively, and the other two monomer molecules of the HA trimer are shown in dark gray. Antibody epitopes are shown in red The epitope of S5V2-29 is hidden between the two HA head interfaces of the trimeric HA. Reproduced with permission from ref. [62], copyright (2023) Elsevier B.V.
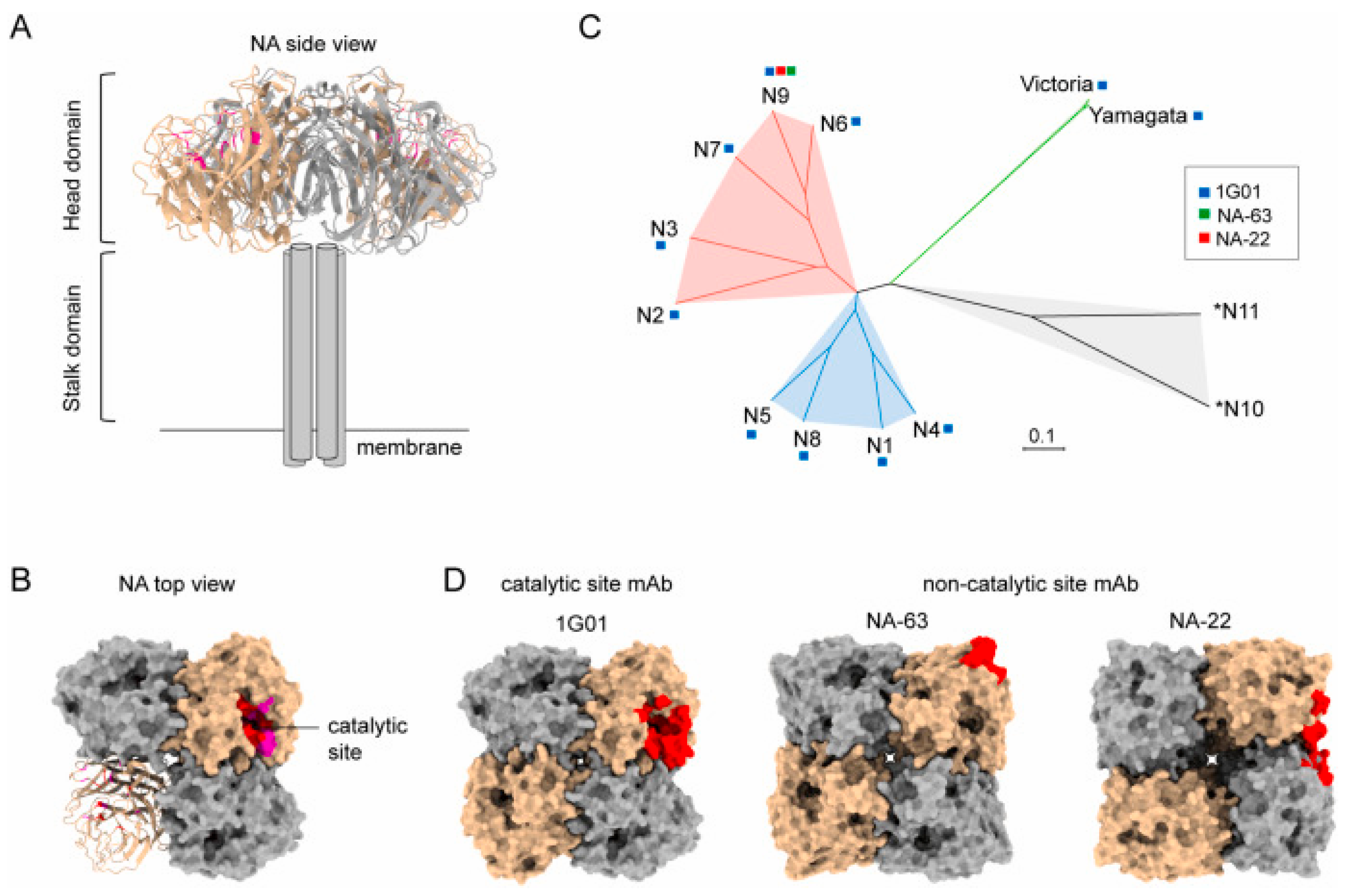
NA, a crucial influenza surface glycoprotein, facilitates viral release through the cleavage of sialic acid residues anchoring nascent virions to host cells [45,66]. While NA exhibits inter-subtype variability, the catalytic site residues remain highly conserved across N1 to N9 subtypes, making it a viable target for universal vaccine development (Figure 3) [62,67]. Recent studies demonstrate that NA-specific monoclonal antibodies (mAbs) confer substantial protective efficacy in animal models through dual mechanisms, namely, the direct inhibition of NA enzymatic activity and Fc-mediated effector mechanisms [68,69]. These findings reinforce NA’s potential as a complementary component in broad-spectrum influenza vaccine formulations.
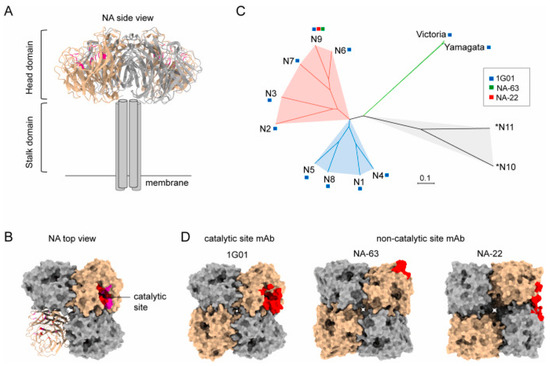
Figure 3.
Structural basis for the induction of broadly neutralizing antibodies against NA: (A) The side view structure of N1 NA (PDB 6Q23). (B) The top view structure of N1 NA (PDB 6Q23). (C) Phylogenetic tree of influenza A and influenza B NA subtypes. Based on sequence variations of NA, IAV NA can be divided into three genetically distinct subgroups: group 1 consists of N1, N4, N5, and N8; group 2 consists of N2, N3, N6, N7, and N9; and group 3 consists of two bats-derived *N10 and *N11 subtypes. (D) Anti-NA antibody epitopes include catalytic (1G01, 6Q23) and non-catalytic binders (NA-63, 6U02; NA-22, 6PZW). The protomers of tetramer NA are shown as surface in tan and dark gray, respectively. The epitopes of antibodies are shown as red.© 2023 Elsevier B.V. All rights reserved. Reproduced with permission from ref. [62], copyright (2023) Elsevier B.V.
M2e is the exposed portion of the M2 protein in influenza A virus, consisting of 24 amino acid residues. M2, an ion channel protein, plays a critical role in the viral uncoating process [70]. M2e is relatively conserved across various influenza A strains, and it can induce broad inhibitory effects against multiple subtypes, making it a promising target for universal influenza vaccines. A highly conserved epitope, SLLTEVET, is found within the 2–9 amino acid region of M2e [71]. Monoclonal antibodies targeting this epitope have been shown to effectively inhibit H1 and H3 subtype influenza A viruses in vitro. Two key amino acids in this epitope—threonine at position 5 and glutamic acid at position 6—have been identified as associated with antibody escape variants [72]. Despite some variability, the specific region of M2e continues to be considered a potential conserved epitope for universal influenza vaccines, offering promising prospects for broad-spectrum protection [73].
2.3. Computational Design and Epitope Screening Strategies
Traditional vaccine development often spans several years, making it difficult to respond swiftly to emerging infectious diseases or rapidly mutating viruses. In recent years, advances in structural biology, artificial intelligence, and big data have accelerated the rise of computational vaccinology, which offers a powerful means to shorten vaccine development timelines [74,75]. For example, during the COVID-19 pandemic, candidate vaccines entered clinical trials within months of the virus’s identification—an achievement enabled in part by computational strategies.
In the design of broadly protective influenza vaccines, computational methods are extensively used to identify highly conserved T or B cell epitopes that possess strong immunogenic potential across diverse viral subtypes. A representative platform is the Integrated Vaccine Design Toolkit (iVAX), developed by a team based in Rhode Island, USA [76,77]. iVAX provides a comprehensive suite of immunoinformatic tools, including algorithms for scoring and ranking candidate antigens, selecting conserved and immunogenic T cell epitopes, redesigning or removing regulatory T cell epitopes, and engineering antigens to enhance immunogenicity and confer protection in both humans and animals [76,78]. The platform is accessible online and has been applied to a range of human and zoonotic pathogens.
In collaboration with Saint Louis University, iVAX has been used to identify conserved CD4+ and CD8+ T cell epitopes across various IAV strains [79]. These epitopes were shown to be immunogenic in individuals with diverse MHC genotypes and were further validated in HLA-transgenic mouse models. The resulting vaccines elicited robust T cell responses and provided cross-strain protection, supporting the feasibility of T cell–targeted universal influenza vaccines.
Furthermore, antigens identified through computational screening can be structurally optimized using emerging protein structure technologies. Methods such as X-ray crystallography, cryo-electron microscopy (cryo-EM), cryo-electron tomography (cryo-ET), and antibody-binding assays allow the precise characterization of antigen conformation [80,81]. These approaches help eliminate misfolded or non-functional antigen designs, reducing the risk of vaccine failure. Structure-based vaccine design is poised to play a central role in accelerating the development of next-generation influenza vaccines.
3. Research Progress of Vaccine Platform
Influenza vaccines have long been a critical tool in the fight against seasonal flu outbreaks, and they continue to be a major focus of public health research. However, as the influenza virus rapidly evolves and new strains emerge, traditional vaccines face challenges in terms of limited adaptability and immune protection. In response to these challenges, recent scientific and technological advancements have led to the development of novel influenza vaccine platforms, including inactivated vaccines, subunit vaccines, viral vector vaccines, and mRNA vaccines. These innovative platforms offer greater options and possibilities for the development and production of influenza vaccines. They not only enhance vaccine efficacy but also provide new solutions to address viral antigen variation and improve global vaccine accessibility. This review will explore the research advancements of various influenza vaccine platforms, analyzing their contributions to improving vaccine effectiveness, combating antigenic drift, addressing the challenges in vaccine development, including the hurdles encountered in translating animal model successes to human applications.
3.1. Characteristics of Inactivated Influenza Vaccines
Inactivated influenza vaccines (IIVs) are among the most widely used flu vaccines worldwide (Table 1). They are produced by chemically or physically inactivating the virus while preserving its main antigenic properties to induce immune protection [82,83]. The production process includes virus cultivation (e.g., in embryonated chicken eggs or MDCK cells), purification, and inactivation. Common inactivation agents include formaldehyde, which cross-links viral genomes to render them non-infectious, and beta-propiolactone (BPL), which chemically modifies nucleic acids and membrane proteins to inhibit viral activity [84].

Table 1.
