Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects
Abstract
1. Introduction
2. Types of Substances and Its Effects
2.1. Benzodiazepines
2.2. Opioids
2.3. Cocaine
2.4. Alcohol
2.5. Cannabis
2.6. Methamphetamines and Other Synthetic Drugs
3. Pathophysiological Mechanisms
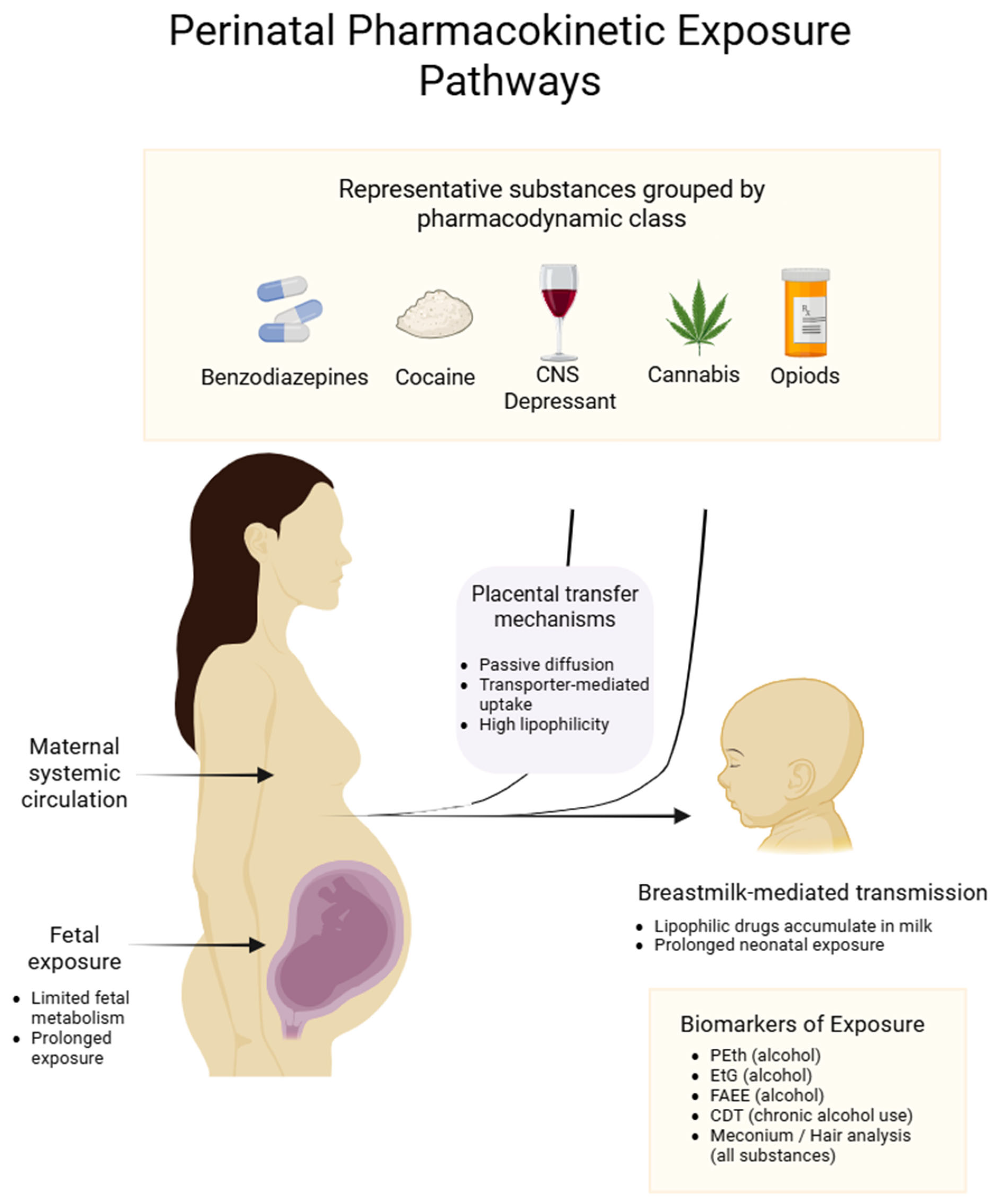
3.1. Trans-Placental Passage
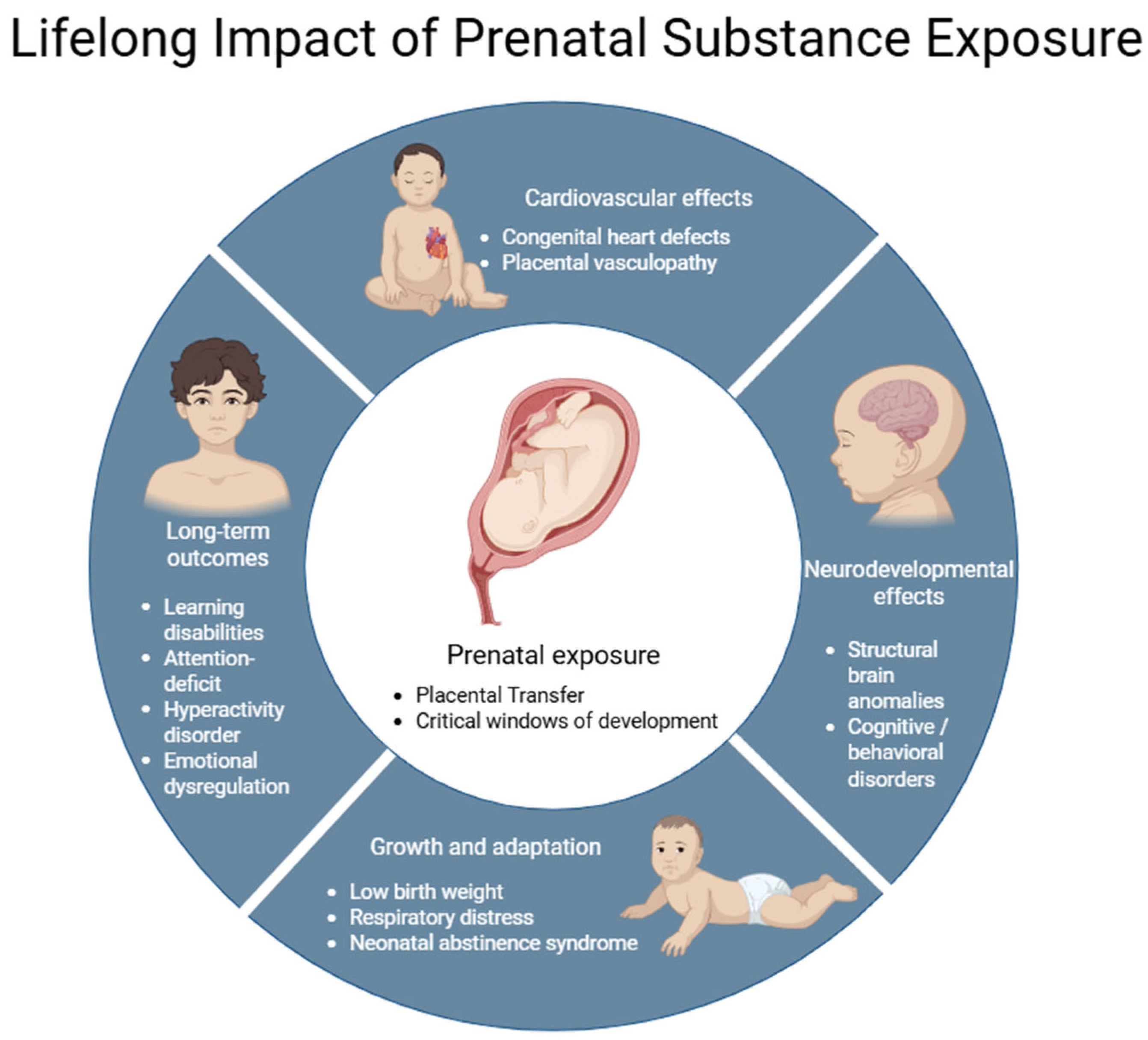
3.2. Effects on Fetal Development
3.3. Placental Dysfunction: Blood Flow and Nutrient Delivery
- Hypoxia and catecholaminergic vasoconstriction: Vasoactive agents (e.g., cocaine, methamphetamines) potentiate placental hypoxia, up-regulate hypoxia-inducible factor-1 α (HIF-1α), and, consequently, overexpress P-glycoprotein (ABCB1). This blunts extravillous trophoblast invasion and spiral-artery remodeling [62].
