3.1. Chemistry
3.1.1. General
Commercially available reagents were used without additional purification. Melting points were determined with an SM-LUX-POL Leitz hot-stage microscope (Leitz GMBH, Midland, ON, USA) and are uncorrected.
IR spectra were recorded on a NICOLET 380FT-IR spectrophotometer (Bruker BioSpin, Wissembourg, France). NMR spectra were recorded with tetramethylsilane as an internal standard using a BRUKER AVANCE 300 spectrometer (Bruker BioSpin, Wissembourg, France). Splitting patterns have been reported as follows: s = singlet; bs = broad singlet; d = doublet; t = triplet; q = quartet; dd = double doublet; ddd = double double doublet; qt = quintuplet; and m = multiplet.
Analytical TLCs were carried out on 0.25 precoated silica gel plates (POLYGRAM SIL G/UV254) (Merck KGaA, Darmstadt, Germany) and compounds were visualized after UV light irradiation. Silica gel 60 (70–230 mesh) was used for column chromatography. Mass spectra were recorded on an ESI LTQ Orbitrap Velos mass spectrometer (ThermoFisher, Bremen, Germany). Ionization was performed using an Electrospray ion source operating in positive ion mode with a capillary voltage of 3.80 kV and a capillary temperature of 250 °C. The scan type analyzed was full scan, and all MS recordings were in the m/z range between 150 to 2000 m/z. No fragmentation was carried out and the resolution used for the analysis was 60,000.
3.1.2. Synthesis of 1,3,-bis[(4-formylphenyl)methyl]benzene 3
To a suspension of α,α’-dibromo-m-xylene (2 mmol), 4-formylphenylboronic acid (5 mmol), and Pd(PPh3)4 (0.225 mmol) in THF (40 mL) under nitrogen were added 4 mL of a 2 M aqueous solution of Na2CO3. The reaction mixture was refluxed for 24 h. The suspension was then evaporated to dryness and extracted with AcOEt (2 × 30 mL). The organic layer was filtered and washed with water (2 × 40 mL). The organic layer was dried over sodium sulfate, filtered, and evaporated under reduced pressure. The crude residue was purified by column chromatography on silica gel using EtOAc/petroleum ether (v/v: 30/70) as eluent to give the pure product 2. Colourless oil (59%): 1H NMR (CDCl3) δ ppm: 9.97 (s, 2H, CHO), 7.80 (d, 4H, J = 8.10 Hz, H-3phen and H-5phen), 7.29 (d, 4H, J = 8.10 Hz, H-2phen and H-6phen), 7.25 (t, 1H, J = 7.2 Hz, Hbenz), 7.02 (s, 1H, Hbenz), 7.05 (d, 2H, J = 6.1 Hz, Hbenz), 3.92 (s, 4H, CH2); 13C NMR (CDCl3) δ ppm: 193.3 (CO), 149.6 (C-1phen), 141.6 (Cq-benz), 136.1 (C-4phen), 131.4 (C-3phen and C-5phen), 130.9 (C-2phen and C-6phen), 130.43 (CHbenz),129.4 (CHbenz), 128.6 (CHbenz), 43.4 (CH2).
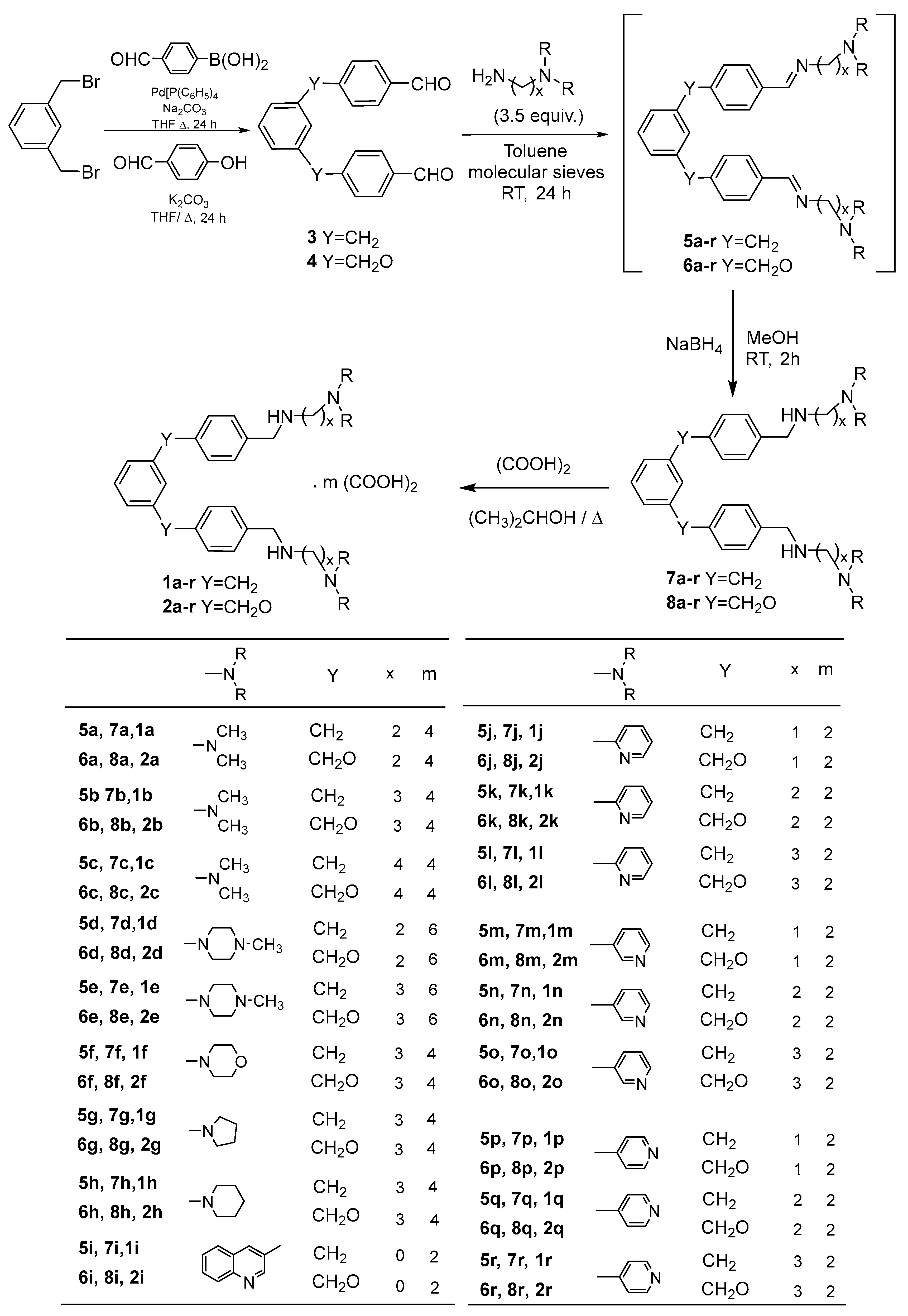
3.1.3. General Procedure for the Synthesis of 1,3,-bis[(4-(substituted-iminomethyl)phenyl)methyl]benzenes 5a–r
The 1,3-bis[(4-formylphenyl)methyl]benzene 3 (62 mg, 0.2 mmol) was dissolved in 6 mL of toluene. Activated molecular sieves 4 Å (800 mg) were then introduced in the reaction mixture, after which dialkylamine (0.5 mmol) was added. The reaction mixture was stirred in a stoppered flask for 24 h. The suspension that was obtained was filtered and washed with dichloromethane, and the solvent was removed under reduced pressure to afford the di-imine 5. The crude products were then used without further purification.
1,3,-bis[(4-(2-dimethylaminoethyl)iminomethyl)phenyl)methyl]benzene (5a)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.28 (s, 2H, CH=N), 7.65 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.22 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.18 (m, 1H, Hbenz), 7.03 (s, 2H, Hbenz), 7.02 (s, 1H, Hbenz), 3.96 (s, 4H, CH2), 3.75 (t, 4H, J = 6.90 Hz, NCH2), 2.67 (t, 4H, J = 6.90 Hz, NCH2), 2.33 (s, 12H, N(CH3)2).
1,3,-bis[(4-(3-dimethylaminopropyl)iminomethyl)phenyl)methyl]benzene (5b)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.30 (s, 2H, CH=N), 7.70 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.30 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.18 (m, 1H, Hbenz), 7.21 (s, 2H, Hbenz), 7.20 (s, 1H, Hbenz), 4.00 (s, 4H, CH2), 3.68 (t, 4H, J = 6.90 Hz, NCH2), 2.40 (t, 6H, J = 6.90 Hz, NCH2), 2.18 (s, 12H, N(CH3)2), 1.92 (qt, 4H, J = 8.40 Hz, CH2).
1,3-bis[(4-(4-dimethylaminobutyl)iminomethyl)phenyl)methyl]benzene (5c)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.24 (s, 2H, CH=N), 7.64 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.19 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.18 (m, 1H, Hbenz), 7.02 (s, 2H, Hbenz), 7.01 (s, 1H, Hbenz), 3.96 (s, 4H, CH2), 3.62 (t, 4H, J = 6.90 Hz, NCH2), 2.33 (t, 4H, J = 6.90 Hz, NCH2), 2.24 (s, 12H, N(CH3)2), 1.72-1.69 (m, 4H, CH2), 1.58–1.55 (m, 4H, CH2).
1,3-bis[(4-(2-(4-methylpiperazin-1-yl)ethyl)iminomethyl)phenyl)methyl]benzene (5d)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.25 (s, 2H, CH=N), 7.62 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.20 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.19 (m, 1H, Hbenz), 6.99 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 3.94 (s, 4H, CH2), 3.74 (t, 4H, J = 6.0 Hz, NCH2), 2.72 (t, 4H, J = 8.40 Hz, NCH2), 2.46 (s, 16H, NCH2pip), 2.28 (s, 6H, NCH3).
1,3,-bis[(4-(3-(4-methylpiperazin-1-yl)propyl)iminomethyl)phenyl)methyl]benzene (5e)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.25 (s, 2H, CH=N), 7.64 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.27 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.28 (m, 1H, Hbenz), 7.03 (s, 2H, Hbenz), 7.01 (s, 1H, Hbenz), 3.96 (s, 4H, CH2), 3.63 (t, 4H, J = 6.00 Hz, NCH2), 2.48 (t, 4H, J = 6.00 Hz, NCH2), 2.33 (s, 16H, NCH2pip), 2.28 (s, 6H, NCH3), 1.90–1.88 (m, 4H, CH2).
1,3,-bis[(4-(3-(morpholin-1-yl)propyl)iminomethyl)phenyl)methyl]benzene (5f)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.24 (s, 2H, CH=N), 7.62 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.19 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.20 (s, 1H, Hbenz), 7.01 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 3.94 (s, 4H, CH2), 3.69 (t, 8H, J = 4.20 Hz, OCH2), 3.58 (t, 4H, J = 6.9 Hz, NCH2), 2.39–2.28 (m, 10H, NCH2 and NCH2morph), 1.83 (qt, 4H, J = 6.90 Hz, CH2).
