Pathophysiology of Alzheimer’s Disease: Focus on H3 Receptor Modulators and Their Implications
Abstract
1. Introduction
2. Drug Targets for the Treatment of AD
3. Beta-Amyloid Plaque and Its Role in AD
4. Involvement of Tau Protein in AD
5. Cholinergic System and Its Effects in AD
6. Serotonergic Pathway Modulation in AD
7. Inflammation and Its Effects in AD
8. Neuroprotective Factors as Regulators of AD
9. Neurogenesis in AD
10. Role of the Genetic Factor in AD
11. Oxidative Stress and Its Role in AD
12. Others
13. Lifestyle and Supportive Interventions
14. Clinical Trials and Research on Histamine Receptors in AD Treatment
15. Histamine
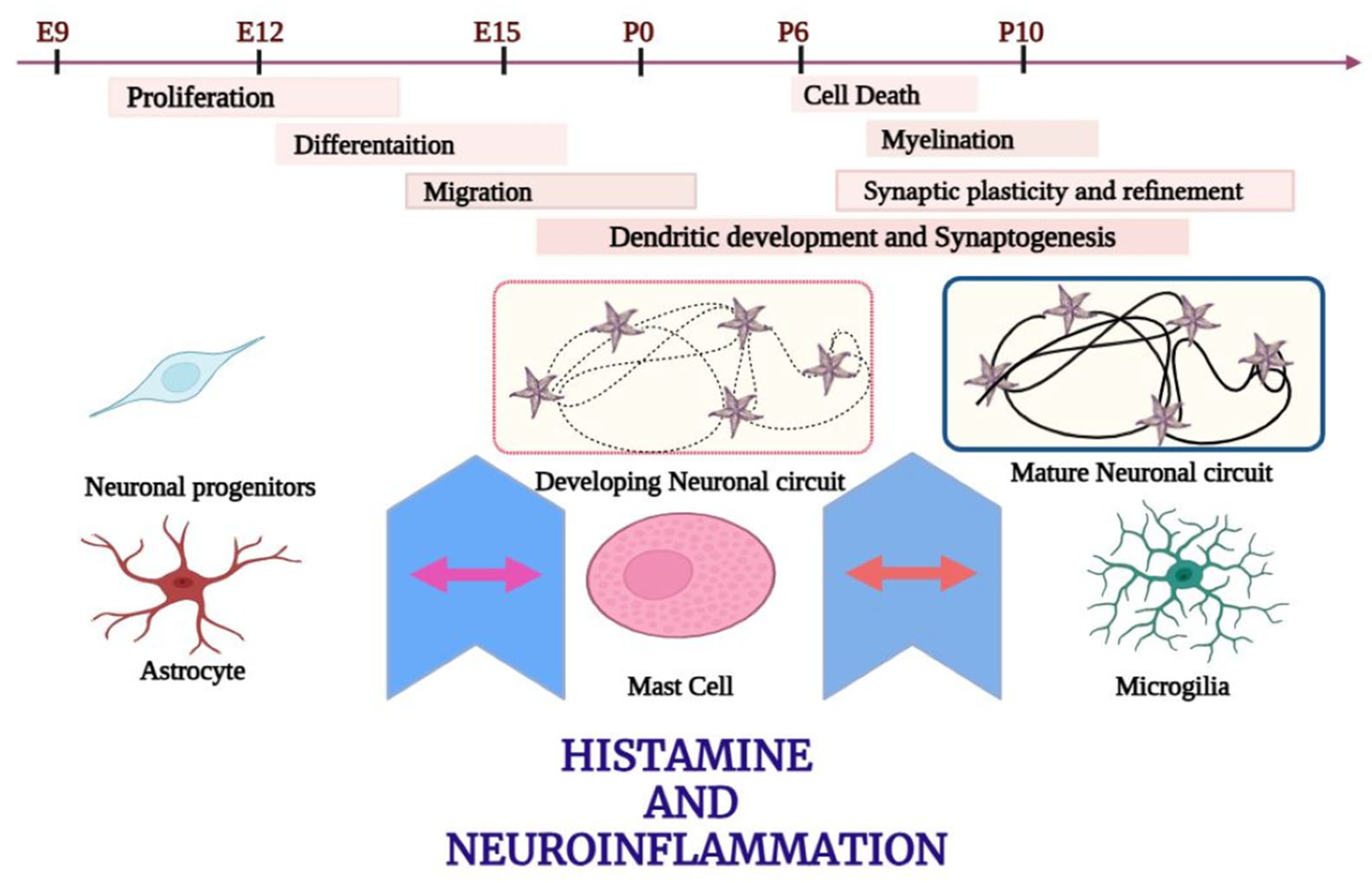
16. Role of Histamine in the Pathogenesis of Dementia
17. Recently Developed Drugs for the Treatment of Dementia by Blocking H3 Receptors
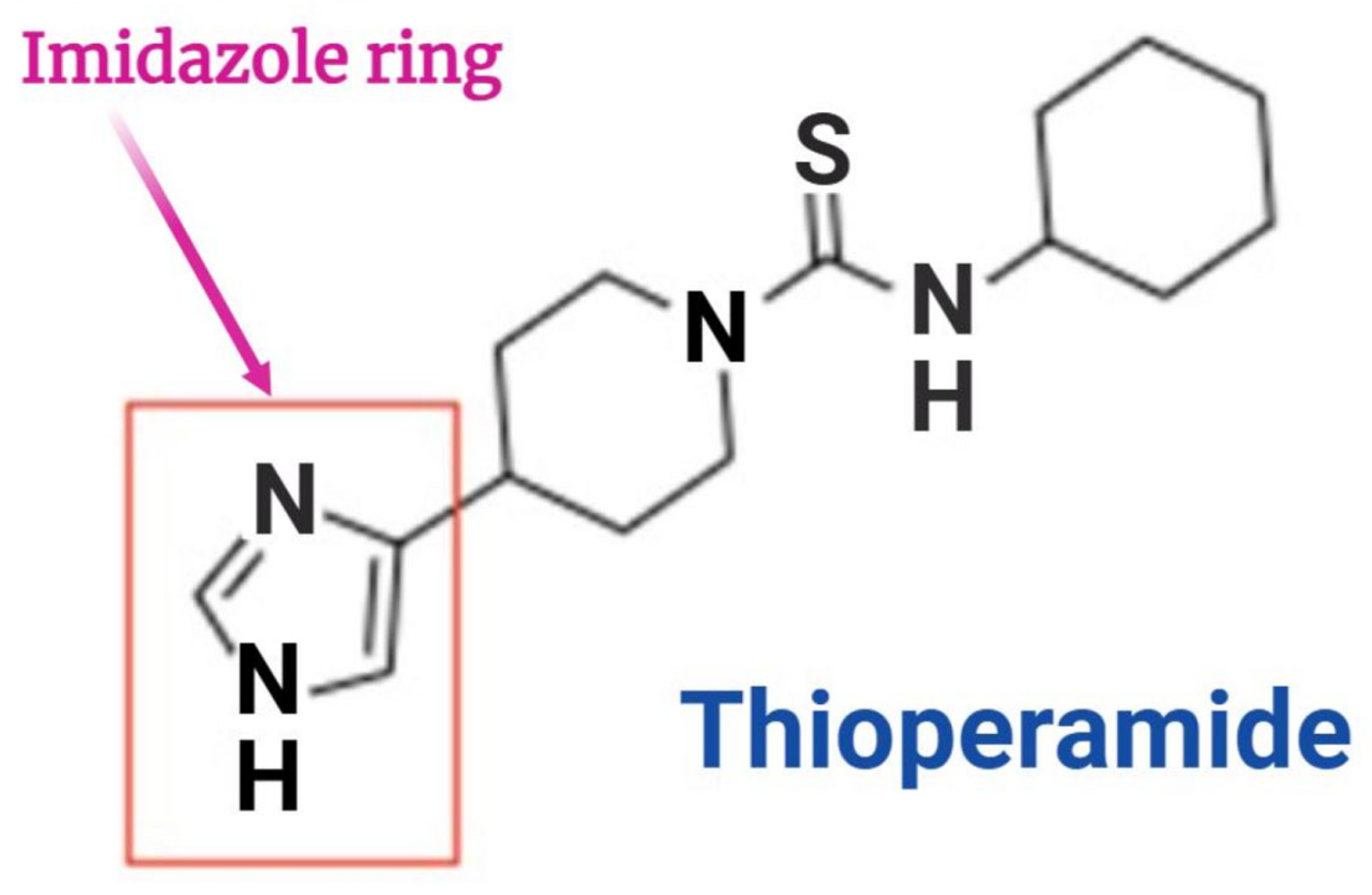
17.1. Thioperamide
17.2. Pitolisant
- GSK189254
- E177
- SAR110894
- ABT-239
- E169
18. Conclusions
19. Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s Disease. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Harris, J.R., Ed.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; Volume 65, pp. 329–352. ISBN 978-94-007-5415-7. Available online: https://link.springer.com/10.1007/978-94-007-5416-4_14 (accessed on 15 March 2025).
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. 2011, 32, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Fisher, A.; Pittel, Z.; Haring, R.; Bar-Ner, N.; Kliger-Spatz, M.; Natan, N.; Egozi, I.; Sonego, H.; Marcovitch, I.; Brandeis, R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: Implications in future therapy. J. Mol. Neurosci. 2003, 20, 349–356. [Google Scholar] [CrossRef]
- Caccamo, A.; Oddo, S.; Billings, L.M.; Green, K.N.; Martinez-Coria, H.; Fisher, A.; LaFerla, F.M. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 2006, 49, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bolon, B.; Kahn, S.; Bennett, B.D.; Babu-Khan, S.; Denis, P.; Fan, W.; Kha, H.; Zhang, J.; Gong, Y.; et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001, 4, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.B.; Frigon, N.L.; Games, D.; Hu, K.; et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kumaragurubaran, N.; Hong, L.; Kulkarni, S.S.; Xu, X.; Chang, W.; Weerasena, V.; Turner, R.; Koelsch, G.; Bilcer, G.; et al. Design, synthesis, and X-ray structure of potent memapsin 2 (beta-secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J. Med. Chem. 2007, 50, 2399–2407. [Google Scholar] [CrossRef]
- Yasojima, K.; McGeer, E.G.; McGeer, P.L. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001, 919, 115–121. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Turner, A.J.; Tanzawa, K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997, 11, 355–364. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E.; Kawashima-Morishima, M.; Lee, H.J.; Hama, E.; Sekine-Aizawa, Y.; et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef]
- Cheng, I.H.; Scearce-Levie, K.; Legleiter, J.; Palop, J.J.; Gerstein, H.; Bien-Ly, N.; Puoliväli, J.; Lesné, S.; Ashe, K.H.; Muchowski, P.J.; et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J. Biol. Chem. 2007, 282, 23818–23828. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008, 283, 32176–32187. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- Lee, V.M.; Trojanowski, J.Q. The disordered neuronal cytoskeleton in Alzheimer’s disease. Curr. Opin. Neurobiol. 1992, 2, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Vogelsberg-Ragaglia, V.; Schuck, T.; Trojanowski, J.Q.; Lee, V.M. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp. Neurol. 2001, 168, 402–412. [Google Scholar] [CrossRef]
- Sontag, E.; Luangpirom, A.; Hladik, C.; Mudrak, I.; Ogris, E.; Speciale, S.; White, C.L. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J. Neuropathol. Exp. Neurol. 2004, 63, 287–301. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G.; Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 2013, 203, 361–364. [Google Scholar] [CrossRef]
- Bracco, L.; Bessi, V.; Padiglioni, S.; Marini, S.; Pepeu, G. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer’s disease patients. J. Alzheimers Dis. 2014, 40, 737–742. [Google Scholar] [CrossRef]