Comparative analysis of influenza vaccine platforms.
To provide broader coverage against co-circulating strains, current influenza vaccines typically include two influenza A strains (H1N1 and H3N2) and one or two influenza B strains, formulated as trivalent (TIV) or quadrivalent (QIV) vaccines based on annual global surveillance. Their primary mechanism of protection relies on neutralizing IgG antibodies targeting the hemagglutinin (HA) head, preventing viral attachment to host cells. A hemagglutination inhibition (HI) antibody titer of ≥40 is generally considered protective [85].
The limitations of inactivated influenza vaccines primarily stem from their reliance on HA head-specific immunity and their mode of administration. Due to the high mutation rate of the HA head, frequent strain updates are required, and vaccine effectiveness can be significantly reduced when mismatches occur, as seen in the 2014–2015 H3N2 season, where protection dropped to 7% [86]. Additionally, these vaccines provide limited cross-protection since they induce primarily HA head-specific antibodies while eliciting weak immune responses against conserved antigens such as the HA stalk, NA, and NP. Inactivated influenza vaccines primarily elicit humoral immune responses dominated by strain-specific antibodies; however, they induce limited cellular immunity, including the weak activation of CD8+ T cells and poor engagement of innate immune pathways, which together restrict their long-term protective efficacy [87]. Overall, despite being a mainstay of influenza prevention, inactivated vaccines are limited by their narrow strain-specific protection and weak cellular immune activation, highlighting the need for next-generation vaccines with broader and longer-lasting efficacy.
3.2. Characteristics of Subunit Influenza Vaccines
Subunit vaccines are produced by extracting or synthesizing key antigenic components of pathogens [88]. These antigens can be derived from purified natural viruses, recombinant expression systems, or chemical synthesis (Table 1). Lacking viral nucleic acids, subunit vaccines are inherently safer, with well-defined compositions and controlled production processes, making them suitable for standardized and large-scale manufacturing [89,90]. Due to their inherently low immunogenicity, subunit vaccines usually require adjuvants to enhance immune responses. Adjuvants function by stimulating and amplifying the immune system, thereby improving vaccine immunogenicity and promoting stronger antibody and cellular responses [91,92]. In addition, the incorporation of multivalent antigen design can broaden the protective scope of the vaccine. For example, the Cui research team developed a multivalent antigen nanoparticle, termed HMNF, by displaying conserved epitopes from influenza surface proteins—HA A α-Helix (H), M2e (M), and NA HCA-2 (N)—on self-assembling human ferritin (F) nanoparticles [93]. This vaccine effectively induced both antibody and cellular responses and provided broad protection in a mouse model.
As a specialized form of subunit vaccines, peptide vaccines use short synthetic peptide epitopes (typically 8–30 amino acids) from pathogen proteins to mimic natural antigen structures and elicit both humoral and cellular immunity [94,95]. Their design usually relies on structural biology and bioinformatics to precisely identify conserved epitopes such as those in the HA stalk region and nucleoprotein (NP). Compared with protein-based vaccines, peptide vaccines can be produced via recombinant systems or entirely through chemical synthesis, avoiding potential expression system contaminants and offering greater batch-to-batch consistency and safety. FLU-v, developed by PepTcell (now SEEK), is a synthetic peptide vaccine composed of four peptides derived from conserved internal antigens of influenza viruses [96,97], namely, M1 (32 amino acids), M2 (24 amino acids), the NP of influenza A (20 amino acids), and the NP of influenza B (19 amino acids). These peptides are engineered to be recognized by human leukocyte antigen (HLA) molecules, immune proteins that present antigens and activate T cells, thus inducing virus-specific T cell responses [97,98,99]. In both preclinical and clinical studies, FLU-v has been shown to induce interferon-γ (IFN-γ) and granzyme B-secreting cells and to confer protection in challenge models. In a randomized, double-blind, placebo-controlled phase IIb clinical trial, FLU-v demonstrated favorable safety and immunogenicity and provided protection against strains such as H3N2.
In summary, subunit vaccines, particularly peptide-based ones, are emerging as a promising avenue toward broad-spectrum influenza protection by leveraging the precise design of conserved antigenic sites in combination with adjuvant enhancement strategies.
3.3. Characteristics of Viral Vector Influenza Vaccines
Viral vector vaccines are a type of vaccine that uses viruses as “delivery vehicles” to introduce specific pathogen genes—typically those encoding antigenic proteins—into host cells to elicit an immune response (Table 1). Viral vector vaccines employ adenoviruses (Ad5s), Newcastle disease virus (NDV), herpesviruses (HVTs), or other vectors to deliver influenza antigen genes (HA, NA) via genetic recombination. For example, adenoviral vectors delete replication-essential genes (e.g., E1/E3 regions) to insert HA genes, forming replication-defective recombinant viruses [100].
After entering host cells via natural infection routes (e.g., respiratory tract or intramuscular injection), recombinant vectors utilize host transcription/translation machinery to express influenza antigens. Adenoviral double-stranded DNA enters the nucleus, where host RNA polymerase II transcribes HA mRNA for cytoplasmic ribosome translation, ultimately presenting HA proteins on cell surfaces or secreting them extracellularly [101,102]. Vectors like Pichinde virus (PICV) naturally target dendritic cells (DCs) and macrophages, enhancing antigen presentation efficiency [103]. Experimental studies have shown that PICV vectors induce robust CD8+ T-cell responses in murine lungs and spleens via MHC-I antigen presentation [104]. HVT vectors sustain low-level antigen expression, eliciting long-term tissue-resident memory T cells (TRM). For instance, HVT-H9 vaccines maintain high IFN-γ+/CD8+ T-cell levels in chickens 35 days post-vaccination [105]. Limitations involve pre-existing Ad5 antibodies in populations that may neutralize vectors (requiring high-dose compensation) [106,107] and risks of HVT reactivation due to latent persistence [108].
3.4. Characteristics of mRNA Influenza Vaccines
mRNA vaccines encapsulate influenza antigen-encoding mRNA (HA, NA) within lipid nanoparticles (LNPs) for delivery to the host cytoplasm (Table 1). Since the mRNA sequence is modular, it can be updated with new sequences encoding antigens from evolving viral strains. This enables the rapid development of variant-specific vaccines. In addition, mRNA’s simplicity as a small molecule (~2–3 kb) and highly specific immune responses due to s encode only one or a few target proteins [109].
Current mRNA vaccine platforms include both conventional non-replicating mRNA and self-amplifying mRNA (saRNA). Conventional non-replicating mRNA contains antigen-coding sequences, 5′ cap, and 3′ poly-A tail, relying on host ribosomes for direct antigen translation [110,111]. Self-amplifying mRNA (saRNA) uses alphavirus or flavivirus-derived genomes (Figure 4), replacing nonstructural (NS) genes with antigen-coding sequences. After cytoplasmic replication, subgenomic RNA fragments express antigens without producing infectious particles, enabling full protection with 0.1–1.0 µg doses (vs. 10 µg for conventional vaccines) and reduced reactogenicity [112,113].
Key challenges include balancing interferon (IFN) signaling: Moderate IFN enhances DC maturation and antigen presentation; however, excessive activation inhibits translation and causes inflammation. Double-stranded RNA (dsRNA) contaminants in in vitro-transcribed mRNA trigger TLR3/RIG-I sensing, inducing rapid IFN-α/β secretion and the degradation of cellular/exogenous RNA [114,115]. Even dsRNA-free single-stranded mRNA activates TLR7/8 as pathogen-associated molecular patterns (PAMPs), suppressing antigen translation via IFN [116]. Current strategies employ naturally modified nucleotides to alter RNA secondary structures or block TLR7/8 binding [117]. To address these challenges, current strategies include the use of chemically modified nucleotides (e.g., pseudouridine) and purification methods that reduce dsRNA contaminants, as well as sequence design that minimizes immune stimulation [108].
However, mRNA vaccines typically require ultra-cold storage conditions (e.g., –70 °C), due to the temperature sensitivity of both the mRNA and the LNP carriers. This presents logistical challenges for global distribution and long-term stockpiling.
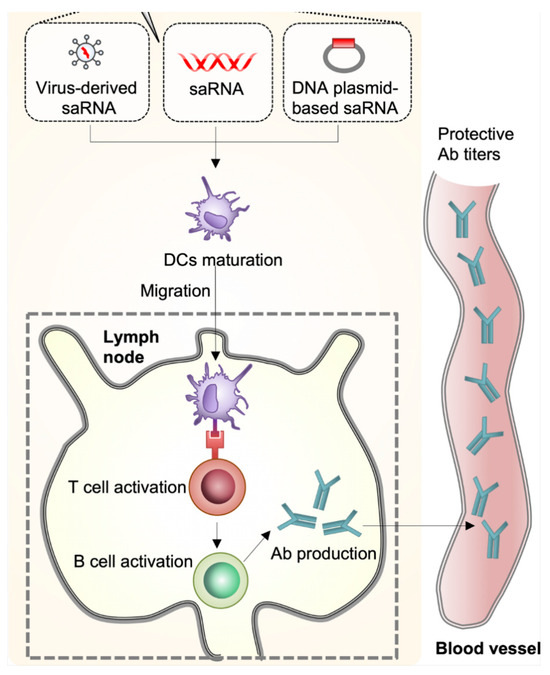
Figure 4.
Self-amplified RNA (Sarna) vaccine can be delivered in the form of virus-like RNA particles, in vitro transcriptional RNA, and plasmid DNA. DCs recognize Sarna in muscle and cells differentiate. Differentiated DCs act as antigen-presenting cells and migrate to lymph nodes, resulting in the activation of T cells and B cells and the production of antibodies. Reproduced with permission from ref. [118], copyright (2021) Lee and Ryu.
Figure 4.
Self-amplified RNA (Sarna) vaccine can be delivered in the form of virus-like RNA particles, in vitro transcriptional RNA, and plasmid DNA. DCs recognize Sarna in muscle and cells differentiate. Differentiated DCs act as antigen-presenting cells and migrate to lymph nodes, resulting in the activation of T cells and B cells and the production of antibodies. Reproduced with permission from ref. [118], copyright (2021) Lee and Ryu.
3.5. Key Challenges in Influenza Vaccine Development
A wide range of influenza vaccines is being developed globally. Table 2 summarizes both licensed vaccines and those currently in clinical trials. However, compared to the numerous candidates being studied, only a small fraction ever make it to market. This gap between research and real-world applicability underscores the high attrition rate in influenza vaccine development. Failures occur for multiple reasons, often unrelated to a single technical flaw. A major issue is the insufficient immune response in humans. For example, the MVA-NP+M1 vaccine developed by Vaccitech showed strong immune cell activation in animal models but failed to reduce clinical symptoms of influenza in a Phase II trial. Another common reason is safety or reactogenicity concerns: the adjuvanted vaccine made by Acambis (ACAM-FLU-A) was halted after reports of adverse events during early human testing. Manufacturing and stability issues have also contributed to setbacks—for example, certain VLP-based vaccines faced scalability problems and antigen degradation during storage. Lastly, regulatory or commercial viability can lead to discontinuation; BiondVax’s universal vaccine candidate (M-001) progressed to Phase III but was terminated after failing to show efficacy, despite extensive investment and early-phase success.