1,3-bis[(4-(3-(pyrrolidin-1-yl)propyl)iminomethyl)phenyl)methyl]benzene (5g)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.24 (s, 2H, CH=N), 7.62 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.20 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.19 (s, 1H, Hbenz), 7.01 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 3.95 (s, 4H, CH2), 3.64 (t, 4H, J = 6.60 Hz, NCH2), 2.54–2.47 (m, 12H, NCH2 and NCH2pyrrol), 1.94–1.92 (m, 4H, J = 6.90 Hz, CH2), 1.79–1.76 (m, 8H, CH2pyrrol).
1,3-bis[(4-(3-(piperidin-1-yl)propyl)iminomethyl)phenyl)methyl]benzene (5h)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.23 (s, 2H, CH=N), 7.62 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.19 (s, 1H, Hbenz), 7.18 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.01 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 3.94 (s, 4H, CH2), 3.60 (t, 4H, J = 6.90 Hz, NCH2), 2.42–2.31 (m, 12H, NCH2 and NCH2pip), 1.62–1.54 (m, 16H, CH2 and CH2pip).
1,3-bis{[4-((quinolin-3-yl)iminomethyl)phenyl]methyl}benzene (5i)
Pale-yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.92 (d, 2H, J = 2.70 Hz, H-2quinol), 8.59 (s, 2H, CH=N), 8.15-8.12 (m, 2H, H-8quinol), 8.02-7.99 (m, 2H, H-5quinol), 7.97 (d, 2H, J = 2.70 Hz, H-4quinol), 7.89 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.50–7.39 (m, 4H, H-6quinol, and H-7quinol), 7.23 (d, 4H, J = 7.80 Hz, H-2phen, and H-6phen), 7.19 (s, 1H, Hbenz), 7.01 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 4.05 (s, 4H, CH2).
1,3-bis[(4-(pyridin-2-ylmethyliminomethyl)phenyl)methyl]benzene (5j)
Pale-yellow oil (93%); 1H NMR (CDCl3) δ ppm: 8.58 (d, 2H, J = 6.90 Hz, H-6pyrid), 8.45 (s, 2H, CH=N), 7.75-7.67 (m, 6H, H-3phen, H-5phen and H-4pyrid), 7.42 (d, 2H, J = 6.90 Hz, H-5pyrid), 7.25–7.17 (m, 7H, H-2phen, H-6phen, H-3pyrid and Hbenz), 7.04 (s, 2H, Hbenz), 7.01 (s, 1H, Hbenz), 4.95 (s, 4H, NCH2), 3.98 (s, 4H, CH2).
1,3-bis[(4-(pyridin-2-ylethyliminomethyl)phenyl)methyl]benzene (5k)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.55 (d, 2H, J = 5.90 Hz, H-6pyrid), 8.17 (s, 2H, CH=N), 7.61-7.54 (m, 6H, H-3phen, H-5phen, and H-4pyrid), 7.20-7.09 (m, 9H, J = 8.10 Hz, H-2phen, H-6phen, H-3pyrid, Hbenz, and H-5pyrid), 7.01 (s, 2H, Hbenz), 7.00 (s, 1H, Hbenz), 4.00 (t, 4H, J = 7.20 Hz, NCH2), 3.94 (s, 4H, CH2), 3.18 (t, 4H, J = 7.20 Hz, CH2Pyrid).
1,3-bis[(4-(pyridin-2-ylpropyliminomethyl)phenyl)methyl]benzene (5l)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.54 (d, 2H, J = 5.80 Hz, H-6pyrid), 8.25 (s, 2H, CH=N), 7.61-7.54 (m, 6H, H-3phen, H-5phen, and H-4pyrid), 7.23–7.17 (m, 8H, J = 8.10 Hz, H-2phen, H-6phen, H-3pyrid, and H-5pyrid), 7.10 (m, 1H, Hbenz), 7.03 (s, 2H, Hbenz), 7.01 (s, 1H, Hbenz), 3.96 (s, 4H, CH2), 3.67 (t, 4H, J = 6.60 Hz, NCH2), 2.88 (t, 4H, J = 6.60 Hz, CH2Pyrid), 2.16 (qt, 4H, J = 6.60 Hz, CH2).
1,3-bis[(4-(pyridin-3-ylmethyliminomethyl)phenyl)methyl]benzene (5m)
Pale-yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.61 (d, 2H, J = 2.20 Hz, H-2pyrid), 8.54 (dd, 2H, J = 4.80 and 1.60 Hz, H-6pyrid), 8.40 (s, 2H, CH=N), 7.72–7.68 (m, 6H, H-3phen, H-5phen and H-4pyrid), 7.28–7.22 (m, 7H, H-2phen, H-6phen, Hbenz, and H-5pyrid), 7.04 (s, 2H, Hbenz), 7.03 (s, 1H, Hbenz), 4.80 (s, 4H, NCH2), 3.98 (s, 4H, CH2).
1,3-bis[(4-(pyridin-3-ylethyliminomethyl)phenyl)methyl]benzene (5n)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.52-8.44 (m, 4H, H-2pyrid and H-6pyrid), 8.13 (s, 2H, CH=N), 7.63 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.53-7.54 (d, 2H, J = 7.80 Hz H-4pyrid), 7.27–7.20 (m, 7H, H-2phen, H-6phen, Hbenz, and H-5pyrid), 7.05 (s, 2H, Hbenz), 7.02 (s, 1H, Hbenz), 3.97 (s, 4H, CH2), 3.85 (t, 2H, J = 7.20 Hz, NCH2), 3.02 (t, 4H, J = 7.20 Hz, CH2Pyrid).
1,3-bis[(4-(pyridin-3-ylpropyliminomethyl)phenyl)methyl]benzene (5o)
Yellow oil (91%); 1H NMR (CDCl3) δ ppm: 8.52 (d, 2H, J = 2.20 Hz, H-2pyrid), 8.49 (dd, 2H, J = 4.80 and 1.60 Hz, H-6pyrid), 8.28 (s, 2H, CH=N), 7.70 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.56–7.53 (m, 2H, H-4pyrid), 7.32-7.18 (m, 7H, H-5pyrid, Hbenz, H-2phen, and H-6phen), 7.04 (s, 2H, Hbenz), 7.03 (s, 1H, Hbenz), 4.01 (s, 4H, CH2), 3.65 (t, 4H, J = 6.60 Hz, NCH2), 2.74 (t, 4H, J = 6.60 Hz, CH2Pyrid), 2.08 (qt, 4H, J = 6.60 Hz, CH2).
1,3-bis[(4-(pyridin-4-ylmethyliminomethyl)phenyl)methyl]benzene (5p)
Pale-yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.54 (d, 4H, J = 5.40 Hz, H-2pyrid, and H-6pyrid), 8.37 (s, 2H, CH=N), 7.72 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.28–7.23 (m, 9H, H-2phen, H-6phen, Hbenz, H-3pyrid, and H-5pyrid), 7.04 (s, 2H, Hbenz), 7.03 (s, 1H, Hbenz), 4.78 (s, 4H, NCH2), 3.98 (s, 4H, CH2).
1,3-bis[(4-(pyridin-4-ylethyliminomethyl)phenyl)methyl]benzene (5q)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.50–8.48 (m, 4H, H-2pyrid, and H-6pyrid), 8.12 (s, 2H, CH=N), 7.62 (d, 4H, J = 8.20 Hz, H-3phen, and H-5phen), 7.20 (d, 4H, J = 8.20 Hz, H-2phen, and H-6phen), 7.16–7.14 (m, 5H, H-3pyrid, and H-5pyrid, Hbenz), 7.03 (s, 2H, Hbenz), 7.02 (s, 1H, Hbenz), 3.96 (s, 4H, CH2), 3.85 (t, 4H, J = 7.20 Hz, NCH2), 3.00 (t, 4H, J = 7.20 Hz, CH2Pyrid).
1,3-bis[(4-(pyridin-4-ylpropyliminomethyl)phenyl)methyl]benzene (5r)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.50 (d, 4H, J = 5.40 Hz, H-2pyrid, and H-6pyrid), 8.25 (s, 2H, CH=N), 7.68 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.28–7.24 (m, 4H, H-3pyrid, and H-5pyrid),), 7.18 (s, 1H, Hbenz), 7.15 (d, 4H, J = 8.20 Hz, H-2phen, and H-6phen), 7.06 (s, 2H, Hbenz), 7.05 (s, 1H, Hbenz) 3.99 (s, 4H, CH2), 3.64 (t, 4H, J = 7.20 Hz, NCH2), 2.72 (t, 4H, J = 7.20 Hz, CH2Pyrid), 2.07 (qt, 6H, J = 7.20 Hz, CH2).
3.1.4. General Procedure for the Synthesis of 1,3-bis[(4-(Substituted-Aminomethyl)Phenyl)Methyl]Benzenes 7a–r
To a solution of compounds 5a–r (0.4 mmol) in methanol (10 mL) was added, portion-wise at 0 °C, sodium borohydride (2.4 mmol, 6 eq.). The reaction mixture was then stirred at room temperature for 2 h. Then, it was evaporated to dryness under reduced pressure. After cooling, the residue was triturated in water and extracted with dichloromethane (40 mL). The organic layer was separated, dried over sodium sulfate and activated charcoal, and evaporated to dryness. The residue was then purified by column chromatography on silica gel using dichloromethane/methanol (90/10: v/v) as eluent to give the pure products 7a–r.
1,3-bis[(4-(2-dimethylaminoethyl)aminomethyl)phenyl)methyl]benzene (7a)
Yellow oil (44%); 1H NMR (CDCl3) δ ppm: 7.38 (m, 1H, Hbenz), 7.23 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.11 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.01 (s, 2H, Hbenz), 6.98 (s, 1H, Hbenz), 3.92 (s, 4H, CH2), 3.78 (s, 4H, NCH2), 2.70 (t, 4H, J = 6.90 Hz, NCH2), 2.43 (t, 4H, J = 6.90 Hz, NCH2), 2.21 (s, 12H, N(CH3)2); 13C NMR (CDCl3) δ ppm: 141.29 (C-4phenyl), 141.17 (Cqbenz), 137.81 (C-1phenyl), 130.24 (C-3phen and C-5phen), 129.65 (C-2phen and C-6phen), 128.81 (CHbenz), 126.66 (CHbenz), 58.90 (NCH2), 53.66 (NCH2), 46.47 (NCH2), 45.44 (N(CH3)2), 41.53 (CH2). ESI-MS m/z [M+H]+ calculated for C30H43N4: 459.3488, found: 459.3474.
