Microbial Influences on Amyotrophic Lateral Sclerosis: The Gut–Brain Axis and Therapeutic Potential of Microbiota Modulation
Abstract
1. Introduction
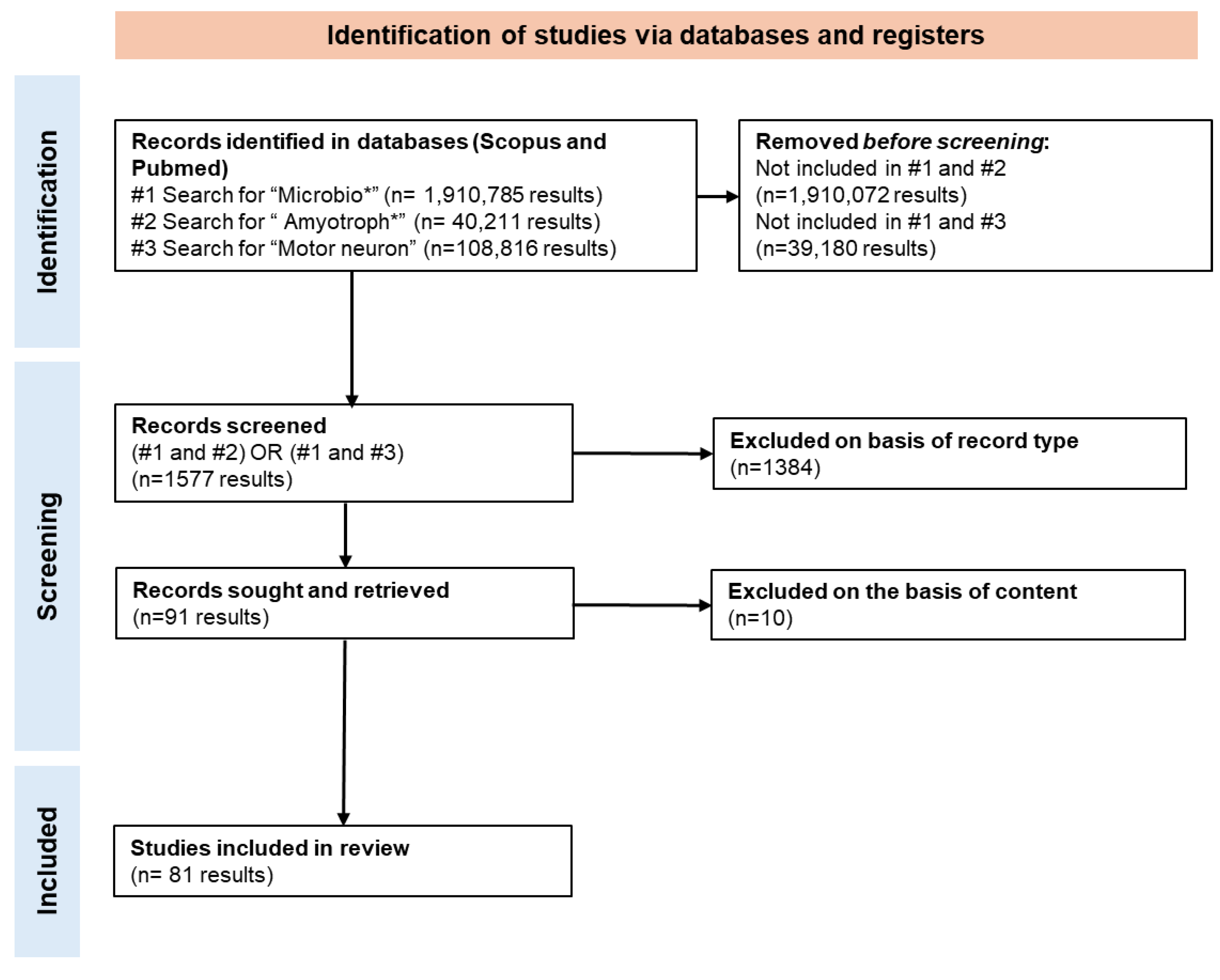
2. Methods
3. Results and Discussion
3.1. Gut Microbiota and ALS: Current Understanding
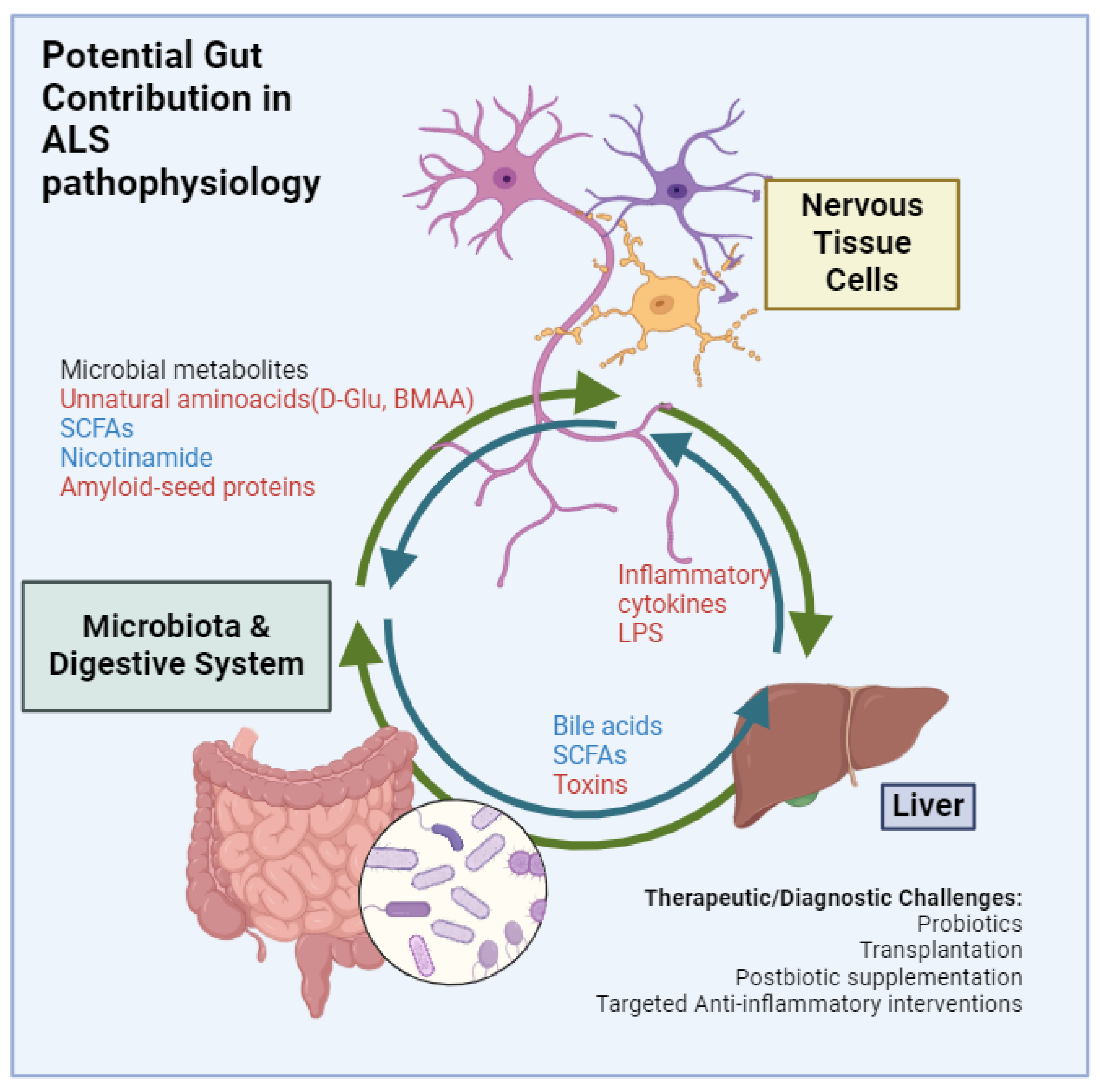
The Gut Microbiota’s Influence on Liver Health
3.2. Key Microbial Metabolites and Factors and Their Influence on ALS
| Metabolomic Change | Cases | Controls | Exclusion and Inclusion Criteria | Methodology | Geographical Background | Reference |
|---|---|---|---|---|---|---|
| ALS patients displayed a significantly higher serum load of Torque Tenoviurs-DNA compared to healthy controls, potentially suggesting enteral permeability changes; patients with ALS exhibited a higher total FFA level, while their SCFA level was generally lower; 10 out of 14 tested cytokines showed a lower expression in ALS patients compared to healthy controls and IL-8 (CXCL8) being more highly expressed in ALS patients. | 100 diagnosed ALS patients (35 females) | 34 controls among spouses (13 females) | Dementia or any other condition that compromised the ability to consent; known organic gastrointestinal disease; celiac disease and/or documented food intolerances; autoimmune disorders; severe comorbidities; history of complicated gastrointestinal surgery; and acute infections at the time of sampling | Torque Tenovirus load in serum determined by a real-time PCR assay; serum levels of fatty acids determined by GC/MS; serum cytokines by Milliplex MAP kits | Italy | Niccolai et al., 2024 [37] |
| The degree of upper motor neuron damage in the ALS group was inversely correlated with the plasma levels of carnitine, betaine, choline, and TMAO. ALS patients and their spouses exhibit disruptions in the gut microbiota’s TMAO metabolic pathway, which may indicate that the alterations in the gut microbiota took place before the development of the disease. | 160 patients with ALS (62 females) | 148 healthy controls | Exclusion of pregnant or breastfeeding women. Also, abnormal findings on electrodiagnostic, neurophysiologic, neuroimaging, or clinical laboratory studies that could not be explained by ALS, the presence of dementia, or psychiatric disorders; the presence of gastrointestinal disorders or the performance of gastrointestinal surgery that might affect gastrointestinal absorption; severe diseases of the heart, liver, kidney, or other organs; and treatment with antibiotics, L-carnitine, or intestinal flora regulation within 3 months before enrollment | TMAO and its precursors were quantified using stable isotope dilution liquid chromatography-tandem mass spectrometry | China | Chen et al., 2020 [38] |
| The fecal metabolome showed increased levels of specific amino acids and their metabolic products in patients with ALS after FMT, particularly in the arginine biosynthesis pathway. | 2 patients undergoing fecal microbial transplantation | 1 healthy donor | No specified | Non-targeted liquid chromatography–mass spectrometry | Japan | Yan et al., 2024 [19] |
| Fecal metabolites suggested changes in retrograde endocannabinoid signaling, inflammatory mediator regulation of the transient receptor potential channels, sphingolipid, nicotinamide, and thiamine metabolism in ALS. Several fecal metabolites differed between patients with cognitive impairment, including lower cholic acid and chenodeoxycholic acid, besides other metabolites. | 35 ALS patients (14 females) | 35 healthy controls, age- and sex-matched (14 females) | Any possible evidence for familial amyotrophic lateral sclerosis (fALS) with other neurodegenerative diseases with definite gastrointestinal diseases, heart failure, acute infection, tumor, immunodeficiency, and autoimmune diseases; any use of antibiotics within the last month, regular drinking of commercial probiotics within the previous year, or any drinking of commercial probiotics within the last week: obvious symptoms of dysphagia or dyspnea | Liquid chromatography–mass spectrometry (untargeted metabolomics) | China | Gong et al., 2022 [39] |
| Kynurenine metabolites related to ALS risk. | 20,806 cases of ALS | 59,804 controls (GWAS summary statistics from IALSC); 18,340 participants (GWAS summary statistics from MiBioGen); 7824 participants (GWAS summary statistics from TwinsUK and KORA) | Not specified | Not specified | Worldwide | Ning et al., 2022 [40] |
| Metabolomics in será in ALS suggest alterations in the tryptophan–nicotinamide metabolism as indole acetate, kynurenine, serotonin, and circulating nicotinamide. Other changes included increased levels of riluzole creatine and 3-hydroxy-2-ethyl propionate and reduced levels of methyl indole 3-acetate and triethanolamine in ALS patients. | 37 ALS patients (8 females) | 29 healthy, BMI- and age-matched family members (20 females) | Pregnancy or fertility treatments; use of antibiotics or antifungals in the three previous months; consumption of probiotics 1 month before; active inflammatory or neoplastic disease three years before enrolment; chronic gastrointestinal disorder, myocardial infarction, or cerebrovascular accident in the six months before participation; coagulation disorders; chronic immunosuppressive medication usage; or pre-diagnosed type I or type II diabetes mellitus or treatment with anti-diabetic medication | Metabolon-based profiling | Israel | Blacher et al., 2019 [41] |
| Microbiome-related variables in plasma (human endotoxin, SCFA, NO2-N/NO3-N, and γ-aminobutyric acid) showed some tendences in patients with ALS potentially compatible with dysbiosis. | 8 ALS patients (4 females) | 8 healthy controls (4 females) with no declared age, sex, or dietary regimes match | ALS-like illnesses, severe systemic disorders, and excessive eating or drinking throughout the previous two weeks | Non-targeted mass spectrometry for selected metabolites, according Yang et al. [42] | China | Zhai et al., 2019 [43] |
| Local (gut or oral) bacterial translocation was associated with more severe symptoms. | 36 Patients with bulbar or spinal ALS (24 spinal onset with 15 females; 12 bulbar onset with 8 females) | 20 healthy controls living with patients | Exposure to antibiotics/probiotics, immunocompromising illness/therapy, previous abdominal/anorectal surgery, GI-/respiratory-/gynecological-tract infection, food poisoning, or major epistaxis requiring treatment; active/persistent primary disease of the GI-/respiratory-/gynecological-tract, endocrinal disease, heart failure, severe renal-insufficiency, current pregnancy, drug/alcohol abuse, and active smoking within 6-months | Bacterial translocation to the blood was assessed by evaluating lipopolysaccharide binding protein (LBP) and 16S rRNA copies in the blood | USA | Kim et al., 2022 [44] |