Table 2.
Marketed and clinical development of influenza vaccines by platform type.
One of the most persistent challenges is the gap between animal models and human responses. While mice and ferrets are commonly used in preclinical studies, they differ significantly from humans in terms of immune receptor distribution and pre-existing immunity. Vaccines that show promise in these animal models, particularly in animals with no prior exposure to the virus, often fail to provide the same protective effect in humans with more complex and diverse immune systems. This issue is particularly pronounced for newer vaccine platforms, such as viral vectors or mRNA vaccines, which rely on precise control of antigen expression and immune modulation. To improve translation to humans, there is an urgent need for more relevant preclinical models, including humanized mice, organoids, or human lymphoid tissue cultures, as well as early-phase clinical trials that focus on immune biomarkers, not just antibody titers.
4. Advances in Vaccine Adjuvant Research
Adjuvants are substances that enhance the immune response to vaccines, typically by promoting the immunogenicity of the antigen or boosting the immune system’s response to the antigen [119]. Adjuvants do not directly trigger an immune response; rather, they assist in the effectiveness of the vaccine by modulating various mechanisms of the immune system, which is particularly important in the development of vaccines against emerging or variant pathogens. Traditional adjuvants, such as aluminum salts (e.g., aluminum hydroxide) and oil-in-water emulsions (e.g., MF59), have been used in vaccines for many years and have been shown to significantly enhance immune responses. However, as vaccine development progresses, the limitations of traditional adjuvants are becoming more apparent. The effectiveness of traditional adjuvants may be constrained by the specific antigen type or the characteristics of the pathogen, creating a need to develop novel adjuvants to overcome these challenges. New adjuvants can not only improve the immunogenicity of vaccines but also optimize immune responses through multiple pathways, thereby enhancing the broad-spectrum and long-term protective effects of vaccines.
4.1. Conventional Adjuvants
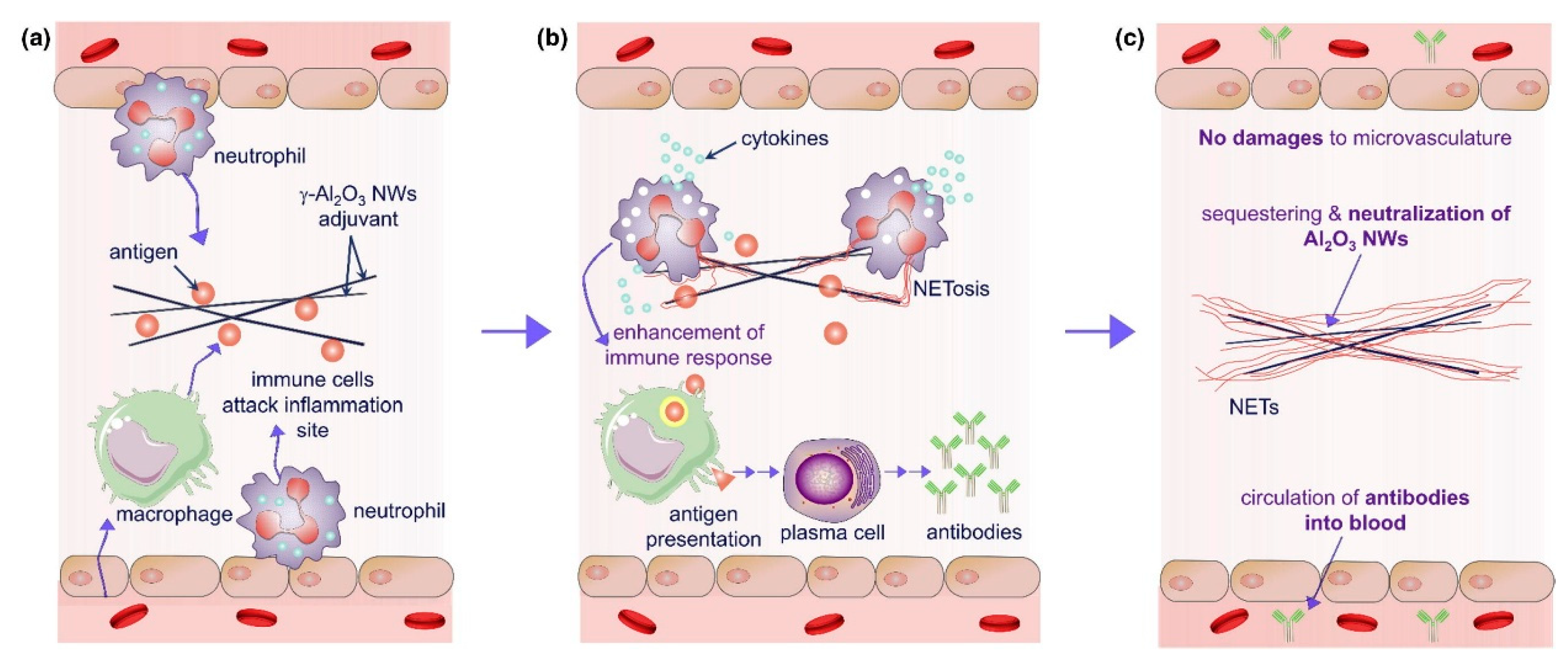
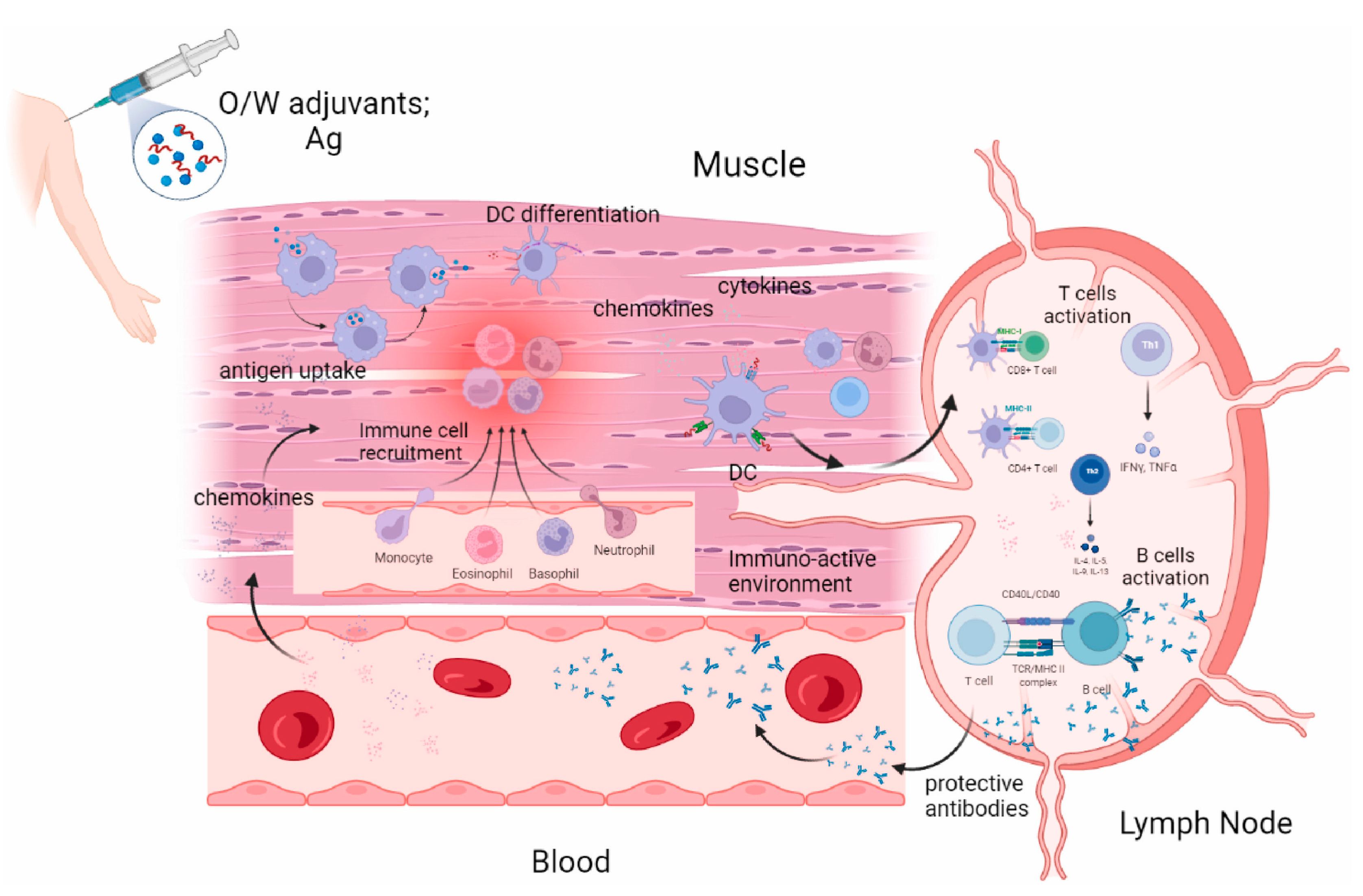
Aluminum-based adjuvants (e.g., aluminum hydroxide, aluminum oxyhydroxide) function as immunostimulants through their unique antigen adsorption capacity [120]. Aluminum salts form localized depots by binding antigens, enabling sustained release at injection sites for days to weeks (Figure 5). This mechanism was first validated in diphtheria toxin experiments: the removal of aluminum-containing injection sites abolished immunity in animals, while transplanting these sites transferred specific immunity to recipients [121]. This physical retention prolongs antigen exposure, creating a window for immune recognition and processing. Aluminum adjuvants also activate the NLRP3 inflammasome to trigger innate immunity [122], recruiting neutrophils, inflammatory monocytes, and other immune cells to injection sites [123]. They induce cellular damage to release endogenous danger signals (e.g., DNA) [124], stimulating pro-inflammatory cytokines (IL-5, IL-6) and fostering a Th2-biased immune microenvironment [125]. This depot-inflammation synergy ultimately generates high-affinity IgG antibody responses [126]. However, their Th2 bias limits utility in vaccines requiring robust cellular immunity, and formulation differences (e.g., Alhydrogel® vs. Imject® alum) yield variable immunogenicity [127,128].