1,3-bis[(4-(3-dimethylaminopropyl)aminomethyl)phenyl)methyl]benzene (7b)
Yellow oil (51%); 1H NMR (CDCl3) δ ppm: 7.25 (m, 1H, Hbenz), 7.20 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.12 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 6.98 (m, 3H, Hbenz), 3.95 (s, 4H, CH2), 3.76 (s, 4H, NCH2), 2.68 (t, 4H, J = 7.10 Hz, NCH2), 2.33 (t, 4H, J = 7.10 Hz, NCH2), 2.22 (s, 12H, N(CH3)2), 1.69 (qt, 4H, J = 7.10 Hz, CH2); 13C NMR (CDCl3) δ ppm: 142.60 (C-4phenyl), 141.13 (Cqbenz), 139.41 (C-1phenyl), 130.94 (CHbenz), 130.29 (C-3phen and C-5phen), 129.91 (CHbenz), 129.61 (C-2phen and C-6phen), 128.84 (CHbenz), 59.40 (NCH2), 55.10 (NCH2), 49.22 (NCH2), 46.90 (N(CH3)2), 42.91 (CH2), 29.32 (CH2). ESI-MS m/z [M + 2H]+ calculated for C32H47N4: 487.3722, found: 487.3784.
1,3-bis[(4-(4-dimethylaminobutyl)aminomethyl)phenyl)methyl]benzene (7c)
Yellow oil (85%); 1H NMR (CDCl3) δ ppm: 7.22 (d, 4H, J = 7.90 Hz, H-3phen, and H-5phen), 7.17 (m, 1H, Hbenz), 7.13 (d, 4H, J = 7.90 Hz, H-2phen, and H-6phen), 7.00 (m, 3H, Hbenz), 3.91 (s, 4H, CH2), 3.75 (s, 4H, NCH2), 2.65 (t, 4H, J = 6.90 Hz, NCH2), 2.24 (t, 4H, J = 6.90 Hz, NCH2), 2.20 (s, 12H, N(CH3)2), 1.54–1.51 (m, 8H, CH2); 13C NMR (CDCl3) δ ppm: 142.61 (C-4phenyl), 141.30 (Cqbenz), 138.97 (C-1phenyl), 130.35 (C-3phen and C-5phen), 130.95 (CHbenz), 129.92 (C-2phen and C-6phen), 128.41 (CHbenz), 128.06 (CHbenz), 60.88 (NCH2), 54.87 (NCH2), 50.49 (NCH2), 46.87 (N(CH3)2), 42.91 (CH2), 29.14 (CH2), 26.77 (CH2). ESI-MS m/z [M + H]+ calculated for C34H51N4: 515.4035, found: 515.5112.
1,3-bis[(4-(2-(4-methylpiperazin-1-yl)ethyl)aminomethyl)phenyl)methyl]benzene (7d)
Yellow oil (61%); 1H NMR (CDCl3) δ ppm: 7.21 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.14 (m, 1H, Hbenz), 7.13 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.03 (m, 3H, Hbenz), 3.90 (s, 4H, CH2), 3.64 (s, 4H, NCH2), 2.46 (t, 4H, J = 6.90 Hz, NCH2), 2.50 (t, 4H, J = 6.90 Hz, NCH2), 2.48–2.35 (m, 16H, NCH2piperazine), 2.27 (s, 6H, NCH3); 13C NMR (CDCl3) δ ppm: 142.65 (C-4phenyl), 141.17 (Cqbenz), 139.39 (C-1phenyl), 130.92 (CHbenz), 130.29 (C-3phen and C-5phen), 129.58 (C-2phen and C-6phen), 128.38 (CHbenz),128.03 (CHbenz), 58.98 (NCH2), 56.30 (NCH2piperazine), 54.97 (NCH2), 54.44 (NCH2piperazine), 47.37 (NCH3), 46.90 (NCH2), 42.90 (CH2). ESI-MS m/z [M + H]+ calculated for C36H53N6: 569.4253, found: 569.43.
1,3-bis[(4-(3-(4-methylpiperazin-1-yl)propyl)aminomethyl)phenyl)methyl]benzene (7e)
Yellow oil (75%); 1H NMR (CDCl3) δ ppm: 7.20 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.16 (m, 3H, Hbenz), 7.12 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 6.99 (m, 3H, Hbenz), 3.91 (s, 4H, CH2), 3.72 (s, 4H, NCH2), 2.65 (t, 4H, J = 7.10 Hz, NCH2), 2.37 (t, 4H, J = 7.10 Hz, NCH2), 2.41–2.34 (m, 16H, NCH2piperazine), 2.25 (s, 6H, NCH3), 1.72 (qt, 4H, J = 7.10 Hz, CH2); 13C NMR (CDCl3) δ ppm: 142.59 (C-4phenyl), 141.10 (Cqbenz), 139.35 (C-1phenyl), 130.90 (CHbenz), 130.25 (C-3phen and C-5phen), 130.26 (CHbenz), 129.55 (C-2phen and C-6phen), 128.07 (CHbenz), 59.10 (NCH2), 56.45 (NCH2piperazine), 55.05 (NCH2), 54.55 (NCH2piperazine), 49.46 (NCH2), 47.39 (NCH3), 42.88 (CH2), 28.20 (CH2). ESI-MS m/z [M + H]+ calculated for C38H57N6: 597.456, found: 597.4630.
1,3-bis[(4-(3-(morpholin-1-yl) propyl)aminomethyl)phenyl)methyl]benzene (7f)
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 7.40 (m, 1H, Hbenz), 7.27 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.11 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 6.99 (m, 3H, Hbenz), 3.93 (s, 4H, CH2), 3.71 (s, 4H, NCH2), 3.69 (t, 8H, J = 4.65 Hz, OCH2), 2.69 (t, 4H, J = 6.90 Hz, NCH2), 2.44–2.37 (m, 12H, NCH2 morpholine, NCH2), 1.71 (qt, 4H, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 142.64 (C-4phenyl), 141.23 (Cqbenz), 139.30 (C-1phenyl), 130.94 (CHbenz), 130.32 (C-3phen and C-5phen), 130.01 (CHbenz), 129.94 (C-2phen and C-6phen), 128.82 (CHbenz), 68.35 (OCH2), 58.76 (NCH2), 55.16 (NCH2morpholine), 55.04 (NCH2), 49.35 (NCH2), 42.82 (CH2), 27.94 (CH2). ESI-MS m/z [M + H]+ calculated for C36H51N4O2: 571.3934, found: 571.40.
1,3-bis[(4-(3-(pyrrolidin-1-yl)propyl)aminomethyl)phenyl)methyl]benzene (7g)
Yellow oil (84%); 1H NMR (CDCl3) δ ppm: 7.24 (m, 1H, Hbenz), 7.20 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.17 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.05 (m, 3H, Hbenz), 3.92 (s, 4H, CH2), 3.76 (s, 4H, NCH2), 2.70 (t, 4H, J = 6.90 Hz, NCH2), 2.53–2.48 (m, 12H, NCH2, and NCH2pyrrolidine), 1.79–1.72 (m, 12H, CH2, and CH2pyrrolidine); 13C NMR (CDCl3) δ ppm: 141.29 (C-4phenyl), 139.74 (Cqbenz), 138.11 (C-1phenyl), 129.58 (CHbenz), 128.92 (C-3phen and C-5phen), 128.55 (CHbenz), 128.21 (C-2phen and C-6phen), 126.67 (CHbenz), 54.77 (NCH2), 54.25 (NCH2pyrrolidine), 53.67 (NCH2), 48.07 (NCH2), 41.55 (CH2), 29.19 (CH2), 23.44 (CH2pyrrolidine). ESI-MS m/z [M + H]+ calculated for C36H51N4: 539.035, found: 539.41.
1,3-bis[(4-(3-(piperidin-1-yl)propyl)aminomethyl)phenyl)methyl]benzene (7h)
Yellow oil (95%); 1H NMR (CDCl3) δ ppm: 7.24 (m, 1H, Hbenz), 7.20 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.17 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 7.01–6.98 (m, 3H, Hbenz), 3.92 (s, 4H, CH2), 3.75 (s, 4H, NCH2), 2.69 (t, 4H, J = 6.90 Hz, NCH2), 2.39–2.32 (m, 12H, NCH2 and NCH2piperidine), 1.71 (qt, 4H, J = 6.90 Hz, CH2), 1.61–1.50 (m, 8H, CH2piperidine), 1.44–1.40 (m, 4H, CH2piperidine); 13C NMR (CDCl3) δ ppm: 142.67 (C-4phenyl), 141.11 (Cqbenz), 139.45 (C-1phenyl), 130.95 (CHbenz), 130.30 (C-3phen and C-5phen), 129.93 (CHbenz), 129.61 (C-2phen and C-6phen), 128.05 (CHbenz), 59.15 (NCH2), 56.04 (NCH2 piperidine), 55.06 (NCH2), 49.63 (NCH2), 42.92 (CH2), 28.34 (CH2), 27.35 (CH2piperidine), 25.84 (CH2piperidine). ESI-MS m/z [M + H]+ calculated for C38H55N4: 567.4348, found: 567.4424.
1,3-bis[(4-((quinolin-3-yl)aminomethyl)phenyl)methyl]benzene (7i)
Pale-yellow oil (87%); 1H NMR (CDCl3) δ ppm: 8.47 (d, 2H, J = 2.70 Hz, H-2quinol), 7.98 (dd, 2H, J = 6.90 and 3.60 Hz, H-8quinol), 7.57 (dd, 2H, J = 6.90 and 3.60 Hz, H-5quinol), 7.46–7.39 (m, 4H, H-6quinol and H-7quinol), 7.29 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.24 (m, 1H, Hbenz), 7.15 (d, 4H, J = 7.80 Hz, H-2phen, and H-6phen), 7.13 (d, 2H, J = 2.70 Hz, H-4quinol), 6.98 (m, 3H, Hbenz), 4.34 (d, 4H, J = 5.1 Hz, NCH2); 3.44 (s, 4H, CH2); 13C NMR (CDCl3) δ ppm: 144.70 (C-2quinol), 144.50 (C-8aquinol), 143.4 (C-4phenyl and C-3quinol), 142.93 (Cqbenz), 137.43 (C-1phenyl), 137.3 (C-4aquinol), 130.61 (C-3phen and C-5phen), 130.35 (C-4quinol), 129.02 (C-2phen and C-6phen), 129.00 (C-8quinol), 128.33 (C-5quinol), 127.37 (C-6quinol), 127.30 (C-7quinol), 116.32, (CHbenz), 111.75 (CHbenz), 48.96 (NCH2), 42.94 (CH2). ESI-MS m/z [M + H]+ calculated for C40H35N4: 571.2783, found: 571.2860.