- Miranda, M.I.; Bermúdez-Rattoni, F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc. Natl. Acad. Sci. USA 1999, 96, 6478–6482. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A.; Honig, W.; Raaijmakers, W.G.M. Effects of intra-hippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology 1992, 109, 373–376. [Google Scholar] [CrossRef]
- Winters, B.D.; Bussey, T.J. Removal of cholinergic input to perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur. J. Neurosci. 2005, 21, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Power, A. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 2003, 80, 178–193. [Google Scholar] [CrossRef]
- Boccia, M.M.; Acosta, G.B.; Blake, M.G.; Baratti, C.M. Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: Effects of i.c.v. injections of hemicholinium-3. Neuroscience 2004, 124, 735–741. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Baratti, C.M.; McGaugh, J.L. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol. Learn. Mem. 2009, 91, 93–97. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Acosta, G.B.; Baratti, C.M. Atropine, an anticholinergic drug, impairs memory retrieval of a high consolidated avoidance response in mice. Neurosci. Lett. 2003, 345, 97–100. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Whitehouse, P.; Price, D.; Struble, R.; Clark, A.; Coyle, J.; DeLong, M.R. Alzheimer’s Disease and Senile Dementia: Loss of Neurons in the Basal Forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Ducatenzeiler, A.; Ribeiro-da-Silva, A.; Duff, K.; Bennett, D.A.; Cuello, A.C. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol. Aging 2006, 27, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T. On Neurodegenerative Diseases, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Rogers, J.L.; Kesner, R.P. Cholinergic Modulation of the Hippocampus During Encoding and Retrieval of Tone/Shock-Induced Fear Conditioning. Learn. Mem. 2004, 11, 102–107. [Google Scholar] [CrossRef]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Egawa, G.; Doi, H.; Miyachi, Y.; Kabashima, K. Histamine modulates the responsiveness of keratinocytes to IL-17 and TNF-α through the H1-receptor. J. Dermatol. Sci. 2011, 61, 79–81. [Google Scholar] [CrossRef]
- Tagawa, M.; Kano, M.; Okamura, N.; Higuchi, M.; Matsuda, M.; Mizuki, Y.; Arai, H.; Fujii, T.; Komemushi, S.; Itoh, M.; et al. Differential cognitive effects of ebastine and (+)-chlorpheniramine in healthy subjects: Correlation between cognitive impairment and plasma drug concentration. Br. J. Clin. Pharmacol. 2002, 53, 296–304. [Google Scholar] [CrossRef]
- Verdurand, M.; Bérod, A.; Le Bars, D.; Zimmer, L. Effects of amyloid-β peptides on the serotoninergic 5-HT1A receptors in the rat hippocampus. Neurobiol. Aging 2011, 32, 103–114. [Google Scholar] [CrossRef]
- Elliott, M.S.J.; Ballard, C.G.; Kalaria, R.N.; Perry, R.; Hortobágyi, T.; Francis, P.T. Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain J. Neurol. 2009, 132, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa, V.; Duchatelle, P.; Boulouard, M.; Dauphin, F. Selective 5-HT6 receptor blockade improves spatial recognition memory and reverses age-related deficits in spatial recognition memory in the mouse. Neuropsychopharmacology 2009, 34, 488–500. [Google Scholar] [CrossRef]
- Upton, N.; Chuang, T.T.; Hunter, A.J.; Virley, D.J. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurother. J. Am. Soc. Exp. Neurother. 2008, 5, 458–469. [Google Scholar] [CrossRef]
- Patat, A.; Parks, V.; Raje, S.; Plotka, A.; Chassard, D.; Le Coz, F. Safety, tolerability, pharmacokinetics and pharmacodynamics of ascending single and multiple doses of lecozotan in healthy young and elderly subjects. Br. J. Clin. Pharmacol. 2009, 67, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Lanni, C.; Racchi, M.; Govoni, S. Targeting dementias through cancer kinases inhibition. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12044. [Google Scholar] [CrossRef]
