Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review
Abstract
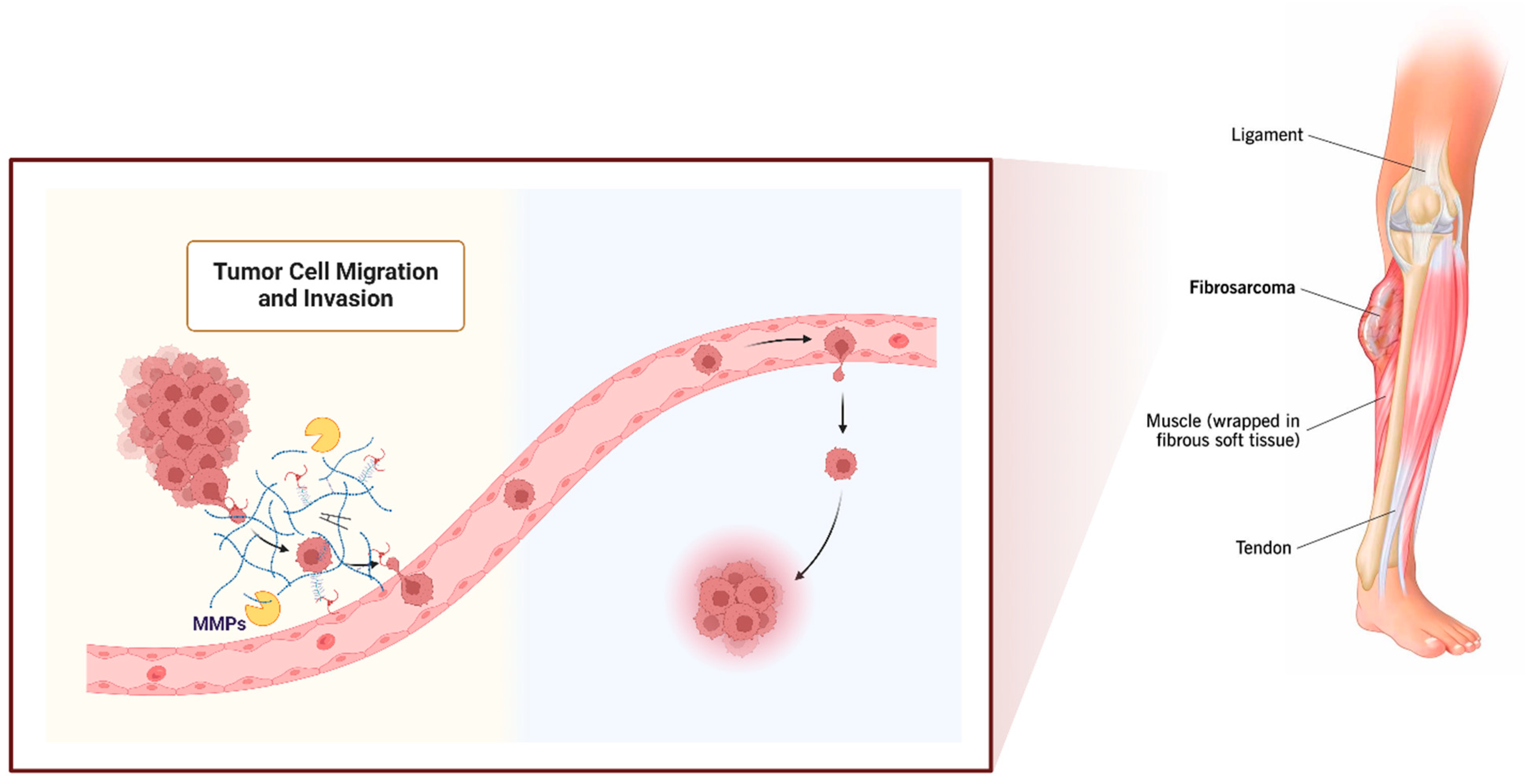
1. Fibrosarcoma
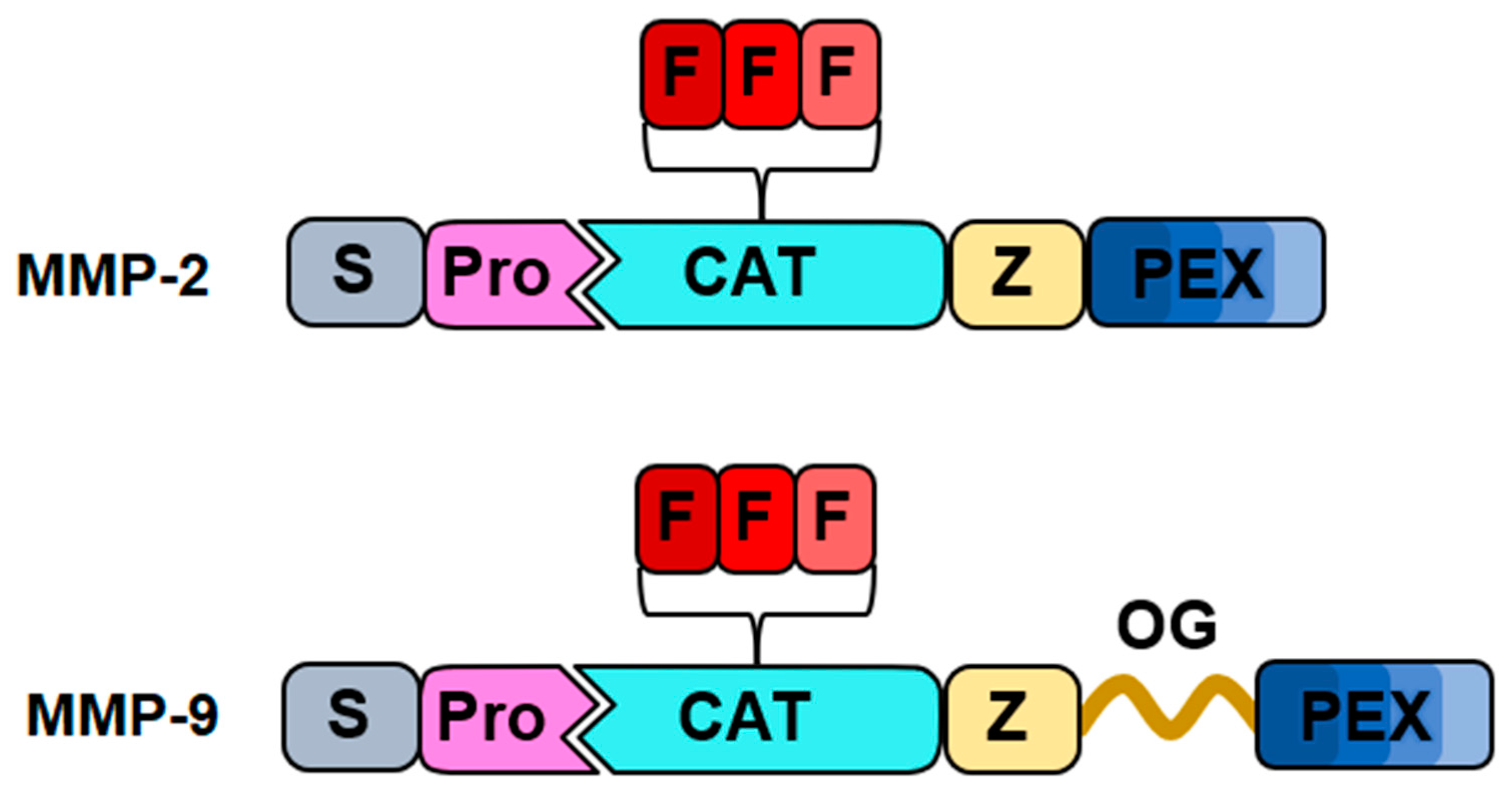
2. Matrix Metalloproteinases (MMPs)
3. MMP Roles in Fibrosarcoma
4. Therapeutic Implications of Gelatinase Inhibition in Fibrosarcoma
5. Natural-Based Inhibitors
6. Synthetic Small-Molecule Inhibitors
7. Protein- and Peptide-Based Inhibitors
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knosel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef] [PubMed]
- Genadry, K.C.; Pietrobono, S.; Rota, R.; Linardic, C.M. Soft Tissue Sarcoma Cancer Stem Cells: An Overview. Front. Oncol. 2018, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Huvos, A.G.; Higinbotham, N.L. Primary fibrosarcoma of bone. A clinicopathologic study of 130 patients. Cancer 1975, 35, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Atalay, I.B.; Togral, G. Unusual localization and aggressive progression of large infantile fibrosarcoma. Acta Orthop. Traumatol. Turc. 2019, 53, 507–511. [Google Scholar] [CrossRef]
- Scott, S.M.; Reiman, H.M.; Pritchard, D.J.; Ilstrup, D.M. Soft tissue fibrosarcoma. A clinicopathologic study of 132 cases. Cancer 1989, 64, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; da Fonseca, L.G.; Piotto, G.H.M.; Geyer, F.C.; de Alcantara, P.S.M. Fibrosarcoma: A challenging diagnosis. Autops. Case Rep. 2013, 3, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dernell, W.S.; Withrow, S.J.; Kuntz, C.A.; Powers, B.E. Principles of treatment for soft tissue sarcoma. Clin. Tech. Small Anim. Pract. 1998, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Windeyer, B.; Dische, S.; Mansfield, C.M. The place of radiotherapy in the management of fibrosarcoma of the soft tissues. Clin. Radiol. 1966, 17, 32–40. [Google Scholar] [CrossRef]
- Chew, W.; Benson, C.; Thway, K.; Hayes, A.; Miah, A.; Zaidi, S.; Lee, A.T.J.; Messiou, C.; Fisher, C.; van der Graaf, W.T.; et al. Clinical Characteristics and efficacy of chemotherapy in sclerosing epithelioid fibrosarcoma. Med. Oncol. 2018, 35, 138. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, L.; Zhu, Y.J.; Qiu, B.; Guo, S.P.; Li, Y.; Liu, Q.; Liu, M.Z.; Xi, M. Prognosis of Fibrosarcoma in Patients With and Without a History of Radiation for Nasopharyngeal Carcinoma. Ann. Surg. Oncol. 2017, 24, 434–440. [Google Scholar] [CrossRef]
- Cantin, J.; McNeer, G.P.; Chu, F.C.; Booher, R.J. The problem of local recurrence after treatment of soft tissue sarcoma. Ann. Surg. 1968, 168, 47–53. [Google Scholar] [CrossRef]
