Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Definitions of Comorbidities
2.3. Diabetes Mortality
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
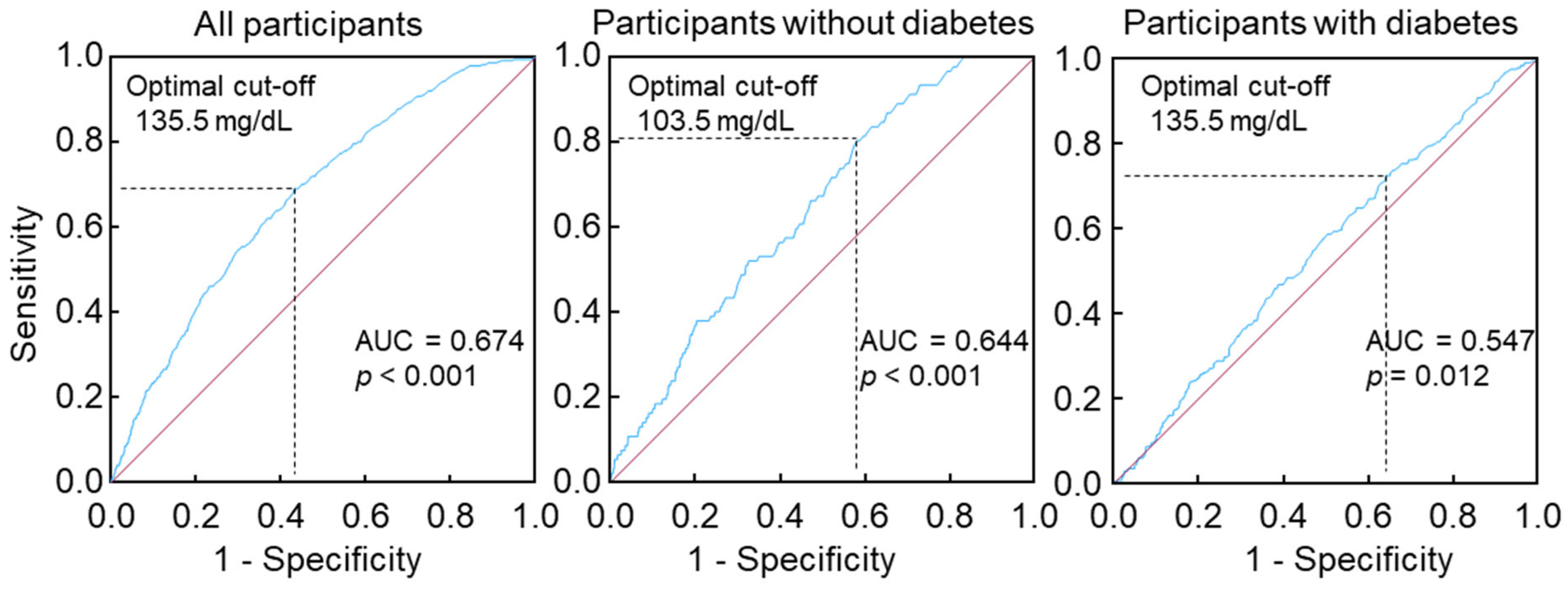
3.2. Association of Non-Fasting Triglycerides with Plasma Glucose, Blood Hemoglobin A1c, Serum Insulin, and Diabetes
3.3. Association of Non-Fasting Triglycerides with Diabetes Mortality
3.4. Sensitivtiy Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Diabetes. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 31 August 2023).
- Centers for Disease Control and Prevention. Diabetes. 2024. Available online: https://www.cdc.gov/nchs/fastats/diabetes.htm (accessed on 17 May 2024).