- Li, T.; Martin, E.; Abada, Y.; Boucher, C.; Cès, A.; Youssef, I.; Fenaux, G.; Forand, Y.; Legrand, A.; Nachiket, N.; et al. Effects of Chronic Masitinib Treatment in APPswe/PSEN1dE9 Transgenic Mice Modeling Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1339–1345. [Google Scholar] [CrossRef]
- Reading, C.L.; Ahlem, C.N.; Murphy, M.F. NM101 Phase III Study of NE3107 in Alzheimer’s Disease: Rationale, Design and Therapeutic Modulation of Neuroinflammation and Insulin Resistance. Neurodegener. Dis. Manag. 2021, 11, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Balzano, T.; Esteban-García, N.; Blesa, J. Neuroinflammation, immune response and α-synuclein pathology: How animal models are helping us to connect dots. Expert Opin. Drug Discov. 2023, 18, 13–23. [Google Scholar] [CrossRef]
- Nørgaard, C.H.; Friedrich, S.; Hansen, C.T.; Gerds, T.; Ballard, C.; Møller, D.V.; Knudsen, L.B.; Kvist, K.; Zinman, B.; Holm, E.; et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Bercovier, H.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med. Hypotheses 2019, 123, 95–97. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Italiani, P.; Puxeddu, I.; Napoletano, S.; Scala, E.; Melillo, D.; Manocchio, S.; Angiolillo, A.; Migliorini, P.; Boraschi, D.; Vitale, E.; et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: New markers of disease progression? J. Neuroinflamm. 2018, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.; Privat, A.-L.; Bressac, L.; Toulorge, D. CD38 in Neurodegeneration and Neuroinflammation. Cells 2020, 9, 471. [Google Scholar] [CrossRef]
- Blacher, E.; Dadali, T.; Bespalko, A.; Haupenthal, V.J.; Grimm, M.O.W.; Hartmann, T.; Lund, F.E.; Stein, R.; Levy, A. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol. 2015, 78, 88–103. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Syed, Y.Y. Lenalidomide: A Review in Newly Diagnosed Multiple Myeloma as Maintenance Therapy After ASCT. Drugs 2017, 77, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mei, Z.L.; Wang, H.; Hu, M.; Long, Y.; Miao, M.X.; Li, N.; Hong, H. Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochem. Int. 2014, 75, 26–31. [Google Scholar] [CrossRef]
- Michael, J.; Zirknitzer, J.; Unger, M.S.; Poupardin, R.; Rieß, T.; Paiement, N.; Zerbe, H.; Hutter-Paier, B.; Reitsamer, H.; Aigner, L. The Leukotriene Receptor Antagonist Montelukast Attenuates Neuroinflammation and Affects Cognition in Transgenic 5xFAD Mice. Int. J. Mol. Sci. 2021, 22, 2782. [Google Scholar] [CrossRef] [PubMed]
- Feigin, A.; Evans, E.E.; Fisher, T.L.; Leonard, J.E.; Smith, E.S.; Reader, A.; Mishra, V.; Manber, R.; Walters, K.A.; Kowarski, L.; et al. Pepinemab antibody blockade of SEMA4D in early Huntington’s disease: A randomized, placebo-controlled, phase 2 trial. Nat. Med. 2022, 28, 2183–2193. [Google Scholar] [CrossRef]
- Alves, S.; Churlaud, G.; Audrain, M.; Michaelsen-Preusse, K.; Fol, R.; Souchet, B.; Braudeau, J.; Korte, M.; Klatzmann, D.; Cartier, N. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain J. Neurol. 2017, 140, 826–842. [Google Scholar] [CrossRef]
- Faridar, A.; Vasquez, M.; Thome, A.D.; Yin, Z.; Xuan, H.; Wang, J.H.; Wen, S.; Li, X.; Thonhoff, J.R.; Zhao, W.; et al. Ex vivo expanded human regulatory T cells modify neuroinflammation in a preclinical model of Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 144. [Google Scholar] [CrossRef]
- LiCausi, F.; Hartman, N.W. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, Z.; Li, Y.; Luo, M.; Luo, S.; He, G. Alteration of the Wnt/GSK3β/β-catenin signalling pathway by rapamycin ameliorates pathology in an Alzheimer’s disease model. Int. J. Mol. Med. 2019, 44, 313–323. [Google Scholar] [CrossRef]
- Kiyota, T.; Machhi, J.; Lu, Y.; Dyavarshetty, B.; Nemati, M.; Yokoyama, I.; Mosley, R.L.; Gendelman, H.E. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. J. Neuroimmunol. 2018, 319, 80–92. [Google Scholar] [CrossRef]
- Potter, H.; Woodcock, J.H.; Boyd, T.D.; Coughlan, C.M.; O’Shaughnessy, J.R.; Borges, M.T.; Thaker, A.A.; Raj, B.A.; Adamszuk, K.; Scott, D.; et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement. 2021, 7, e12158. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-W.; Lucente, J.D.; Nguyen, H.M.; Singh, V.; Singh, L.; Chavez, M.; Bushong, T.; Wulff, H.; Maezawa, I. Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; García-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennström, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef]