| ALS was linked to specific lipids related to fatty acid and acylcarnitine metabolism by mendelian randomization assay. | 75 ALS patients (32 females) | 110 controls (66 females), matched for sex and age | For controls, neurodegenerative condition or family history of ALS | Metabolon-based profiling of serum. Metabolite–gut microbiome associations using weighted gene co-expression network analysis and two-way orthogonal partial least square with discriminant analysis | USA | Guo et al., 2024 [45] |
| ALS was associated with increased plasma formaldehyde. Trimethylamine and trimethylamine oxide did not show differences, but patients with increased formaldehyde levels also showed increased concentrations of trimethyl amine and its oxide. | 50 ALS patients (19 females) | 40 healthy controls (17 females) | Not specified | Plasma formaldehyde levels were quantified using a commercial kit. Trimethylamine and trimethylamine oxide in the plasma were quantified by multiple reactions monitoring mass spectrometry | Australia | Lee et al., 2019 [46] |
3.2.1. SCFAs, LPS, and Other Lipids
3.2.2. Microbiota Influence on Protein Aggregation in ALS: Potential Parallels in Bacterial Systems
3.2.3. β-Methylamino-L-Alanine (BMAA) and Other Amines as Postbiotic Neurotoxic Factors
3.2.4. Microbiota Differences in Spinal vs. Bulbar ALS
3.2.5. Impact of Dysphagia on Oral and Gut Microbiota
3.2.6. The Role of Antibiotics and Dietary Changes in ALS Progression
3.3. Therapeutic Potential of Microbiota Modulation
3.3.1. Probiotics and Prebiotics
3.3.2. Fecal Microbiota Transplantation (FMT)
3.3.3. Dietary Interventions
3.3.4. Targeting Specific Bacterial Metabolites
3.4. Current Gaps and Future Directions
3.4.1. Need for Larger and Longitudinal Studies
3.4.2. Heterogeneity in ALS Phenotypes and Microbiota Response
| Potential Microbiota Alterations in ALS | Cases | Controls | Exclusion Criteria | Methodology for Metagenome | Geographical Background | Reference |
|---|---|---|---|---|---|---|
| Decreased Firmicutes-to-Bacteroidetes ratio in ALS cases; increased Dorea; decreased Oscillibacter, Anaerostipes, Lachnospiraceae at genus level for ALS cases. | 6 ALS patients | 5 healthy controls, with apparently no matching in BMI, sex, or age | FVC 1 < 70%, mental illness or neurological disorders, or nocturnal hypoventilation | Bacterial 16S rRNA (V3–V4 region) sequencing for gut microbiome profiling | China | Fang et al., 2016 [62] |
| ALS cases showed decreased diversity, with 3 of 5 ALS patients having a low Firmicutes-to-Bacteroidetes ratio. | 5 ALS patients (4 females) | 96 healthy controls, with apparently no matching in BMI, sex, or age | Cases with concurrent intestinal diseases or abdominal symptoms | Bacterial 16S rRNA-based PCR with multiple primer design aimed at phylum- and class-level classification | USA | Rowin et al., 2017 [65] |
| A higher OTU number in cases, though indexes of neither alpha nor beta diversity differed significantly; only one OTU (uncultured Ruminococcaceae) at the genus level differed significantly. Authors concluded that ALS patients do not exhibit a substantial alteration of gut microbiota composition. | 25 ALS patients (13 females) | 32 healthy controls (16 females) matched for age and sex | Recent antibiotic use, neoplastic disease, autoimmune disease, gastrointestinal disorders, or active infections | Bacterial 16S rRNA sequencing (454 pyrosequencing) | Germany | Brenner et al., 2018 [26] |
| Increased Firmicutes-to-Bacteroidetes ratio in cases; ALS associated with increased Methanobrevibacter, and decreased Faecalibacterium and Bacteroides, at the genus level. | 8 ALS patients (4 females) | 8 healthy controls (4 females) with no declared age, sex, or dietary regimes match | ALS-like illnesses, severe systemic disorders, and excessive eating or drinking throughout the previous two weeks | Bacterial 16S rRNA (V4–V5 region) sequencing; unclear declaration of methods employed for statistical analyses | China | Zhai et al., 2019 [43] |
| Several microbiome differences between ALS cases and controls, with Bifidobacterium pseudocatenulatum being correlated with serum nicotinamide levels, with alterations in gene content for tryptophan and nicotinamide metabolism in cases. | 37 ALS patients | 29 healthy controls consisting of family members; matched for age and BMI | Pregnancy, fertility therapies, antibiotics, probiotics, and inflammatory or malignant diseases were among the exclusion criteria | Shotgun metagenomic sequencing | Israel | Blacher et al., 2019 [41] |
| Increased alpha diversity (evaluated by Shannon index) but not beta diversity in ALS; increased in Bacteroidetes; decreased in Firmicutes, at phylum level; Increased in Kineothrix, Parabacteroides, Odoribacter, Sporobacter, Eisenbergiella, Mannheimia, Anaerotruncus, and unclassified Porphyromonadaceae; decreased in Megamonas at the genus level. | 20 probable or definite ALS patients (8 females) | 20 healthy controls (8 females) with overall similar living conditions and dietary structure; probable age and sex matching | Diseases and drugs of the gastrointestinal tract, gastrointestinal surgical history, and nutritional imbalances in the diet | Two methods: 16S rRNA (V4 region) sequencing for gut bacterial microbiome profiling, and shotgun metagenomic sequencing for gut microbiome profiling and functional measure | China | Zeng et al., 2020 [24] |
| Similar alpha and beta diversities; increased in Escherichia (unclassified) and Streptococcus; decreased in Bilophila (unclassified) at the genus level; Clostridiaceae bacterium JC118, Coprobacter fastidiosus, Eubacterium eligens, and Ruminococcus sp 5 1 39 BF, with two butyrate-producing bacteria (Eubacterium rectale and Roseburia intestinalis) significantly lower in ALS; total relative abundance of the eight dominant butyrate producers significantly lower in ALS. | 66 at least suspected ALS (26 females) | 61 healthy controls (36 females) consisting of caregivers and other healthy individuals; 12 neurodegenerative controls (7 females) | Adults (older than 18 years), not employing probiotics for 14 days, no use of antibiotics or immune suppressants in the last three months, and no active inflammatory bowel disease, GI malignancy, irritable bowel syndrome, or other GI sickness needing treatment (apart from gastroesophageal reflux) for more than 18 years | Two methods: 16S rRNA (V4 region) sequencing for gut bacterial microbiome profiling, and shotgun metagenomic sequencing for gut microbiome profiling and functional measure | USA | Nicholson et al., 2020 [100] |