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras-Roubin, S.; Rosselló, X.; Oliva, B.; Fernández-Friera, L.; Mendiguren, J.M.; Andrés, V.; Bueno, H.; Sanz, J.; Martínez de Vega, V.; Abu-Assi, E.; et al. Triglycerides and Residual Atherosclerotic Risk. J. Am. Coll. Cardiol. 2021, 77, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Magliano, D.J.; Charchar, F.J.; Sobey, C.G.; Drummond, G.R.; Golledge, J. Fasting triglycerides are positively associated with cardiovascular mortality risk in people with diabetes. Cardiovasc. Res. 2023, 119, 826–834. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y. Fasting status modifies the association between triglyceride and all-cause mortality: A cohort study. Health Sci. Rep. 2022, 5, e642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Higher fasting triglyceride predicts higher risks of diabetes mortality in US adults. Lipids Health Dis. 2021, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association, 2. 10. American Diabetes Association, 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care. [CrossRef]
- Liu, L.; Miura, K.; Kadota, A.; Fujiyoshi, A.; Gracely, E.J.; Xue, F.; Liu, Z.; Takashima, N.; Miyagawa, N.; Ohkubo, T.; et al. The impact of sex on risk of cardiovascular disease and all-cause mortality in adults with or without diabetes mellitus: A comparison between the U.S. and Japan. J. Diabetes Complicat. 2019, 33, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, I.; Martín-Nieto, A.; Martínez, R.; Casanovas-Marsal, J.O.; Aguayo, A.; Del Olmo, J.; Arana, E.; Fernandez-Rubio, E.; Castaño, L.; Gaztambide, S. Incidence of diabetes mellitus and associated risk factors in the adult population of the Basque country, Spain. Sci. Rep. 2021, 11, 3016. [Google Scholar] [CrossRef]
- Al-Mawali, A.; Al-Harrasi, A.; Jayapal, S.K.; Morsi, M.; Pinto, A.D.; Al-Shekaili, W.; Al-Kharusi, H.; Al-Balushi, Z.; Idikula, J. Prevalence and risk factors of diabetes in a large community-based study in the Sultanate of Oman: STEPS survey 2017. BMC Endocr. Disord. 2021, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Dyck, R.; Janzen, B.; Karunanayake, C.; Dosman, J.; Pahwa, P. Risk factors, incidence, and prevalence of diabetes among rural farm and non-farm residents of Saskatchewan, Canada; A population-based longitudinal cohort study. J. Diabetes Metab. Disord. 2020, 19, 1563–1582. [Google Scholar] [CrossRef]
- Wang, Y. Stage 1 hypertension and risk of cardiovascular disease mortality in United States adults with or without diabetes. J. Hypertens. 2022, 40, 794–803. [Google Scholar] [CrossRef]
- Turner, N. Chi-squared test. J. Clin. Nurs. 2000, 9, 93. [Google Scholar] [PubMed]
- Elkahwagy, D.; Kiriacos, C.J. Logistic regression and other statistical tools in diagnostic biomarker studies. Clin. Transl. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Brancato, D.; Saura, G.; Fleres, M.; Ferrara, L.; Scorsone, A.; Aiello, V.; Di Noto, A.; Spano, L.; Provenzano, V. Prognostic accuracy of continuous glucose monitoring in the prediction of diabetes mellitus in children with incidental hyperglycemia: Receiver operating characteristic analysis. Diabetes Technol. Ther. 2013, 15, 580–585. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y. Postabsorptive homeostasis model assessment for insulin resistance is a reliable biomarker for cardiovascular disease mortality and all-cause mortality. Diabetes Epidemiol. Manag. 2021, 6, 100045. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Hu, N.; Liu, Y.; Wang, H.; Wang, Z.; Zhang, K.; Wang, L.; Chen, B.; Wu, F.; Rong, W.; et al. Long-term outcome of adjuvant radiotherapy upon postoperative relapse of centrally located hepatocellular carcinoma: A real-world study. Sci. Rep. 2024, 14, 8506. [Google Scholar] [CrossRef]
- Wang, Y. Fasting triglycerides in the upper normal range are independently associated with an increased risk of diabetes mortality in a large representative US population. J. Cardiovasc. Dev. Dis. 2024, 11, 128. [Google Scholar] [CrossRef]
- Aung, H.H.; Lame, M.W.; Gohil, K.; An, C.I.; Wilson, D.W.; Rutledge, J.C. Induction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2088–2096. [Google Scholar] [CrossRef]