- Oxidative stress: Excess reactive oxygen species and mitochondrial impairment compromise intervillous perfusion and heighten the risk of placental abruption, partly via altered SLC transporters [63]. Overproduction of reactive oxygen species (ROS) is initiated by the activation of cytochrome P450 (CYP2E1), NADPH oxidase (NOX), and nitric oxide synthase pathways [64]. Concurrently, down-regulation of the Nrf2-dependent antioxidant axis weakens the cell’s ability to buffer oxidative stress. Excess ROS impairs mitochondrial function, triggers cytochrome c release, and activates pro-apoptotic proteins such as Drp1, catalyzing the caspase cascade—most notably caspase-3 and caspase-9—and culminating in apoptosis. Oxidative stress also promotes the release of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) and the opening of ROS-responsive cation channels such as TRPM2, thereby exacerbating cell death [65,66].
- Down-regulation of key transporters: Alcohol and opioids suppress pivotal ABC and SLC nutrient/gas carriers, whereas cannabinoids inhibit placental 11β-HSD-2, thereby amplifying fetal glucocorticoid exposure [67].
3.4. Fetal Susceptibility and Teratogenic Mechanisms
3.5. Inflammatory and Immunological Pathways
4. Obstetric, Fetal, and Neonatal Complications Associated with Prenatal Exposure to Drugs of Abuse
4.1. Obstetric Complications
4.2. Fetal Complications
4.3. Neonatal Complications
5. Psychosocial and Ethical Dimensions of Substance Use in Pregnancy
6. Clinical Approach and Treatment
Biomarkers of Exposure
7. Public Health Policy and Prevention
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4 P’s Plus | Parents, Peers, Partner, Past, Pregnancy Plus (substance-use screening tool) |
| ARND | Alcohol-Related Neurodevelopmental Disorder |
| AST | Aspartate Aminotransferase |
| AUDIT-C | Alcohol Use Disorders Identification Test—Consumption |
| CB1 | Cannabinoid Receptor Type 1 |
| CDT | Carbohydrate-Deficient Transferrin |
| CRAFFT | Car, Relax, Alone, Forget, Family, Trouble (adolescent substance-use screener) |
| CYP2E1 | Cytochrome P450 2E1 |
| DAMPs | Danger-Associated Molecular Patterns |
| EtG | Ethyl Glucuronide |
| FAEE | Fatty Acid Ethyl Esters |
| FAS | Fetal Alcohol Syndrome |
| FASD | Fetal Alcohol Spectrum Disorder |
| GDM | Gestational Diabetes Mellitus |
| GGT | Gamma-Glutamyltransferase |
| IUGR | Intra-Uterine Growth Restriction |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| MCV | Mean Corpuscular Volume |
| MOUD | Medication for Opioid Use Disorder |
| NAS | Neonatal Abstinence Syndrome |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NICU | Neonatal Intensive Care Unit |
| OGTT | Oral Glucose Tolerance Test |
| OUD | Opioid Use Disorder |
| PEth | Phosphatidylethanol |
| PNC | Prenatal Care |
| SIDS | Sudden Infant Death Syndrome |
| T-ACE | Tolerance, Annoyed, Cut Down, Eye-Opener (alcohol screener) |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TWEAK | Tolerance, Worry, Eye-Opener, Amnesia, Kut Down (alcohol screener) |
References
- Aderoba, A.K.; Adu-Bonsaffoh, K. Antenatal and Postnatal Care. Obstet. Gynecol. Clin. N. Am. 2022, 49, 665–692. [Google Scholar] [CrossRef]
- Soares Goncalves, A.; Maria Ferreira, I.; Pestana-Santos, M.; McCourt, C.; Paula Prata, A. Antenatal Care Policy in High-Income Countries with a Universal Health System: A Scoping Review. Sex. Reprod. Healthc. 2022, 32, 100717. [Google Scholar] [CrossRef]
- Ota, E.; Da Silva Lopes, K.; Middleton, P.; Flenady, V.; Wariki, W.M.; Rahman, M.O.; Tobe-Gai, R.; Mori, R. Antenatal Interventions for Preventing Stillbirth, Fetal Loss and Perinatal Death: An Overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2020, 12, CD009599. [Google Scholar] [CrossRef] [PubMed]
- Vince, K.; Perković, P.; Matijević, R. What Is Known and What Remains Unresolved Regarding Gestational Diabetes Mellitus (GDM). J. Perinat. Med. 2020, 48, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Peahl, A.F.; Turrentine, M.; Srinivas, S.; King, T.; Zahn, C.M. Routine Prenatal Care. Obstet. Gynecol. Clin. N. Am. 2023, 50, 439–455. [Google Scholar] [CrossRef]
- Ibañez-Cuevas, M.; Heredia-Pi, I.B.; Fuentes-Rivera, E.; Andrade-Romo, Z.; Alcalde-Rabanal, J.; Cacho, L.B.-B.; Guzmán-Delgado, X.; Jurkiewicz, L.; Darney, B.G. Atención Prenatal En Grupo En México: Perspectivas y Experiencias Del Personal de Salud. Rev. Saúde Pública 2020, 54, 140. [Google Scholar] [CrossRef]
- Grangé, G.; Vayssiere, C.; Borgne, A.; Ouazana, A.; L’Huillier, J.-P.; Valensi, P.; Peiffer, G.; Aubin, H.-J.; Renon, D.; Thomas, D.; et al. Description of Tobacco Addiction in Pregnant Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 146–151. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Einstein, E.B.; Elder, E.; Volkow, N.D. Pregnancy and Postpartum Drug Overdose Deaths in the US Before and During the COVID-19 Pandemic. JAMA Psychiatry 2024, 81, 270–283. [Google Scholar] [CrossRef]
- Gómez-Ruiz, L.-M.; Marchei, E.; Rotolo, M.C.; Brunetti, P.; Mannocchi, G.; Acosta-López, A.; Ramos-Gutiérrez, R.-Y.; Varela-Busaka, M.-B.; Pichini, S.; Garcia-Algar, O. Prevalence of Licit and Illicit Drugs Use during Pregnancy in Mexican Women. Pharmaceuticals 2022, 15, 382. [Google Scholar] [CrossRef]
- Wallace, M.E.; Jahn, J.L. Pregnancy-Associated Mortality Due to Homicide, Suicide, and Drug Overdose. JAMA Netw. Open 2025, 8, e2459342. [Google Scholar] [CrossRef]
- Tavella, R.A.; De Abreu, V.O.M.; Muccillo-Baisch, A.L.; Da Silva Júnior, F.M.R. Prevalence of Illicit Drug Use During Pregnancy: A Global Perspective. An. Acad. Bras. Ciênc. 2020, 92, e20200302. [Google Scholar] [CrossRef]
- Desiron, M.; Saad, C.; Tecco, J.M. Caroline Kadji Substance Use During Pregnancy. Psychiatr. Danub. 2024, 36 (Suppl. S2), 241–249. [Google Scholar] [PubMed]