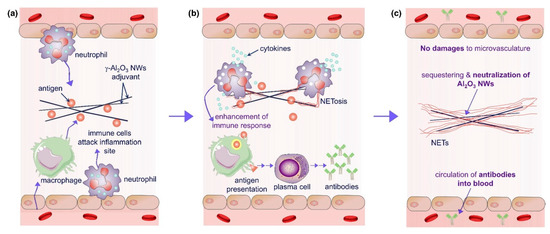
Figure 5.
The enhancement mechanism of γ-Al2O3 nanowires (NWS) aluminum adjuvant+antigen: (a) γ-Al2O3 nanoparticles adsorb antigen and release antigen after injection, resulting in the activation of local macrophages and neutrophils. Macrophages process antigen and stimulate antibody production through plasma cells; (b) In the early stage of neutrophils contacting with γ-Al2O3 NWs, cytokines were released to enhance antibody production; (c) γ-Al2O3 NWs were isolated by neutrophil extracellular traps, which limited the inflammation caused by the presence of exogenous γ-Al2O3 NW particles; γ-Al2O3 NWs locally exist in the trap and will not damage the microvessels or cause local inflammation. Reproduced with permission from ref. [120], copyright (2018) Elsevier Ltd. All rights reserved.
MF59, an oil-in-water emulsion adjuvant, comprises 4.3% squalene, surfactants Tween 80 and Span 85 in citrate buffer, forming stable ~160 nm oil droplets. Licensed in over 30 countries for influenza vaccines (e.g., Fluad®), it is particularly effective in children, pregnant women, and the elderly [129,130]. Upon intramuscular injection, MF59 activates immunity via the following three mechanisms: Inducing a local chemokine storm (CCL2, IL-8) to recruit neutrophils and monocytes, which capture antigens and migrate to lymph nodes [131,132]; Promoting monocyte differentiation into dendritic cells (DCs) within lymph nodes [133,134]; Driving IgG class-switching and CD8+ T cell responses via double-negative T cells (CD4−CD8−) and apoptotic cell-derived uric acid signals, even in CD4+ T cell-deficient mice [135,136]. Clinical data show that MF59-adjuvanted H1N1 vaccines elevate hemagglutination inhibition (HI) antibodies two-to-five-fold, confer cross-protection against variants, and exhibit maternal-fetal safety profiles comparable to non-adjuvanted vaccines [137,138]. Compared to alum, MF59 recruits significantly more neutrophils, monocytes, eosinophils, macrophages, and DCs. Its “low antigen, high response” property enables protective thresholds with 3.75 µg antigen in elderly subjects, making it critical for pandemic preparedness [139,140].
AS03, another oil-in-water emulsion, contains 4.3% squalene, 11.86 mg/dose α-tocopherol, and surfactants Tween 80/Span 85 in phosphate buffer, forming ~160 nm droplets (Figure 6) [141]. Approved in >30 countries for seasonal/pandemic influenza vaccines (e.g., Pandemrix®, Arepanrix®) [142,143,144], it shares MF59’s chemokine-driven recruitment mechanisms but differs via α-tocopherol’s MyD88-dependent, TLR-independent signaling. This enhances antigen-specific antibodies (IgG1, IgG2a) and CD8+ T cell responses [145]. AS03 induces apoptosis in DCs and macrophages, releasing endogenous danger signals (e.g., uric acid) to amplify immunity [146]. In H5N1/H1N1 vaccines, AS03 reduces antigen doses to 3.75 µg HA/dose (vs. 15–30 µg in conventional vaccines) while maintaining high HI titers [147]. In CD4+ T cell-deficient models, it drives IgG isotype switching and CD8+ T cell activation via double-negative T cells and MHC II upregulation [145]. However, the 2009 Pandemrix® formulation was linked to increased narcolepsy risk in children, potentially due to H1N1 HA-derived CD4+ T cell-mimicking epitopes [148]. Reformulated versions mitigate this risk, and current research explores combining AS03 with TLR agonists to balance Th1/Th2 responses [149].
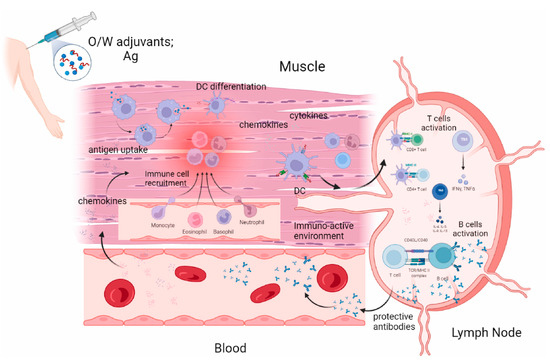
Figure 6.
Possible mechanism of action of oil in water emulsion (MF59, AS03). Reproduced with permission from ref. [141], copyright (2024) Elsevier Ltd. All rights reserved.
4.2. Novel Adjuvants Based on Pattern Recognition Receptor Agonists
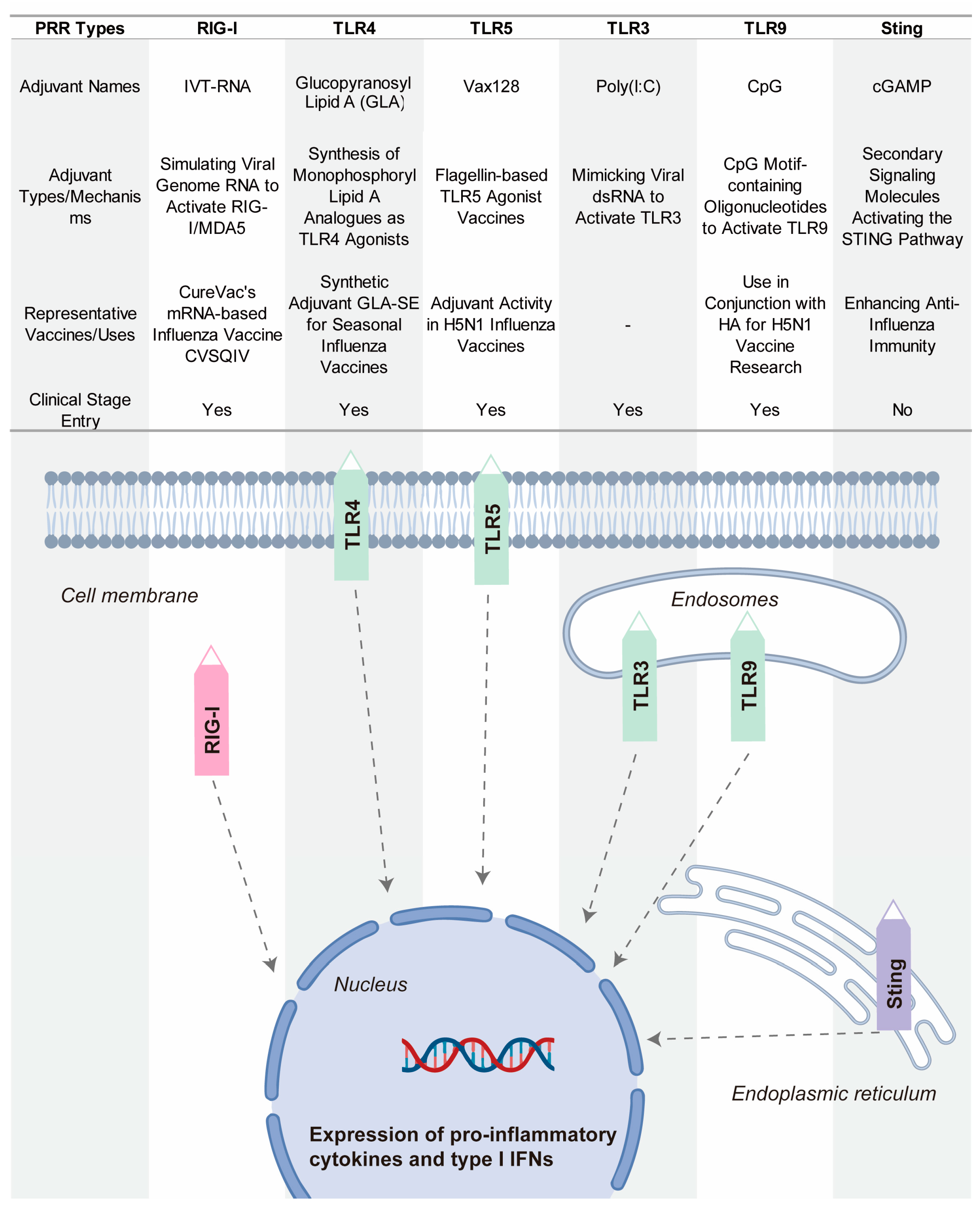
Pattern recognition receptors (PRRs) constitute essential proteins that enable the immune recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [150,151,152]. Recent advancements highlight the emerging potential of PRR-targeting strategies in adjuvant development, with growing attention to their application in enhancing vaccine efficacy [153]. PRR activation potentiates immune responses by improving immunogenicity and critically bridging innate and adaptive immunity. Major PRR families include Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs), as well as the cGAS–STING pathway, which senses cytosolic DNA and triggers potent type I interferon responses [154]. Figure 7 show some key PRR agonists currently being researched or used in influenza vaccines.
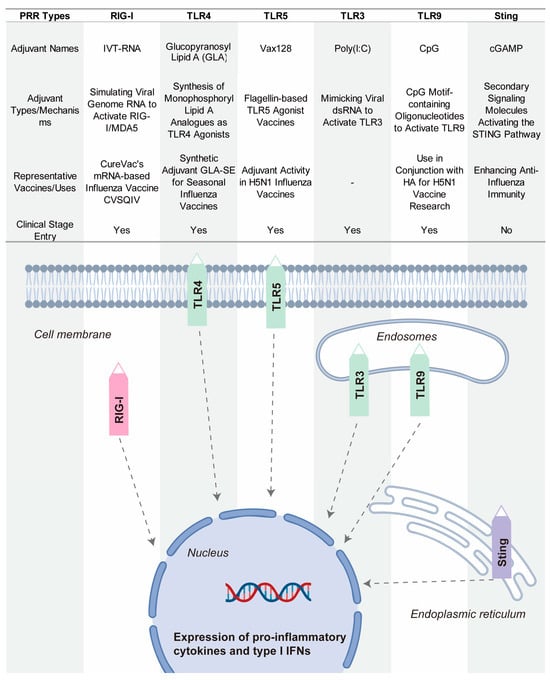
Figure 7.
Representative PRR-targeting adjuvants in influenza vaccine development.
Glucopyranosyl Lipid A–Stable Emulsion (GLA-SE) is a synthetic TLR4 agonist that has been tested in seasonal influenza vaccines [155]. By activating TLR4, GLA-SE induces a potent immune response that promotes both innate and adaptive immunity. This activation results in a strong Th1-biased immune response, which is particularly beneficial for enhancing the immune system’s ability to recognize and fight the influenza virus [156]. GLA-SE has shown promise in enhancing immune protection and is being investigated for its potential to improve the overall efficacy of influenza vaccines.