1,3-bis[(4-(pyridin-2-ylmethylaminomethyl)phenyl)methyl]benzene (7j)
Yellow oil (80%); 1H NMR (CDCl3) δ ppm: 8.57 (d, 2H, J = 6.90 Hz, H-6pyrid), 7.65–7.60 (m, 2H, H-4pyrid), 7.33–7.30 (m, 6H, H-3phen, H-5phen, and H-3pyrid), 7.21 (s, 1H, Hbenz), 7.20–7.13 (m, 6H, H-6phen, H-2phen, and H-5pyrid), 7.07–7.00 (m, 3H, Hbenz), 3.94 (m, 8H, NCH2), 3.82 (s, 4H, CH2). 13C NMR (CDCl3) δ ppm: 161.18 (C-2pyrid), 150.70 (C-6pyrid), 142.69 (C-4phenyl), 141.25 (Cqbenz), 139.24 (C-1phenyl), 137.82 (C-4pyrid), 130.98 (CHbenz), 130.34 (C-3phen and C-5phen), 129.95 (C-2phen and C-6phen), 128.46 (CHbenz), 128.08 (CHbenz), 123.75 (C-3pyrid), 123.32 (C-5pyrid), 55.92 (NCH2), 54.61 (NCH2), 42.94 (CH2). ESI-MS m/z [M + H]+ calculated for C34H35N4: 499.2783, found: 499.2860.
1,3-bis[(4-(pyridin-2-ylethylaminomethyl)phenyl)methyl]benzene (7k)
Yellow oil (95%); 1H NMR (CDCl3) δ ppm: 8.54-8.51 (m, 2H, H-6pyrid), 7.60–7.52 (m, 2H, H-4pyrid), 7.39–7.36 (m, 1H, Hbenz), 7.20-7.11 (m, 6H, H-3phen, H-5phen, and H-3pyrid), 7.09–7.05 (m, 6H, H-6phen, H-2phen, and H-5pyrid), 7.02–6.98 (m, 3H, Hbenz), 3.92 (s, 4H, NCH2), 3.79 (s, 4H, CH2), 3.05–3.01 (m, 8H, NCH2, and CH2Pyrid); 13C NMR (CDCl3) δ ppm: 161.61 (C-2pyrid), 150.69 (C-6pyrid), 142.67 (C-4phenyl), 141.17 (Cqbenz), 139.30 (C-1phenyl), 137.78 (C-4pyrid), 130.96 (CHbenz), 129.94 (C-3phen and C-5phen), 129.65 (C-2phen and C-6phen), 128.40 (CHbenz), 128.06 (CHbenz), 124.71 (C-3pyrid), 122.66 (C-5pyrid), 54.93 (NCH2), 50.22 (NCH2), 42.92 (CH2), 39.74 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4:: 527.3096, found: 527.3166.
1,3-bis[(4-(pyridin-2-ylpropylaminomethyl)phenyl)methyl]benzene (7l).
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 8.51–8.49 (m, 2H, H-6pyrid), 7.58–7.53 (m, 2H, H-4pyrid), 7.24 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.24–7.22 (m, 1H, Hbenz), 7.23–7.07 (m, 8H, H-6phen, H-2phen, H-5pyrid, and H-3pyrid), 7.01–6.99 (m, 3H, Hbenz), 3.92 (s, 4H, NCH2), 3.74 (s, 4H, CH2), 2.83 (t, 4H, J = 6.90 Hz, NCH2), 2.68 (t, 4H, J = 6.90 Hz, CH2Pyrid), 1.95 (qt,4H J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 163.17 (C-2pyrid), 150.72 (C-6pyrid), 142.69 (C-4phenyl), 141.16 (Cqbenz), 139.44 (C-1phenyl), 137.86 (C-4pyrid), 130.96 (CHbenz), 130.31 (C-3phen and C-5phen), 128.76 (C-2phen and C-6phen), 128.73 (CHbenz), 128.03 (CHbenz), 124.19 (C-3pyrid), 122.44 (C-5pyrid), 54.98 (NCH2), 50.12 (NCH2), 42.92 (CH2), 37.34 (CH2), 31.41 (CH2). ESI-MS m/z [M + H]+ calculated for C38H43N4: 555.3409, found: 555.3476.
1,3-bis[(4-(pyridin-3-ylmethylaminomethyl)phenyl)methyl]benzene (7m)
Pale-yellow oil (80%); 1H NMR (CDCl3) δ ppm: 8.58–8.51 (m, 4H, H-6pyrid, and H-3pyrid), 7.72 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.28–7.26 (m, 6H, H-5pyrid, H-3phen, and H-5phen), 7.21 (m, 1H, Hbenz), 7.15 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 7.07–7.01 (m, 3H, Hbenz), 3.92 (s, 4H, NCH2), 3.82 (s, 4H, NCH2), 3.78 (s, 4H, CH2); 13C NMR (CDCl3) δ ppm: 151.15 (C-2pyrid), 149.89 (C-6pyrid), 142.63 (C-4phenyl), 141.43 (Cqbenz), 138.99 (C-1phenyl), 137.25 (C-4pyrid), 137.24 (C-3pyrid), 130.98 (CHbenz), 130.40 (C-3phen and C-5phen), 129.66 (C-2phen and C-6phen), 128.52 (CHbenz), 128.11 (CHbenz), 124.81 (C-5pyrid), 54.28 (NCH2), 51.82 (NCH2), 42.93 (CH2). ESI-MS m/z [M + H]+ calculated for C34H35N4: 499.2783, found: 499.2855.
1,3-bis[(4-(pyridin-3-ylethylaminomethyl)phenyl)methyl]benzene (7n)
Yellow oil (66%); 1H NMR (CDCl3) δ ppm: 8.48–8.46 (m, 4H, H-6pyrid and H-3pyrid), 7.53 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.23–7.18 (m, 6H, H-5pyrid, H-3phen and H-5phen), 7.20–7.12 (m, 1H, Hbenz), 7.12 (d, 6H, J = 7.80 Hz, H-6phen and H-2phen), 7.03–7.00 (m, 3H, Hbenz), 3.93 (s, 4H, NCH2), 3.78 (s, 4H,NCH2), 2.91-2.82 (m, 8H,CH2 and CH2Pyrid); 13C NMR (CDCl3) δ ppm: 151.53 (C-2pyrid), 149.06 (C-6pyrid), 142.64 (C-4phenyl), 141.32 (Cqbenz), 139.16 (C-1phenyl), 137.58 (C-4pyrid), 136.84 (C-3pyrid), 130.96 (CHbenz), 130.36 (C-3phen and C-5phen), 129.97 (C-2phen and C-6phen), 129.19 (CHbenz), 129.09 (CHbenz), 124.77 (C-5pyrid), 54.94 (NCH2), 51.53 (NCH2), 42.92 (CH2), 34.97 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4: 527.3096, Found: 527.3169.
1,3-bis[(4-(pyridin-3-ylpropylaminomethyl)phenyl)methyl]benzene (7o).
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 8.46–8.41 (m, 4H, H-6pyrid and H-3pyrid), 7.8 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.21 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.23–7.21 (m, 2H, H-5pyrid), 7.20–7.12 (m, 1H, Hbenz), 7.09 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 7.01–6.99 (m, 3H, Hbenz), 3.92 (s, 4H, NCH2), 3.74 (s, 4H, CH2), 2.68–2.63 (m, 8H, NCH2, and CH2Pyrid), 1.82 (qt, 4H, J = 7.20 Hz, CH2); 13C NMR (CDCl3) δ ppm: 151.24 (C-2pyrid), 148.68 (C-6pyrid), 142.65 (C-4phenyl), 141.26 (Cqbenz), 139.28 (C-1phenyl), 138.76 (C-3pyrid), 137.23 (C-4pyrid), 130.96 (CHbenz), 130.33 (C-3phen and C-5phen), 129.97 (CHbenz), 129.65 (C-2phen and C-6phen), 128.08 (CHbenz), 124.71 (C-5pyrid), 55.08 (NCH2), 49.96 (NCH2), 42.91 (CH2), 32.76 (CH2), 32.00 (CH2). ESI-MS m/z [M + H]+ calculated for C38H43N4: 555.3409, found: 555.3480.
1,3-bis[(4-(pyridin-4-ylmethylaminomethyl)phenyl)methyl]benzene (7p).
Yellow oil (60%); 1H NMR (CDCl3) δ ppm: 8.54 (d, 4H, J = 6.00 Hz, H-2pyrid, and H-6pyrid), 7.72 (d, 4H, J = 6.00 Hz, H-3pyrid, and H-5pyrid), 7.28–7.23 (m, 8H, H-3phen, H-5phen, H-6phen, and H-2phen), 7.21 (m, 1H, Hbenz), 7.04 (m, 3H, Hbenz), 4.77 (s, 4H, NCH2), 3.98 (s, 4H, NCH2), 2.34 (s, 4H, CH2); 13C NMR (CDCl3) δ ppm: 151.16 (C-2pyrid and C-6pyrid), 150.6 (C-4pyrid), 142.61 (C-4phenyl), 141.47 (Cqbenz), 138.89 (C-1phenyl), 130.96 (CHbenz), 130.39 (C-3phen and C-5phen), 130.00 (CHbenz), 129.65 (C-2phen and C-6phen), 128.11 (CHbenz), 124.39 (C-3pyrid and C-5pyrid), 54.29 (NCH2), 53.21 (NCH2), 42.96 (CH2). ESI-MS m/z [M + H]+ calculated for C34H35N4: 499.2783, found: 499.2853.
1,3-bis[(4-(pyridin-4-ylethylaminomethyl)phenyl)methyl]benzene (7q).
Yellow oil (89%); 1H NMR (CDCl3) δ ppm: 8.47–8.45 (m, 6H, H-2pyrid, and H-6pyrid), 7.56 (d, 4H, J = 6.00 Hz, H-3pyrid, and H-5pyrid), 7.28–7.23 (m, 9H, H-3phen, H-5phen, Hbenz H-6phen, and H-2phen), 7.02–7.00 (m, 3H, Hbenz), 3.93 (s, 4H, NCH2), 3.77 (s, 4H, CH2), 2.92 (t, 4H, J = 6.90 Hz, NCH2), 2.80 (t, 4H, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 151.06 (C-2pyrid and C-6pyrid), 150.62 (C-4pyrid), 142.65 (C-4phenyl), 141.38 (Cqbenz), 139.01 (C-1phenyl), 130.95 (CHbenz), 130.37 (C-3phen and C-5phen), 129.62 (C-2phen and C-6phen), 128.74 (CHbenz), 128.48 (CHbenz), 125.59 (C-3pyrid and C-5pyrid), 54.85 (NCH2), 50.65 (NCH2), 42.92 (CH2), 37.08 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4: 527.3096, found: 527.3164.
1,3-bis[(4-(pyridin-4-ylpropylaminomethyl)phenyl)methyl]benzene (7r).