- Mburu, G.; Ayon, S.; Mahinda, S.; Kaveh, K. Determinants of Women’s Drug Use During Pregnancy: Perspectives from a Qualitative Study. Matern. Child Health J. 2020, 24, 1170–1178. [Google Scholar] [CrossRef]
- Bangxang, N.; Sudjai, D.; Arthayukti, V.; Cheepo, S.; Pipatjarussakul, K. Maternal Substance Used during Labor and Neonatal Outcome. Eur. Psychiatry 2024, 67, S352. [Google Scholar] [CrossRef]
- Forray, A.; Foster, D. Substance Use in the Perinatal Period. Curr. Psychiatry Rep. 2015, 17, 91. [Google Scholar] [CrossRef]
- Andrade, C. Gestational Exposure to Benzodiazepines and Z-Hypnotics and the Risk of Major Congenital Malformations, Ectopic Pregnancy, and Other Adverse Pregnancy Outcomes. J. Clin. Psychiatry 2023, 84, 46490. [Google Scholar] [CrossRef]
- Meng, L.; Lin, C.-W.; Lin, Y.-C.; Huang, S.-T.; Chen, Y.-Y.; Shang, C.; Wu, C.-Y.; Chen, L.; Chan, K.; Hsiao, F. Association between Maternal Benzodiazepine or Z-Hypnotic Use in Early Pregnancy and the Risk of Stillbirth, Preterm Birth, and Small for Gestational Age: A Nationwide, Population-Based Cohort Study in Taiwan. Lancet Psychiatry 2023, 10, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, C.W.; Chuang, H.M.; Chen, L.K.; Hsiao, F. Benzodiazepine Use During Pregnancy and Risk of Miscarriage. JAMA Psychiatry 2023, 81, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, E.J. The Prevalence of Anxiety Disorders During Pregnancy and the Postpartum Period: A Multivariate Bayesian Meta-Analysis. Available online: https://www.psychiatrist.com/jcp/anxiety-disorders-in-pregnant-and-postpartum-women/ (accessed on 29 July 2025).
- Roddy Mitchell, A.; Gordon, H.; Atkinson, J.; Lindquist, A.; Walker, S.P.; Middleton, A.; Tong, S.; Hastie, R. Prevalence of Perinatal Anxiety and Related Disorders in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2343711. [Google Scholar] [CrossRef]
- Szpunar, M.; Freeman, M.; Kobylski, L.; Caplin, P.; Gaccione, P.; Viguera, A.; Chitayat, D.; Hernández-Díaz, S.; Cohen, L.S. Risk of Major Malformations in Infants After First-Trimester Exposure to Benzodiazepines. Depress. Anxiety 2022, 39, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Lee, H.; Choi, A.; Kwon, J.S.; Choe, S.A.; Chae, J.; Kim, J.-M.; Shin, J.-Y. First-Trimester Exposure to Benzodiazepines and Risk of Congenital Malformations. PLoS Med. 2022, 19, e1003945. [Google Scholar] [CrossRef] [PubMed]
- Krans, E.; Kim, J.; Chen, Q.; Rothenberger, S.; James, A.; Kelley, D.; Jarlenski, M.P. Outcomes Associated with the Use of Medications for Opioid Use Disorder During Pregnancy. Addiction 2021, 116, 3504–3514. [Google Scholar] [CrossRef]
- Banks, D.; Fentem, A.; Li, X.; Paschke, M.; Filiatreau, L.; Woolfolk, C.; Patricia, C.-R. Attitudes Toward Medication for Opioid Use Disorder Among Pregnant and Postpartum Women. J. Addict. Med. 2022, 17, 356–359. [Google Scholar] [CrossRef]
- Henkhaus, L.; Buntin, M.; Henderson, S.; Lai, P.; Patrick, S. Disparities in Receipt of Medications for Opioid Use Disorder Among Pregnant Women. Subst. Abus. 2021, 43, 508–513. [Google Scholar] [CrossRef]
- Hohman, E.E.; Corr, T.E.; Kawasaki, S.; Savage, J.S.; Downs, D.S. Nutritional Status Differs by Prescription Opioid Use among Women of Reproductive Age: NHANES 1999–2018. Nutrients 2023, 15, 1891. [Google Scholar] [CrossRef]
- Nowakowski, E.; Dayananda, S.; Morgan, M.; Jarvis, O.; Altamirano, V.; LaSorda, K.R.; Krans, E.; Lim, G. Obstetric Pain Management for Pregnant Women with Opioid Use Disorder: A Qualitative and Quantitative Comparison of Patient and Provider Perspectives (QUEST Study). Addiction 2023, 118, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Kelty, E.; Pyle, A.; Preen, D. Opioid Poisoning During Pregnancy: Prevalence, Characteristics, and Neonatal Outcomes. Arch. Women’s Ment. Health 2022, 25, 957–963. [Google Scholar] [CrossRef]
- Nielsen, S.; Tse, W.C.; Larance, B. Opioid Agonist Treatment for People Who Are Dependent on Pharmaceutical Opioids. In Cochrane Library; Nielsen, S., Ed.; John Wiley & Sons: London, UK, 2022. [Google Scholar]
- Whelan, P.J.; Remski, K. Buprenorphine vs Methadone Treatment: A Review of Evidence in Both Developed and Developing Worlds. J. Neurosci. Rural. Pract. 2012, 3, 45–50. [Google Scholar] [CrossRef]
- Zedler, B.K.; Mann, A.L.; Kim, M.M.; Amick, H.R.; Joyce, A.R.; Murrelle, E.L.; Jones, H.E. Buprenorphine Compared with Methadone to Treat Pregnant Women with Opioid Use Disorder: A Systematic Review and Meta-Analysis of Safety in the Mother, Fetus and Child. Addiction 2016, 111, 2115–2128. [Google Scholar] [CrossRef]
- Ahamad, K.; Hayashi, K.; Nguyen, P.; Dobrer, S.; Kerr, T.; Schütz, C.G.; Montaner, J.S.; Wood, E. Effect of Low-Threshold Methadone Maintenance Therapy for People Who Inject Drugs on HIV Incidence in Vancouver, BC, Canada: An Observational Cohort Study. Lancet HIV 2015, 2, e445–e450. [Google Scholar] [CrossRef]
- Vieiros, M.; Mirahi, A.; Villarreal, M.; Ramos-Triguero, A.; Fernández-Rubal, I.; Andreu-Férnández, V.; Simona, P.; García-Algar, Ó.; Marchei, E. Prevalence of Psychoactive Substance Use During Pregnancy in Argentine Women. Ther. Drug Monit. 2024, 46, 530–536. [Google Scholar] [CrossRef]
- Jarlenski, M.; Krans, E.; Chen, Q.; Rothenberger, S.; Cartus, A.; Zivin, K.; Bodnar, L. Substance Use Disorders and Risk of Severe Maternal Morbidity in the United States. Drug Alcohol Depend. 2020, 216, 108236. [Google Scholar] [CrossRef]
- Salemi, J.; Raza, S.; Modak, S.; Fields-Gilmore, J.A.; De Grubb, M.M.; Zoorob, R. Opiates, Cocaine, and Amphetamines Use During Pregnancy and Maternal Postpartum Readmission. Drug Alcohol Depend. 2020, 210, 107963. [Google Scholar] [CrossRef]