- Pradhan, A.D. A New Beginning for Triglyceride-Lowering Therapies. Circulation 2019, 140, 167–169. [Google Scholar] [CrossRef]
- Tirosh, A.; Shai, I.; Bitzur, R.; Kochba, I.; Tekes-Manova, D.; Israeli, E.; Shochat, T.; Rudich, A. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care 2008, 31, 2032–2037. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wei, F.; Song, J.; Cao, Z.; Chen, C.; Zhang, K.; Feng, S.; Li, W.-D. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: A prospective study with 8-year follow-ups in two cohorts. J. Transl. Med. 2019, 17, 403. [Google Scholar] [CrossRef] [PubMed]
- Beshara, A.; Cohen, E.; Goldberg, E.; Lilos, P.; Garty, M.; Krause, I. Triglyceride levels and risk of type 2 diabetes mellitus: A longitudinal large study. J. Investig. Med. 2016, 64, 383–387. [Google Scholar] [CrossRef]
- Guo, R.; Wei, L.; Cao, Y.; Zhao, W. Normal triglyceride concentration and the risk of diabetes mellitus type 2 in the general population of China. Front. Endocrinol. 2024, 15, 1330650. [Google Scholar] [CrossRef]
- Szili-Torok, T.; Bakker, S.J.L.; Tietge, U.J.F. Normal fasting triglyceride levels and incident type 2 diabetes in the general population. Cardiovasc. Diabetol. 2022, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Nakagawa, Y.; Oishi, A.; Han, S.I.; Kumagai, K.; Ohno, H.; Mizunoe, Y.; Iwasaki, H.; Sekiya, M.; Matsuzaka, T.; et al. The peroxisome proliferator-activated receptor α (PPARα) agonist pemafibrate protects against diet-induced obesity in mice. Int. J. Mol. Sci. 2018, 19, 2148. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, A.; Motro, M.; Fisman, E.Z.; Schwammenthal, E.; Adler, Y.; Goldenberg, I.; Leor, J.; Boyko, V.; Mandelzweig, L.; Behar, S. Peroxisome proliferator–activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004, 109, 2197–2202. [Google Scholar] [CrossRef]
- The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Hoy, A.J.; Brandon, A.E.; Turner, N.; Watt, M.J.; Bruce, C.R.; Cooney, G.J.; Kraegen, E.W. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E67–E75. [Google Scholar] [CrossRef] [PubMed]
- Høeg, L.D.; Sjøberg, K.A.; Jeppesen, J.; Jensen, T.E.; Frøsig, C.; Birk, J.B.; Bisiani, B.; Hiscock, N.; Pilegaard, H.; Wojtaszewski, J.F.P.; et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes 2010, 60, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, S.; Lee, M.; Yoon, M. Fenofibrate alleviates insulin resistance by reducing tissue inflammation in obese ovariectomized mice. Nutr. Diabetes 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; Ferranti, S.d.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Vrablik, M. Homeostasis model assessment for insulin resistance mediates the positive association of triglycerides with diabetes. Diagnostics 2024, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Al-Shali, K.Z.; Hegele, R.A. Hypertriglyceridemia: Its etiology, effects and treatment. CMAJ 2007, 176, 1113–1120. [Google Scholar] [CrossRef]
- Simha, V. Management of hypertriglyceridemia. BMJ 2020, 371, m3109. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.ø.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Triglyceride concentration and ischemic heart disease. Circulation 1998, 97, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Tikhonoff, V.; Casiglia, E.; Virdis, A.; Grassi, G.; Angeli, F.; Arca, M.; Barbagallo, C.M.; Bombelli, M.; Cappelli, F.; Cianci, R.; et al. Prognostic value and relative cutoffs of triglycerides predicting cardiovascular outcome in a large regional-based italian database. J. Am. Heart Assoc. 2024, 13, e030319. [Google Scholar] [CrossRef]
- Klempfner, R.; Erez, A.; Sagit, B.-Z.; Goldenberg, I.; Fisman, E.; Kopel, E.; Shlomo, N.; Israel, A.; Tenenbaum, A. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 100–108. [Google Scholar] [CrossRef]
- MacMahon, S.; Peto, R.; Cutler, J.; Collins, R.; Sorlie, P.; Neaton, J.; Abbott, R.; Godwin, J.; Dyer, A.; Stamler, J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 1990, 335, 765–774. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics; Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index—2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. 2019. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm (accessed on 9 July 2021).