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.45 (d, 4H, J = 6.00 Hz, H-2pyrid, and H-6pyrid), 7.23 (d, 6H, J = 8.10 Hz, H-3phen, and H-5phen), 7.14 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 7.19 (m, 1H, Hbenz), 7.08–7.03 (m, 7H, H-3pyrid, H-5pyrid, and Hbenz), 3.91 (s, 4H, NCH2), 3.72 (s, 4H, CH2), 2.63 (t, 8H, J = 7.20 Hz, NCH2, and CH2Pyrid), 1.81 (qt, 4H, J = 7.20 Hz, CH2); 13C NMR (CDCl3) δ ppm: 152.57 (C-4pyrid), 150.97 (C-2pyrid and C-6pyrid), 142.63 (C-4phenyl), 141.27 (Cqbenz), 139.29 (C-1phenyl), 130.96 (CHbenz), 130.33 (C-3phen and C-5phen), 129.98 (CHbenz), 129.65 (C-2phen and C-6phen), 128.08 (CHbenz), 125.30 (C-3pyrid and C-5pyrid), 55.99 (NCH2), 49.87 (NCH2), 42.92 (CH2), 34.22 (CH2), 31.88 (CH2). ESI-MS m/z [M + H]+ calculated for C38H43N4: 555.3409, found: 555.3489.
3.1.5. Synthesis of the 1,3-bis[(4-Formylphenoxy)methyl]benzene 4
To a suspension of 1,3-bis(bromomethyl)benzene (2.5 mmol) and 4-hydroxybenzaldehydel (7.5 mmol) in THF (25 mL) were added 17.5 mmol of powder K
2CO
3. The reaction mixture was refluxed for 24 h. The suspension was then filtered, evaporated to dryness, and extracted with DCM (2 × 30 mL). The organic layer was filtered and washed with a solution of NaOH 2.5M (2 × 20 mL). The organic layer was dried over sodium sulfate, filtered, and evaporated under reduced pressure to give the pure product
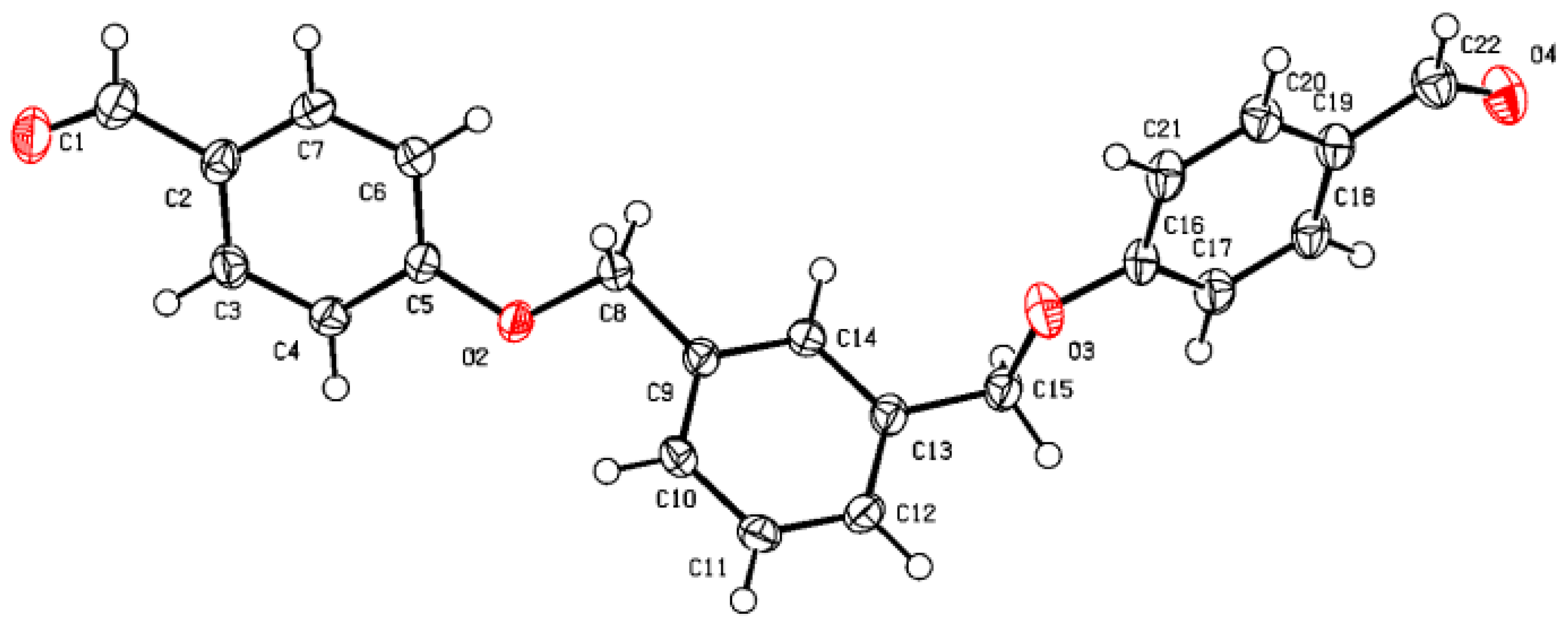
2. Beige crystals (90%); Mp = 90 °C [
38].
1H NMR (CDCl
3) δ ppm: 9.89 (s, 2H, CHO), 7.84 (d, 4H, J = 8.10 Hz, H-3
phen and H-5
phen), 7.52 (s, 3H, H
benz), 7.42 (s, 1H, H
benz), 7.43 (s, 2H, H
benz), 7.07 (d, 4H, J = 8.10 Hz, H-2
phen, and H-6
phen), 5.16 (s, 4H, CH
2);
13C NMR (CDCl
3) δ ppm: 192.1(CO), 164.97(C-4
phen), 138.03 (Cq-
benz), 133.37 (C-3
phen and C-5
phen), 132.3 (C-1
phen), 130.54 (CH
benz) 128.76 (CH
benz), 127.82 (CH
benz), 116.54 (C-2
phen and C-6
phen), 71.35 (CH
2). Colorless single crystal
4 was obtained by slow evaporation from a methanol/chloroform solution (
v/
v: 20/80): monoclinic, space group P21/n, a = 16.0383(7) Å, b =5.9521(3) Å, c = 18.1048(9) Å, α = 90°, β = 90.005(2)°, γ = 90°, V = 1728.31(14) Å
3, Z = 4, δ(calcd) = 1.331 Mg.m
−3, FW = 346.36 for C
22H
18O
4, F(000) = 728.0. Full crystallographic results were deposited at the Cambridge Crystallographic Data Centre (CCDC-2427200), UK [
39].
3.1.6. General Procedure for the Synthesis of 1,3-bis[(4-(Substituted-iminomethyl)phenoxy)methyl]benzenes 6a-r
The 1,3-bis[(4-formylphenoxy)methyl]benzene 4 (69 mg, 0.2 mmol) was dissolved in 6 mL of toluene. Activated molecular sieves of 4 Å (800 mg) were introduced, followed by dialkylamine (0.5 mmol). The reaction mixture was stirred for 24 h. The obtained suspension was filtered and washed with dichloromethane and the solvent was removed under reduced pressure to afford the di-imine 6. The crude products were then used without further purification.
1,3-bis[(4-(2-dimethylaminoethyl)iminomethyl)phenoxy)methyl]benzene (6a)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.23 (s, 2H, CH = N), 7.65 (d, 6H, J = 8.10 Hz, H-3phen, and H-5phen), 7.49 (s, 1H, Hbenz), 7.39 (s, 2H, Hbenz), ), 7.28 (s, 1H, Hbenz), 6.97 (d, 6H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, CH2), 3.72 (t, 4H, J = 6.90 Hz, NCH2), 2.62 (t, 4H, J = 6.90 Hz, NCH2), 2.30 (s, 12H, N(CH3)2).
1,3-bis[(4-(3-dimethylaminopropyl)iminomethyl)phenoxy)methyl]benzene (6b)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.35 (s, 2H, CH=N), 7.86 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.62 (s, 1H, Hbenz), 7.53 (s, 2H, Hbenz), 7.44 (s, 1H, Hbenz), 7.13 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.17 (s, 4H, CH2), 3.79 (t, 4H, J = 6.90 Hz, NCH2), 2.54 (t, 4H, J = 6.90 Hz, NCH2), 2.41 (s, 12H, N(CH3)2), 2.06 (qt, 4H, J = 8.40 Hz, CH2).
1,3-bis[(4-(4-dimethylaminobutyl)iminomethyl)phenoxy)methyl]benzene (6c)
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.67 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.40 (s, 2H, Hbenz), 7.23 (s, 1H, Hbenz), 6.98 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.10 (s, 4H, CH2), 3.60 (t, 4H, J = 6.90 Hz, NCH2), 2.30 (t, 4H, J = 6.90 Hz, NCH2), 2.22 (s, 12H, N(CH3)2, 1.71 (qt, 4H, J = 8.40 Hz, CH2), 1.55 (qt, 4H, J = 8.40 Hz, CH2).
1,3-bis[(4-(2-(4-methylpiperazin-1-yl)ethyl)iminomethyl)phenoxy)methyl]benzene (6d)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.64 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.47 (s, 1H, Hbenz), 7.37 (s, 2H, Hbenz), 7.23 (s, 1H, Hbenz), 6.98 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.06 (s, 4H, CH2), 3.72 (t, 4H, J = 6.0 Hz, NCH2), 2.8 (t, 4H, J = 8.40 Hz, NCH2), 2.23 (s, 16H, NCH2pip), 2.26 (s, 6H, NCH3).
1,3-bis[(4-(3-(4-methylpiperazin-1-yl)propyl)iminomethyl)phenoxy)methyl]benzene (6e)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.65 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.39 (s, 2H, Hbenz), 7.28 (s, 1H, Hbenz), 6.98 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.10 (s, 4H, CH2), 3.60 (t, 6H, J = 6.00 Hz, NCH2), 2.47–2.34 (m, 20H, NCH2, NCH2pip), 2.28 (s, 6H, NCH3), 1.91–1.86 (qt, 4H, J = 8.40 Hz, CH2).
1,3-bis[(4-(3-(morpholin-1-yl)propyl)iminomethyl)phenoxy)methyl]benzene (6f)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.65 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.39 (s, 2H, Hbenz), 7.17 (s, 1H, Hbenz), 6.98 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, CH2), 3.72 (t, 8H, J = 4.20 Hz, OCH2), 3.63 (t, 4H, J = 6.9 Hz, NCH2), 2.44–2.34 (m, 12H, NCH2, and NCH2morph), 1.86 (qt, 6H, J = 6.90 Hz, CH2).
1,3,5-bis[(4-(3-(pyrrolidin-1-yl)propyl)iminomethyl)phenoxy)methyl]benzene (6g)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.65 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.49 (s, 1H, Hbenz), 7.39 (s, 2H, Hbenz), 7.17 (s, 1H, Hbenz), 6.99 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, CH2) 3.62 (t, 4H, J = 6.60 Hz, NCH2), 2.54–2.48 (m, 12H, NCH2, and NCH2pyrrol), 1.93 (t, 4H, J = 6.60 Hz, CH2), 1.79–1.74 (m, 8H, CH2pyrrol).