Vax128 is a TLR5 agonist based on bacterial flagellin, which is the major component of bacterial flagella [157]. Flagellin activates TLR5, triggering a strong innate immune response. When fused with influenza hemagglutinin (HA), Vax128 enhances the immunogenicity of the vaccine. This combination boosts the body’s ability to recognize and respond to the H5N1 influenza virus. Vax128 has been used as an adjuvant in H5N1 vaccines, demonstrating its ability to increase the effectiveness of influenza vaccines.
CpG 1018 is a TLR9 agonist that has been licensed for use in the hepatitis B vaccine Heplisav-B [158,159]. This synthetic oligonucleotide contains CpG motifs that activate TLR9, stimulating B cells and promoting the production of antibodies. In the context of influenza vaccines, CpG 1018 has been explored in combination with HA antigens. It has shown promise in rapidly inducing protective antibodies, enhancing the immune response, and providing quicker protection against influenza viruses. Its potential use in influenza vaccines offers a powerful tool for improving vaccine efficacy.
Poly(I:C) is a synthetic TLR3 ligand that mimics viral double-stranded RNA (dsRNA), an intermediate produced by viruses during replication [160,161]. By activating TLR3, Poly(I:C) triggers antiviral responses, preparing the immune system to recognize and fight viral infections. Poly(I:C) has been used as an adjuvant in influenza vaccines, enhancing immune activation and improving the body’s defense against influenza viruses. Its application highlights the potential of using TLR3 agonists to boost immune responses in vaccine development.
IVT-RNA is a RIG-I agonist used in mRNA-based influenza vaccines. RIG-I detects RNA viruses, including influenza, by recognizing viral RNA patterns [162]. IVT-RNA mimics the viral genome, activating RIG-I to stimulate an immune response. By including IVT-RNA in mRNA vaccines, researchers aim to enhance the immune system’s recognition of the virus and improve the effectiveness of the vaccine [163]. This strategy is being explored in the development of next-generation influenza vaccines that leverage the power of RNA-based platforms.
The STING pathway is increasingly recognized as a promising target to enhance vaccine efficacy [164]. Although influenza is an RNA virus and lacks DNA, it can indirectly activate the cGAS-STING pathway through cellular stress during infection, such as mitochondrial damage or nuclear DNA leakage [165]. This activation triggers type I interferon responses, which play a crucial role in boosting both innate and adaptive immune responses [166]. The activation of STING not only enhances the breadth and specificity of immune responses but also facilitates antigen presentation and immune cell activation. Upon recognizing cytosolic double-stranded DNA, cGAS (cyclic GMP-AMP synthase) produces cGAMP, a second messenger that activates STING [167]. Recently, cGAMP-loaded pulmonary surfactant-mimetic nanoparticles have been developed as inhalable STING agonists. These nanoparticles effectively deliver cGAMP to the pulmonary immune system, activating the STING pathway to enhance both mucosal and systemic immunity against influenza [168]. Studies in animal models have demonstrated that this approach can significantly improve immune responses against influenza, highlighting the potential of STING agonists as nanocarrier-based adjuvants. Although STING agonists have been clinically tested in cancer vaccines, their application in influenza vaccines is still in the early stages of research.
Manganese, an essential micronutrient, plays critical roles in physiological and immune processes. In anti-influenza vaccines, manganese-based nano-adjuvants enhance cGAS sensitivity to DNA [169]. Mn2+ significantly strengthens cGAS-DNA binding, enabling efficient detection of even low concentrations of mtDNA or nuclear DNA fragments [170,171]. Studies also indicate that Mn2+ acts as a co-activator of STING, promoting its conformational changes and TBK1-IRF3 signaling independent of DNA [172]. This makes manganese-based nanoparticles a promising tool in immune modulation and adjuvant development for influenza vaccines. In addition to enhancing cGAS-STING pathway activation, manganese-based nanoparticles also enhance antigen presentation and immune cell activation. Mn3O4 nanoparticles, for instance, promote dendritic cell (DC) antigen uptake, upregulate MHC-II and co-stimulatory molecules (CD80/CD86), and amplify T cell activation [173]. Manganese adjuvants also induce B cell surface markers (CD69, CD86) and the transcription factor Blimp-1, driving antibody secretion [174]. Beyond virology, manganese reverses tumor multidrug resistance (MDR) in cancer microenvironments by inducing reactive oxygen species (ROS) and inhibiting ABC transporters, enhancing chemotherapy efficacy [175]. Similar mechanisms may regulate antiviral immune evasion. Manganese nano-adjuvants (e.g., MnJ) stimulate mucosal immunity in the respiratory or intestinal tract, inducing secretory IgA (sIgA) to form localized barriers against influenza and other mucosal pathogens [176].
5. Towards Universal Influenza Vaccines: Challenges, Innovations, and the Path Forward
5.1. The Urgency and Challenges in the Development of Broad-Spectrum Influenza Vaccines
The high mutability of influenza viruses is the primary factor limiting the long-term efficacy and broad protection of vaccines. Each year, antigenic drift and shift drive the continuous evolution of circulating strains, necessitating frequent updates of seasonal vaccines. Current influenza vaccines mainly target the hemagglutinin (HA) and neuraminidase (NA) antigens and rely heavily on predictions of prevalent strains. However, these vaccines typically confer limited strain-specific protection and often fail to cover rapidly emerging viral lineages. In cases of mismatched predictions or substantial viral mutations, their protective efficacy can be significantly reduced. Moreover, influenza viruses employ various immune evasion mechanisms, such as glycosylation modification, interference with antigen presentation, and the induction of immunosuppression, further undermining the breadth of protection. For example, the glycosylation of HA epitopes can hinder antibody recognition and reduce the efficiency of humoral immune responses. Therefore, in addition to inducing neutralizing antibodies, effective vaccines must also elicit robust memory T cell responses to clear infected cells and compensate for immune escape, thereby achieving broader and more durable protection.
5.2. Key Strategies for Universal Vaccine Design
To counter the challenges of antigenic variation and immune evasion, vaccine strategies targeting conserved epitopes have emerged as a promising approach for achieving broad-spectrum protection. Conserved regions such as the HA stem, M2e, and NP have demonstrated strong cross-reactivity potential; however, their practical application remains challenging. These conserved epitopes are often structurally shielded and inherently poorly immunogenic, making it difficult to induce potent immune responses. Furthermore, their conformational stability and presentation efficiency across different delivery platforms directly influence vaccine performance. Current strategies, including structure-based optimization, multivalent design, and epitope reconfiguration, are widely used to enhance epitope exposure and immune recognition. Nevertheless, balancing structural stability with immunological accessibility remains a key technical bottleneck. Several vaccine candidates based on conserved epitopes have entered clinical trials and show promising potential; yet, their applicability across diverse populations, consistency in immune responses, and durability of protection remain to be validated. Adjuvants play a crucial role in enhancing the immunogenicity of vaccines, particularly in the context of broad-spectrum influenza vaccine development. Pattern recognition receptor (PRR) agonists, including those targeting TLR7/8, STING, and RIG-I pathways, are widely used to potentiate innate immunity, promote Th1-biased responses, and facilitate the formation of memory T cells. These adjuvants have shown significant potential in augmenting the immunogenicity of conserved epitopes. Some are also capable of inducing mucosal immunity, providing an added layer of defense against respiratory infections. However, the clinical translation of these potent immunostimulatory adjuvants still faces considerable hurdles. These include strong dose dependency, the risk of excessive inflammatory responses, limited suitability across different population groups, and variable delivery efficiency depending on the vaccine platform. Achieving controllable immune activation, particularly ensuring safety in high-risk populations, remains a critical challenge that must be addressed to facilitate its broader clinical application.
5.3. Translational Bottlenecks and Practical Challenges
Although various universal influenza vaccine strategies have shown promising results in animal models, transitioning from laboratory research to clinical application remains fraught with challenges. Firstly, there is a lack of comprehensive systems for evaluating cross-protective efficacy. The diversity and rapid evolution of influenza viruses render traditional immune protection metrics insufficient to accurately assess a vaccine’s broad-spectrum performance across multiple subtypes. Currently, no unified and systematic criteria exist to evaluate the breadth of protection provided by different vaccine candidates, which limits clinical advancement. Secondly, significant differences exist between animal models and the human immune system. While mouse and ferret models are widely used for preliminary validation, their immune system structure and response patterns do not fully mimic those of humans. Some vaccines elicit robust T cell or antibody responses in animal studies but perform poorly in human trials. Thus, improving the predictive power of preclinical models—such as through humanized models and AI-based tools—has become a critical step toward enhancing clinical translation. Thirdly, population adaptability remains unresolved. Individuals of different ages, health conditions, and immune statuses respond differently to vaccination. In particular, elderly individuals, infants, and immunocompromised patients often exhibit weaker immune responses. A universal influenza vaccine must be designed with these varied immunological characteristics in mind and should be tested across diverse demographic groups during clinical trials.
Moreover, the industrialization of broad-spectrum influenza vaccines presents substantial technical hurdles. Each vaccine type faces distinct challenges in large-scale production, quality control, and supply chain logistics. For subunit protein vaccines, conserved epitopes often exhibit inherently low immunogenicity and expression efficiency. Their complex three-dimensional structures are difficult to replicate accurately in expression systems, complicating antigen purification and leading to variability between production batches, making consistency and stability in scaled production difficult to control. For viral vector vaccines, despite their strong immunogenic potential, issues such as the genetic stability of the vector, maintaining high viral titers, and compatibility with different host systems increase manufacturing demands. As for mRNA vaccines, the encoding of conserved antigens requires precise nucleic acid sequence optimization to enhance translation efficiency and accurate protein folding. These vaccines also rely heavily on lipid nanoparticle (LNP) delivery systems, which pose challenges including batch-to-batch variability, stringent cold-chain requirements, and high manufacturing costs—major barriers to commercialization.