- Rubin, K.; Glazer, S. The pertussis hypothesis: Bordetella pertussis colonization in the pathogenesis of Alzheimer’s disease. Immunobiology 2017, 222, 228–240. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Jacobs, C.; Morley, J.E.; Hoft, D.F. Lower Risk for Dementia Following Adult Tetanus, Diphtheria, and Pertussis (Tdap) Vaccination. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1436–1443. [Google Scholar] [CrossRef]
- Weidung, B.; Hemmingsson, E.-S.; Olsson, J.; Sundström, T.; Blennow, K.; Zetterberg, H.; Ingelsson, M.; Elgh, F.; Lövheim, H. VALZ-Pilot: High-dose valacyclovir treatment in patients with early-stage Alzheimer’s disease. Alzheimers Dement. 2022, 8, e12264. [Google Scholar] [CrossRef]
- MacPherson, K.P.; Sompol, P.; Kannarkat, G.T.; Chang, J.; Sniffen, L.; Wildner, M.E.; Norris, C.M.; Tansey, M.G. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol. Dis. 2017, 102, 81–95. [Google Scholar] [CrossRef]
- Patel, A.G.; Nehete, P.N.; Krivoshik, S.R.; Pei, X.; Cho, E.L.; Nehete, B.P.; Ramani, M.D.; Shao, Y.; Williams, L.E.; Wisniewski, T.; et al. Innate immunity stimulation via CpG oligodeoxynucleotides ameliorates Alzheimer’s disease pathology in aged squirrel monkeys. Brain J. Neurol. 2021, 144, 2146–2165. [Google Scholar] [CrossRef]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Ghareghani, M.; Rivest, S. The Synergistic Potential of Combining PD-1/PD-L1 Immune Checkpoint Inhibitors with NOD2 Agonists in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2023, 24, 10905. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.A.; et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-K.; Vázquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732.e23. [Google Scholar] [CrossRef]
- Stym-Popper, G.; Matta, K.; Chaigneau, T.; Rupra, R.; Demetriou, A.; Fouquet, S.; Dansokho, C.; Toly-Ndour, C.; Dorothée, G. Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology. J. Neuroinflamm. 2023, 20, 64. [Google Scholar] [CrossRef]
- Bakker, A.; Albert, M.S.; Krauss, G.; Speck, C.L.; Gallagher, M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 2015, 7, 688–698. [Google Scholar] [CrossRef]
- Rosenzweig-Lipson, S.; Barton, R.; Gallagher, M.; Edgar, C.J.; Maruff, P.T.; Mohs, R. HOPE4MCI trial: First trial targeting reduction of hippocampal overactivity to treat mild cognitive impairment due to Alzheimer’s disease with AGB101: Human/Human trials: Other. Alzheimers Dement. 2020, 16, e045331. [Google Scholar] [CrossRef]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2014, 8, 463. [Google Scholar] [CrossRef]
- Hamasaki, H.; Honda, H.; Suzuki, S.O.; Hokama, M.; Kiyohara, Y.; Nakabeppu, Y.; Iwaki, T. Down-regulation of MET in hippocampal neurons of Alzheimer’s disease brains. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2014, 34, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Church, K.; Walker, W.; L’Hostis, P.; Viardot, G.; Danjou, P.; Hendrix, S.; Moebius, H.J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Positive Modulator of HGF/MET, Fosgonimeton, in Healthy Volunteers and Subjects with Alzheimer’s Disease: Randomized, Placebo-Controlled, Double-Blind, Phase I Clinical Trial. J. Alzheimers Dis. 2022, 86, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Reda, S.M.; Setti, S.E.; Taylor, R.W.; Berthiaume, A.-A.; Walker, W.E.; Wu, W.; Moebius, H.J.; Church, K.J. Fosgonimeton, a Novel Positive Modulator of the HGF/MET System, Promotes Neurotrophic and Procognitive Effects in Models of Dementia. Neurother. J. Am. Soc. Exp. Neurother. 2023, 20, 431–451. [Google Scholar] [CrossRef]