1,3-bis[(4-(3-(piperidin-1-yl)propyl)iminomethyl)phenoxy)methyl]benzene (6h)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.20 (s, 2H, CH=N), 7.65 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.39 (s, 2H, Hbenz), 7.17 (s, 1H, Hbenz), 6.99 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, CH2), 3.60 (t, 4H, J = 6.90 Hz, NCH2), 2.40–2.35 (m, 12H, NCH2, and NCH2pip), 1.88 (t, 4H, J = 6.90 Hz, CH2), 1.62–1.54 (m, 8H, CH2pip), 1.45–1.42 (m, 4H, CH2pip).
1,3-bis{[4-((quinolin-3-yl)iminomethyl)phenoxy]methyl}benzene (6i)
Pale-yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.91 (d, 2H, J = 2.70 Hz, H-2quinol), 8.57–8.53 (m, 2H, H-8quinol), 8.15 (s, 2H, CH=N), 7.99–7.92 (m, 4H, H-5quinol, H-4quinol), 7.81 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.69–7.24 (m, 8H, H-6quinol, and H-7quinol, Hbenz), 7.12 (d, 4H, J = 7.80 Hz, H-2phen and H-6phen), 5.19 (s, 4H, CH2).
1,3-bis[(4-(pyridin-2-ylmethyliminomethyl)phenoxy)methyl]benzene (6j)
Pale-yellow oil (93%); 1H NMR (CDCl3) δ ppm: 8.53 (d, 2H, J = 6.90 Hz, H-6pyrid), 8.40 (s, 2H, CH=N), 7.76 (d, 4H, J = 8.1 Hz, H-3phen, and H-5phen), 7.69–7.66 (m, 2H, H-4pyrid), 7.44–7.41 (m, 3H, J = 6.10 Hz, H-3pyrid), 7.19–7.17 (m, 5H, Hbenz, H-5pyrid), 7.03 (d, 4H, J = 8.1 Hz, H-2phen, and H-6phen), 5.10 (s, 4H, OCH2), 4.93 (s, 4H, NCH2).
1,3-bis[(4-(pyridin-2-ylethyliminomethyl)phenoxy)methyl]benzene (6k)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.55 (d, 2H, J = 5.90 Hz, H-6pyrid), 8.14 (s, 2H, CH=N), 7.63 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.71–7.50 (m, 3H, H-4pyrid, Hbenz), 7.39 (s, 3H, Hbenz), 7.22–7.11 (m, 4H, H-3pyrid, and H-5pyrid), 6.99 (t, 4H, J = 8.10 Hz, H-2phen, H-6phen), 5.09 (s, 4H, J = 7.20 Hz, OCH2), 4.01 (s, 4H, NCH2), 3.18 (m, 4H, CH2Pyrid).
1,3-bis[(4-(pyridin-2-ylpropyliminomethyl)phenoxy)methyl]benzene (6l)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.53 (d, 2H, J = 5.80 Hz, H-6pyrid), 8.21 (s, 2H, CH=N), 7.68 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.58–7.57 (m, 2H, H-4pyrid), 7.22 (s, 1H, Hbenz), 7.41 (s, 3H, Hbenz), 7.12–7.03 (m, 2H, H-3pyrid ) 7.10–7.09 (m, 2H, H-5pyrid), 6.99 (t, 4H, J = 8.10 Hz, H-2phen, H-6phen), 5.11 (s, 4H, OCH2), 3.65 (t, 4H, J = 6.60 Hz, NCH2), 2.89 (t, 4H, J = 6.60 Hz, CH2Pyrid), 2.15 (qt, 4H, J = 6.60 Hz, CH2).
1,3-bis[(4-(pyridin-3-ylmethyliminomethyl)phenoxy)methyl]benzene (6m)
Pale-yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.61–8.50 (m, 4H, H-2pyrid, and H-6pyrid), 8.34 (s, 2H, CH=N), 7.73 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.67–7.64 (m, 2H, H-4pyrid), 7.50 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.18 (m, 2H, H-5pyrid), 7.02 (t, 4H, J = 8.10 Hz, H-2phen, H-6phen), 5.09 (s, 4H, OCH2), 4.76 (s, 4H, NCH2).
1,3-bis[(4-(pyridin-3-ylethyliminomethyl)phenoxy)methyl]benzene (6n)
Pale Yellow oil (97%): 1H NMR (CDCl3) δ ppm: 8.51–8.44 (m, 4H, H-2pyrid, and H-6pyrid), 8.09 (s, 2H, CH=N), 7.65 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.56–7.52 (m, 3H, H-4pyrid, Hbenz), 7.51 (s, 3H, Hbenz), 7.26–7.21 (m, 2H, H-5pyrid), 7.00 (t, 4H, J = 8.10 Hz, H-2phen, H-6phen), 5.10 (s, 4H, OCH2), 3.79 (t, 4H, J = 7.20 Hz, NCH2), 3.00 (t, 4H, J = 7.20 Hz, CH2Pyrid).
1,3-bis[(4-(pyridin-3-ylpropyliminomethyl)phenoxy)methyl]benzene (6o)
Yellow oil (91%); 1H NMR (CDCl3) δ ppm: 8.47–8.43 (m, 4H, H-2pyrid, and H-6pyrid), 8.20 (s, 2H, CH=N), 7.65 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.54–7.50 (m, 3H, H-4pyrid, Hbenz), 7.51 (s, 3H, Hbenz), 7.26–7.21 (m, 2H, H-5pyrid), 7.00 (t, 4H, J = 8.10 Hz, H-2phen, H-6phen), 5.10 (s, 4H, OCH2), 3.60 (t, 4H, J = 7.20 Hz, NCH2), 2.71 (t, 4H, J = 7.20 Hz, CH2Pyrid), 2.04 (qt, 6H, J = 6.60 Hz, CH2).
1,3-bis[(4-(pyridin-4-ylmethyliminomethyl)phenoxy)methy]benzene (6p)
Pale-yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.53 (d, 4H, J = 5.40 Hz, H-2pyrid, and H-6pyrid), 8.33 (s, 2H, CH=N), 7.74 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.52 (s, 1H, Hbenz), 7.41 (s, 3H, Hbenz), 7.28–7.11 (m, 4H, H-4pyrid, and H-5pyrid), 7.00 (d, 4H, J = 8.10 Hz, H-2phen and H-6phen), 5.12 (s, 4H, OCH2), 4.74 (s, 4H, NCH2).
1,3-bis[(4-(pyridin-4-ylethyliminomethyl)phenoxy)methyl]benzene (6q)
Yellow oil (97%); 1H NMR (CDCl3) δ ppm: 8.50 (d, 4H, J = 5.40 Hz, H-2pyrid, and H-6pyrid), 8.07 (s, 2H, CH=N), 7.63 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.18–7.14 (m, 4H, H-4pyrid, and H-5pyrid), 7.00 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, OCH2), 3.82 (s, 4H, NCH2), 2.99 (t, 6H, J = 7.20 Hz, CH2Pyrid).
1,3-bis[(4-(pyridin-4-ylpropyliminomethyl)phenoxy)methyl]benzene (6r)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 8.48 (d, 4H, J = 5.40 Hz, H-2pyrid, and H-6pyrid), 8.17 (s, 2H, CH=N), 7.67 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.50 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.18–7.14 (m, 4H, H-4pyrid, and H-5pyrid), 7.00 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.09 (s, 4H, OCH2), 3.59 (s, 4H, NCH2), 2.69 (t, 6H, J = 7.20 Hz, CH2Pyrid), 2.03 (qt, 4H, J = 7.20 Hz, CH2).
3.1.7. General Procedure for the Synthesis of 1,3-bis[(4-(Substituted-aminomethyl)phenoxy)methyl]benzenes 8a–r
Compounds 6a–r (0.4 mmol) were dissolved in methanol (10 mL), and sodium borohydride (2.4 mmol, 6 eq.) was added gradually at 0 °C. The resulting mixture was stirred at room temperature for 2 h. Subsequently, the solvent was removed under reduced pressure, and the residue obtained was cooled, triturated in water, and then extracted with dichloromethane (40 mL). After separating, the organic layer was dried with sodium sulfate, filtered, and evaporated to dryness, yielding the products 8a–r.
1,3-bis[(4-(2-dimethylaminoethyl)aminomethyl)phenoxy)methyl]benzene (8a)
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 7.49 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.92 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.04 (s, 4H, OCH2), 3.74 (t, 4H, J = 6.90 Hz, NCH2), 2.68 (t, 4H, J = 6.90 Hz, NCH2), 2.41 (t, 4H, J = 6.90 Hz, NCH2), 2.18 (s, 12H, N(CH3)2); 13C NMR (CDCl3) δ ppm: 159.14 (C-4phenyl), 138.88 (Cqbenz), 134.07 (C-1phenyl), 130.80 (C-3phen and C-5phen), 130.20 (CHbenz), 128.36 (CHbenz), 127.79 (CHbenz), 116.13 (C-2phen and C-6phen), 71.26 (OCH2), 60.31 (NCH2), 54.80 (NCH2), 46.84 (NCH2). 46.87 (N(CH3)2), ESI-MS m/z [M + H]+ calculated for C30H43N4O2: 490.3308, found: 491.3386.
1,3-bis[(4-(3-dimethylaminopropyl)aminomethyl)phenoxy)methyl]benzene (8b)
Yellow oil (81%); 1H NMR (CDCl3) δ ppm: 7.42 (s, 1H, Hbenz), 7.36 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.92 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.02 (s, 4H, OCH2), 3.70 (t, 4H, J = 6.90 Hz, NCH2), 2.68 (t, 4H, J = 6.90 Hz, NCH2), 2.29 (t, 4H, J = 6.90 Hz, NCH2), 2.19 (s, 12H, N(CH3)2); 1.66 (qt, 4H, J = 7.10 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.12(C-4phenyl), 138.88 (Cqbenz), 134.24 (C-1phenyl), 130.67 (C-3phen and C-5phen), 130.67 (CHbenz), 128.31 (CHbenz), 127.74 (CHbenz), 116.26 (C-2phen and C-6phen), 71.22 (OCH2), 59.38 (NCH2), 54.75 (NCH2), 49.11 (NCH2), 46.86 (N(CH3)2), 29.28 (CH2). ESI-MS m/z [M + H]+ calculated for C32H47N4: 519.3621, found: 519.3703.