| No difference in alpha and beta diversities. | 49 Motor Neuron Disease patients (15 females) | 51 healthy controls (21 females) consisting of spouses, friends, and family members; age, sex, and BMI matching | Individuals with a history of diabetes, gastrostomy use, antibiotic or probiotic use, or FVC < 60% | Bacterial 16S rRNA (V6–V8 region) sequencing for gut microbiome profiling | Australia | Ngo et al., 2020 [101] |
| Increased alpha diversity in cases (Chao1 index), also with changes in beta diversity; no changes in the Firmicutes-to-Bacteroidetes ratio; increased in Cyanobacteria at the phylum level; increased in Lactobacillus, Citrobacter, and Coprococcus at the genus level; the PCR-denaturing gradient gel electrophoresis analysis demonstrated a distinct cluster split between the bacterial profiles of ALS patients and healthy people. Compared to Eubacteria, the profiles of yeast were significantly simpler, and there was no clear correlation between the two that indicated the presence or absence of illness; lower DNA content in feces from ALS patients; lower amount of Clostridium cluster I and yeasts and a higher concentration of E. coli and Enterobacteriaceae were detected in ALS patients. | 50 probable or defined ALS patients (22 females) | 50 controls (22 females) of unrelated subjects, unrelated family members, or friends; matched for sex, age, origin, eating habits, and geographic region | Individuals with noninvasive ventilation, gastrostomy, illnesses, antibiotic or medication use during the last eight weeks, or FVC < 50% | Bacterial 16S rRNA (V3–V4 region) sequencing for gut microbiome profiling; PCR-Denaturing Gradient Gel Electrophoresis for the first 38 control and 38 diseased subjects recruited in order to have a preliminary investigation of total Eubacteria and yeast populations. DNA was amplified using primers targeting the V2–V3 region of 16S rDNA and the D1 region of 26S rDNA; absolute quantification of Lactobacillus spp., Bifidobacterium spp., Clostridium cluster I (including C. baratii, C. hystoliticum, C. butyricum, C. prefringens, C. botulinum, and C. tetani), Escherichia coli, Enterobacteriaceae, and total yeasts was performed with qPCR | Italy | Di Gioia et al., 2020 [102]; Mazzini et al., 2018 [128] |
| ALS was associated with decreased Succinivibrionaceae and Lachnospiraceae family abundance, with dominance of Streptococcaceae and Ruminococcaceae in controls. LEfSE analyses showed that Atopobiaceae, Actinomycetaceae, Erysipelatoclostridiaceae, and Peptococcacceae families differed between ALS and controls. | 6 ALS patients (1 female) | 6 family members living in the same house (4 females); 8 unrelated individuals (3 females) were also chosen | Participants with a history of diarrhea or antimicrobial drug use in the past 3 weeks and with a history of inflammatory bowel disease or history of bowel operations were excluded | Bacterial primers selected from the 16S rRNA region (ITS1 and ITS4) and next-generation sequencing | Turkey | Özaydin Aksun et al., 2024 [129] |
| Patient microbiomes showed a higher diversity with a higher number of taxa. ALS patients were also deficient in Prevotella spp. | 10 ALS patients (3 females) | 30 healthy controls (20 females) with overall similar living conditions and dietary structure; probable age and sex matching | Patients receiving enteral nutrition as well as those with a history of bowel disease other than constipation, malignancy, dementia/other cognitive disorders, or Parkinson’s disease/other neurodegenerative diseases | 16S rRNA (V4 region) gene sequencing | USA | Hertzberg et al., 2022 [130] |
| Nasal microbiome changes over ALS, with a lower alpha diversity. Gaiella, Sphingomonas, Polaribacter_1, Lachnospiraceae_NK4A136_group, Klebsiella, and Alistipes were higher in ALS cases at the genus level. No significant differences in nasal microbiota richness and evenness were detected in ALS patients. | 66 ALS patients (29 females) | 40 healthy controls, caregivers (the spouses of the ALS patients) who lived in close proximity with the patients, potentially matched for diet, daily schedule, pollution exposure, and other related factors. | Human immunodeficiency virus infection, primary immunodeficiency, systemic inflammatory disorder, or history of intranasal drug administration, including antibiotics, immune suppressants, or probiotics within the prior 3 years, and oral administration or infusion of antibiotics in the prior 2 months | 16S rRNA (V3–V4 region) gene sequencing | China | Liu et al., 2024 [131] |
| No changes in alpha diversity associated with ALS. Lower Bifidobacterium in ALS at the genus level | 27 ALS patients (12 females) | 15 healthy controls chosen as donors in a fecal microbiota transplantation procedure | FVC < 70%, having a first-degree relative or more than one relative with ALS, a diagnosis of major depression or psychosis acute infection or inflammatory conditions within the preceding 4 weeks, history of abdominal surgery, autoimmune or chronic inflammatory conditions, probiotic or antibiotic use in the past 3 months, active malignancy, pregnancy, and drug abuse | 16S rRNA (V3–V4 region) gene sequencing | China | Feng et al., 2024 [132] |
| Decreased abundance of Fusicatenibacter and Catenibacterium; increased abundance of Lachnospira. | 20,806 cases with ALS | 59,804 controls (GWAS summary statistics from IALSC); 18,340 participants (GWAS summary statistics from MiBioGen); 7824 participants (GWAS summary statistics from TwinsUK and KORA) | Not specified | Not specified | Worldwide | Ning et al., 2022 [40] |
| Increased abundance of the Soutella and Lactobacillales orders in ALS; interaction with genetically predicted increased susceptibility to ALS; increased risk for ALS linked to unclassified Enterobacteriaceae and unclassified Acidaminococcaceae. | 20,806 cases with ALS | 59,804 controls (GWAS summary statistics from IALSC); 1812 samples (GWAS summary statistics); 7824 adult individuals (GWAS summary statistics from 2 European cohorts) | Not specified | Not specified | Worldwide | Zhang et al., 2022 [133] |