- Burns, L.H.; Pei, Z.; Wang, H.-Y. Targeting α7 nicotinic acetylcholine receptors and their protein interactions in Alzheimer’s disease drug development. Drug Dev. Res. 2023, 84, 1085–1095. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lee, K.-C.; Pei, Z.; Khan, A.; Bakshi, K.; Burns, L.H. PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer’s disease pathogenesis. Neurobiol. Aging 2017, 55, 99–114. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Pei, Z.; Lee, K.-C.; Lopez-Brignoni, E.; Nikolov, B.; Crowley, C.A.; Marsman, M.R.; Barbier, R.; Friedmann, N.; Burns, L.H. PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J. Prev. Alzheimers Dis. 2020, 7, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Yu, H.-J.; Kim, S.; Kim, G.; Choi, N.-Y.; Lee, E.-H.; Lee, Y.J.; Yoon, M.-Y.; Lee, K.-Y.; Koh, S.-H. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology 2016, 55, 131–141. [Google Scholar] [CrossRef]
- Koh, S.-H.; Kwon, H.S.; Choi, S.H.; Jeong, J.H.; Na, H.R.; Lee, C.N.; Yang, Y.; Lee, A.Y.; Lee, J.-H.; Park, K.W.; et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: A phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res. Ther. 2021, 13, 66. [Google Scholar] [CrossRef]
- Hampel, H.; Lista, S.; Mango, D.; Nisticò, R.; Perry, G.; Avila, J.; Hernandez, F.; Geerts, H.; Vergallo, A. Alzheimer Precision Medicine Initiative (APMI) Lithium as a Treatment for Alzheimer’s Disease: The Systems Pharmacology Perspective. J. Alzheimers Dis. 2019, 69, 615–629. [Google Scholar] [CrossRef]
- Zhang, X.; Heng, X.; Li, T.; Li, L.; Yang, D.; Zhang, X.; Du, Y.; Doody, R.S.; Le, W. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J. Alzheimers Dis. 2011, 24, 739–749. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Appukuttan, A.T.; Moon, S.; Duce, J.A.; Volitakis, I.; Cherny, R.; Wood, S.J.; Greenough, M.; Berger, G.; et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol. Psychiatry 2017, 22, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-K.; Nelson, T.J.; Alkon, D.L. Towards universal therapeutics for memory disorders. Trends Pharmacol. Sci. 2015, 36, 384–394. [Google Scholar] [CrossRef]
- Farlow, M.R.; Thompson, R.E.; Wei, L.-J.; Tuchman, A.J.; Grenier, E.; Crockford, D.; Wilke, S.; Benison, J.; Alkon, D.L. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Assessing Safety, Tolerability, and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer’s Disease. J. Alzheimers Dis. 2019, 67, 555–570. [Google Scholar] [CrossRef]
- Correia, S.S.; Iyengar, R.R.; Germano, P.; Tang, K.; Bernier, S.G.; Schwartzkopf, C.D.; Tobin, J.; Lee, T.W.-H.; Liu, G.; Jacobson, S.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CY6463 Reveals its Therapeutic Potential in Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 656561. [Google Scholar] [CrossRef]
- Gamba, P.; Giannelli, S.; Staurenghi, E.; Testa, G.; Sottero, B.; Biasi, F.; Poli, G.; Leonarduzzi, G. The Controversial Role of 24-S-Hydroxycholesterol in Alzheimer’s Disease. Antioxidants 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Ono, K.; Matsumoto, J.; Yamada, M.; Nishijo, H. Effects of the neurotrophic agent T-817MA on oligomeric amyloid-β-induced deficits in long-term potentiation in the hippocampal CA1 subfield. Neurobiol. Aging 2014, 35, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Yuede, C.M.; LaBarbera, K.M.; Limegrover, C.S.; Rehak, C.; Yurko, R.; Waybright, L.; Look, G.; Rishton, G.; Safferstein, H.; et al. Preclinical and clinical biomarker studies of CT1812: A novel approach to Alzheimer’s disease modification. Alzheimers Dement. J. Alzheimers Assoc. 2021, 17, 1365–1382. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, P.-K.; Chang, Y.-C.; Chuo, L.-J.; Chen, Y.-S.; Tsai, G.E.; Lane, H.-Y. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biol. Psychiatry 2014, 75, 678–685. [Google Scholar] [CrossRef]
- Tari, A.R.; Berg, H.H.; Videm, V.; Bråthen, G.; White, L.R.; Røsbjørgen, R.N.; Scheffler, K.; Dalen, H.; Holte, E.; Haberg, A.K.; et al. Safety and efficacy of plasma transfusion from exercise-trained donors in patients with early Alzheimer’s disease: Protocol for the ExPlas study. BMJ Open 2022, 12, e056964. [Google Scholar] [CrossRef]
- Rutigliano, G.; Stazi, M.; Arancio, O.; Watterson, D.M.; Origlia, N. An isoform-selective p38α mitogen-activated protein kinase inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 70, 86–91. [Google Scholar] [CrossRef]