- Steane, S.E.; Young, S.L.; Clifton, V.L.; Gallo, L.A.; Akison, L.K.; Moritz, K.M. Prenatal Alcohol Consumption and Placental Outcomes: A Systematic Review and Meta-Analysis of Clinical Studies. Am. J. Obstet. Gynecol. 2021, 225, 607.e1–607.e22. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Strandberg-Larsen, K.; Gerds, T.A.; Torp-Pedersen, C.; Kesmodel, U.S. Heavy Prenatal Alcohol Exposure and Overall Morbidities: A Danish Nationwide Cohort Study from 1996 to 2018. Lancet Public Health 2023, 8, e36–e46. [Google Scholar] [CrossRef]
- Tin Tin, S.; Smith-Byrne, K.; Ferrari, P.; Rinaldi, S.; McCullough, M.L.; Teras, L.R.; Manjer, J.; Giles, G.; Le Marchand, L.; Haiman, C.A.; et al. Alcohol Intake and Endogenous Sex Hormones in Women: Meta-Analysis of Cohort Studies and Mendelian Randomization. Cancer 2024, 130, 3375–3386. [Google Scholar] [CrossRef]
- Andrade, C. Maternal Cannabis Use During Pregnancy and Maternal and Neonatal Adverse Outcomes. J. Clin. Psychiatry 2024, 85, 57493. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Slama, N.E.; Sarovar, V.; Terplan, M.; Ansley, D.; Adams, S.R.; Alexeeff, S.E. Trends in Self-Reported and Biochemically Verified Cocaine and Methamphetamine Use Among Pregnant Individuals in Northern California, 2011–2019. JAMA Netw. Open 2022, 5, e2248055. [Google Scholar] [CrossRef] [PubMed]
- Kendall-Tackett, K.; Poulin, S.; Garner, C. Health Problems Mediate the Effects of Adverse Childhood Experiences on Frequency of Cannabis Use in Pregnant and Breastfeeding Women. J. Interpers. Violence 2024, 40, 2518–2537. [Google Scholar] [CrossRef]
- Hayer, S.; Garg, B.; Wallace, J.; Prewitt, K.C.; Lo, J.O.; Caughey, A.B. Prenatal Methamphetamine Use Increases Risk of Adverse Maternal and Neonatal Outcomes. Am. J. Obstet. Gynecol. 2024, 231, 356.e1–356.e15. [Google Scholar] [CrossRef]
- Alsanie, W.F.; Abdelrahman, S.; Felimban, R.I.; Alkhatabi, H.A.; Gaber, A.; Alosimi, E.A.; Alhomrani, M.; Habeeballah, H.; Hauser, C.A.E.; Alamri, A.S.; et al. The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain. Int. J. Mol. Sci. 2023, 24, 5668. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Tinajero, Y.; Mo, L.; Schulkin, J.; Schmidt, L.; Wakeman, B.; Kremer, M. Obstetrical and Perinatal Outcomes of Patients with Methamphetamine-Positive Drug Screen on Labor and Delivery. Am. J. Obstet. Gynecol. MFM 2020, 2, 100195. [Google Scholar] [CrossRef]
- ELNahas, G.; Thibaut, F. Perinatal Psychoactive Substances Use: A Rising Perinatal Mental Health Concern. J. Clin. Med. 2023, 12, 2175. [Google Scholar] [CrossRef]
- Šlamberová, R. Drugs in Pregnancy: The Effects on Mother and Her Progeny. Physiol. Res. 2012, 61 (Suppl. S1), S123–S135. [Google Scholar] [CrossRef]
- Jarque, P.; Roca, A.; Gomila, I.; Marchei, E.; Tittarelli, R.; Elorza, M.; Sanchís, P.; Barceló, B. Role of Neonatal Biomarkers of Exposure to Psychoactive Substances to Identify Maternal Socio-Demographic Determinants. Biology 2021, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Breit, K.; Thomas, J. Prenatal Alcohol and Tetrahydrocannabinol Exposure: Effects on Spatial and Working Memory. Front. Neurosci. 2023, 17, 1192786. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Traccis, F.; Frau, R. Gender Differences in the Outcome of Offspring Prenatally Exposed to Drugs of Abuse. Front. Behav. Neurosci. 2020, 14, 72. [Google Scholar] [CrossRef]
- Ruisch, H.; Buitelaar, J.; Dietrich, A.; Glennon, J.; Hoekstra, P. Maternal Substance Use during Pregnancy and Offspring Conduct Problems: A Meta-Analysis. Neurosci. Biobehav. Rev. 2018, 84, 325–336. [Google Scholar] [CrossRef]
- Narkowicz, S.; Płotka, J.; Polkowska, Ż.; Biziuk, M.; Namieśnik, J. Prenatal Exposure to Substance of Abuse: A Worldwide Problem. Environ. Int. 2013, 54, 141–163. [Google Scholar] [CrossRef]
- Kalinina, A.; Semenova, M.; Bruter, A.; Varlamova, E.; Kubekina, M.; Pavlenko, N.; Silaeva, Y.; Deikin, A.; Antoshina, E.; Gorkova, T.; et al. Cyclophilin A as a Pro-Inflammatory Factor Exhibits Embryotoxic and Teratogenic Effects during Fetal Organogenesis. Int. J. Mol. Sci. 2023, 24, 11279. [Google Scholar] [CrossRef]
- Basal, W.; Ahmed, A.R.; Mahmoud, A.; Omar, A. Lufenuron Induces Reproductive Toxicity and Genotoxic Effects in Pregnant Albino Rats and Their Fetuses. Sci. Rep. 2020, 10, 19544. [Google Scholar] [CrossRef]
- Elangovan, V.R.; Saadat, N.; Ghnenis, A.; Padmanabhan, V.; Vyas, A. Developmental Programming: Adverse Sexually Dimorphic Transcriptional Programming of Gestational Testosterone Excess in Cardiac Left Ventricle of Fetal Sheep. Sci. Rep. 2023, 13, 2682. [Google Scholar] [CrossRef]
- Breton-Larrivée, M.; Elder, E.; Legault, L.M.; Langford-Avelar, A.; MacFarlane, A.J.; McGraw, S. Mitigating the Detrimental Developmental Impact of Early Fetal Alcohol Exposure Using a Maternal Methyl Donor-Enriched Diet. FASEB J. 2023, 37, e22829. [Google Scholar] [CrossRef]
- Gao, J.; Yan, D.; Yao, X. Risk of Teratogenicity in Continued Pregnancy after Gestational Exposure to Mifepristone and/or Misoprostol: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2024, 10, 1331–1342. [Google Scholar] [CrossRef]
- Madesh, S.; Sudhakaran, G.; Ramamurthy, K.; Sau, A.; Kathiravan, M.; Almutairi, M.; Almutairi, B.; Palaniappan, S.; Arockiaraj, J. Protective Role of 2-Aminothiazole Derivative against Ethanol-Induced Teratogenic Effects in-Vivo Zebrafish. Biochem. Pharmacol. 2024, 230, 116601. [Google Scholar] [CrossRef]
- Cui, M.; Qi, C.; Yang, L.; Zhang, M.; Wang, H.; She, G.; Yu, R.; Miao, T.; Sun, J. A Pregnancy Complication-Dependent Change in SIgA-Targeted Microbiota during Third Trimester. Food Funct. 2020, 11, 1513–1524. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational Diabetes Is Associated with Change in the Gut Microbiota Composition in Third Trimester of Pregnancy and Postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M. Regulation of Drug Transporter Expression and Function in the Placenta. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Cerveny, L.; Ceckova, M. Pharmacotherapy in Pregnancy; Effect of ABC and SLC Transporters on Drug Transport across the Placenta and Fetal Drug Exposure. J. Drug Target. 2012, 20, 736–763. [Google Scholar] [CrossRef] [PubMed]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.; Tulen, C.; Janssen, G.; Kaminski, I.; Geomini, I.; Lemmens, T.; Schiffers, P.; et al. Hypoxia-Induced Mitochondrial Abnormalities in Cells of the Placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef]