| Lower alpha diversity in ALS patients, beta-diversity significantly different as well, and Firmicutes and Cyanobacteria differed in ALS patients, at the phylum level. Higher relative abundance in ALS cases of Bacteroides, Parasutterella, and Lactococcus and higher relative abundance in control of Faecalibacterium and Bifidobacterium at the genus level. Lower abundance of butyrate-producing species in ALS. | 75 ALS patients (32 females) | 110 controls (66 females), matched for sex and age | For controls, neurodegenerative condition or family history of ALS | 16S rRNA gene sequencing (V4 region) | USA | Guo et al., 2024 [45] |
| No differences in Firmicutes-to-Bacteroidetes ratios. α diversity did not differ between ALS patients and healthy controls. β diversity differed between ALS patients and healthy controls. ALS patients had lower phylum Proteobacteria, and from genus Escherichia, Shigella, Klebsiella, Lachnoclostridium, Sutterella, and Catenibacterium; and genus unclassified family Enterobacteriaceae with increased Subdoligranulum, order Coriobacteriales, Olsenella, Pygmaiobacter, Ralstonia, Gordonibacter, Ezakiella, and families Coriobacteriales and Atopobiaceae. Cognitive impairment affected microbiome abundances. | 35 ALS patients (14 females) | 35 healthy controls, age- and sex-matched (14 females) | Any possible evidence for familial amyotrophic lateral sclerosis (fALS); with other neurodegenerative diseases; with definite gastrointestinal diseases, heart failure, acute infection, tumor, immunodeficiency and autoimmune diseases; any use of antibiotics within the last month; regular drinking of commercial probiotics within the last year or any drinking of commercial probiotics within the last week; obvious symptoms of dysphagia or dyspnea | 16S rRNA gene sequencing; the cognitive function of the ALS patients was evaluated using the Edinburgh Cognitive and Behavioral ALS Screen | China | Gong et al., 2022 [39] |
| Alpha and beta diversity varied between patients with ALS and healthy donors. Firmicutes was predominant in patients with ALS, resulting in an elevated F/B ratio. Some species with beneficial profiles (B. stercoris, B. uniformis, B. vulgatus, and F. prausnitzii) were lower in ALS patients. | 2 patients undergoing fecal microbial transplantation | 1 healthy donor | Not specified | Metagenomic sequencing | Japan | Yan et al., 2024 [19] |
| Any antibiotics use—especially two prescriptions of beta-lactamase-sensitive penicillin—was associated with a higher risk of ALS. | 2484 ALS patients according to the Swedish national registers | 12,420 healthy individuals matched by sex, birth year, and area of residence | Eligible controls were individuals who were alive and free of ALS diagnosis on the diagnosis date of their corresponding cases | Nested case-control study, conditional logistic regression model to calculate odds ratios | Sweden | Sun et al., 2019 [110] |
| Marginally significant difference in the abundances of specific bacterial species after FDR correction; Anaerostipes hadrus, Bacteroidales bacterium ph8, Bifidobacterium pseudocatenulatum (correlating with serum nicotinamide), Clostridium leptum, and Escherichia coli. Decreased content in several key genes participating in the metabolism of tryptophan and nicotinamide, focused on Akkermasia muciniphila. | 37 ALS patients (8 females) | 29 healthy, BMI- and age-matched family members (20 females) | Pregnancy or fertility treatments; use of antibiotics or antifungals in the 3 previous months; consumption of probiotics 1 month before; active inflammatory or neoplastic disease three years before enrolment; chronic gastrointestinal disorder, myocardial infarction, or cerebrovascular accident in the six months before participation; coagulation disorders; chronic immunosuppressive medication usage; or pre-diagnosed type I or type II diabetes mellitus or treatment with anti-diabetic medication | Shotgun metagenomics sequencing | Israel | Blacher et al., 2019 [41] |
| No changes in alpha or beta diversity in ALS, nor in the Firmicutes-to-Bacteroidetes ratio; ALS patients showed higher Fusobacteria and Acidobacteria at the phylum level. | 16 diagnosed ALS patients (8 females) | 12 controls (6 females) matched for age and sex, among spouses and caregivers | Cases with GI diseases or those treated with drugs (such as antibiotics) that could alter nutritional balance and affect intestinal microbiota. Antibiotic use within 2 months | 16S rRNA gene sequencing (V3–V4 region) | Spain | Fontdevila et al., 2024 [13] |
| Spinal ALS patients showed higher fecal F/B ratios in contrast with bulbar ALS patients who had decreased oral F/B. The Shannon index in ALS patients evidenced decreased richness. In spinal ALS, there was an enrichment of the Ruminococcaceae and a depletion of the Bacteroidaceae. In the oral microbiota of bulbar ALS, there was a depletion of Veillonellaceae and an increased abundance of the Prevotellaceae family. | 36 Patients with bulbar or spinal ALS (24 spinal onset with 15 females; 12 bulbar onset with 8 females) | 20 healthy controls living with patients | Exposure to antibiotics/probiotics, immunocompromising illness/therapy, previous abdominal/anorectal surgery, GI-/respiratory-/gynecological-tract infection, food poisoning, or major epistaxis requiring treatment; active/persistent primary disease of GI-/respiratory-/gynecological-tract, endocrinal disease, heart failure, severe renal-insufficiency, current pregnancy, drug/alcohol abuse, and active smoking within 6 months | Deep sequencing the V4 hypervariable region of bacterial 16S rRNA | USA | Kim et al., 2022 [44] |
3.4.3. Mechanistic Understanding of Microbiota’s Role in ALS
3.4.4. Therapeutic Trials of Microbiota-Based Interventions
3.4.5. Role of Environmental and Dietary Factors in Modulating Microbiota
3.4.6. Developing Personalized Microbiota-Based Therapies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS genetics, mechanisms, and therapeutics: Where are we now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Chen, H.; Kankel, M.W.; Su, S.C.; Han, S.W.S.; Ofengeim, D. Exploring the genetics and non-cell autonomous mechanisms underlying ALS/FTLD. Cell Death Differ. 2018, 25, 648–662. [Google Scholar] [CrossRef]