- Menke, A.; Muntner, P.; Batuman, V.; Silbergeld, E.K.; Guallar, E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation 2006, 114, 1388–1394. [Google Scholar] [CrossRef]


| All | Without Diabetes | With Diabetes | p Value | |
|---|---|---|---|---|
| Participant number | 7312 | 6132 | 1180 | NA |
| Non-fasting triglycerides, mg/dL, mean (SD) | 159 (126) | 147 (108) | 220 (180) | <0.001 |
| Age, y, mean (SD) | 50 (19) | 48 (19) | 62 (14) | <0.001 |
| PG, mg/dL, mean (SD) | 107 (49) | 94 (13) | 176 (93) | <0.001 |
| HbA1c, %, mean (SD) | 5.7 (1.3) | 5.3 (0.5) | 7.8 (1.9) | <0.001 |
| Insulin, µU/mL, mean (SD) | 17.5 (39.6) | 13.4 (15.3) | 39.2 (89.1) | |
| Gender (male), % | 46.7 | 46.9 | 46.0 | 0.58 |
| Ethnicity, % | ||||
| Hispanic | 27.5 | 27.2 | 29.6 | <0.001 |
| Non-Hispanic white | 44.3 | 45.7 | 36.9 | |
| Non-Hispanic black | 25.9 | 24.9 | 31.2 | |
| Other | 2.3 | 2.3 | 2.3 | |
| Obesity, % | ||||
| Underweight | 2.2 | 2.5 | 0.6 | <0.001 |
| Normal | 35.3 | 38.6 | 17.8 | |
| Overweight | 35.0 | 34.7 | 36.4 | |
| Obese | 26.7 | 23.5 | 43.4 | |
| Unknown | 0.8 | 0.7 | 1.8 | |
| Poverty–income ratio, % | ||||
| <130% | 29.9 | 28.7 | 35.7 | <0.001 |
| 130%–349% | 38.5 | 38.7 | 37.4 | |
| ≥350% | 22.3 | 23.6 | 15.3 | |
| Unknown | 9.4 | 8.9 | 11.7 | |
| Education status, % | ||||
| <High School | 40.7 | 37.6 | 56.9 | <0.001 |
| High School | 29.0 | 30.0 | 24.0 | |
| >High School | 30.2 | 32.4 | 19.1 | |
| Physical activity, % | ||||
| Inactive | 32.3 | 34.1 | 23.3 | <0.001 |
| Insufficiently active | 38.9 | 39.5 | 35.6 | |
| Active | 28.8 | 26.5 | 41.1 | |
| Alcohol consumption, % | <0.001 | |||
| 0 drink/week | 18.7 | 16.5 | 30.2 | |
| <1 drink/week | 12.2 | 12.9 | 8.3 | |
| 1–6 drinks/week | 17.7 | 19.6 | 7.8 | |
| ≥7 drinks/week | 12.1 | 13.3 | 5.9 | |
| Unknown | 39.4 | 37.8 | 47.8 | |
| Cigarette smoker, % | 51.4 | 51.1 | 53.5 | 0.13 |
| Hypercholesterolemia, % | 33.5 | 30.7 | 48.2 | <0.001 |
| Hypertension, % | 41.6 | 36.3 | 69.6 | <0.001 |
| Diabetes, % | 16.1 | 0 | 100 | <0.001 |
| Family diabetes history, % | 44.0 | 40.3 | 63.3 | <0.001 |
| All Participants (n = 7312) | Without Diabetes (n = 6132) | With Diabetes (n = 1180) | ||||
|---|---|---|---|---|---|---|
| β | p Value | β | p Value | β | p Value | |
| Unadjusted | ||||||
| Plasma glucose | 0.258 | <0.001 | 0.170 | <0.001 | 0.191 | <0.001 |
| Hemoglobin A1c | 0.257 | <0.001 | 0.145 | <0.001 | 0.151 | <0.001 |
| Serum insulin | 0.396 | <0.001 | 0.362 | <0.001 | 0.267 | <0.001 |
| Adjusted 2 | ||||||
| Plasma glucose | 0.106 | <0.001 | 0.087 | <0.001 | 0.235 | <0.001 |
| Hemoglobin A1c | 0.067 | <0.001 | 0.051 | <0.001 | 0.163 | <0.001 |
| Serum insulin | 0.286 | <0.001 | 0.318 | <0.001 | 0.247 | <0.001 |
| All (n = 7312) | Without Diabetes (n = 6132) | With Diabetes (n = 1180) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Model 1 | 2.50 | 2.17–2.87 | <0.001 | 2.29 | 1.69–3.11 | <0.001 | 1.20 | 1.01–1.42 | 0.038 |
| Model 2 | 2.32 | 1.98–2.72 | <0.001 | 1.87 | 1.32–2.65 | <0.001 | 1.31 | 1.08–1.57 | 0.005 |
| Model 3 | 2.21 | 1.87–2.61 | <0.001 | 1.68 | 1.17–2.43 | 0.006 | 1.37 | 1.13–1.65 | 0.001 |
| Model 4 | 2.14 | 1.81–2.53 | <0.001 | 1.65 | 1.15–2.39 | 0.007 | 1.35 | 1.12–1.64 | 0.002 |