- Wibmer, C.; Leithner, A.; Zielonke, N.; Sperl, M.; Windhager, R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann. Oncol. 2010, 21, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Coffin, C.M.; Dehner, L.P. Fibroblastic-myofibroblastic tumors in children and adolescents: A clinicopathologic study of 108 examples in 103 patients. Pediatr. Pathol. 1991, 11, 569–588. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Folpe, A.L. Adult-type fibrosarcoma: A reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am. J. Surg. Pathol. 2010, 34, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L. Fibrosarcoma: A review and update. Histopathology 2014, 64, 12–25. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland. Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, Y.; Liu, W.J.; Li, L.; Li, Y.; Wang, X.F.; Yi, H.F.; Shan, C.K.; Xia, G.M.; Liu, X.J.; et al. Intensive fibrosarcoma-binding capability of the reconstituted analog and its antitumor activity. Drug Deliv. 2018, 25, 102–111. [Google Scholar] [CrossRef]
- Benassi, M.S.; Gamberi, G.; Magagnoli, G.; Molendini, L.; Ragazzini, P.; Merli, M.; Chiesa, F.; Balladelli, A.; Manfrini, M.; Bertoni, F.; et al. Metalloproteinase expression and prognosis in soft tissue sarcomas. Ann. Oncol. 2001, 12, 75–80. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front. Oncol. 2019, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Yang, H.K.; Jeong, K.C.; Kim, Y.K.; Jung, S.T. Role of matrix metalloproteinase (MMP) 2 and MMP-9 in soft tissue sarcoma. Clin. Orthop. Surg. 2014, 6, 443–454. [Google Scholar] [CrossRef]
- Roebuck, M.M.; Helliwell, T.R.; Chaudhry, I.H.; Kalogrianitis, S.; Carter, S.; Kemp, G.J.; Ritchie, D.A.; Jane, M.J.; Frostick, S.P. Matrix metalloproteinase expression is related to angiogenesis and histologic grade in spindle cell soft tissue neoplasms of the extremities. Am. J. Clin. Pathol. 2005, 123, 405–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Martino, J.S.; Akhter, T.; Bravo-Cordero, J.J. Remodeling the ECM: Implications for Metastasis and Tumor Dormancy. Cancers 2021, 13, 4916. [Google Scholar] [CrossRef] [PubMed]
- Partridge, J.J.; Madsen, M.A.; Ardi, V.C.; Papagiannakopoulos, T.; Kupriyanova, T.A.; Quigley, J.P.; Deryugina, E.I. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J. Biol. Chem. 2007, 282, 35964–35977. [Google Scholar] [CrossRef]
- Nguyen, M.; Arkell, J.; Jackson, C.J. Human endothelial gelatinases and angiogenesis. Int. J. Biochem. Cell Biol. 2001, 33, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, M.; Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta 2005, 1755, 37–69. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Ardiani, A.; Farsaci, B.; Kwilas, A.R.; Gameiro, S.R. The tipping point for combination therapy: Cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin. Oncol. 2012, 39, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Moliere, S.; Jaulin, A.; Tomasetto, C.L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef]