- Gros-Louis, F.; Gaspar, C.; Rouleau, G.A. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2006, 1762, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Lewis, C.M. Modelling the effects of penetrance and family size on rates of sporadic and familial disease. Hum. Hered. 2011, 71, 281–288. [Google Scholar] [CrossRef]
- Povedano, M.; Saez, M.; Martínez-Matos, J.-A.; Barceló, M.A. Spatial Assessment of the Association between Long-Term Exposure to Environmental Factors and the Occurrence of Amyotrophic Lateral Sclerosis in Catalonia, Spain: A Population-Based Nested Case-Control Study. Neuroepidemiology 2018, 51, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Proaño, B.; Cuerda-Ballester, M.; Daroqui-Pajares, N.; Del Moral-López, N.; Seguí-Sala, F.; Martí-Serer, L.; Calisaya Zambrana, C.K.; Benlloch, M.; de la Rubia Ortí, J.E. Clinical and sociodemographic factors related to amyotrophic lateral sclerosis in spain: A pilot study. J. Clin. Med. 2024, 13, 5800. [Google Scholar] [CrossRef] [PubMed]
- Fabi, J.P. The connection between gut microbiota and its metabolites with neurodegenerative diseases in humans. Metab. Brain Dis. 2024, 39, 967–984. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y. Microbiome and micronutrient in ALS: From novel mechanisms to new treatments. Neurotherapeutics 2024, 21, e00441. [Google Scholar] [CrossRef]
- Frick, J.-S.; Autenrieth, I.B. The gut microflora and its variety of roles in health and disease. Curr. Top. Microbiol. Immunol. 2013, 358, 273–289. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef] [PubMed]
- Fontdevila, L.; Povedano, M.; Domínguez, R.; Boada, J.; Serrano, J.C.; Pamplona, R.; Ayala, V.; Portero-Otín, M. Examining the complex Interplay between gut microbiota abundance and short-chain fatty acid production in amyotrophic lateral sclerosis patients shortly after onset of disease. Sci. Rep. 2024, 14, 23497. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Galaction, A.I.; Turnea, M.; Blendea, C.D.; Rotariu, M.; Poștaru, M. Redox homeostasis, gut microbiota, and epigenetics in neurodegenerative diseases: A systematic review. Antioxidants 2024, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R. Introduction: Unraveling the complex contributions of indigenous microbes to neurological health and disease. Int. Rev. Neurobiol. 2022, 167, xi. [Google Scholar] [CrossRef]
- Willyard, C. How gut microbes could drive brain disorders. Nature 2021, 590, 22–25. [Google Scholar] [CrossRef]
- Lefèvre-Arbogast, S.; Chaker, J.; Mercier, F.; Barouki, R.; Coumoul, X.; Miller, G.W.; David, A.; Samieri, C. Assessing the contribution of the chemical exposome to neurodegenerative disease. Nat. Neurosci. 2024, 27, 812–821. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites 2022, 12, 1052. [Google Scholar] [CrossRef]
- Yan, J.; Chen, H.; Zhang, Y.; Peng, L.; Wang, Z.; Lan, X.; Yu, S.; Yang, Y. Fecal microbiota transplantation significantly improved respiratory failure of amyotrophic lateral sclerosis. Gut Microbes 2024, 16, 2353396. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediators Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef]
- Sun, P.; Su, L.; Zhu, H.; Li, X.; Guo, Y.; Du, X.; Zhang, L.; Qin, C. Gut microbiota regulation and their implication in the development of neurodegenerative disease. Microorganisms 2021, 9, 2281. [Google Scholar] [CrossRef]
- Niccolai, E.; Di Pilato, V.; Nannini, G.; Baldi, S.; Russo, E.; Zucchi, E.; Martinelli, I.; Menicatti, M.; Bartolucci, G.; Mandrioli, J.; et al. The Gut Microbiota-Immunity Axis in ALS: A Role in Deciphering Disease Heterogeneity? Biomedicines 2021, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Shen, J.; Chen, K.; Zhou, J.; Liao, Q.; Lu, K.; Yuan, J.; Bi, F. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 2020, 10, 12998. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D.; Lee, A.; Lee, J.D.; Woodruff, T.M.; Restuadi, R.; McRae, A.; Wray, N.R.; Ngo, S.; Steyn, F.J. Gut microbiota in ALS: Possible role in pathogenesis? Expert Rev. Neurother. 2019, 19, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Hiergeist, A.; Adis, C.; Mayer, B.; Gessner, A.; Ludolph, A.C.; Weishaupt, J.H. The fecal microbiome of ALS patients. Neurobiol. Aging 2018, 61, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Banerjee, S. Microbiome and motor neuron diseases. Prog. Mol. Biol. Transl. Sci. 2020, 176, 111–122. [Google Scholar] [CrossRef]
- Chauhan, V.; Chauhan, N.K.; Dutta, S.; Pathak, D.; Nongthomba, U. Comparative in-silico analysis of microbial dysbiosis discern potential metabolic link in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1153422. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mazmanian, S.K. Microbiota-brain axis: Context and causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Lucchetti, M.; Kaminska, M.; Oluwasegun, A.K.; Mosig, A.S.; Wilmes, P. Emulating the gut-liver axis: Dissecting the microbiome’s effect on drug metabolism using multiorgan-on-chip models. Curr. Opin. Endocr. Metab. Res. 2021, 18, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Y.; Yu, X.; Chen, Y.; Zhang, J.; Pang, C.; Xie, J.; Gao, L.; Du, L.; Cao, W.; et al. Fighting amyotrophic lateral sclerosis by protecting the liver? A prospective cohort study. Ann. Neurol. 2024, 97, 270–280. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.-H. Disorders of the enteric nervous system—A holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Kurlawala, Z.; McMillan, J.D.; Singhal, R.A.; Morehouse, J.; Burke, D.A.; Sears, S.M.; Duregon, E.; Beverly, L.J.; Siskind, L.J.; Friedland, R.P. Mutant and curli-producing E. coli enhance the disease phenotype in a hSOD1-G93A mouse model of ALS. Sci. Rep. 2023, 13, 5945. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, Y.; Niu, K.; Zeng, W.; Wang, R.; Guo, X.; Lin, C.; Hu, L. Oleanolic acid alleviate intestinal inflammation by inhibiting Takeda G-coupled protein receptor (TGR) 5 mediated cell apoptosis. Food Funct. 2024, 15, 1963–1976. [Google Scholar] [CrossRef]
- Niccolai, E.; Pedone, M.; Martinelli, I.; Nannini, G.; Baldi, S.; Simonini, C.; Di Gloria, L.; Zucchi, E.; Ramazzotti, M.; Spezia, P.G.; et al. Amyotrophic lateral sclerosis stratification: Unveiling patterns with virome, inflammation, and metabolism molecules. J. Neurol. 2024, 271, 4310–4325. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Zhao, M.; Zheng, L.; Fan, D. Changes in the concentrations of trimethylamine N-oxide (TMAO) and its precursors in patients with amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 15198. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Ba, L.; Tang, J.; Yang, Y.; Li, Z.; Liu, M.; Yang, C.; Ding, F.; Zhang, M. Gut microbiota links with cognitive impairment in amyotrophic lateral sclerosis: A multi-omics study. J. Biomed. Res. 2022, 37, 125–137. [Google Scholar] [CrossRef]