1,3-bis[(4-(4-dimethylaminobutyl)aminomethyl)phenoxy)methyl]benzene (8c)
Yellow oil (91%); 1H NMR (CDCl3) δ ppm: 7.50 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.92 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.05 (s, 4H, OCH2), 3.71 (t, 4H, J = 6.90 Hz, NCH2), 2.63 (t, 4H, J = 6.90 Hz, NCH2), 2.25 (t, 4H, J = 6.90 Hz, NCH2), 2.19 (s, 12H, N(CH3)2); 1.66 (m, 8H, CH2); 13C NMR (CDCl3) δ ppm: 159.16 (C-4phenyl), 138.88 (Cqbenz), 134.19 (C-1phenyl), 130.74 (C-3phen and C-5phen), 130.21 (CHbenz), 128.37 (CHbenz), 127.8 (CHbenz), 116.13 (C-2phen and C-6phen), 71.23 (OCH2), 61.01 (NCH2), 54.72 (NCH2), 50.55 (NCH2), 46.78 (N(CH3)2), 29.27 (CH2), 26.87 (CH2). ESI-MS m/z [M + H]+ calculated for C34H51N4: 547.3934, found: 547.40.
1,3-bis[(4-(2-(4-methylpiperazin-1-yl)ethyl)aminomethyl)phenoxy)methyl]benzene (8d)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 7.48 (s, 1H, Hbenz), 7.36 (s, 3H, Hbenz), 7.20 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.92 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.03 (s, 4H, OCH2), 3.71 (s, 4H, NCH2), 2.66 (t, 4H, J = 6.90 Hz, NCH2), 2.48–2.40 (m, 20H, NCH2piperazine, NCH2), 2.25 (s, 6H, NCH3); 13C NMR (CDCl3) δ ppm: 159.11 (C-4phenyl), 138.87 (Cqbenz), 134.29 (C-1phenyl), 130.69 (C-3phen and C-5phen), 116.09 (C-2phen and C-6phen), 71.22 (OCH2), 59.06 (NCH2), 56.49 (NCH2piperazine), 54.49(NCH2piperazine), 47.40 (NCH3), 46.88 (CH2). ESI-MS m/z [M + H]+ calculated for C36H53N6 O2: 601.42, found: 601.42.
1,3bis[(4-(3-(4-methylpiperazin-1-yl) propyl)aminomethyl)phenoxy)methyl]benzene (8e)
Yellow oil (63%); 1H NMR (CDCl3) δ ppm: 7.48 (s, 1H, Hbenz), 7.36 (s, 3H, Hbenz), 7.24 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.93 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.06 (s, 4H, OCH2), 3.70 (s, 4H, NCH2), 2.66 (t, 4H, J = 7.10 Hz, NCH2), 2.37 (t, 6H, J = 7.10 Hz, NCH2), 2.47–2.36 (m, 20H, NCH2, and NCH2piperazine), 2.26 (s, 6H, NCH3), 1.67 (qt, 4H, J = 7.10 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.15 (C-4phenyl), 138.81 (Cqbenz), 134.21 (C-1phenyl), 130.71 (C-3phen and C-5phen), 130.23 (CHbenz), 128.40 (CHbenz), 127.82 (CHbenz), 116.12 (C-2phen and C-6phen), 71.25 (OCH2), 58.32 (NCH2), 56.47 (NCH2piperazine), 54.76 (NCH2), 54.57 (NCH2piperazine), 49.39 (NCH2), 47.40 (NCH3), 28.27 (CH2). ESI-MS m/z [M + H]+ calculated for C38H57N6O2: 629.4465, found: 629.45.
1,3-bis[(4-(3-(morpholin-1-yl)propyl)aminomethyl)phenoxy)methyl]benzene (8f)
Yellow oil (83%); 1H NMR (CDCl3) δ ppm: 7.50 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen and H-5phen), 6.91 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.06 (s, 4H, OCH2), 3.71–3.67 (m, 12H, NCH2, and OCH2), 2.66 (t, 4H, J = 6.90 Hz, NCH2), 2.43–2.36 (m, 12H, NCH2, and NCH2 morpholine), 1.69 (qt, 4H, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.13 (C-4phenyl), 138.88 (Cqbenz), 134.27 (C-1phenyl), 130.65 (C-3phen and C-5phen), 130.21 (CHbenz), 128.37 (CHbenz), 127.80 (CHbenz), 116.12 (C-2phen and C-6phen), 71.23 (OCH2), 68.35 (OCH2morpholine), 58.76 (NCH2), 55.16 (NCH2morpholine), 54.80 (NCH2), 49.27 (NCH2), 28.02 (CH2). ESI-MS m/z [M + H]+ calculated for C36H51N4O4: 603,3832, found: 603.39.
1,3-bis[(4-(3-(pyrrolidin-1-yl)propyl)aminomethyl)phenoxy)methyl]benzene (8g)
Yellow oil (70%); 1H NMR (CDCl3) δ ppm: 7.49 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.93 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.05 (s, 4H,OCH2), 3.72 (s, 4H, NCH2), 2.7 (t, 4H, J = 6.90 Hz, NCH2), 251–2.46 (m, 12H, NCH2, and NCH2pyrrolidine), 1.78–1.70 (m, 12H, CH2, and CH2pyrrolidine); 13C NMR (CDCl3) δ ppm: 159.10 (C-4phenyl), 138.88 (Cqbenz), 134.37 (C-1phenyl), 130.64 (C-3phen and C-5phen), 130.19 (CHbenz), 128.35 (CHbenz), 127.79 (CHbenz), 116.09 (C-2phen and C-6phen), 71.23 (OCH2), 56.15 (NCH2), 55.64 (NCH2pyrrolidine), 54.76 (NCH2), 49.37 (NCH2), 30.67 (CH2), 24.79 (CH2pyrrolidine). ESI-MS m/z [M + H]+ calculated for C36H51N4O2: 571.3934, found: 571.40.
1,3-bis[(4-(3-(piperidin-1) propyl)aminomethyl)phenoxy)methyl]benzene (8h)
Yellow oil (98%); 1H NMR (CDCl3) δ ppm: 7.50 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.22 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 6.93 (d, 4H, J = 8.10 Hz, H-2phen, and H-6phen), 5.05 (s, 4H,OCH2), 3.71 (s, 4H, NCH2), 2.64 (t, 6H, J = 6.90 Hz, NCH2), 2.36–2.31 (m, 12H, NCH2, and NCH2piperidine), 1.70 (qt, 6H, J = 6.90 Hz, CH2), 1.58–1.54 (m, 8H, CH2piperidine), 1.43–1.41 (m, 4H, CH2piperidine); 13C NMR (CDCl3) δ ppm: 159.10 (C-4phenyl), 138.88 (Cqbenz), 134.37 (C-1phenyl), 130.66 (C-3phen and C-5phen), 130.20 (CHbenz), 128.37 (CHbenz), 127.81 (CHbenz), 116.09 (C-2phen and C-6phen), 71.23 (OCH2), 56.17 (NCH2), 56.06 (NCH2 piperidine), 54.78 (NCH2), 49.56 (NCH2), 28.41 (CH2), 27.37 (CH2piperidine), 25.85 (CH2piperidine). ESI-MS m/z [M + H]+ calculated for C38H55N4 O2: 599.4247, found: 599.4321.
1,3-bis[(4-((quinolin-3-yl)aminomethyl)phenoxy)methyl]benzene (8i)
Pale-yellow oil (85%); 1H NMR (CDCl3) δ ppm: 8.47 (d, 2H, J = 2.70 Hz, H-2quinol), 7.98 (dd, 2H, J = 6.90 and 3.60 Hz, H-8quinol), 7.57 (dd, 2H, J = 6.90 and 3.60 Hz, H-5quinol), 7.50 (m, 1H, Hbenz), 7.46–7.39 (m, 7H, Hbenz, H-6quinol, and H-7quinol), 7.35 (d, 4H, J = 7.80 Hz, H-3phen, and H-5phen), 7.00 (d, 4H, J = 7.80 Hz, H-2phen, and H-6phen), 6.96 (d, 2H, J = 2.70 Hz, H-4quinol), 5.08 (s, 4H, OCH2), 4.34 (d, 4H, J = 5.1 Hz, NCH2). 13C NMR (CDCl3) δ ppm: 159.59 (C-4phenyl), 144.54 (C-2quinol), 143.48 (Cqbenz), 142.83 (C-4aquinol), 138.76 (C-8aquinol), 131.97 (C-1phenyl), 130.86 (C-3quinol), 130.30 (C-3phen and C-5phen), 128.51 (CHbenz), 128.34 (CHbenz), 127.87 (CHbenz), 127.41 (C-4quinol), 126.99 (C-6quinol), 126.38 (C-5quinol), 116.54 (C-2phen and C-6phen), 111.77 (C-7quinol), 71.28 (OCH2), 48.77 (NCH2). ESI-MS m/z [M + H]+ calculated for C40H35N4 O2: 603.2782, found: 603.2756.
1,3-bis[(4-(pyridin-2-ylmethylaminomethyl) phenoxy)methyl]benzene (8j)
Yellow oil (75%); 1H NMR (CDCl3) δ ppm: 8.57–8.55 (m, 2H, H-6pyrid), 7.67–7.60 (m, 2H, H-4pyrid), 7.52 (s, 1H, Hbenz), 7.39 (s, 3H, Hbenz), 7.33–7.25 (m, 6H, H-3phen, H-5phen, and H-3pyrid), 7.18–7.15 (m, 2H, H-5pyrid), 6.98-6.95 (d, 4H, J = 7.80 Hz, H-6phen, and H-2phen), 5.07 (s, 4H, OCH2), 3.92 (s, 4H, NCH2), 3.79 (s, 4H, NCH2); 13C NMR (CDCl3) δ ppm: 161.19 (C-4phenyl), 159.21 (C-2pyrid), 150.69 (C-6pyrid), 138.91 (Cqbenz), 137.81 (C-4pyrid), 134.06 (C-1phenyl), 130.88 (C-3phen and C-5phen), 130.24 (CHbenz), 128.39 (CHbenz), 127.82 (CHbenz), 123.75 (C-5pyrid), 123.32 (C-3pyrid), 116.15 (C-2phen and C-6phen), 71.25 (OCH2), 55.83 (NCH2), 54.29 (NCH2). ESI-MS m/z [M + H]+ calculated for C34H35N4O2: 531.2682, found: 531.2760.
1,3-bis[(4-(pyridin-2-ylethylaminomethyl)phenoxy)methyl]benzene (8k)
Orange oil (89%); 1H NMR (CDCl3) δ ppm: 8.51–8.49 (m, 2H, H-6pyrid), 7.59–7.53 (m, 2H, H-4pyrid), 7.49 (s, 1H, Hbenz), 7.37 (s, 3H, Hbenz), 7.20–7.18 (m, 6H, H-3phen, H-5phen, and H-3pyrid), 7.18–7.15 (m, 2H, H-5pyrid), 6.96-6.90 (d, 4H, J = 7.80 Hz, H-6phen, and H-2phen), 5.03 (s, 4H, OCH2), 3.74 (s, 4H, NCH2), 3.01 (m, 4H, CH2); 13C NMR (CDCl3) δ ppm: 161.19 (C-4phenyl), 159.21 (C-2pyrid), 150.65 (C-6pyrid), 138.91 (Cqbenz), 137.81 (C-4pyrid), 134.10 (C-1phenyl), 130.76 (C-3phen and C-5phen), 130.22 (CHbenz), 128.37 (CHbenz), 127.80 (CHbenz), 124.71 (C-5pyrid), 122.67 (C-3pyrid), 116.14 (C-2phen and C-6phen), 71.22 (OCH2), 54.57 (NCH2), 50.11 (NCH2 39.68 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4O2: 559.2995, found: 559.3061.