- Rasheed, S.; Nelson-Rees, W.A.; Toth, E.M.; Arnstein, P.; Gardner, M.B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer 1974, 33, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hill, R.P. Hypoxia enhances metastatic efficiency in HT1080 fibrosarcoma cells by increasing cell survival in lungs, not cell adhesion and invasion. Cancer Res. 2007, 67, 7789–7797. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.F. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Su, H.; Zhou, B.; Liu, S. The function of natural compounds in important anticancer mechanisms. Front. Oncol. 2022, 12, 1049888. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, M.M. Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, Y.J.; Choi, Y.J.; Jang, J.W.; Kim, J.H.; Rho, Y.K.; Kim, I.J.; Kim, H.J.; Leem, M.J.; Lee, S.T. Fisetin inhibits matrix metalloproteinases and reduces tumor cell invasiveness and endothelial cell tube formation. Nutr. Cancer 2013, 65, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Chung, M.Y.; Lim, T.G.; Huh, W.B.; Lee, H.J.; Lee, K.W. Quercetin suppresses intracellular ROS formation, MMP activation, and cell motility in human fibrosarcoma cells. J. Food Sci. 2013, 78, H1464–H1469. [Google Scholar] [CrossRef]
- Filipiak, K.; Hidalgo, M.; Silvan, J.M.; Fabre, B.; Carbajo, R.J.; Pineda-Lucena, A.; Ramos, A.; de Pascual-Teresa, B.; de Pascual-Teresa, S. Dietary gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct. 2014, 5, 381–389. [Google Scholar] [CrossRef]
- Shi, X.; Chen, G.; Liu, X.; Qiu, Y.; Yang, S.; Zhang, Y.; Fang, X.; Zhang, C.; Liu, X. Scutellarein inhibits cancer cell metastasis in vitro and attenuates the development of fibrosarcoma in vivo. Int. J. Mol. Med. 2015, 35, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, Y.H.; Lee, S.T. Galangin and kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells. Mol. Cells 2015, 38, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.; Sato, T. Novel Antitumor Invasive Actions of p-Cymene by Decreasing MMP-9/TIMP-1 Expression Ratio in Human Fibrosarcoma HT-1080 Cells. Biol. Pharm. Bull. 2016, 39, 1247–1253. [Google Scholar] [CrossRef]
- Gong, F.; Zhang, Y.; Lin, J.; Li, C.; Zhou, C.; Hong, P.; Ryu, B.; Qian, Z.J. 1-(5-Bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] Inhibits MMP-9 Expression by Regulating NF-kappaB and MAPKs Signaling Pathways in HT1080 Human Fibrosarcoma Cells. Evid. Based Complement. Alternat Med. 2018, 2018, 5639486. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Bhanap, B.; Niedzwiecki, A.; Rath, M. Chlorophyllin suppresses growth, mmp secretion, invasion and cell migration of fibrosarcoma cell line ht-1080. Med. Res. Arch. 2018, 6. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, Y.P.; Jin, S.W.; Lee, G.H.; Kim, H.G.; Han, E.H.; Kim, S.K.; Kang, K.W.; Chung, Y.C.; Jeong, H.G. Suppression of PMA-induced human fibrosarcoma HT-1080 invasion and metastasis by kahweol via inhibiting Akt/JNK1/2/p38 MAPK signal pathway and NF-kappaB dependent transcriptional activities. Food Chem. Toxicol. 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.M. Effect of Lapachol on the Inhibition of Matrix Metalloproteinase Related to the Invasion of Human Fibrosarcoma Cells. Curr. Mol. Pharmacol. 2021, 14, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kong, C.S.; Seo, Y. Salidroside, 8(E)-Nuezhenide, and Ligustroside from Ligustrum japonicum Fructus Inhibit Expressions of MMP-2 and -9 in HT 1080 Fibrosarcoma. Int. J. Mol. Sci. 2022, 23, 2660. [Google Scholar] [CrossRef]
- Jo, H.W.; Kim, M.M. beta-Caryophyllene oxide inhibits metastasis by downregulating MMP-2, p-p38 and p-ERK in human fibrosarcoma cells. J. Food Biochem. 2022, 46, e14468. [Google Scholar] [CrossRef]
- Jo, A.I.; Kim, M.M. Silibinin Inhibits Cell Invasion through the Inhibition of MMPs, p-p38, and IL-1beta in Human Fibrosarcoma Cells. Front. Biosci. (Landmark Ed.) 2023, 28, 64. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol-A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of u-PA, MMPs and their inhibitors by a novel nutrient mixture in adult human sarcoma cell lines. Int. J. Oncol. 2013, 43, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yue, G.G.; Lee, J.K.; Gao, S.; Yuen, K.K.; Cheng, W.; Li, X.; Lau, C.B. Scutellarin, a flavonoid compound from Scutellaria barbata, suppresses growth of breast cancer stem cells in vitro and in tumor-bearing mice. Phytomedicine 2024, 128, 155418. [Google Scholar] [CrossRef] [PubMed]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Karadeniz, F.; Ahn, B.N.; Kong, C.S. Evaluation of Effective MMP Inhibitors from Eight Different Brown Algae in Human Fibrosarcoma HT1080 Cells. Prev. Nutr. Food Sci. 2015, 20, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adh. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Lee, S.G.; Seo, Y.; Kong, C.S. Inhibition of MMP-2 and MMP-9 Activities by Limonium tetragonum Extract. Prev. Nutr. Food Sci. 2016, 21, 38–43. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Kong, C.S. Inhibitory Effect of Hizikia fusiformis Solvent-Partitioned Fractions on Invasion and MMP Activity of HT1080 Human Fibrosarcoma Cells. Prev. Nutr. Food Sci. 2017, 22, 184–190. [Google Scholar] [CrossRef]