5.4. Future Directions and Technological Frontiers
Vaccine development is typically a long and complex process, encompassing antigen selection, platform design, animal testing, and clinical trials. In the case of influenza vaccines, even those showing promising results in animals often fall short in human trials. One key reason is the discrepancy between animal and human immune responses, leading to divergent outcomes across species. Additionally, varying levels of vaccine responsiveness among different age groups and immune statuses further complicate development. To accelerate vaccine development and improve success rates, computational epitope prediction and structure-based design are emerging as powerful tools. Computational modeling enables researchers to identify conserved surface epitopes early and optimize their immunogenicity. For instance, simulations can quickly pinpoint and assess multiple potential conserved epitopes and guide antigen optimization based on the virus’s 3D structure. This approach not only improves immunogenicity but also helps streamline vaccine platform selection, shortening design timelines. Moreover, advances in structural biology and artificial intelligence are driving more precise and efficient vaccine design. Structure-guided antigen design helps overcome issues like low immunogenicity and epitope masking seen in traditional approaches. In virus-like particle (VLP) or nanoparticle delivery systems, structural optimization ensures the effective presentation of multiple conserved epitopes, boosting cross-protective capacity. Beyond computational strategies, humanized mouse models and organoid platforms offer new opportunities in vaccine development. These systems more accurately mimic human immune responses and enable more precise evaluations during early development stages. Humanized mice incorporate human immune cells, making their responses more reflective of human physiology. Similarly, organoids can replicate microenvironments of human tissues, such as the respiratory tract, providing valuable insights into localized immune responses. These tools may significantly reduce the time from laboratory research to clinical application.
The route of vaccine administration also significantly influences immune outcomes. Traditional influenza vaccines are mostly delivered via intramuscular injection, which primarily induces systemic IgG responses in peripheral blood. However, this method weakly stimulates local respiratory immunity, resulting in low antibody concentrations in the airway and insufficient frontline defense. In contrast, intranasal vaccines can directly target the respiratory mucosa, inducing strong local IgA responses and activating T cell immunity. Mucosal immunity not only forms a robust first line of defense against influenza viruses but also enables rapid immune responses upon re-exposure, thanks to memory T cells. This delivery method offers unique potential in preventing respiratory infections and could be particularly beneficial in controlling influenza.
In summary, the development of a universal influenza vaccine is both an urgent need in response to viral evolution and a major public health challenge. With continuous advances in technologies such as computational modeling, structural biology, and innovative delivery strategies, we have reason to believe that future broad-spectrum vaccines will overcome current technical hurdles and offer more effective, long-lasting protection. Cross-disciplinary collaboration and innovation will be key to achieving breakthroughs and ensuring comprehensive protection against diverse influenza strains.
Author Contributions
Conceptualization, M.-Q.Z. and J.-W.B.; Writing—original draft preparation, M.-Q.Z. and J.-W.B.; Data curation, M.-Q.Z. and J.-W.B.; Project administration, Z.-G.W. and S.-L.L.; Funding acquisition, S.-L.L.; Writing—review and editing, M.-Q.Z., Z.-G.W. and S.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2024YFA1210003), the National Natural Science Foundation of China (Nos. 22293032, 22374138, and 21977054), and the Tianjin Natural Science Foundation (Nos. 24JCZDJC01240 and 23JCYBJC01880).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Wang, L.; Si, H.; Yu, Z.; Tian, S.; Xiang, R.; Deng, X.; Liang, R.; Jiang, S.; Yu, F. Influenza virus glycoprotein-reactive human monoclonal antibodies. Microb. Infect. 2020, 22, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Isolation of Avian Influenza A(H5N1) Viruses from Humans—Hong Kong, May–December 1997. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 1204–1207. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00050459.htm (accessed on 12 May 2025).
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Wei, C.J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.V.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Vaccine approaches conferring cross-protection against influenza viruses. Expert Rev. Vaccines 2017, 16, 1141–1154. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef]
- Ullah, S.; Ross, T.M. Next generation live-attenuated influenza vaccine platforms. Expert Rev. Vaccines 2022, 21, 1097–1110. [Google Scholar] [CrossRef]
- Heiny, A.T.; Miotto, O.; Srinivasan, K.N.; Khan, A.M.; Zhang, G.L.; Brusic, V.; Tan, T.W.; August, J.T. Evolutionarily conserved protein sequences of influenza a viruses, avian and human, as vaccine targets. PLoS ONE 2007, 2, e1190. [Google Scholar] [CrossRef]
- Tan, P.T.; Khan, A.M.; August, J.T. Highly conserved influenza A sequences as T cell epitopes-based vaccine targets to address the viral variability. Hum. Vaccin. 2011, 7, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.; Ben-Yedidia, T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014, 54, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.D.; Poh, C.L. Development of Universal Influenza Vaccines Targeting Conserved Viral Proteins. Vaccines 2019, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ma, J.; Zhao, C. Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza. Vaccines 2024, 12, 1382. [Google Scholar] [CrossRef]
- Bedi, R.; Bayless, N.L.; Glanville, J. Challenges and Progress in Designing Broad-Spectrum Vaccines Against Rapidly Mutating Viruses. Annu. Rev. Biomed. Sci. 2023, 6, 419–441. [Google Scholar] [CrossRef]
- Lim, C.M.L.; Komarasamy, T.V.; Adnan, N.; Radhakrishnan, A.K.; Balasubramaniam, V. Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines. Influenza Other Respir. Viruses 2024, 18, e13276. [Google Scholar] [CrossRef] [PubMed]
- Vogel, O.A.; Manicassamy, B. Broadly Protective Strategies Against Influenza Viruses: Universal Vaccines and Therapeutics. Front. Microbiol. 2020, 11, 135. [Google Scholar] [CrossRef]
- Viboud, C.; Gostic, K.; Nelson, M.I.; Price, G.E.; Perofsky, A.; Sun, K.; Sequeira Trovão, N.; Cowling, B.J.; Epstein, S.L.; Spiro, D.J. Beyond clinical trials: Evolutionary and epidemiological considerations for development of a universal influenza vaccine. PLoS Pathog. 2020, 16, e1008583. [Google Scholar] [CrossRef]
- Berry, C.M.; Penhale, W.J.; Sangster, M.Y. Passive broad-spectrum influenza immunoprophylaxis. Influenza Res. Treat. 2014, 2014, 267594. [Google Scholar] [CrossRef]
- Kerstetter, L.J.; Buckley, S.; Bliss, C.M.; Coughlan, L. Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses. Front. Immunol. 2020, 11, 607333. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Alqazlan, N.; Astill, J.; Raj, S.; Sharif, S. Strategies for enhancing immunity against avian influenza virus in chickens: A review. Avian Pathol. 2022, 51, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tumpey, T.M.; Park, H.J.; Byun, Y.H.; Tran, L.D.; Nguyen, V.D.; Kilgore, P.E.; Czerkinsky, C.; Katz, J.M.; Seong, B.L.; et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE 2010, 5, e10152. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2021, 12, 790121. [Google Scholar] [CrossRef] [PubMed]
- Hendy, D.A.; Amouzougan, E.A.; Young, I.C.; Bachelder, E.M.; Ainslie, K.M. Nano/microparticle Formulations for Universal Influenza Vaccines. AAPS J. 2022, 24, 24. [Google Scholar] [CrossRef]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kaushal, A.; Gupta, S. Vaccine development: Current trends and technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef]
- Cappellano, G.; Abreu, H.; Casale, C.; Dianzani, U.; Chiocchetti, A. Nano-Microparticle Platforms in Developing Next-Generation Vaccines. Vaccines 2021, 9, 660. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef]
- Lopez, C.E.; Legge, K.L. Influenza A Virus Vaccination: Immunity, Protection, and Recent Advances Toward A Universal Vaccine. Vaccines 2020, 8, 434. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, C.; Wei, L.; Wang, B.Z. Promising Adjuvants and Platforms for Influenza Vaccine Development. Pharmaceutics 2021, 13, 68. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Stepanova, E.; Mezhenskaya, D.; Matyushenko, V.; Prokopenko, P.; Sychev, I.; Wong, P.F.; Rudenko, L. Influenza vaccine: Progress in a vaccine that elicits a broad immune response. Expert Rev. Vaccines 2021, 20, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Y.; Li, Y.; Chen, X. Adjuvantation of Influenza Vaccines to Induce Cross-Protective Immunity. Vaccines 2021, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Jia, W.; Xie, Y.; Yu, M.; Chen, Y. Adjuvant physiochemistry and advanced nanotechnology for vaccine development. Chem. Soc. Rev. 2023, 52, 5172–5254. [Google Scholar] [CrossRef] [PubMed]
- Goff, P.H.; Hayashi, T.; Martínez-Gil, L.; Corr, M.; Crain, B.; Yao, S.; Cottam, H.B.; Chan, M.; Ramos, I.; Eggink, D.; et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J. Virol. 2015, 89, 3221–3235. [Google Scholar] [CrossRef]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef]
- Zeigler, D.F.; Gage, E.; Clegg, C.H. Epitope-targeting platform for broadly protective influenza vaccines. PLoS ONE 2021, 16, e0252170. [Google Scholar] [CrossRef]
- Nagashima, K.A.; Mousa, J.J. Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine. Viruses 2021, 13, 546. [Google Scholar] [CrossRef]
- Sia, Z.R.; Miller, M.S.; Lovell, J.F. Engineered Nanoparticle Applications for Recombinant Influenza Vaccines. Mol. Pharm. 2021, 18, 576–592. [Google Scholar] [CrossRef]
- Boulo, S.; Akarsu, H.; Ruigrok, R.W.H.; Baudin, F. Nuclear traffic of influenza virus proteins and ribonucleoprotein complexes. Virus Res. 2007, 124, 12–21. [Google Scholar] [CrossRef]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.K.; Kephart, S.M.; Benhaim, M.A.; Matsui, T.; Mileant, A.; Guttman, M.; Lee, K.K. Structural dynamics reveal subtype-specific activation and inhibition of influenza virus hemagglutinin. J. Biol. Phys. Chem. 2023, 299, 104765. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef]