- Vaka, V.; McMaster, K.; Cunningham, M.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.; Amaral, L.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Osmanlıoğlu, H.Ö.; Yıldırım, M.K.; Akyuva, Y.; Yıldızhan, K.; Nazıroğlu, M. Morphine Induces Apoptosis, Inflammation, and Mitochondrial Oxidative Stress via Activation of TRPM2 Channel and Nitric Oxide Signaling Pathways in the Hippocampus. Mol. Neurobiol. 2020, 57, 3376–3389. [Google Scholar] [CrossRef]
- Nie, Q.-Y.; Yang, G.-M.; Zhang, P.; Dong, W.-J.; Jing, D.; Hou, Z.-P.; Peng, Y.-X.; Yu, Y.; Li, L.-H.; Hong, S.-J. Nrf2 Expression, Mitochondrial Fission, and Neuronal Apoptosis in the Prefrontal Cortex of Methamphetamine Abusers and Rats. Brain Res. 2024, 1837, 148973. [Google Scholar] [CrossRef]
- Darbinian, N.; Merabova, N.; Tatevosian, G.; Adele, S.; Darbinyan, A.; Morrison, M.; DeVane, C.; Ramamoorthy, S.; Goetzl, L.; Selzer, M. Prenatal Opioid and Alcohol Exposures: Association with Altered Placental Serotonin Transporter Structure and/or Expression. Int. J. Mol. Sci. 2024, 25, 11570. [Google Scholar] [CrossRef]
- Yu, J.-T.; Hu, X.-W.; Chen, H.-Y.; Yang, Q.; Li, H.-D.; Dong, Y.-H.; Zhang, Y.; Wang, J.-N.; Jin, J.; Wu, Y.-G.; et al. DNA Methylation of FTO Promotes Renal Inflammation by Enhancing m6A of PPAR-α in Alcohol-Induced Kidney Injury. Pharmacol. Res. 2020, 163, 105286. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C. Non-Canonical Nitric Oxide Signalling and DNA Hypomethylation: Inflammation Induced Epigenetic Alterations and Potential Drug Targets. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef]
- De Ganzo Suárez, T.; De Paco Matallana, C.; Plasencia, W. Spiral, Uterine Artery Doppler and Placental Ultrasound in Relation to Preeclampsia. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 92, 102426. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of Physiological Transformation and Spiral Artery Atherosis: Their Roles in Preeclampsia. Am. J. Obstet. Gynecol. 2020, 226, S895–S906. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssière, C.; Salvayre, R.; Nègre-Salvayre, A. Role of Oxidative Stress in the Dysfunction of the Placental Endothelial Nitric Oxide Synthase in Preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Choi, D.K. Epigenetic Regulation of Angiogenesis and Its Therapeutics. Genom. Inform. 2025, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S. Prenatal Substance Use and Developmental Disorders: Overview and Highlights. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Fahmy, C.; Jackson, D.B. Incarceration Exposure and Prescription Opioid Use During Pregnancy. Drug Alcohol Depend. 2022, 235, 109434. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, N.; Marchetti, D. Cocaine and Its Metabolites in the Placenta: A Systematic Review of the Literature. Reprod. Toxicol. 2012, 33, 1–14. [Google Scholar] [CrossRef]
- Nash, A.; Davies, L. Fetal Alcohol Spectrum Disorders: What Pediatric Providers Need to Know. J. Pediatr. Health Care 2017, 31, 594–606. [Google Scholar] [CrossRef]
- Syvertsen, J.L.; Toneff, H.; Howard, H.; Spadola, C.; Madden, D.; Clapp, J. Conceptualizing Stigma in Contexts of Pregnancy and Opioid Misuse: A Qualitative Study with Women and Healthcare Providers in Ohio. Drug Alcohol Depend. 2021, 222, 108677. [Google Scholar] [CrossRef]
- Gonzalez-Flores, D.; Marquez, A.; Casimiro, I. Oxidative Effects in Early Stages of Embryo Development Due to Alcohol Consumption. Int. J. Mol. Sci. 2024, 25, 4100. [Google Scholar] [CrossRef]
- Hasselström, J.; Mataix-Cols, D.; D’Onofrio, B.; Wang, X.; Zhang, T.; Jayaram-Lindström, N.; Chang, Z.; Ekheden, I.; Hellner, C.; Sidorchuk, A.; et al. Prenatal Exposure to Benzodiazepines and Z-Drugs in Humans and Risk of Adverse Neurodevelopmental Outcomes in Offspring: A Systematic Review. Neurosci. Biobehav. Rev. 2022, 137, 104647. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Wells, P.G. Developmental Role of Nuclear Factor E2-Related Factor 2 in Mitigating Methamphetamine Fetal Toxicity and Postnatal Neurodevelopmental Deficits. Free. Radic. Biol. Med. 2013, 65, 620–631. [Google Scholar] [CrossRef]
- Kaushal, N.; Elliott, M.; Robson, M.J.; Iyer, A.K.V.; Rojanasakul, Y.; Coop, A. AC927, a σ Receptor Ligand, Blocks Methamphetamine-Induced Release of Dopamine and Generation of Reactive Oxygen Species in NG108-15 Cells. Mol. Pharmacol. 2012, 81, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.; Qin, L.; Boyadjieva, N.; Crews, F.; Sarkar, D.; Zou, J. Neuroimmune Function and the Consequences of Alcohol Exposure. UNC Institutional Repos. 2025, 37, 331–351. [Google Scholar]
- Abdelwahab, M.; Petrich, M.; Wang, H.; Walker, E.; Cleary, E.M.; Rood, K.M. Risk factors for preterm birth among gravid individuals receiving buprenorphine for opioid use disorder. Am. J. Obstet. Gynecol. MFM 2022, 4, 100582. [Google Scholar] [CrossRef]
- Pfeifer, L.; Haile, Z. Unmet Mental Health Care Needs and Illicit Drug Use During Pregnancy. J. Addict. Med. 2020, 15, 233–240. [Google Scholar] [CrossRef]
- Yao, H.; Hu, D.; Wang, J.; Wu, W.; Zhao, H.H.; Wang, L.; Gleeson, J.; Haddad, G.G. Buprenorphine and methadone differentially alter early brain development in human cortical organoids. Neuropharmacology 2023, 239, 109683. [Google Scholar] [CrossRef]