- Ning, J.; Huang, S.-Y.; Chen, S.-D.; Zhang, Y.-R.; Huang, Y.-Y.; Yu, J.-T. Investigating casual associations among gut microbiota, metabolites, and neurodegenerative diseases: A mendelian randomization study. J. Alzheimers Dis. 2022, 87, 211–222. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lv, X.; Du, H.; Wu, D.; Wang, M. Causal effects of serum metabolites on amyotrophic lateral sclerosis: A Mendelian randomization study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 97, 109771. [Google Scholar] [CrossRef]
- Zhai, C.-D.; Zheng, J.-J.; An, B.-C.; Huang, H.-F.; Tan, Z.-C. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: Establishment of bacterial and archaeal communities analyses. Chin. Med. J. 2019, 132, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Figueroa-Romero, C.; Noureldein, M.H.; Murdock, B.J.; Savelieff, M.G.; Hur, J.; Goutman, S.A.; Feldman, E.L. Gut microbiome correlates with plasma lipids in amyotrophic lateral sclerosis. Brain 2024, 147, 665–679. [Google Scholar] [CrossRef]
- Lee, A.; Arachchige, B.J.; Reed, S.; Henderson, R.; Aylward, J.; McCombe, P.A. Plasma from some patients with amyotrophic lateral sclerosis exhibits elevated formaldehyde levels. J. Neurol. Sci. 2020, 409, 116589. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Zhai, S.; Qin, S.; Li, L.; Zhu, L.; Zou, Z.; Wang, L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol. Lett. 2019, 366, fnz153. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef]
- Ghosh, A.; Gorain, B. Mechanistic insight of neurodegeneration due to micro/nano-plastic-induced gut dysbiosis. Arch. Toxicol. 2025, 99, 83–101. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mustafa, A.N.; Mustafa, M.A.; Renuka Jyothi, S.; Dabis, H.K.; Prasad, G.V.S.; Mohammad, I.J.; Adnan, A.; Idan, A.H. The role of gut-derived short-chain fatty acids in Parkinson’s disease. Neurogenetics 2024, 25, 307–336. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: From gut development to neuroprotection. Front. Microbiol. 2024, 15, 1456793. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, X.; Lao, L.; Wang, Y.; Su, H.; Sun, H. Brain-Gut-Microbiota Axis in Amyotrophic Lateral Sclerosis: A Historical Overview and Future Directions. Aging Dis. 2024, 15, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Föh, B.; Buhre, J.S.; Lunding, H.B.; Moreno-Fernandez, M.E.; König, P.; Sina, C.; Divanovic, S.; Ehlers, M. Microbial metabolite butyrate promotes induction of IL-10+IgM+ plasma cells. PLoS ONE 2022, 17, e0266071. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef]
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The role of altered bdnf/trkb signaling in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2019, 13, 368. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhang, S.-Y.; Wen, R.; Zhang, T.-N.; Yang, N. Role of histone deacetylases and their inhibitors in neurological diseases. Pharmacol. Res. 2024, 208, 107410. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Sun, J.; Huang, T.; Debelius, J.W.; Fang, F. Gut microbiome and amyotrophic lateral sclerosis: A systematic review of current evidence. J. Intern. Med. 2021, 290, 758–788. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, X.; Yang, S.; Meng, F.; Wang, X.; Wei, H.; Chen, T. Evaluation of the Microbial Diversity in Amyotrophic Lateral Sclerosis Using High-Throughput Sequencing. Front. Microbiol. 2016, 7, 1479. [Google Scholar] [CrossRef]
- Torres, P.; Rico-Rios, S.; Ceron-Codorniu, M.; Santacreu-Vilaseca, M.; Seoane-Miraz, D.; Jad, Y.; Ayala, V.; Mariño, G.; Beltran, M.; Miralles, M.P.; et al. TDP-43 regulates LC3ylation in neural tissue through ATG4B cryptic splicing inhibition. Acta Neuropathol. 2024, 148, 45. [Google Scholar] [CrossRef]
- Torres, P.; Ramírez-Núñez, O.; Romero-Guevara, R.; Barés, G.; Granado-Serrano, A.B.; Ayala, V.; Boada, J.; Fontdevila, L.; Povedano, M.; Sanchís, D.; et al. Cryptic exon splicing function of TARDBP interacts with autophagy in nervous tissue. Autophagy 2018, 14, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Rowin, J.; Xia, Y.; Jung, B.; Sun, J. Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 2017, 5, e13443. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vello, P.; Tytgat, H.L.P.; Elzinga, J.; Van Hul, M.; Plovier, H.; Tiemblo-Martin, M.; Cani, P.D.; Nicolardi, S.; Fragai, M.; De Castro, C.; et al. The lipooligosaccharide of the gut symbiont Akkermansia muciniphila exhibits a remarkable structure and TLR signaling capacity. Nat. Commun. 2024, 15, 8411. [Google Scholar] [CrossRef]
- Li, C.; Liang, Y.; Qiao, Y. Messengers From the Gut: Gut Microbiota-Derived Metabolites on Host Regulation. Front. Microbiol. 2022, 13, 863407. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Shrestha, A.; Duff, A.F.; Kontic, D.; Brewster, P.C.; Kasperek, M.C.; Lin, C.-H.; Wainwright, D.A.; Hernandez-Saavedra, D.; Woods, J.A.; et al. Aging amplifies a gut microbiota immunogenic signature linked to heightened inflammation. Aging Cell 2024, 23, e14190. [Google Scholar] [CrossRef]
- Erny, D.; Prinz, M. How microbiota shape microglial phenotypes and epigenetics. Glia 2020, 68, 1655–1672. [Google Scholar] [CrossRef]
- Mossad, O.; Erny, D. The microbiota-microglia axis in central nervous system disorders. Brain Pathol. 2020, 30, 1159–1177. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jin, H.K.; Bae, J.-S. Sphingolipids in neuroinflammation: A potential target for diagnosis and therapy. BMB Rep. 2020, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Girolamo, F.; Coppola, C.; Ribatti, D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav. Immun. 2017, 65, 68–89. [Google Scholar] [CrossRef]
- Mohanty, I.; Allaband, C.; Mannochio-Russo, H.; El Abiead, Y.; Hagey, L.R.; Knight, R.; Dorrestein, P.C. The changing metabolic landscape of bile acids—Keys to metabolism and immune regulation. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 493–516. [Google Scholar] [CrossRef]
- Li, Y.R.; King, O.D.; Shorter, J.; Gitler, A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013, 201, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.; Cone, A.; Ju, S. Supersaturated proteins in ALS. Proc. Natl. Acad. Sci. USA 2017, 114, 5065–5066. [Google Scholar] [CrossRef]