- Varghese, J.N.; Laver, W.G.; Colman, P.M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 Å resolution. Nature 1983, 303, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Noton, S.L.; Medcalf, E.; Fisher, D.; Mullin, A.E.; Elton, D.; Digard, P. Identification of the domains of the influenza A virus M1 matrix protein required for NP binding, oligomerization and incorporation into virions. J. Gen. Mol. Virol. 2007, 88, 2280–2290. [Google Scholar] [CrossRef]
- Watanabe, K.; Fuse, T.; Asano, I.; Tsukahara, F.; Maru, Y.; Nagata, K.; Kitazato, K.; Kobayashi, N. Identification of Hsc70 as an influenza virus matrix protein (M1) binding factor involved in the virus life cycle. FEBS Lett. 2006, 580, 5785–5790. [Google Scholar] [CrossRef]
- Pinto, L.H.; Lamb, R.A. The M2 proton channels of influenza A and B viruses. J. Biol. Phys. Chem. 2006, 281, 8997–9000. [Google Scholar] [CrossRef]
- Hao, W.; Wang, L.; Li, S. Roles of the Non-Structural Proteins of Influenza A Virus. Pathogens 2020, 9, 812. [Google Scholar] [CrossRef]
- Malik, G.; Zhou, Y. Innate Immune Sensing of Influenza A Virus. Viruses 2020, 12, 755. [Google Scholar] [CrossRef]
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef]
- Chen, J.; Skehel, J.J.; Wiley, D.C. N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc. Natl. Acad. Sci. USA 1999, 96, 8967–8972. [Google Scholar] [CrossRef] [PubMed]
- Guthmiller, J.J.; Han, J.; Utset, H.A.; Li, L.; Lan, L.Y.; Henry, C.; Stamper, C.T.; McMahon, M.; O’Dell, G.; Fernández-Quintero, M.L.; et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 2022, 602, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Sharma, S.; Kumar, B.; Rajput, R. Protective immunity based on the conserved hemagglutinin stalk domain and its prospects for universal influenza vaccine development. Biomed. Res. 2014, 2014, 546274. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C.; Wang, T.T.; Yondola, M.; Gao, Q.; Haye, K.; García-Sastre, A.; Palese, P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 2010, 1, 10–1128. [Google Scholar] [CrossRef]
- Xu, R.; McBride, R.; Nycholat, C.M.; Paulson, J.C.; Wilson, I.A. Structural characterization of the hemagglutinin receptor specificity from the 2009 H1N1 influenza pandemic. J. Virol. 2012, 86, 982–990. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef]
- Mair, C.M.; Meyer, T.; Schneider, K.; Huang, Q.; Veit, M.; Herrmann, A. A histidine residue of the influenza virus hemagglutinin controls the pH dependence of the conformational change mediating membrane fusion. J. Virol. 2014, 88, 13189–13200. [Google Scholar] [CrossRef]
- Byrd-Leotis, L.; Galloway, S.E.; Agbogu, E.; Steinhauer, D.A. Influenza hemagglutinin (HA) stem region mutations that stabilize or destabilize the structure of multiple HA subtypes. J. Virol. 2015, 89, 4504–4516. [Google Scholar] [CrossRef]
- Sun, X.; Ma, H.; Wang, X.; Bao, Z.; Tang, S.; Yi, C.; Sun, B. Broadly neutralizing antibodies to combat influenza virus infection. Antivir. Res. 2024, 221, 105785. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, V.V.A.; Citron, M.; Ferrara, F.; Lu, X.; Callahan, C.; Heidecker, G.J.; Sarma, S.P.; Flynn, J.A.; Temperton, N.J.; Liang, X.; et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl. Acad. Sci. USA 2014, 111, E2514–E2523. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Jagadesh, A.; Salam, A.A.; Mudgal, P.P.; Arunkumar, G. Influenza virus neuraminidase (NA): A target for antivirals and vaccines. Arch. Virol 2016, 161, 2087–2094. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef]
- Creytens, S.; Pascha, M.N.; Ballegeer, M.; Saelens, X.; de Haan, C.A.M. Influenza Neuraminidase Characteristics and Potential as a Vaccine Target. Front. Immunol. 2021, 12, 786617. [Google Scholar] [CrossRef]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and function of the influenza A M2 proton channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef]
- Zharikova, D.; Mozdzanowska, K.; Feng, J.; Zhang, M.; Gerhard, W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J. Virol. 2005, 79, 6644–6654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, L.; Shi, H.; Xu, H.; Yao, H.; Xi, X.G.; Toyoda, T.; Wang, X.; Wang, T. Monoclonal antibody recognizing SLLTEVET epitope of M2 protein potently inhibited the replication of influenza A viruses in MDCK cells. Biochem. Biophys. Res. Commun. 2009, 385, 118–122. [Google Scholar] [CrossRef]
- Neirynck, S.; Deroo, T.; Saelens, X.; Vanlandschoot, P.; Jou, W.M.; Fiers, W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999, 5, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Brusic, V. Computational immunology: The coming of age. Immunol. Cell Biol. 2002, 80, 248–254. [Google Scholar] [CrossRef]
- De Groot, A.S.; Sbai, H.; Aubin, C.S.; McMurry, J.; Martin, W. Immuno-informatics: Mining genomes for vaccine components. Immunol. Cell Biol. 2002, 80, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Moise, L.; Gutierrez, A.; Kibria, F.; Martin, R.; Tassone, R.; Liu, R.; Terry, F.; Martin, B.; De Groot, A.S. iVAX: An integrated toolkit for the selection and optimization of antigens and the design of epitope-driven vaccines. Hum. Vaccines Immunother. 2015, 11, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.S.; Moise, L.; Terry, F.; Gutierrez, A.H.; Hindocha, P.; Richard, G.; Hoft, D.F.; Ross, T.M.; Noe, A.R.; Takahashi, Y.; et al. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front. Immunol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Bounds, C.E.; Terry, F.E.; Moise, L.; Hannaman, D.; Martin, W.D.; De Groot, A.S.; Suschak, J.J.; Dupuy, L.C.; Schmaljohn, C.S. An immunoinformatics-derived DNA vaccine encoding human class II T cell epitopes of Ebola virus, Sudan virus, and Venezuelan equine encephalitis virus is immunogenic in HLA transgenic mice. Hum. Vaccines Immunother. 2017, 13, 2824–2836. [Google Scholar] [CrossRef]
- Eickhoff, C.S.; Terry, F.E.; Peng, L.; Meza, K.A.; Sakala, I.G.; Van Aartsen, D.; Moise, L.; Martin, W.D.; Schriewer, J.; Buller, R.M.; et al. Highly conserved influenza T cell epitopes induce broadly protective immunity. Vaccine 2019, 37, 5371–5381. [Google Scholar] [CrossRef]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. De novo design of protein structure and function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Jenni, S.; Raymond, D.D.; Caradonna, T.; Do, K.T.; Schmidt, A.G.; Harrison, S.C.; Grigorieff, N. CryoEM Structure of an Influenza Virus Receptor-Binding Site Antibody-Antigen Interface. J. Mol. Biol. 2017, 429, 1829–1839. [Google Scholar] [CrossRef]
- Taylor, R.M.; Dreguss, M. An Experiment in Immunization Against Influenza with a Formaldehyde-Inactivated Virus. Am. J. Epidemiol. 1940, 31, 31–35. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Bast, C.; Hengartner, H.; Zinkernagel, R.M. Immunogenicity of a viral model vaccine after different inactivation procedures. Microbiol. Immunol. 1994, 183, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bonnafous, P.; Nicolaï, M.C.; Taveau, J.C.; Chevalier, M.; Barrière, F.; Medina, J.; Le Bihan, O.; Adam, O.; Ronzon, F.; Lambert, O. Treatment of influenza virus with beta-propiolactone alters viral membrane fusion. Biochim. Biophys. Acta 2014, 1838, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.L.; Betts, R.F.; Tierney, E.L.; Murphy, B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986, 24, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, B.J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Xu, W.; Huo, X.; Wang, J.; Xu, Y.; Ding, W.; Guo, Z.; Liu, R. Advances in protein subunit vaccines against H1N1/09 influenza. Front. Immunol. 2024, 15, 1499754. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, Z.; Leonard, R.A.; Hamele, C.E.; Spreng, R.L.; Heaton, N.S. Administration of antigenically distinct influenza viral particle combinations as an influenza vaccine strategy. PLoS Pathog. 2025, 21, e1012878. [Google Scholar] [CrossRef]
- Puig Barbera, J.; Gonzalez Vidal, D. MF59-adjuvanted subunit influenza vaccine: An improved interpandemic influenza vaccine for vulnerable populations. Expert Rev. Vaccines 2007, 6, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, J.; Kim, D.I.; Avila, J.P.; Nakaya, H.; Kwak, K.; Kim, E.H. Emulsion adjuvant-induced uric acid release modulates optimal immunogenicity by targeting dendritic cells and B cells. npj Vaccines 2025, 10, 72. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Q.; Qi, M.; Chen, J.; Wu, X.; Zhang, X.; Li, W.; Zhang, X.E.; Cui, Z. An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses. ACS Nano 2023, 17, 13474–13487. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D. Medicinal Chemistry and Methodological Advances in the Development of Peptide-Based Vaccines. J. Med. Chem. 2020, 63, 14184–14196. [Google Scholar] [CrossRef]
- Hamley, I.W. Peptides for Vaccine Development. ACS Appl. Bio Mater. 2022, 5, 905–944. [Google Scholar] [CrossRef]
- Abbasi, J. FLU-v, a Universal Flu Vaccine Candidate, Advances in Trial. JAMA 2020, 323, 1336. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, O.; James, E.; Fernandez, A.; Lopes, V.; Rosas, L.A.; Cervantes-Medina, A.; Cleath, J.; Edwards, K.; Neitzey, D.; Gu, W.; et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. npj Vaccines 2020, 5, 22. [Google Scholar] [CrossRef]
- Pleguezuelos, O.; Dille, J.; de Groen, S.; Oftung, F.; Niesters, H.G.M.; Islam, M.A.; Naess, L.M.; Hungnes, O.; Aldarij, N.; Idema, D.L.; et al. Immunogenicity, Safety, and Efficacy of a Standalone Universal Influenza Vaccine, FLU-v, in Healthy Adults: A Randomized Clinical Trial. Ann. Intern. Med. 2020, 172, 453–462. [Google Scholar] [CrossRef]
- Pleguezuelos, O.; Robinson, S.; Stoloff, G.A.; Caparros-Wanderley, W. Synthetic Influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomised, placebo-controlled Phase I trial. Vaccine 2012, 30, 4655–4660. [Google Scholar] [CrossRef]
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr. Top. Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Wingerath, J.; Ostroumov, D.; Woller, N.; Manns, M.P.; Pinschewer, D.D.; Orlinger, K.; Berka, U.; Kühnel, F.; Wirth, T.C. Recombinant LCMV Vectors Induce Protective Immunity following Homologous and Heterologous Vaccinations. Mol. Ther. 2017, 25, 2533–2545. [Google Scholar] [CrossRef]