- Alam, J.J. Selective Brain-Targeted Antagonism of p38 MAPKα Reduces Hippocampal IL-1β Levels and Improves Morris Water Maze Performance in Aged Rats. J. Alzheimers Dis. 2015, 48, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Prins, N.; Lammertsma, A.; Yaqub, M.; Gouw, A.; Wink, A.M.; Chu, H.-M.; van Berckel, B.N.M.; Alam, J. An exploratory clinical study of p38α kinase inhibition in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2018, 5, 464–473. [Google Scholar] [CrossRef]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica—Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.M.; Irwin, R.W.; Yao, J.; Liu, L.; Brinton, R.D. Allopregnanolone promotes regeneration and reduces β-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE 2011, 6, e24293. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 3146. [Google Scholar] [CrossRef]
- Liu, C.-C.; Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Samant, N.P.; Gupta, G.L. Novel therapeutic strategies for Alzheimer’s disease targeting brain cholesterol homeostasis. Eur. J. Neurosci. 2021, 53, 673–686. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.-T.; Tan, L.; Wang, Y.-L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. BioMed Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.-S.; Yao, X.-Q.; Liu, Y.-H.; Wang, Q.-H.; Zeng, F.; Lu, J.-J.; Liu, J.; Zhu, C.; Shen, L.-L.; Liu, C.-H.; et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc. Natl. Acad. Sci. USA 2015, 112, 5225–5230. [Google Scholar] [CrossRef]
- He, P.; Ouyang, X.; Zhou, S.; Yin, W.; Tang, C.; Laudon, M.; Tian, S. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm. Behav. 2013, 64, 1–7. [Google Scholar] [CrossRef]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- Tiligada, E.; Kyriakidis, K.; Chazot, P.L.; Passani, M.B. Histamine Pharmacology and New CNS Drug Targets. CNS Neurosci. Ther. 2011, 17, 620–628. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M. Antihistamines and Mental Status. J. Clin. Psychopharmacol. 2016, 36, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S127–S135. [Google Scholar] [CrossRef]
- Leurs, R.; Bakker, R.A.; Timmerman, H.; De Esch, I.J.P. The histamine H3 receptor: From gene cloning to H3 receptor drugs. Nat. Rev. Drug Discov. 2005, 4, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, J.; Carruthers, N.; Thurmond, R. The Histamine H4 Receptor and Potential Therapeutic Uses for H4 Ligands. Mini-Rev. Med. Chem. 2004, 4, 993–1000. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chien, W.-C.; Chung, C.-H.; Lai, C.-Y.; Tzeng, N.-S. The Usage of Histamine Type 1 Receptor Antagonist and Risk of Dementia in the Elderly: A Nationwide Cohort Study. Front. Aging Neurosci. 2022, 14, 811494. [Google Scholar] [CrossRef]
- Gemkow, M.J.; Davenport, A.J.; Harich, S.; Ellenbroek, B.A.; Cesura, A.; Hallett, D. The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov. Today 2009, 14, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wellendorph, P.; Goodman, M.W.; Burstein, E.S.; Nash, N.R.; Brann, M.R.; Weiner, D.M. Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H(3) receptor. Neuropharmacology 2002, 42, 929–940. [Google Scholar] [CrossRef]
- Wieland, K.; Bongers, G.; Yamamoto, Y.; Hashimoto, T.; Yamatodani, A.; Menge, W.M.B.P.; Timmerman, H.; Lovenberg, T.W.; Leurs, R. Constitutive Activity of Histamine H3 Receptors Stably Expressed in SK-N-MC Cells: Display of Agonism and Inverse Agonism by H3 Antagonists. J. Pharmacol. Exp. Ther. 2001, 299, 908–914. [Google Scholar] [CrossRef]
- Thompson, C.M.; Troche, K.; Jordan, H.L.; Barr, B.L.; Wyatt, C.N. Evidence for functional, inhibitory, histamine H3 receptors in rat carotid body Type I cells. Neurosci. Lett. 2010, 471, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, G.; Bakker, R.A.; Leurs, R. Molecular aspects of the histamine H3 receptor. Biochem. Pharmacol. 2007, 73, 1195–1204. [Google Scholar] [CrossRef]
- Mariottini, C.; Scartabelli, T.; Bongers, G.; Arrigucci, S.; Nosi, D.; Leurs, R.; Chiarugi, A.; Blandina, P.; Pellegrini-Giampietro, D.E.; Beatrice Passani, M. Activation of the histaminergic H3 receptor induces phosphorylation of the Akt/GSK-3β pathway in cultured cortical neurons and protects against neurotoxic insults. J. Neurochem. 2009, 110, 1469–1478. [Google Scholar] [CrossRef]