- Karadeniz, F.L.S.; Oh, J.H.; Kim, J.A.; Kong, C.S. Inhibition of MMP-2 and MMP-9 activities by solvent-partitioned Sargassum horneri extracts. Fish. Aquat. Sci. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Karadeniz, F.L.S.; Oh, J.H.; Yu, G.H.; Jang, M.S.; Seo, Y.; Kong, C.S. Solvent-partitioned fractions from Ishige okamurae extract inhibit MMP-2 and MMP-9 activities in human fibrosarcoma cells in vitro. J. Appl. Phycol. 2018, 30, 121–127. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sak, K.; Adhikary, S.; Kaur, G.; Aggarwal, D.; Kaur, J.; Kumar, M.; Parashar, N.C.; Parashar, G.; Sharma, U.; et al. Galangin: A metabolite that suppresses anti-neoplastic activities through modulation of oncogenic targets. Exp. Biol. Med. 2022, 247, 345–359. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Huang, B. The genus Hippocampus—A review on traditional medicinal uses, chemical constituents and pharmacological properties. J. Ethnopharmacol. 2015, 162, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H.; et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Mendes Miranda, S.E.; Alcantara Lemos, J.; Fernandes, R.S.; Silva, J.O.; Ottoni, F.M.; Townsend, D.M.; Rubello, D.; Alves, R.J.; Cassali, G.D.; Ferreira, L.A.M.; et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed. Pharmacother. 2021, 133, 110936. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ray, P.P.; Islam, M.A.; Islam, M.S.; Han, A.; Geng, P.; Aziz, M.A.; Mamun, A.A. A comprehensive evaluation of the therapeutic potential of silibinin: A ray of hope in cancer treatment. Front. Pharmacol. 2024, 15, 1349745. [Google Scholar] [CrossRef]
- Tavakoli, J.; Miar, S.; Majid Zadehzare, M.; Akbari, H. Evaluation of effectiveness of herbal medication in cancer care: A review study. Iran. J. Cancer Prev. 2012, 5, 144–156. [Google Scholar] [PubMed]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Monterrey, J.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in adult human sarcoma cell lines by cytokines, inducers and inhibitors. Int. J. Oncol. 2013, 43, 1787–1798. [Google Scholar] [CrossRef]
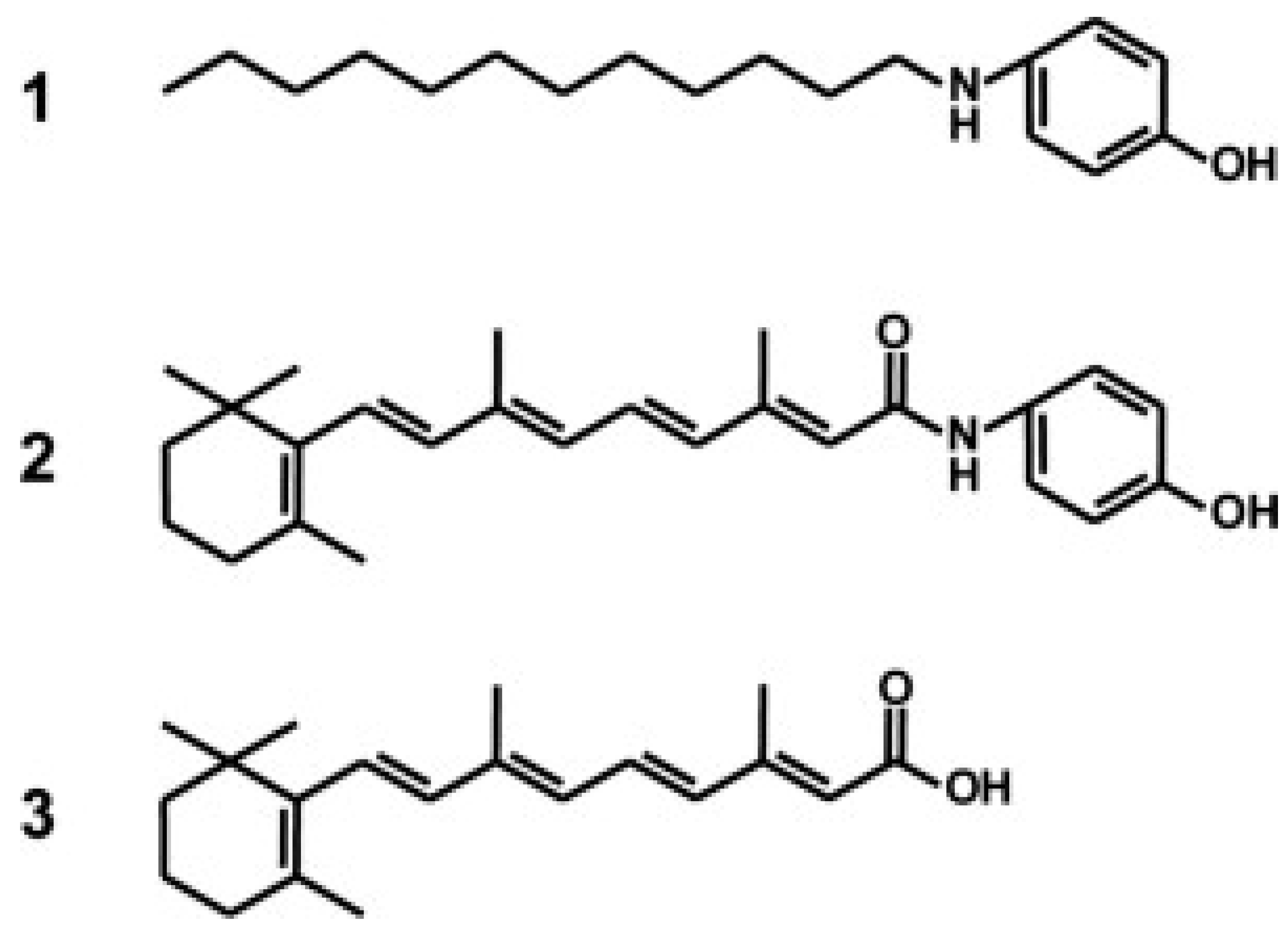
- Takahashi, N.; Watanabe, Y.; Maitani, Y.; Yamauchi, T.; Higashiyama, K.; Ohba, T. p-Dodecylaminophenol derived from the synthetic retinoid, fenretinide: Antitumor efficacy in vitro and in vivo against human prostate cancer and mechanism of action. Int. J. Cancer 2008, 122, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Takeda, K.; Imai, M. Inhibitory effects of p-dodecylaminophenol on the invasiveness of human fibrosarcoma cell line HT1080. Bioorg Med. Chem. 2013, 21, 6015–6021. [Google Scholar] [CrossRef] [PubMed]
- Nanjan, P.; Nambiar, J.; Nair, B.G.; Banerji, A. Synthesis and discovery of (I-3,II-3)-biacacetin as a novel non-zinc binding inhibitor of MMP-2 and MMP-9. Bioorg Med. Chem. 2015, 23, 3781–3787. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.; Dai, X. A Review of the Preparation, Analysis and Biological Functions of Chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef]