- Bonilla, W.V.; Kirchhammer, N.; Marx, A.F.; Kallert, S.M.; Krzyzaniak, M.A.; Lu, M.; Darbre, S.; Schmidt, S.; Raguz, J.; Berka, U.; et al. Heterologous arenavirus vector prime-boost overrules self-tolerance for efficient tumor-specific CD8 T cell attack. Cell Rep. Med. 2021, 2, 100209. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Wang, M.; Tong, Q.; Sun, Y.; Pu, J.; Sun, H.; Liu, J. Recombinant turkey herpesvirus expressing H9 hemagglutinin providing protection against H9N2 avian influenza. Virology 2019, 529, 7–15. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, Y.; Wu, H.; Wang, Z.; Zhan, Y.; Feng, X.; Geng, R.; Wu, Y.; Kong, W.; Yu, X. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J. Med. Virol. 2012, 84, 1408–1414. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, N.; Vemula, S.V.; Couëtil, L.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS ONE 2012, 7, e33428. [Google Scholar] [CrossRef]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Iavarone, C.; O’Hagan, D.T.; Yu, D.; Delahaye, N.F.; Ulmer, J.B. Mechanism of action of mRNA-based vaccines. Expert Rev. Vaccines 2017, 16, 871–881. [Google Scholar] [CrossRef]
- Sandbrink, J.B.; Shattock, R.J. RNA Vaccines: A Suitable Platform for Tackling Emerging Pandemics? Front. Immunol. 2020, 11, 608460. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Reina, J. The new generation of messenger RNA (mRNA) vaccines against influenza. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2023, 41, 301–304. [Google Scholar] [CrossRef]
- Rockman, S.; Laurie, K.L.; Parkes, S.; Wheatley, A.; Barr, I.G. New Technologies for Influenza Vaccines. Microorganisms 2020, 8, 1745. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef]
- Liang, S.L.; Quirk, D.; Zhou, A. RNase L: Its biological roles and regulation. IUBMB Life 2006, 58, 508–514. [Google Scholar] [CrossRef]
- Tanji, H.; Ohto, U.; Shibata, T.; Taoka, M.; Yamauchi, Y.; Isobe, T.; Miyake, K.; Shimizu, T. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 2015, 22, 109–115. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.-H. Influenza Viruses: Innate Immunity and mRNA Vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Bilyy, R.; Paryzhak, S.; Turcheniuk, K.; Dumych, T.; Barras, A.; Boukherroub, R.; Wang, F.; Yushin, G.; Szunerits, S. Aluminum oxide nanowires as safe and effective adjuvants for next-generation vaccines. Mater. Today 2019, 22, 58–66. [Google Scholar] [CrossRef]
- Harrison, W.T. Some Observations on the Use of Alum Precipitated Diphtheria Toxoid. Am. J. Public Health Nations Health 1935, 25, 298–300. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
- Cain, D.W.; Snowden, P.B.; Sempowski, G.D.; Kelsoe, G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS ONE 2011, 6, e19957. [Google Scholar] [CrossRef]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28 (Suppl. S3), C25–C36. [Google Scholar] [CrossRef]
- Hem, S.L.; Johnston, C.T.; HogenEsch, H. Imject Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine 2007, 25, 4985–4986. [Google Scholar] [CrossRef]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59(®) adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Cioncada, R.; Maddaluno, M.; Vo, H.T.M.; Woodruff, M.; Tavarini, S.; Sammicheli, C.; Tortoli, M.; Pezzicoli, A.; De Gregorio, E.; Carroll, M.C.; et al. Vaccine adjuvant MF59 promotes the intranodal differentiation of antigen-loaded and activated monocyte-derived dendritic cells. PLoS ONE 2017, 12, e0185843. [Google Scholar] [CrossRef]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef]
- Seubert, A.; Calabro, S.; Santini, L.; Galli, B.; Genovese, A.; Valentini, S.; Aprea, S.; Colaprico, A.; D’Oro, U.; Giuliani, M.M.; et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc. Natl. Acad. Sci. USA 2011, 108, 11169–11174. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Lee, Y.T.; Kim, K.H.; Jung, Y.J.; Lee, Y.; Denning, T.L.; Kang, S.M. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. J. Virol. 2016, 90, 6976–6988. [Google Scholar] [CrossRef]
- Belshe, R.B.; Frey, S.E.; Graham, I.L.; Anderson, E.L.; Jackson, L.A.; Spearman, P.; Edupuganti, S.; Mulligan, M.J.; Rouphael, N.; Winokur, P.; et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: A randomized clinical trial. J. Am. Med. Assoc. 2014, 312, 1420–1428. [Google Scholar] [CrossRef]
- van der Maas, N.; Dijs-Elsinga, J.; Kemmeren, J.; van Lier, A.; Knol, M.; de Melker, H. Safety of vaccination against influenza A (H1N1) during pregnancy in the Netherlands: Results on pregnancy outcomes and infant’s health: Cross-sectional linkage study. BJOG 2016, 123, 709–717. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Edwards, K.M.; Dekker, C.L.; Belshe, R.; Talbot, H.K.; Graham, I.L.; Noah, D.L.; He, F.; Hill, H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 2008, 197, 667–675. [Google Scholar] [CrossRef]
- Reisinger, K.S.; Holmes, S.J.; Pedotti, P.; Arora, A.K.; Lattanzi, M. A dose-ranging study of MF59(®)-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum. Vaccines Immunother. 2014, 10, 2395–2407. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Beran, J.; Devaster, J.M.; Esen, M.; Launay, O.; Leroux-Roels, G.; Ruiz-Palacios, G.M.; van Essen, G.A.; Caplanusi, A.; Claeys, C.; et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: A phase 3 randomised trial. Lancet Infect. Dis. 2013, 13, 485–496. [Google Scholar] [CrossRef]
- Madan, A.; Collins, H.; Sheldon, E.; Frenette, L.; Chu, L.; Friel, D.; Drame, M.; Vaughn, D.W.; Innis, B.L.; Schuind, A. Evaluation of a primary course of H9N2 vaccine with or without AS03 adjuvant in adults: A phase I/II randomized trial. Vaccine 2017, 35, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Frenette, L.; Chu, L.; McNeil, S.; Halperin, S.; Li, P.; Vaughn, D. A randomized, controlled non-inferiority trial comparing A(H1N1)pmd09 vaccine antigen, with and without AS03 adjuvant system, co-administered or sequentially administered with an inactivated trivalent seasonal influenza vaccine. BMC Infect. Dis. 2012, 12, 279. [Google Scholar] [CrossRef]
- Sarkanen, T.O.; Alakuijala, A.P.E.; Dauvilliers, Y.A.; Partinen, M.M. Incidence of narcolepsy after H1N1 influenza and vaccinations: Systematic review and meta-analysis. Sleep Med. Rev. 2018, 38, 177–186. [Google Scholar] [CrossRef]
- Nazareth, I.; Tavares, F.; Rosillon, D.; Haguinet, F.; Bauchau, V. Safety of AS03-adjuvanted split-virion H1N1 (2009) pandemic influenza vaccine: A prospective cohort study. BMJ Open 2013, 3, e001912. [Google Scholar] [CrossRef]
- Xiang, M.; Fan, J. Pattern recognition receptor-dependent mechanisms of acute lung injury. Mol. Med. 2010, 16, 69–82. [Google Scholar] [CrossRef]
- Murugaiah, V.; Tsolaki, A.G.; Kishore, U. Collectins: Innate Immune Pattern Recognition Molecules. Adv. Exp. Med. Biol. 2020, 1204, 75–127. [Google Scholar]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine 2014, 9, 2657–2669. [Google Scholar] [CrossRef]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Coler, R.N.; Baldwin, S.L.; Shaverdian, N.; Bertholet, S.; Reed, S.J.; Raman, V.S.; Lu, X.; DeVos, J.; Hancock, K.; Katz, J.M.; et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS ONE 2010, 5, e13677. [Google Scholar] [CrossRef] [PubMed]
- Behzad, H.; Huckriede, A.L.; Haynes, L.; Gentleman, B.; Coyle, K.; Wilschut, J.C.; Kollmann, T.R.; Reed, S.G.; McElhaney, J.E. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J. Infect. Dis. 2012, 205, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Treanor, J.J.; Sheldon, E.A.; Johnson, C.; Umlauf, S.; Song, L.; Kavita, U.; Liu, G.; Tussey, L.; Ozer, K.; et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine 2012, 30, 5761–5769. [Google Scholar] [CrossRef]
- Campbell, J.D. Development of the CpG Adjuvant 1018: A Case Study. Methods Mol. Biol. 2017, 1494, 15–27. [Google Scholar]
- Hoxie, I.; Vasilev, K.; Clark, J.J.; Bushfield, K.; Francis, B.; Loganathan, M.; Campbell, J.D.; Yu, D.; Guan, L.; Gu, C.; et al. A recombinant N2 neuraminidase-based CpG 1018(R) adjuvanted vaccine provides protection against challenge with heterologous influenza viruses in mice and hamsters. Vaccine 2024, 42, 126269. [Google Scholar] [CrossRef]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef]
- Le, C.T.T.; Ahn, S.Y.; Ho, T.L.; Lee, J.; Lee, D.H.; Hwang, H.S.; Kang, S.M.; Ko, E.J. Adjuvant effects of combination monophosphoryl lipid A and poly I:C on antigen-specific immune responses and protective efficacy of influenza vaccines. Sci. Rep. 2023, 13, 12231. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gil, L.; Goff, P.H.; Hai, R.; Garcia-Sastre, A.; Shaw, M.L.; Palese, P. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J. Virol. 2013, 87, 1290–1300. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Abe, T.; Marutani, Y.; Shoji, I. Cytosolic DNA-sensing immune response and viral infection. Microbiol. Immunol. 2019, 63, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Amurri, L.; Horvat, B.; Iampietro, M. Interplay between RNA viruses and cGAS/STING axis in innate immunity. Front. Cell. Infect. Microbiol. 2023, 13, 1172739. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Kamynina, M.; Sorokin, M.; Zolotovskaia, M.; Koroleva, E.; Kremenchutckaya, K.; Gudkov, A.; Buzdin, A.; Borisov, N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines 2022, 10, 1072. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, H. The cGAS-STING-mediated NLRP3 inflammasome is involved in the neurotoxicity induced by manganese exposure. Biomed. Pharmacother. 2022, 154, 113680. [Google Scholar] [CrossRef]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Li, J.; Park, K.S.; Han, K.; Zhou, X.; Xu, Y.; Nam, J.; Xu, J.; Shi, X.; et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 2021, 16, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, Z.; Lin, X.; Wang, L.; Zhu, H.; Su, Z.; Zhang, S. In situ bio-mineralized Mn nanoadjuvant enhances anti-influenza immunity of recombinant virus-like particle vaccines. J. Control. Release 2024, 368, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, S.; Lu, W.; Wang, Y.; Li, J.; Qu, K.; Yang, M.; Wang, L.; Yu, Y. The adjuvanticity of manganese for microbial vaccines via activating the IRF5 signaling pathway. Biochem. Pharmacol. 2021, 192, 114720. [Google Scholar] [CrossRef]
- Pei, M.; Liu, K.; Qu, X.; Wang, K.; Chen, Q.; Zhang, Y.; Wang, X.; Wang, Z.; Li, X.; Chen, F.; et al. Enzyme-catalyzed synthesis of selenium-doped manganese phosphate for synergistic therapy of drug-resistant colorectal cancer. Nanobiotechnology 2023, 21, 72. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Guan, Y.; Wei, X.; Sha, M.; Yi, M.; Jing, M.; Lv, M.; Guo, W.; Xu, J.; et al. Manganese salts function as potent adjuvants. Cell. Mol. Immunol. 2021, 18, 1222–1234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).