- Lai, X.; Ye, L.; Liao, Y.; Jin, L.; Ma, Q.; Lu, B.; Sun, Y.; Shi, Y.; Zhou, N. Agonist-induced activation of histamine H3 receptor signals to extracellular signal-regulated kinases 1 and 2 through PKC-, PLD-, and EGFR-dependent mechanisms. J. Neurochem. 2016, 137, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Novoa, L.; Cacabelos, R. Histamine function in brain disorders. Behav. Brain Res. 2001, 124, 213–233. [Google Scholar] [CrossRef]
- Wulff, B.S.; Hastrup, S.; Rimvall, K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur. J. Pharmacol. 2002, 453, 33–41. [Google Scholar] [CrossRef]
- Zarrindast, M.-R.; Eidi, M.; Eidi, A.; Oryan, S. Effects of histamine and opioid systems on memory retention of passive avoidance learning in rats. Eur. J. Pharmacol. 2002, 452, 193–197. [Google Scholar] [CrossRef]
- Higuchi, M.; Yanai, K.; Okamura, N.; Meguro, K.; Arai, H.; Itoh, M.; Iwata, R.; Ido, T.; Watanabe, T.; Sasaki, H. Histamine H1 receptors in patients with Alzheimer’s disease assessed by positron emission tomography. Neuroscience 2000, 99, 721–729. [Google Scholar] [CrossRef]
- Motawaj, M.; Burban, A.; Davenas, E.; Gbahou, F.; Faucard, R.; Morisset, S.; Arrang, J.-M. Le système histaminergique : Une cible pour de nouveaux traitements des deficits cognitifs. Therapies 2010, 65, 415–422. [Google Scholar] [CrossRef]
- Brioni, J.D.; Esbenshade, T.A.; Garrison, T.R.; Bitner, S.R.; Cowart, M.D. Discovery of Histamine H 3 Antagonists for the Treatment of Cognitive Disorders and Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2011, 336, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Motawaj, M.; Peoc’h, K.; Callebert, J.; Arrang, J.-M. CSF Levels of the Histamine Metabolite tele-Methylhistamine are only Slightly Decreased in Alzheimer’s Disease. J. Alzheimers Dis. 2010, 22, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Da Silva, W.C.; Benetti, F.; Bonini, J.S. Histaminergic Mechanisms for Modulation of Memory Systems. Neural Plast. 2011, 2011, 328602. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Roberts, J.C.; Lee, J.; Chen, C.P.L.-H.; Brown, S.H.; Roman, S.; Lai, M.K.P. Characterization of histamine H3 receptors in Alzheimer’s Disease brain and amyloid over-expressing TASTPM mice. Br. J. Pharmacol. 2009, 157, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chazot, P.L. Therapeutic potential of histamine H3 receptor antagonists in dementias. Drug News Perspect. 2010, 23, 99. [Google Scholar] [CrossRef]
- Breitner, J. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol. Aging 1995, 16, 523–530. [Google Scholar] [CrossRef]
- Fu, Q.-L.; Dai, H.-B.; Shen, Y.; Chen, Z. Reversing effect of histamine on neurotoxicity induced by beta-amyloid1-42. J. Zhejiang Univ. Med. Sci. 2007, 36, 146–149. [Google Scholar] [CrossRef]
- Dere, E.; Zlomuzica, A.; Viggiano, D.; Ruocco, L.A.; Watanabe, T.; Sadile, A.G.; Huston, J.P.; De Souza-Silva, M.A. Episodic-like and procedural memory impairments in histamine H1 Receptor knockout mice coincide with changes in acetylcholine esterase activity in the hippocampus and dopamine turnover in the cerebellum. Neuroscience 2008, 157, 532–541. [Google Scholar] [CrossRef]
- Dai, H.; Kaneko, K.; Kato, H.; Fujii, S.; Jing, Y.; Xu, A.; Sakurai, E.; Kato, M.; Okamura, N.; Kuramasu, A.; et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci. Res. 2007, 57, 306–313. [Google Scholar] [CrossRef]
- Bandaru, N.; Komavari, C.; Gorla, U.S.; Koteswarao, G.; Kulandaivelu, U.; Ankarao, A. Neuroprotective effect of Conessinin on Elevated oxidative stress induced Alzheimers’disease in rats. Res. J. Pharm. Technol. 2020, 13, 2703. [Google Scholar] [CrossRef]
- Simon, T.; Gogolák, P.; Kis-Tóth, K.; Jelinek, I.; László, V.; Rajnavölgyi, É. Histamine modulates multiple functional activities of monocyte-derived dendritic cell subsets via histamine receptor 2. Int. Immunol. 2012, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Gao, Y.; Yang, J.; Zou, J.; Guo, W. Potential role of store-operated Ca2+ entry in Th2 response induced by histamine in human monocyte-derived dendritic cells. Int. Immunopharmacol. 2012, 12, 358–367. [Google Scholar] [CrossRef]