| Model 5 | 1.41 | 1.19–1.67 | <0.001 | 1.62 | 1.10–2.38 | 0.014 | 1.33 | 1.10–1.61 | 0.004 |
| All (n = 7312) | Without Diabetes (n = 6132) | With Diabetes (n = 1180) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Model 1 | 3.07 | 2.50–3.78 | <0.001 | 3.24 | 1.94–5.43 | <0.001 | 1.33 | 1.05–1.70 | 0.020 |
| Model 2 | 2.26 | 1.83–2.79 | <0.001 | 2.18 | 1.28–3.69 | 0.004 | 1.31 | 1.02–1.68 | 0.035 |
| Model 3 | 2.04 | 1.64–2.54 | <0.001 | 1.93 | 1.12–3.33 | 0.017 | 1.39 | 1.07–1.80 | 0.014 |
| Model 4 | 2.00 | 1.61–2.48 | <0.001 | 1.93 | 1.12–3.32 | 0.018 | 1.40 | 1.08–1.82 | 0.011 |
| Model 5 | 1.37 | 1.10–1.72 | 0.006 | 1.86 | 1.07–3.23 | 0.028 | 1.38 | 1.06–1.79 | 0.017 |
| Model 1 | Model 1 with Further Adjustment for Total Cholesterol 2 | Model 1 with Further Adjustment for Total Cholesterol + HDL 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Q1 | HR = 1 (reference) | HR = 1 (reference) | HR = 1 (reference) | ||||||
| Q2 | 1.72 | 1.03–2.87 | 0.040 | 1.72 | 1.02–2.88 | 0.040 | 1.72 | 1.02–2.89 | 0.041 |
| Q3 | 1.90 | 1.13–3.19 | 0.016 | 1.90 | 1.13–3.19 | 0.016 | 1.90 | 1.12–3.22 | 0.017 |
| Q4 | 1.92 | 1.16–3.18 | 0.011 | 1.92 | 1.16–3.19 | 0.011 | 1.93 | 1.14–3.24 | 0.014 |
| Q5 | 2.41 | 1.46–3.97 | <0.001 | 2.41 | 1.45–4.01 | <0.001 | 2.42 | 1.40–4.17 | 0.001 |
| Model 1 | Model 1 with Further Adjustment for Total Cholesterol 2 | Model 1 with Further Adjustment for Total Cholesterol + HDL 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Q1 | HR = 1 (reference) | HR = 1 (reference) | HR = 1 (reference) | ||||||
| Q2 | 2.96 | 0.84–10.39 | 0.090 | 2.87 | 0.82–10.08 | 0.100 | 1.33 | 0.89–1.97 | 0.160 |
| Q3 | 4.14 | 1.22–14.06 | 0.023 | 3.90 | 1.15–13.29 | 0.030 | 1.25 | 0.84–1.88 | 0.275 |
| Q4 | 3.22 | 0.92–11.23 | 0.067 | 2.97 | 0.85–10.40 | 0.089 | 1.59 | 1.05–2.42 | 0.030 |
| Q5 | 4.84 | 1.42–16.54 | 0.012 | 4.26 | 1.23–14.78 | 0.022 | 1.82 | 1.16–2.85 | 0.009 |
| Model 1 | Model 1 with Further Adjustment for Total Cholesterol 2 | Model 1 with Further Adjustment for Total Cholesterol + HDL 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Q1 | HR = 1 (reference) | HR = 1 (reference) | HR = 1 (reference) | ||||||
| Q2 | 1.32 | 0.89–1.94 | 0.164 | 1.32 | 0.89–1.94 | 0.167 | 1.33 | 0.89–1.97 | 0.160 |
| Q3 | 1.23 | 0.83–1.81 | 0.299 | 1.24 | 0.84–1.82 | 0.287 | 1.25 | 0.84–1.88 | 0.275 |
| Q4 | 1.54 | 1.05–2.26 | 0.029 | 1.56 | 1.06–2.30 | 0.026 | 1.59 | 1.05–2.42 | 0.030 |
| Q5 | 1.74 | 1.18–2.55 | 0.005 | 1.77 | 1.19–2.62 | 0.005 | 1.82 | 1.16–2.85 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fang, Y.; Zhang, X.; Wu, N.-Q. Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population. Targets 2024, 2, 93-103. https://doi.org/10.3390/targets2020006
Wang Y, Fang Y, Zhang X, Wu N-Q. Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population. Targets. 2024; 2(2):93-103. https://doi.org/10.3390/targets2020006
Chicago/Turabian StyleWang, Yutang, Yan Fang, Xiulin Zhang, and Na-Qiong Wu. 2024. "Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population" Targets 2, no. 2: 93-103. https://doi.org/10.3390/targets2020006
APA StyleWang, Y., Fang, Y., Zhang, X., & Wu, N.-Q. (2024). Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population. Targets, 2(2), 93-103. https://doi.org/10.3390/targets2020006