- Staszewski, C.L.; Garretto, D.; Garry, E.T.; Ly, V.; Davis, J.A.; Herrera, K.M. Comparison of buprenorphine and methadone in the management of maternal opioid use disorder in full term pregnancies. J. Perinat. Med. 2020, 48, 677–680. [Google Scholar] [CrossRef]
- Connery, H.; Gray, K.; Terplan, M.; Huybrechts, K.; Bateman, B.; Lester, B.; Straub, L.; Davis, J.; Mogun, H.; Hernández-Díaz, S.; et al. Buprenorphine versus Methadone for Opioid Use Disorder in Pregnancy. N. Engl. J. Med. 2022, 387, 2033–2044. [Google Scholar] [CrossRef]
- DeVido, J.; Bogunovic, O.; Weiss, R.D. Alcohol Use Disorders in Pregnancy. Harv. Rev. Psychiatry 2015, 23, 112–121. [Google Scholar] [CrossRef]
- Marchand, G.; Masoud, A.T.; Govindan, M.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Ulibarri, H.; Parise, J.; Arroyo, A.; et al. Birth Outcomes of Neonates Exposed to Marijuana in Utero: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2145653. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.W.; Barfield, W.D.; Poindexter, B.B. Neonatal Opioid Withdrawal Syndrome. Pediatrics 2020, 146, e2020029074. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Allshouse, A.A.; McMillin, G.A.; Greene, T.; Chung, J.H.; Grobman, W.A.; Haas, D.M.; Mercer, B.M.; Parry, S.; Reddy, U.M.; et al. Cannabis Exposure and Adverse Pregnancy Outcomes Related to Placental Function. JAMA 2023, 330, 2191–2199. [Google Scholar] [CrossRef]
- Luke, S.; Hobbs, A.J.; Smith, M.; Riddell, C.; Murphy, P.; Agborsangaya, C.; Cantin, C.; Fahey, J.; Der, K.; Pederson, A.; et al. National Maternal Cannabis Working Group. Cannabis use in pregnancy and maternal and infant outcomes: A Canadian cross-jurisdictional population-based cohort study. PLoS ONE 2022, 17, e0276824. [Google Scholar] [CrossRef]
- Evans, K.; Wu, P.; Mamas, M.A.; Irwin, C.; Kang, P.; Perlow, J.H.; Foley, M.; Gulati, M. Substance Use in Pregnancy and its Association with Cardiovascular Events. JACC Adv. 2023, 2, 100619. [Google Scholar] [CrossRef]
- Hunsaker, J.J.H.; La’ulu, S.L.; LaGrave, D.; Murphy, W.; Reichman, H.A.; Snow, T.M.; McMillin, G.A.; Johnson-Davis, K.L.; Genzen, J.R. Tobacco and Cannabis Use During Pregnancy. Am. J. Clin. Pathol. 2022, 157, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Miskle, B.; Lynch, A.; Arndt, S.; Acion, L. Substance Use in Pregnancy: Identifying Stigma and Improving Care. Subst. Abus. Rehabil. 2021, 12, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Trainor, K. Maternal Substance Use Disorder: A Look at Provider Stigma, Attitudes, and Beliefs. Adv. Soc. Work 2022, 22, 67–90. [Google Scholar] [CrossRef]
- Serino, M.A.D.; Peterson, T.S.R.; Rosen, T.S. Psychological Functioning of Women Taking Illicit Drugs During Pregnancy and the Growth and Development of Their Offspring in Early Childhood. J. Dual Diagn. 2018, 14, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.S.; Melbostad, H.S.; Heil, S.H. Prevalence of Psychosocial Issues Among Pregnant Women Who Do and Do Not Use Illicit Substances. Psychol. Addict. Behav. 2024, 38, 205–210. [Google Scholar] [CrossRef]
- Pacho, M.; Aymerich, C.; Pedruzo, B.; Salazar de Pablo, G.; Sesma, E.; Bordenave, M.; Dieguez, R.; Lopez-Zorroza, I.; Herero, J.; Laborda, M.; et al. Substance use during pregnancy and risk of postpartum depression: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1264998. [Google Scholar] [CrossRef]
- Joyce, K.M.; Delaquis, C.P.; Alsaidi, T.; Sulymka, J.; Conway, A.; Garcia, J.; Paton, A.; Kelly, L.E.; Roos, L.E. Treatment for substance use disorder in mothers of young children: A systematic review of maternal substance use and child mental health outcomes. Addict. Behav. 2025, 163, 108241. [Google Scholar] [CrossRef]
- Minkoff, H.; Ecker, J. Balancing risks: Making decisions for maternal treatment without data on fetal safety. Am. J. Obstet. Gynecol. 2021, 224, 479–483. [Google Scholar] [CrossRef]
- Lacroix, T.; Cherry, J.; Grimaldi, M. Improving Early Detection and Management of Prenatal Substance Use Disorder. J. Natl. Med. Assoc. 2024, 116, 444. [Google Scholar] [CrossRef]
- Board, A.; D’Angelo, D.; Von Essen, B.S.; Denny, C.; Miele, K.; Dunkley, J.; Baillieu, R.; Kim, S. Polysubstance Use during Pregnancy: The Importance of Screening, Patient Education, and Integrating a Harm Reduction Perspective. Drug Alcohol Depend. 2023, 247, 109872. [Google Scholar] [CrossRef]
- McLafferty, L.P.; Becker, M.; Dresner, N.; Meltzer-Brody, S.; Gopalan, P.; Glance, J.; Victor, G.S.; Mittal, L.; Marshalek, P.; Lander, L.; et al. Guidelines for the Management of Pregnant Women with Substance Use Disorders. Psychosomatics 2016, 57, 115–130. [Google Scholar] [CrossRef]
- Krans, E.E.; Bobby, S.; England, M.; Gedekoh, R.H.; Chang, J.C.; Maguire, B.; Genday, P.; English, D.H. The Pregnancy Recovery Center: A Women-Centered Treatment Program for Pregnant and Postpartum Women with Opioid Use Disorder. Addict. Behav. 2018, 86, 124–129. [Google Scholar] [CrossRef]
- Frazer, Z.; McConnell, K.; Jansson, L.M. Treatment for Substance Use Disorders in Pregnant Women: Motivators and Barriers. Drug Alcohol Depend. 2019, 205, 107652. [Google Scholar] [CrossRef]
- Sujan, A.C.; Alexeeff, S.E.; Slama, N.E.; Goler, N.; Avalos, L.A.; Adams, S.R.; Conway, A.; Ansley, D.; Pal, A.; Gunn, R.L.; et al. Agreement Between Self-reports and Urine Toxicology Measures of Illicit Methamphetamine and Cocaine Use During Early Pregnancy. J. Addict. Med. 2024, 18, 28–32. [Google Scholar] [CrossRef]
- Chiodo, L.M.; Cosmian, C.; Pereira, K.; Kent, N.; Sokol, R.J.; Hannigan, J.H. Prenatal Alcohol Screening During Pregnancy by Midwives and Nurses. Alcohol. Clin. Exp. Res. 2019, 43, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Ingall, G.B. Alcohol Biomarkers. Clin. Lab. Med. 2012, 32, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Jańczewska, I.; Wierzba, J.; Cichoń-Kotek, M.; Jańczewska, A. Fetal Alcohol Spectrum Disorders Diagnostic Difficulties in the Neonatal Period and New Diagnostic Approaches. Dev. Period Med. 2019, 23, 60–66. [Google Scholar] [PubMed]