1,3-bis[(4-(pyridin-2-ylpropylaminomethyl)phenoxy)methyl]benzene (8l)
Yellow oil (85%); 1H NMR (CDCl3) δ ppm: 8.50–8.48 (m, 2H, H-6pyrid), 7.59–7.53 (m, 2H, H-4pyrid), 7.50 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.24 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.21–7.06 (m, 4H, H-5pyrid, and H-3pyrid), 6.96-6.91 (d, 4H, J = 7.80 Hz, H-6phen, and H-2phen), 5.05 (s, 4H, OCH2), 3.71 (s, 4H, NCH2), 3.71 (s, 4H, NCH2), 2.81 (t, 4H, J = 6.90 Hz, NCH2), 2.66 (t, 4H, J = 6.90 Hz, CH2Pyrid), 1.94 (qt, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 161.19 (C-4phenyl), 159.12 (C-2pyrid), 150.65 (C-6pyrid), 138.87 (Cqbenz), 137.80 (C-4pyrid), 134.21 (C-1phenyl), 130.75 (C-3phen and C-5phen), 130.23 (CHbenz), 128.40 (CHbenz), 127.80 (CHbenz), 124.18 (C-5pyrid), 122.44 (C-3pyrid), 116.09 (C-2phen and C-6phen), 71.22 (OCH2), 54.64 (NCH2), 50.01 (NCH2), 37.33 (CH2), 31.38 (CH2). ESI-MS m/z [M + H]+ calculated for C54H62N6: 841.47, found: 841.48.
1,3-bis[(4-(pyridin-3-ylmethylaminomethyl)phenoxyl)methyl]benzene (8m)
Pale-yellow oil (94%); 1H NMR (CDCl3) δ ppm: 8.57–8.48 (m, 4H, H-6pyrid, and H-2pyrid), 7.70 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.52 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.25–7.23 (m, 6H, H-5pyrid, H-3phen, and H-5phen), 6.93 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 5.05 (s, 4H, OCH2), 3.79 (s, 4H, NCH2), 3.74 (s, 4H, CH2); 13C NMR (CDCl3) δ ppm: 159.28 (C-4phenyl), 151.12 (C-2pyrid), 149.83 (C-6pyrid), 138.86 (Cqbenz), 137.25 (C-3pyrid), 133.80 (C-1phenyl), 130.76 (C-3phen and C-5phen), 130.25 (CHbenz), 128.42 (CHbenz), 127.83 (CHbenz), 124.79 (C-5pyrid), 116.21 (C-2phen and C-6phen), 71.25 (OCH2), 53.96 (NCH2), 51.71 (CH2). ESI-MS m/z [M + H]+ calculated for C34H35N4O2: 531.2682, found: 531.2753.
1,3-bis[(4-(pyridin-3-ylethylaminomethyl)phenoxy)methyl]benzene (8n)
Yellow oil (62%); 1H NMR (CDCl3) δ ppm: 8.48–8.44 (m, 4H, H-6pyrid, and H-2pyrid), 7.70 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.52 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.23–7.19 (m, 6H, H-5pyrid, H-3phen, and H-5phen), 6.91 (d, 4H, J = 7.80 Hz, H-6phen and H-2phen), 5.06 (s, 4H, OCH2), 3.75 (s, 4H, NCH2), 2.90–2.81 (m, 8H, NCH2, and CH2Pyrid); 13C NMR (CDCl3) δ ppm: 159.22 (C-4phenyl), 151.57 (C-2pyrid), 149.04 (C-6pyrid), 138.86 (C-3pyrid), 137.57 (C-4pyrid), 136.87 (Cqbenz), 133.97 (C-1phenyl), 130.68 (C-3phen and C-5phen), 130.24 (CHbenz), 128.41 (CHbenz), 127.83 (CHbenz), 124.76 (C-5pyrid), 116.18 (C-2phen and C-6phen), 71.25 (OCH2), 54.61 (NCH2), 51.42 (NCH2), 34.95 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4O2: 559.2995, found: 559.3065.
1,3-bis[(4-(pyridin-3-ylpropylaminomethyl)phenoxy)methyl]benzene (8o)
Yellow oil (90%); 1H NMR (CDCl3) δ ppm: 8.43–8.39 (m, 4H, H-6pyrid, and H-2pyrid), 7.48 (d, 2H, J = 7.80 Hz, H-4pyrid), 7.31 (s, 3H, Hbenz), 7.23–7.14 (m, 7H, H-5pyrid, Hbenz, H-3phen, and H-5phen), 7.12 (d, 4H, J = 7.80 Hz, H-6phen, and H-2phen), 5.04 (s, 4H, OCH2), 3.69 (s, 4H, NCH2), 2.66–2.61 (m, 8H, NCH2, and CH2Pyrid), 1.80 (qt, 4H, J = 7.20 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.17 (C-4phenyl), 151.24 (C-2pyrid), 148.67 (C-6pyrid), 138.84 (Cqbenz), 138.85 (C-1phenyl), 137.20 (C-4pyrid), 134.23 (C-3pyrid), 130.71 (C-3phen and C-5phen), 130.21 (CHbenz), 128.38 (C-5pyrid), 127.80 (CHbenz), 124.70(CHbenz), 116.14 (C-2phen and C-6phen), 71.23 (OCH2), 54.75 (NCH2), 49.87 (NCH2), 32.75 (CH2), 32.04 (CH2). ESI-MS m/z [M + H]+ calculated for C38H43N4O2: 587.3308, found: 587.3375.
1,3-bis[(4-(pyridin-4-ylmethylaminomethyl)phenoxy) methyl]benzene (8p)
Yellow oil (65%); 1H NMR (CDCl3) δ ppm: 8.54 (d, 4H, J = 6.00 Hz, H-2pyrid, and H-6pyrid), 7.51 (s, 1H, Hbenz), 7.40 (s, 3H, Hbenz), 7.29–7.28 (m, 8H, H-3pyrid, H-5pyrid, H-3phen, and H-5phen), 6.96 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 5.06 (s, 4H, OCH2), 3.79 (s, 6H, NCH2), 3.73 (s, 4H, NCH2); 13C NMR (CDCl3) δ ppm: 159.31 (C-4phenyl), 151.13 (C-2pyrid and C-6pyrid), 150.2 (C-4pyrid), 138.84 (Cqbenz), 133.69. (C-1phenyl), 130.76 (C-3phen and C-5phen), 130.27 (CHbenz), 128.44 (CHbenz), 127.84 (CHbenz), 124.43 (C-3pyrid and C-5pyrid), 116.21 (C-2phen and C-6phen), 71.25 (OCH2), 53.96 (NCH2), 53.09 (NCH2). ESI-MS m/z [M + H]+ calculated for C34H35N4O2: 531.2682, found: 531.2751.
1,3-bis[(4-(pyridin-4-ylethylaminomethyl)phenoxy)methyl]benzene (8q)
Yellow oil (96%); 1H NMR (CDCl3) δ ppm: 8.49–8.47 (m, 4H, H-2pyrid, and H-6pyrid), 7.50 (s, 1H, Hbenz), 7.38 (s, 3H, Hbenz), 7.18 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.12-7.10 (m, 8H, H-3pyrid, H-5pyrid) 6.91 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 5.04 (s, 4H, OCH2), 3.73 (s, 4H, NCH2), 2.89 (t, 4H, J = 6.90 Hz, CH2), 2.78 (t, 4H, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.22 (C-4phenyl), 151.13 (C-2pyrid and C-6pyrid), 150.58 (C-4pyrid), 138.84 (Cqbenz), 133.84 (C-1phenyl), 130.68 (C-3phen and C-5phen), 130.23 (CHbenz), 128.42 (CHbenz), 127.81 (CHbenz), 125.55 (C-3pyrid and C-5pyrid), 116.16 (C-2phen and C-6phen), 71.23 (OCH2), 54.57 (NCH2), 50.60 (NCH2). 37.12 (CH2). ESI-MS m/z [M + H]+ calculated for C36H39N4O2: 559.2995, found: 559.3061.
1,3-bis[(4-(pyridin-4-ylpropylaminomethyl)phenoxy) methyl]benzene (8r)
Yellow oil (99%); 1H NMR (CDCl3) δ ppm: 8.44–8.41 (m, 4H, H-2pyrid, and H-6pyrid), 7.50 (s, 1H, Hbenz), 7.36 (s, 3H, Hbenz), 7.18 (d, 4H, J = 8.10 Hz, H-3phen, and H-5phen), 7.07–7.04 (m, 8H, H-3pyrid, H-5pyrid) 6.91 (d, 4H, J = 8.10 Hz, H-6phen, and H-2phen), 5.03 (s, 4H, OCH2), 3.68 (s, 4H, NCH2), 2.65–2.58 (m, 8H, NCH2, and CH2), 1.78 (t, 4H, J = 6.90 Hz, CH2); 13C NMR (CDCl3) δ ppm: 159.19 (C-4phenyl), 151.58 (C-4pyrid), 150.93 (C-2pyrid and C-6pyrid), 138.84 (Cqbenz), 134.16 (C-1phenyl), 130.71 (C-3phen and C-5phen), 130.20 (CHbenz), 128.42 (CHbenz), 127.78 (CHbenz), 125.27 (C-3pyrid and C-5pyrid), 116.16 (C-2phen and C-6phen), 71.23 (OCH2), 54.68 (NCH2), 49.79 (NCH2). 34.22 (CH2), 31.87 (CH2). ESI-MS m/z [M + H]+ calculated for C38H43N4O2: 587.3308, found: 587.3387.
3.1.8. General Procedure for the Synthesis of 1,3-bis[(4-(Substituted-aminomethyl)phenyl)methyl]benzene ammonium oxalates salts 1a-r and 1,3-bis[(4-(substituted-aminomethyl)phenoxy)methyl]benzene ammonium oxalates salts 2a-r
To a solution of compounds 7–8a–r (0.3 mmol) in isopropanol (11 mL) was added oxalic acid (2.4 mmol, 8 eq.). The reaction mixture was heated under reflux for 30 min. The precipitate was filtered, washed with isopropanol then with diethyl ether, and dried under reduced pressure to give ammonium oxalate salts 1–2, which were crystallized using a mixture of 2-PrOH–H2O solvents.