- Gitler, A.D.; Shorter, J. RNA-binding proteins with prion-like domains in ALS and FTLD-U. Prion 2011, 5, 179–187. [Google Scholar] [CrossRef]
- Smethurst, P.; Sidle, K.C.L.; Hardy, J. Review: Prion-like mechanisms of transactive response DNA binding protein of 43 kDa (TDP-43) in amyotrophic lateral sclerosis (ALS). Neuropathol. Appl. Neurobiol. 2015, 41, 578–597. [Google Scholar] [CrossRef]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef]
- Wang, C.; Lau, C.Y.; Ma, F.; Zheng, C. Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2106504118. [Google Scholar] [CrossRef]
- Van Assche, E.; Van Puyvelde, S.; Vanderleyden, J.; Steenackers, H.P. RNA-binding proteins involved in post-transcriptional regulation in bacteria. Front. Microbiol. 2015, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- McQuail, J.; Switzer, A.; Burchell, L.; Wigneshweraraj, S. The RNA-binding protein Hfq assembles into foci-like structures in nitrogen starved Escherichia coli. J. Biol. Chem. 2020, 295, 12355–12367. [Google Scholar] [CrossRef]
- Bowman, G.R.; Comolli, L.R.; Gaietta, G.M.; Fero, M.; Hong, S.-H.; Jones, Y.; Lee, J.H.; Downing, K.H.; Ellisman, M.H.; McAdams, H.H.; et al. Caulobacter PopZ forms a polar subdomain dictating sequential changes in pole composition and function. Mol. Microbiol. 2010, 76, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, J.E.; Paul, K.R.; Cascarina, S.M.; Ross, E.D. The prion-like protein kinase Sky1 is required for efficient stress granule disassembly. Nat. Commun. 2019, 10, 3614. [Google Scholar] [CrossRef]
- Guzikowski, A.R.; Chen, Y.S.; Zid, B.M. Stress-induced mRNP granules: Form and function of processing bodies and stress granules. Wiley Interdiscip. Rev. RNA 2019, 10, e1524. [Google Scholar] [CrossRef] [PubMed]
- Calloni, G.; Chen, T.; Schermann, S.M.; Chang, H.-C.; Genevaux, P.; Agostini, F.; Tartaglia, G.G.; Hayer-Hartl, M.; Hartl, F.U. DnaK functions as a central hub in the E. coli chaperone network. Cell Rep. 2012, 1, 251–264. [Google Scholar] [CrossRef]
- Zajkowski, T.; Lee, M.D.; Mondal, S.S.; Carbajal, A.; Dec, R.; Brennock, P.D.; Piast, R.W.; Snyder, J.E.; Bense, N.B.; Dzwolak, W.; et al. The Hunt for Ancient Prions: Archaeal Prion-Like Domains Form Amyloid-Based Epigenetic Elements. Mol. Biol. Evol. 2021, 38, 2088–2103. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Banack, S.A.; Cox, P.A. Creating a simian model of guam ALS/PDC which reflects chamorro lifetime BMAA exposures. Neurotox. Res. 2018, 33, 24–32. [Google Scholar] [CrossRef]
- Lance, E.; Arnich, N.; Maignien, T.; Biré, R. Occurrence of β-N-methylamino-l-alanine (BMAA) and Isomers in Aquatic Environments and Aquatic Food Sources for Humans. Toxins 2018, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, β-N-methylamino-L-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H.; et al. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 101–108. [Google Scholar] [CrossRef] [PubMed]
- Torbick, N.; Ziniti, B.; Stommel, E.; Linder, E.; Andrew, A.; Caller, T.; Haney, J.; Bradley, W.; Henegan, P.L.; Shi, X. Assessing cyanobacterial harmful algal blooms as risk factors for amyotrophic lateral sclerosis. Neurotox. Res. 2018, 33, 199–212. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Klug, M.G. Motor neuron disease mortality rates in U.S. states are associated with well water use. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 528–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pierce, E.S. How did Lou Gehrig get Lou Gehrig’s disease? Mycobacterium avium subspecies paratuberculosis in manure, soil, dirt, dust and grass and amyotrophic lateral sclerosis (motor neurone disease) clusters in football, rugby and soccer players. Med. Hypotheses 2018, 119, 1–5. [Google Scholar] [CrossRef]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef]
- Nicholson, K.; Bjornevik, K.; Abu-Ali, G.; Chan, J.; Cortese, M.; Dedi, B.; Jeon, M.; Xavier, R.; Huttenhower, C.; Ascherio, A.; et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 186–194. [Google Scholar] [CrossRef]
- Ngo, S.T.; Restuadi, R.; McCrae, A.F.; Van Eijk, R.P.; Garton, F.; Henderson, R.D.; Wray, N.R.; McCombe, P.A.; Steyn, F.J. Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 2020, 18, 153. [Google Scholar] [CrossRef]
- Wang, I.-F.; Guo, B.-S.; Liu, Y.-C.; Wu, C.-C.; Yang, C.-H.; Tsai, K.-J.; Shen, C.-K.J. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc. Natl. Acad. Sci. USA 2012, 109, 15024–15029. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Corsetti, G. Natural compounds and autophagy: Allies against neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef] [PubMed]
- Noor Eddin, A.; Alfuwais, M.; Noor Eddin, R.; Alkattan, K.; Yaqinuddin, A. Gut-Modulating Agents and Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives. Nutrients 2024, 16, 590. [Google Scholar] [CrossRef]
- Rizos, E.; Pyleris, E.; Pimentel, M.; Triantafyllou, K.; Giamarellos-Bourboulis, E.J. Small intestine bacterial overgrowth can form an indigenous proinflammatory environment in the duodenum: A prospective study. Microorganisms 2022, 10, 960. [Google Scholar] [CrossRef]
- Wright, M.L.; Fournier, C.; Houser, M.C.; Tansey, M.; Glass, J.; Hertzberg, V.S. Potential role of the gut microbiome in ALS: A systematic review. Biol. Res. Nurs. 2018, 20, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kakaroubas, N.; Brennan, S.; Keon, M.; Saksena, N.K. Pathomechanisms of Blood-Brain Barrier Disruption in ALS. Neurosci. J. 2019, 2019, 2537698. [Google Scholar] [CrossRef]
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut microbiota modulation and prevention of dysbiosis as an alternative approach to antimicrobial resistance: A narrative review. Yale J. Biol. Med. 2022, 95, 479–494. [Google Scholar]
- Sun, J.; Zhan, Y.; Mariosa, D.; Larsson, H.; Almqvist, C.; Ingre, C.; Zagai, U.; Pawitan, Y.; Fang, F. Antibiotics use and risk of amyotrophic lateral sclerosis in Sweden. Eur. J. Neurol. 2019, 26, 1355–1361. [Google Scholar] [CrossRef]
- Fondell, E.; O’Reilly, E.J.; Fitzgerald, K.C.; Falcone, G.J.; Kolonel, L.N.; Park, Y.; McCullough, M.L.; Ascherio, A. Dietary fiber and amyotrophic lateral sclerosis: Results from 5 large cohort studies. Am. J. Epidemiol. 2014, 179, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kim, S.H.; Noh, M.-Y.; Lee, S.; Park, Y. Relationship between Dietary Fiber Intake and the Prognosis of Amytrophic Lateral Sclerosis in Korea. Nutrients 2020, 12, 3420. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Harijan, A.K.; Kalaiarasan, R.; Ghosh, A.K.; Jain, R.P.; Bera, A.K. The neuroprotective effect of short-chain fatty acids against hypoxia-reperfusion injury. Mol. Cell. Neurosci. 2024, 131, 103972. [Google Scholar] [CrossRef]