- Wurst, F.M.; Skipper, G.E.; Weinmann, W. Ethyl Glucuronide—The Direct Ethanol Metabolite on the Threshold from Science to Routine Use. Addiction 2003, 98, 51–61. [Google Scholar] [CrossRef]
- Kwak, H.-S.; Han, J.-Y.; Choi, J.-S.; Ahn, H.-K.; Ryu, H.-M.; Chung, H.-J.; Cho, D.-H.; Shin, C.-Y.; Velazquez-Armenta, E.Y.; Nava-Ocampo, A.A. Characterization of Phosphatidylethanol Blood Concentrations for Screening Alcohol Consumption in Early Pregnancy. Clin. Toxicol. 2014, 52, 25–31. [Google Scholar] [CrossRef]
- Ivorra, C.; García-Vicent, C.; Ponce, F.; Ortega-Evangelio, G.; Fernández-Formoso, J.A.; Lurbe, E. High Cotinine Levels Are Persistent during the First Days of Life in Newborn Second Hand Smokers. Drug Alcohol Depend. 2014, 134, 275–279. [Google Scholar] [CrossRef]
- Lowe, R.H.; Abraham, T.T.; Darwin, W.D.; Herning, R.; Cadet, J.L.; Huestis, M.A. Extended Urinary Delta9-Tetrahydrocannabinol Excretion in Chronic Cannabis Users Precludes Use as a Biomarker of New Drug Exposure. Drug Alcohol Depend. 2009, 105, 24–32. [Google Scholar] [CrossRef]
- Vandevenne, M.; Vandenbussche, H.; Verstraete, A. Detection Time of Drugs of Abuse in Urine. Acta Clin. Belg. 2000, 55, 323–333. [Google Scholar] [CrossRef]
- Vikingsson, S.; Krauss, S.T.; Winecker, R.E.; Flegel, R.R.; Hayes, E.D. Update on Urine Adulterants and Synthetic Urine Samples to Subvert Urine Drug Testing. J. Anal. Toxicol. 2022, 46, 697–704. [Google Scholar] [CrossRef]
- McMillin, G.A.; Wood, K.E.; Strathmann, F.G.; Krasowski, M.D. Patterns of Drugs and Drug Metabolites Observed in Meconium: What Do They Mean? Ther. Drug Monit. 2015, 37, 568–580. [Google Scholar] [CrossRef]
- de Jong, L.A.A.; Qurishi, R.; Stams, M.P.J.; Böttcher, M.; de Jong, C.A.J. Prolonged Ketamine and Norketamine Excretion Profiles in Urine After Chronic Use: A Case Series. J. Clin. Psychopharmacol. 2020, 40, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; Qiu, Y.; Kong, J.; Zhang, X. High Sensitive Electrochemical Methamphetamine Detection in Serum and Urine via Atom Transfer Radical Polymerization Signal Amplification. Talanta 2022, 238, 123026. [Google Scholar] [CrossRef]
- Estrada-Gutiérrez, G.; Zambrano, E.; Polo-Oteyza, E.; Cardona-Pérez, A.; Vadillo-Ortega, F. Intervention During the First 1000 Days in Mexico. Nutr. Rev. 2020, 78, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Navarro, S.; Serván-Mori, E.; Heredia-Pi, I.; Pelcastre-Villafuerte, B.; Nigenda, G. Ethnic Disparities in Sexual and Reproductive Health in Mexico After 25 Years of Social Policies. Sex. Res. Soc. Policy 2022, 19, 975–990. [Google Scholar] [CrossRef]
- Missler, M.; Donker, T.; Beijers, R.; Ciharova, M.; Moyse, C.; de Vries, R.; Denissen, J.; van Straten, A. Universal prevention of distress aimed at pregnant women: A systematic review and meta-analysis of psychological interventions. BMC Pregnancy Childbirth 2021, 21, 276. [Google Scholar] [CrossRef]
- Muhamad, Z.; Mahmudiono, T.; Abihail, C.T.; Sahila, N.; Wangi, M.P.; Suyanto, B.; Abdullah, N.A.B. Effectiveness of Nutrition Education Intervention Targeting Short-Statured Pregnant Women to Prevent Gestational Stunting. Nutrients 2023, 15, 4305. [Google Scholar] [CrossRef]
- Rutman, D.; Hubberstey, C.; Poole, N.; Schmidt, R.A.; Van Bibber, M. Multi-Service Prevention Programs for Pregnant and Parenting Women with Substance Use and Multiple Vulnerabilities. BMC Pregnancy Childbirth 2020, 20, 441. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; MacKinnon, K.; Dobbins, M.; Jack, S.M. Nurse-Family Partnership and Geography: An Intersectional Perspective. Glob. Qual. Nurs. Res. 2020, 7, 2333393619900888. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Allen, V.M.; Carson, G.; Graves, L.; Tanguay, R.; Green, C.R.; Cook, J.L. Guideline No. 443b: Opioid Use Throughout Women’s Lifespan: Opioid Use in Pregnancy and Breastfeeding. J. Obstet. Gynaecol. Can. 2023, 45, 102144. [Google Scholar] [CrossRef]
- Sany, S.T.; Eslami, V.; Lael-Monfared, E.; Ghavami, V.; Peyman, N. Self-Efficacy and Health Literacy Intervention on UTI Prevention in Pregnant Women. PLoS ONE 2024, 19, e0306558. [Google Scholar]
- Ott, M.A.; Hunt, A.L.; Katz, A.J.; Zaban, L.S. Community Resiliency in Rural Adolescent Pregnancy Prevention. Behav. Med. 2020, 46, 340–352. [Google Scholar] [CrossRef]
- Pimlott, N. The Legacy of Motherisk. Can. Fam. Physician 2020, 66, 787. [Google Scholar] [CrossRef]
- Kameg, B. Modernizing Perinatal Substance Use Management. Policy Politics Nurs. Pract. 2020, 22, 146–155. [Google Scholar] [CrossRef]
- Meinhofer, A.; Witman, A.; Maclean, J.; Bao, Y. Prenatal Substance Use Policies and Newborn Health. Health Econ. 2022, 31, 1452–1467. [Google Scholar] [CrossRef]
- Faherty, L.; Stein, B.; Terplan, M. Consensus Guidelines and State Policies: Gap Between Principle and Practice. Am. J. Obstet. Gynecol. MFM 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Thompson, T.; Taylor, K. Dismantling the Legacy of Failed Policy Approaches to Pregnant People’s Substance Use. Int. Rev. Psychiatry 2021, 33, 502–513. [Google Scholar] [CrossRef] [PubMed]



| Substance | Placental Transfer Mechanism * | Pharmacotoxicity and Teratogenic Mechanism | Principal Clinical Manifestations |
|---|---|---|---|
| Benzodiazepines [22] | Passive diffusion (lipophilic) | Placental uptake rises late in gestation; potentiation of fetal GABAA leads to sedation and hypotonia; first-trimester exposure occasionally causes orofacial and cardiac malformations | Fetus: Hypotonia, respiratory difficulties, sleep disturbance; early exposure may cause craniofacial and cardiac defects Mother: Sedation, dizziness and fall risk; excretion in breast milk may sedate the neonate via lactation |