- Hong, S.; Ngo, D.N.; Kim, M.M. Inhibitory effect of aminoethyl-chitooligosaccharides on invasion of human fibrosarcoma cells. Environ. Toxicol. Pharmacol. 2016, 45, 309–314. [Google Scholar] [CrossRef] [PubMed]
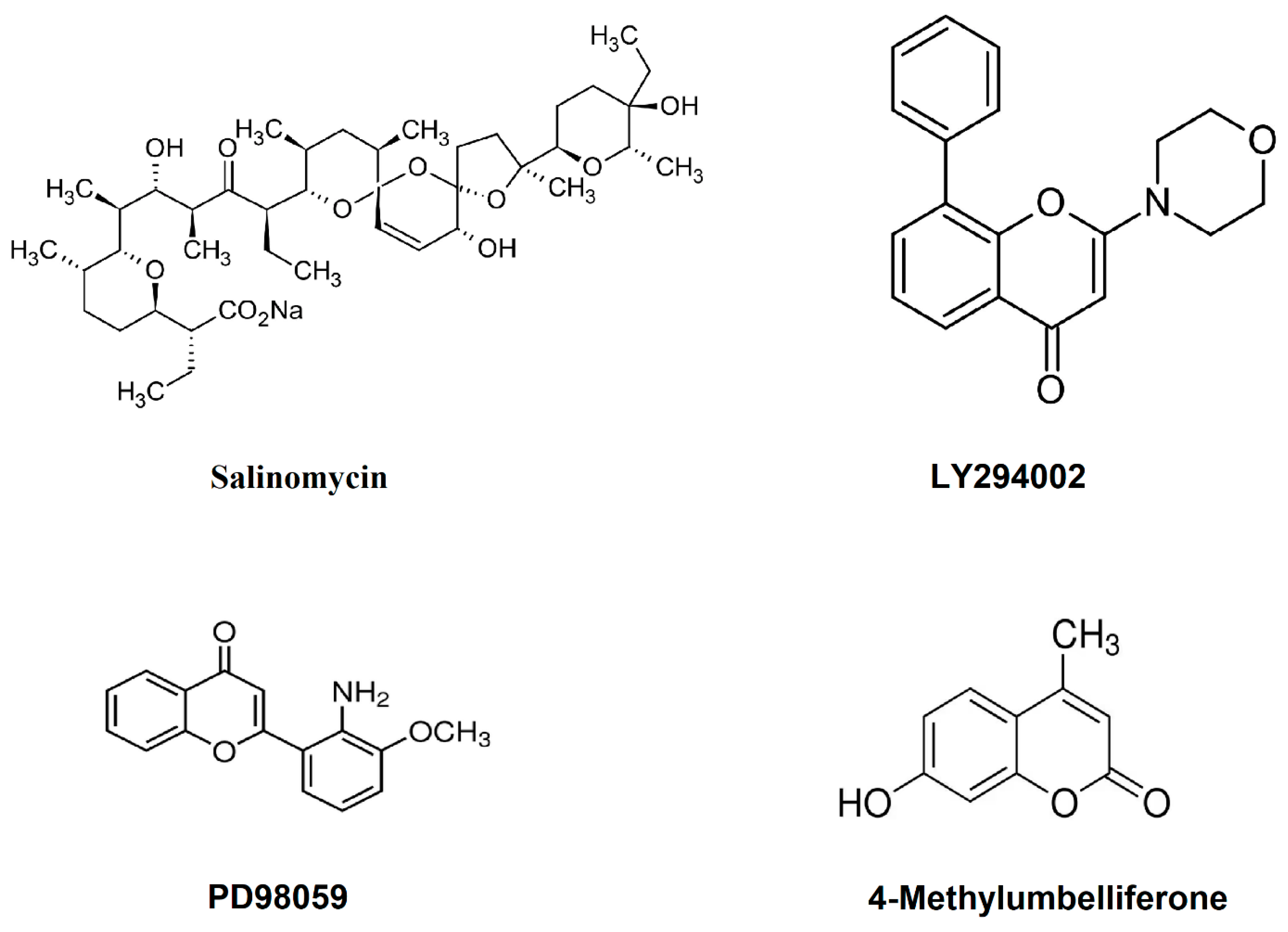
- Bar, J.; Lukaschuk, N.; Zalcenstein, A.; Wilder, S.; Seger, R.; Oren, M. The PI3K inhibitor LY294002 prevents p53 induction by DNA damage and attenuates chemotherapy-induced apoptosis. Cell Death Differ. 2005, 12, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hou, Y.; Gu, C.; Zhao, D.; Duan, Y.; Ran, Z.; Li, Q.; Li, X. Inhibitory effect of the mitogen activated protein kinase specific inhibitor PD98059 on Mtb-Ag-activated gammadeltaTau cells. Int. J. Clin. Exp. Pathol. 2017, 10, 9644–9648. [Google Scholar]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. SB203580 Modulates p38 MAPK Signaling and Dengue Virus-Induced Liver Injury by Reducing MAPKAPK2, HSP27, and ATF2 Phosphorylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, S.J. Salinomycin causes migration and invasion of human fibrosarcoma cells by inducing MMP-2 expression via PI3-kinase, ERK-1/2 and p38 kinase pathways. Int. J. Oncol. 2016, 48, 2686–2692. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Saga, R.; Monzen, S.; Chiba, M.; Yoshino, H.; Nakamura, T.; Hosokawa, Y. Anti-tumor and anti-invasion effects of a combination of 4-methylumbelliferone and ionizing radiation in human fibrosarcoma cells. Oncol. Lett. 2017, 13, 410–416. [Google Scholar] [CrossRef]
- Alford, V.M.; Kamath, A.; Ren, X.; Kumar, K.; Gan, Q.; Awwa, M.; Tong, M.; Seeliger, M.A.; Cao, J.; Ojima, I.; et al. Targeting the Hemopexin-like Domain of Latent Matrix Metalloproteinase-9 (proMMP-9) with a Small Molecule Inhibitor Prevents the Formation of Focal Adhesion Junctions. ACS Chem. Biol. 2017, 12, 2788–2803. [Google Scholar] [CrossRef]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2021, 12, 48. [Google Scholar] [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm (2020) 2023, 4, e265. [Google Scholar] [CrossRef]
- Beck, H.; Harter, M.; Hass, B.; Schmeck, C.; Baerfacker, L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov. Today 2022, 27, 1560–1574. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.S.; Gunassekaran, G.R.; Lee, S.M.; Yoon, J.W.; Lee, Y.K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Mirzaei, H.; Kazemi, B.; Bandehpour, M.; Shoari, A.; Asgary, V.; Ardestani, M.S.; Madadkar-Sobhani, A.; Cohan, R.A. Computational and nonglycosylated systems: A simpler approach for development of nanosized PEGylated proteins. Drug Des. Devel Ther. 2016, 10, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Eom, T.K.; Byun, H.G. Inhibitory effect of the carnosine-gallic acid synthetic peptide on MMP-2 and MMP-9 in human fibrosarcoma HT1080 cells. J. Pept. Sci. 2014, 20, 716–724. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Wang, W.C.; Kuo, W.L.; Chen, H.Y.; Chen, T.C.; Hamann, J.; Lin, H.H. CD97 inhibits cell migration in human fibrosarcoma cells by modulating TIMP-2/MT1- MMP/MMP-2 activity--role of GPS autoproteolysis and functional cooperation between the N- and C-terminal fragments. FEBS J. 2014, 281, 4878–4891. [Google Scholar] [CrossRef]
- Ko, S.C.; Heo, S.Y.; Choi, S.W.; Qian, Z.J.; Heo, S.J.; Kang, D.H.; Kim, N.; Jung, W.K. A heptameric peptide isolated from the marine microalga suppresses PMA-induced secretion of matrix metalloproteinase-9 through the inactivation of the JNK, p38, and NF-κB pathways in human fibrosarcoma cells. J. Appl. Phycol. 2018, 30, 2367–2378. [Google Scholar] [CrossRef]
- Zani, M.L.; Moreau, T. Phage display as a powerful tool to engineer protease inhibitors. Biochimie 2010, 92, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Rasaee, M.J.; Kanavi, M.R.; Daraei, B. Functional mimetic peptide discovery isolated by phage display interacts selectively to fibronectin domain and inhibits gelatinase. J. Cell Biochem. 2019, 120, 19699–19711. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Khalili, S.; Rasaee, M.J.; Löwik, D.W.P.M. A Phage Display Derived Cyclized Peptide Inhibits Fibrosarcoma Cells Invasion via Suppression of MMP-9 Activity. Int. J. Pept. Res. Ther. 2022, 28, 136. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
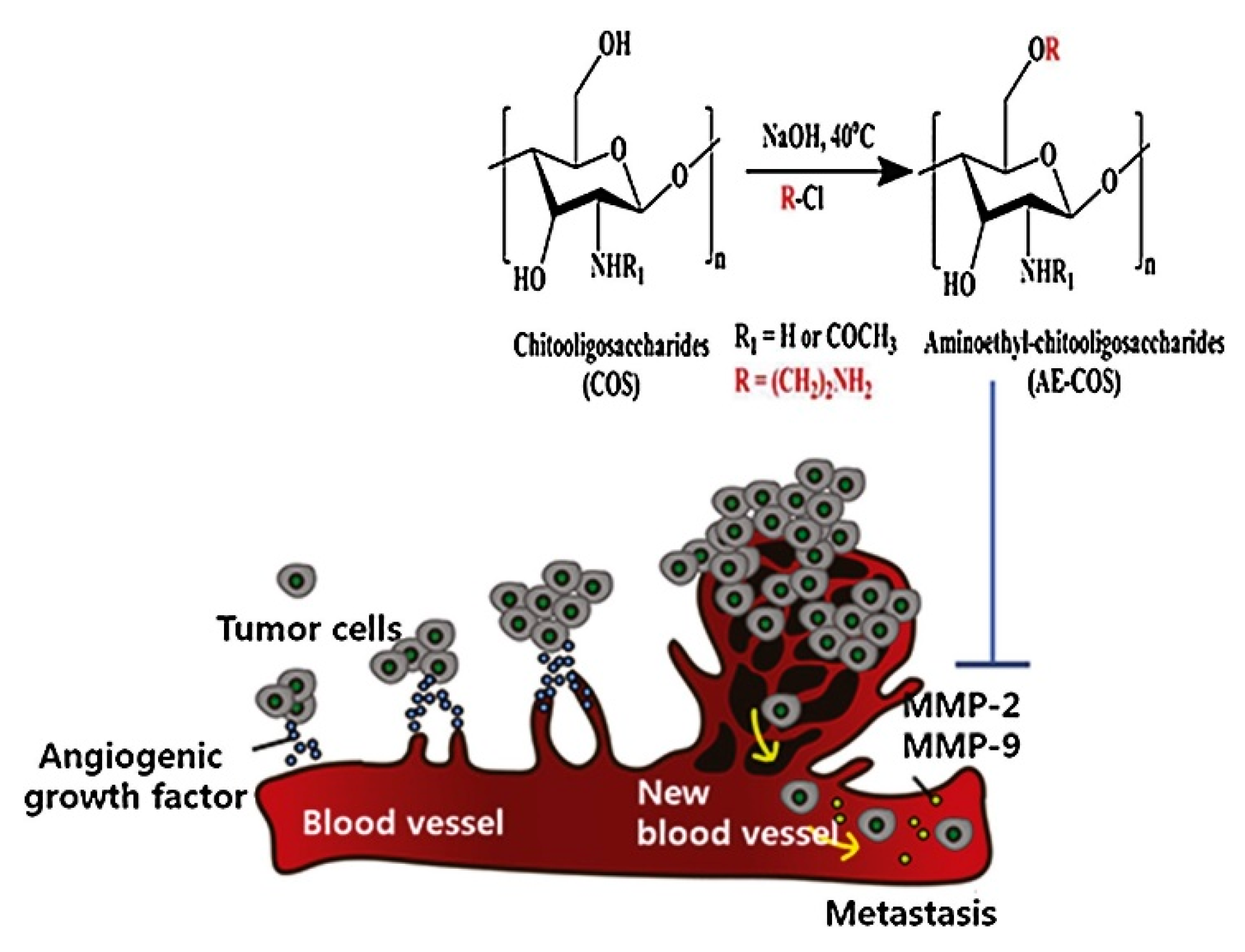
| Name | Source | Structure | Function | Ref. |
|---|---|---|---|---|
| Eugenol | Cloves | 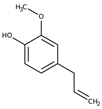 | Inhibited MMP-9 activities related to metastasis in PMA-stimulated HT-1080 cells | [47] |
| Fisetin | Dalbergia odorifera | 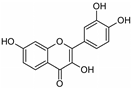 | Inhibited MMP-9 more efficiently than a naturally occurring MMP inhibitor, tetracycline; dose-dependently inhibits proliferation of fibrosarcoma HT-1080 cells | [48] |
| Quercetin | Polyphenol found abundantly in a variety of foods | 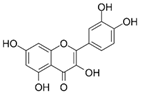 | Inhibited PMS-induced increases in cell motility in HT-1080 cells; attenuated PMS-induced MMP-2 activation | [49] |
| Gallic acid, delphinidin-3-glucoside | Polyphenols found in a variety of foods | 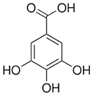 | Anti-invasive activity; inhibitory activity on secreted and activated gelatinases; inhibited MMP-2 and MMP-9 proteolytic activity | [50] |