- Herrera-Rincon, C.; Murciano-Brea, J.; Geuna, S. Can we promote neural regeneration through microbiota-targeted strategies? Introducing the new concept of neurobiotics. Neural Regen. Res. 2022, 17, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Y.; Sun, J. Probiotics and microbial metabolites maintain barrier and neuromuscular functions and clean protein aggregation to delay disease progression in TDP43 mutation mice. Gut Microbes 2024, 16, 2363880. [Google Scholar] [CrossRef]
- Mincic, A.M.; Antal, M.; Filip, L.; Miere, D. Modulation of gut microbiome in the treatment of neurodegenerative diseases: A systematic review. Clin. Nutr. 2024, 43, 1832–1849. [Google Scholar] [CrossRef]
- Niccolai, E.; Martinelli, I.; Quaranta, G.; Nannini, G.; Zucchi, E.; De Maio, F.; Gianferrari, G.; Bibbò, S.; Cammarota, G.; Mandrioli, J.; et al. Fecal microbiota transplantation in amyotrophic lateral sclerosis: Clinical protocol and evaluation of microbiota immunity axis. Methods Mol. Biol. 2024, 2761, 373–396. [Google Scholar] [CrossRef]
- Carrera-Juliá, S.; Obrador, E.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. Ketogenic effect of coconut oil in ALS patients. Front. Nutr. 2024, 11, 1429498. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Johnston, S.E.; Simpson, P.; Chang, D.K.; Mather, D.; Dick, R.J. Time-restricted ketogenic diet in amyotrophic lateral sclerosis: A case study. Front. Neurol. 2023, 14, 1329541. [Google Scholar] [CrossRef]
- Norgren, J.; Kåreholt, I.; Sindi, S. Is there evidence of a ketogenic effect of coconut oil? Commentary: Effect of the Mediterranean diet supplemented with nicotinamide riboside and pterostilbene and/or coconut oil on anthropometric variables in amyotrophic lateral sclerosis. A pilot study. Front. Nutr. 2023, 10, 1333933. [Google Scholar] [CrossRef] [PubMed]
- Moțățăianu, A.; Șerban, G.; Andone, S. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Cross-Talk with a Focus on Amyotrophic Lateral Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15094. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Parkinson’s Disease and Amyotrophic Lateral Sclerosis. ACS Med. Chem. Lett. 2023, 14, 886–888. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 2017, 39, 322–336. [Google Scholar] [CrossRef]
- Li, A.; Yi, J.; Li, X.; Dong, L.; Ostrow, L.W.; Ma, J.; Zhou, J. Distinct transcriptomic profile of satellite cells contributes to preservation of neuromuscular junctions in extraocular muscles of ALS mice. eLife 2024, 12, RP92644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Z.; Ling, Y.; Teng, W.; Cui, J.; Yan, Z.; Hou, X.; Cen, W.; Long, N.; Li, W.; et al. Causal effect of air pollution on the risk of brain health and potential mediation by gut microbiota. Ecotoxicol. Environ. Saf. 2024, 285, 117080. [Google Scholar] [CrossRef]
- Mazzini, L.; Mogna, L.; De Marchi, F.; Amoruso, A.; Pane, M.; Aloisio, I.; Cionci, N.B.; Gaggìa, F.; Lucenti, A.; Bersano, E.; et al. Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S68–S70. [Google Scholar] [CrossRef]
- Özaydin Aksun, Z.; Erdoğan, S.; Kalkanci, A.; Şahin, E.A.; Çuhadar, T.; Şener, H.Ö. Is gut microbiota of patients with ALS different from that of healthy individuals? Turk. J. Med. Sci. 2024, 54, 579–587. [Google Scholar] [CrossRef]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef]
- Liu, K.; Guo, Q.; Ding, Y.; Luo, L.; Huang, J.; Zhang, Q. Alterations in nasal microbiota of patients with amyotrophic lateral sclerosis. Chin. Med. J. 2024, 137, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhu, Q.; Wang, A.; Wang, H.; Wang, J.; Chen, P.; Zhang, R.; Liang, D.; Teng, J.; Ma, M.; et al. Effect of fecal microbiota transplantation on patients with sporadic amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled trial. BMC Med. 2024, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, Z.; Zhang, G.; Huang, T.; Fan, D. Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: A 2-sample Mendelian randomization study. BMC Neurol. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kurilshikov, A.; Radjabzadeh, D.; Turpin, W.; Croitoru, K.; Bonder, M.J.; Jackson, M.A.; Medina-Gomez, C.; Frost, F.; Homuth, G.; et al. Meta-analysis of human genome-microbiome association studies: The MiBioGen consortium initiative. Microbiome 2018, 6, 101. [Google Scholar] [CrossRef]
- Lu, G.; Wen, Q.; Cui, B.; Li, Q.; Zhang, F. Washed microbiota transplantation stopped the deterioration of amyotrophic lateral sclerosis: The first case report and narrative review. J. Biomed. Res. 2022, 37, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Monselise, E.B.-I.; Vyazmensky, M.; Scherf, T.; Batushansky, A.; Fishov, I. D-Glutamate production by stressed Escherichia coli gives a clue for the hypothetical induction mechanism of the ALS disease. Sci. Rep. 2024, 14, 18247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, V.; Fontdevila, L.; Rico-Rios, S.; Povedano, M.; Andrés-Benito, P.; Torres, P.; Serrano, J.C.E.; Pamplona, R.; Portero-Otin, M. Microbial Influences on Amyotrophic Lateral Sclerosis: The Gut–Brain Axis and Therapeutic Potential of Microbiota Modulation. Sclerosis 2025, 3, 8. https://doi.org/10.3390/sclerosis3010008
Ayala V, Fontdevila L, Rico-Rios S, Povedano M, Andrés-Benito P, Torres P, Serrano JCE, Pamplona R, Portero-Otin M. Microbial Influences on Amyotrophic Lateral Sclerosis: The Gut–Brain Axis and Therapeutic Potential of Microbiota Modulation. Sclerosis. 2025; 3(1):8. https://doi.org/10.3390/sclerosis3010008
Chicago/Turabian StyleAyala, Victòria, Laia Fontdevila, Santiago Rico-Rios, Mònica Povedano, Pol Andrés-Benito, Pascual Torres, José C. E. Serrano, Reinald Pamplona, and Manuel Portero-Otin. 2025. "Microbial Influences on Amyotrophic Lateral Sclerosis: The Gut–Brain Axis and Therapeutic Potential of Microbiota Modulation" Sclerosis 3, no. 1: 8. https://doi.org/10.3390/sclerosis3010008
APA StyleAyala, V., Fontdevila, L., Rico-Rios, S., Povedano, M., Andrés-Benito, P., Torres, P., Serrano, J. C. E., Pamplona, R., & Portero-Otin, M. (2025). Microbial Influences on Amyotrophic Lateral Sclerosis: The Gut–Brain Axis and Therapeutic Potential of Microbiota Modulation. Sclerosis, 3(1), 8. https://doi.org/10.3390/sclerosis3010008