| Opioids [75] | Passive diffusion (lipophilic, low protein binding) | μ-receptor agonism produces central nervous system (CNS) depression in the fetus and neuroadaptation that leads to neonatal abstinence syndrome involving CNS, gastrointestinal and autonomic systems | Fetus: Neonatal abstinence syndrome (irritability, hypertonia, tachypnoea, gastrointestinal disturbances) Mother: Risk of preterm labor, fetal growth restriction, high rates of postnatal depression and anxiety, increased perinatal morbidity and mortality |
| Cocaine [41,76] | Rapid passive diffusion (~80% of antipyrine) | Uteroplacental vasoconstriction and acute fetal ischemia; blockade of monoamine reuptake and oxidative stress in the fetal CNS | Fetus: Placental abruption, growth restriction, preterm birth, low birth weight, neurobehavioral deficits (attention, impulsivity) Mother: Risk of preterm labor, premature rupture of membranes and cardiac arrhythmias |
| Alcohol [77] | Passive diffusion (fetal/maternal ≈ 1:1) | Oxidative stress mediated by acetaldehyde accumulation and ROS, triggering apoptosis during organogenesis | Fetus: Microcephaly, facial dysmorphisms, intrauterine growth restriction, neurobehavioral deficits (FASD) Mother: Increased risk of spontaneous abortion, placental abruption, preeclampsia |
| Cannabis (THC) [78] | Active efflux transport limits fetal levels. | Binding to placental and fetal CB1 receptors alters endocannabinoid signaling and placental vascularization (labyrinth), causing vascular defects and symmetrically reduced growth | Fetus: Low birth weight, preterm birth, increased neonatal intensive care admission, neurobehavioral alterations (memory, attention deficits, anxiety) Mother: Gestational hypertension, pre-eclampsia, abnormal weight gain, placental abruption |
| Methamphetamines [45] | Passive diffusion with high placental uptake | Excessive GABAa potentiation triggers fetal sedation, hypotonia; first-trimester exposure linked to orofacial and cardiac malformations in isolated cases | Fetus: Hypotonia, respiratory difficulties, sleep disturbance; early exposure may cause craniofacial and cardiac defects Mother: Sedation, dizziness, fall risk; excretion in breast milk may sedate neonate |
| Substance Class | Principal Direct Biomarker(s) | Optimal Specimen(s) | Detection Window * | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Ethanol [112,113] | • Phosphatidylethanol (PEth) • Ethyl-glucuronide (EtG) • Fatty acid ethyl esters (FAEE) • Carbohydrate-deficient transferrin (CDT) | • Whole blood (PEth) • Urine (EtG) • Meconium/hair (FAEE) • Serum (CDT) | • PEth: ≤4 wk • EtG: ≤80 h urine, ≤3 mo hair • FAEE: ≥3 mo meconium/hair | PEth highly specific; FAEE + EtG extend window | • Incidental alcohol (EtG) • Microbial degradation (EtG) • Hair treatments (FAEE) |
| Nicotine [114] | • Cotinine | • Maternal urine /serum • Meconium • Hair | • ≤48 h (blood) • ≤1 wk (urine) • ≤3 mo (hair) | Quantifies active vs. passive smoke | Rapid clearance; overlap with nicotine-replacement therapy |
| Cannabis [115] | • Δ9-THC-COOH • 11-OH-THC | • Maternal blood /urine • Meconium • Cord tissue • Hair | • ≤36 h (blood) • ≤30 d (urine) • ≤3 mo (hair) | Distinguishes recent vs. historic use | Lipid partitioning leads to variable windows; hair external contamination |
| Cocaine [116] | • Benzoylecgonine • Cocaethylene (with ethanol) | • Maternal urine • Meconium • Hair | • ≤3 d (urine) • ≤3 mo (hair) | Parent + metabolite confirm timing | False positives from anesthetic use (rare) |
| Opioids [117] | • 6-Monoacetylmorphine (6-MAM, heroin) • Norbuprenorphine/nor-methadone | • Maternal urine • Meconium • Cord tissue | • 6-MAM ≤8 h • Metabolites ≤3 d (urine) • ≤3 mo (meconium/hair) | 6-MAM is heroin-specific; metabolite ratios indicate compliance with MOUD | Short 6-MAM window; poppy-seed ingestion confounder (morphine) |
| Benzodiazepines [118] | • Nordiazepam • Desmethyldiazepam | • Maternal urine • Hair | • ≤7 d (urine) • ≤90 d (hair) | Identifies chronic vs. intermittent use | Numerous analogs require panel testing |
| Ketamine/ Synthetic cathinones [119] | Norketamine • Parent cathinone | • Maternal urine • Hair | • ≤3 d (urine) • ≤3 mo (hair) | Distinctive metabolites | Limited obstetric validation |
| Methamphetamine/Amphetamine [120] | • d-Amphetamine • p-Hydroxy-methamphetamine | • Maternal urine • Meconium • Hair | • ≤3–5 d (urine) • ≤3 mo (hair) | Quantifies frequency/intensity | OTC sympathomimetics may cross-react |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Fernández, A.A.; Grajeda-Perez, J.A.; García-Alcázar, S.d.l.P.; Luis-Díaz, M.G.; Granada-Chavez, F.J.; Peña-Durán, E.; García-Galindo, J.J.; Suárez-Rico, D.O. Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects. Drugs Drug Candidates 2025, 4, 40. https://doi.org/10.3390/ddc4030040
Jiménez-Fernández AA, Grajeda-Perez JA, García-Alcázar SdlP, Luis-Díaz MG, Granada-Chavez FJ, Peña-Durán E, García-Galindo JJ, Suárez-Rico DO. Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects. Drugs and Drug Candidates. 2025; 4(3):40. https://doi.org/10.3390/ddc4030040
Chicago/Turabian StyleJiménez-Fernández, Atziri Alejandra, Joceline Alejandra Grajeda-Perez, Sofía de la Paz García-Alcázar, Mariana Gabriela Luis-Díaz, Francisco Javier Granada-Chavez, Emiliano Peña-Durán, Jesus Jonathan García-Galindo, and Daniel Osmar Suárez-Rico. 2025. "Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects" Drugs and Drug Candidates 4, no. 3: 40. https://doi.org/10.3390/ddc4030040
APA StyleJiménez-Fernández, A. A., Grajeda-Perez, J. A., García-Alcázar, S. d. l. P., Luis-Díaz, M. G., Granada-Chavez, F. J., Peña-Durán, E., García-Galindo, J. J., & Suárez-Rico, D. O. (2025). Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects. Drugs and Drug Candidates, 4(3), 40. https://doi.org/10.3390/ddc4030040