| Scutellarein | Scutellaria lateriflora |  | Proliferation rate of cells was significantly suppressed; volume and weight of the tumors were markedly reduced; potently inhibited cell migration, invasion, and the expression and activity of MMP-2 and MMP-9 | [51] |
| Galangin, kaempferol | Flavonoids |  | Efficiently decreased MMP-9 secretion; decreased transcription of MMP-9 mRNA | [52] |
| p- Cymene | Plants | 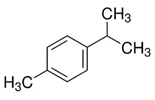 | Dose-dependently inhibited the TPA-augmented production and gene expression of MMP-9; enhanced the TPA-augmented production and gene expression of TIMP-1; TPA-augmented invasiveness was inhibited | [53] |
| SE1 | Hippocampus kuda Bleeler | 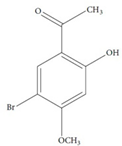 | Potently inhibited gelatin digestion by MMP-9 induced by PMA; inhibited migration of HT-1080 cells in a dose-dependent manner | [54] |
| Chlorophyllin | Plants and some algae | 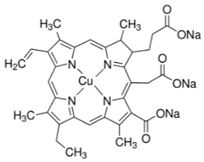 | Cell proliferation was significantly decreased; expression of MMP-2 and MMP-9 decreased; cell invasion through Matrigel and cell migration were also reduced | [55] |
| Kahweol | Coffee | 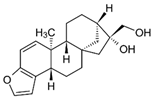 | Inhibited cell proliferation enhanced by PMA; attenuated PMA-induced cell migration; suppressed PMA-enhanced activation of MMP-9 | [56] |
| Lapachol | Bignoniaceae | 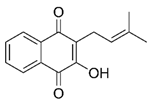 | Inhibited the activation of MMP-2 and MMP-9 stimulated by PMA; protein and gene expression levels of MMP-2 remarkably decreased; increased the expression level of TIMP-1 | [57] |
| Salidroside, 8(E)-nuezhenide, ligustroside | Ligustrum japonicum | 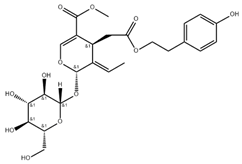 | Significantly lowered the amount of MMP-2 and MMP-9 released; RNA and protein expression levels of MMP-2 and MMP-9 were notably suppressed | [58] |
| β-Caryophyllene oxide | Piper nigrum |  | Reduced MMP-2 and MMP-9 activity; expression level of MMP-2 was reduced; reduced cell invasion | [59] |
| Silibinin | Silybum marianum | 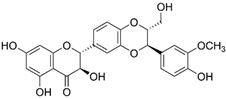 | Showed growth inhibitory effects on fibrosarcoma cells; remarkably inhibited the levels of MMP-2 and MMP-9 activation; inhibited cell invasion on HT-1080 cells | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoari, A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets 2024, 2, 104-125. https://doi.org/10.3390/targets2020007
Shoari A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets. 2024; 2(2):104-125. https://doi.org/10.3390/targets2020007
Chicago/Turabian StyleShoari, Alireza. 2024. "Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review" Targets 2, no. 2: 104-125. https://doi.org/10.3390/targets2020007
APA StyleShoari, A. (2024). Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets, 2(2), 104-125. https://doi.org/10.3390/targets2020007