- Loewenbrueck, K.F.; Tigno-Aranjuez, J.T.; Boehm, B.O.; Lehmann, P.V.; Tary-Lehmann, M. Th1 responses to beta-amyloid in young humans convert to regulatory IL-10 responses in Down syndrome and Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Felaco, P.; Castellani, M.L.; De Lutiis, M.A.; Felaco, M.; Pandolfi, F.; Salini, V.; De Amicis, D.; Vecchiet, J.; Tete, S.; Ciampoli, C.; et al. IL-32: A newly-discovered proinflammatory cytokine. J. Biol. Regul. Homeost. Agents 2009, 23, 141–147. [Google Scholar]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Fox, G.B.; Esbenshade, T.A.; Pan, J.B.; Radek, R.J.; Krueger, K.M.; Yao, B.B.; Browman, K.E.; Buckley, M.J.; Ballard, M.E.; Komater, V.A.; et al. Pharmacological Properties of ABT-239 [4-(2-{2-[(2 R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological Characterization and Broad Preclinical Efficacy in Cognition and Schizophrenia of a Potent and Selective Histamine H3 Receptor Antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 176–190. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, S.H.; Joo, S.H.; Kim, S.-H.; Shin, C.Y. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochem. Biophys. Res. Commun. 2010, 402, 48–53. [Google Scholar] [CrossRef]
- Sakurai, E.; Sakurai, E.; Tanaka, Y.; Watanabe, T.; Singh Jossan, S.; Oreland, L. Effects of histamine H3-receptor ligands on brain monoamine oxidase in various mammalian species. Brain Res. 2001, 906, 180–183. [Google Scholar] [CrossRef]
- Ligneau, X.; Perrin, D.; Landais, L.; Camelin, J.-C.; Calmels, T.P.G.; Berrebi-Bertrand, I.; Lecomte, J.-M.; Parmentier, R.; Anaclet, C.; Lin, J.-S.; et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, Hydrochloride], a Nonimidazole Inverse Agonist/Antagonist at the Human Histamine H3 Receptor: Preclinical Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. The histamine H3 receptor: From discovery to clinical trials with pitolisant. Br. J. Pharmacol. 2011, 163, 713–721. [Google Scholar] [CrossRef]
- Kollb-Sielecka, M.; Demolis, P.; Emmerich, J.; Markey, G.; Salmonson, T.; Haas, M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Michel, A.D.; Kilpatrick, G.J. In vivo occupancy of histamine H3 receptors by thioperamide and (R)-α-methylhistamine measured using histamine turnover and an ex vivo labeling technique. Biochem. Pharmacol. 1992, 44, 1261–1267. [Google Scholar] [CrossRef]
- Alachkar, A.; Khan, N.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Histamine H3 receptor antagonist E177 attenuates amnesia induced by dizocilpine without modulation of anxiety-like behaviors in rats. Neuropsychiatr. Dis. Treat. 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Griebel, G.; Pichat, P.; Pruniaux, M.-P.; Beeské, S.; Lopez-Grancha, M.; Genet, E.; Terranova, J.-P.; Castro, A.; Sánchez, J.A.; Black, M.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays procognitive effects in rodents. Pharmacol. Biochem. Behav. 2012, 102, 203–214. [Google Scholar] [CrossRef]
- Delay-Goyet, P.; Blanchard, V.; Schussler, N.; Lopez-Grancha, M.; Ménager, J.; Mary, V.; Sultan, E.; Buzy, A.; Guillemot, J.; Stemmelin, J.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays disease-modifying activity in a transgenic mouse model of tauopathy. Alzheimers Dement. Transl. Res. Clin. Interv. 2016, 2, 267–280. [Google Scholar] [CrossRef]
- Passani, M.B.; Blandina, P. Cognitive implications for H3 and 5-HT3 receptor modulation ofcortical cholinergic function: A parallel story. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 725. [Google Scholar] [CrossRef] [PubMed]
- Bitner, R.S.; Markosyan, S.; Nikkel, A.L.; Brioni, J.D. In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer’s disease. Neuropharmacology 2011, 60, 460–466. [Google Scholar] [CrossRef]
- Łażewska, D.; Kaleta, M.; Hagenow, S.; Mogilski, S.; Latacz, G.; Karcz, T.; Lubelska, A.; Honkisz, E.; Handzlik, J.; Reiner, D.; et al. Novel naphthyloxy derivatives—Potent histamine H3 receptor ligands. Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2018, 26, 2573–2585. [Google Scholar] [CrossRef]
- Abdalla, S.; Eissa, N.; Jayaprakash, P.; Beiram, R.; Kuder, K.J.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice. Int. J. Mol. Sci. 2023, 24, 12719. [Google Scholar] [CrossRef] [PubMed]
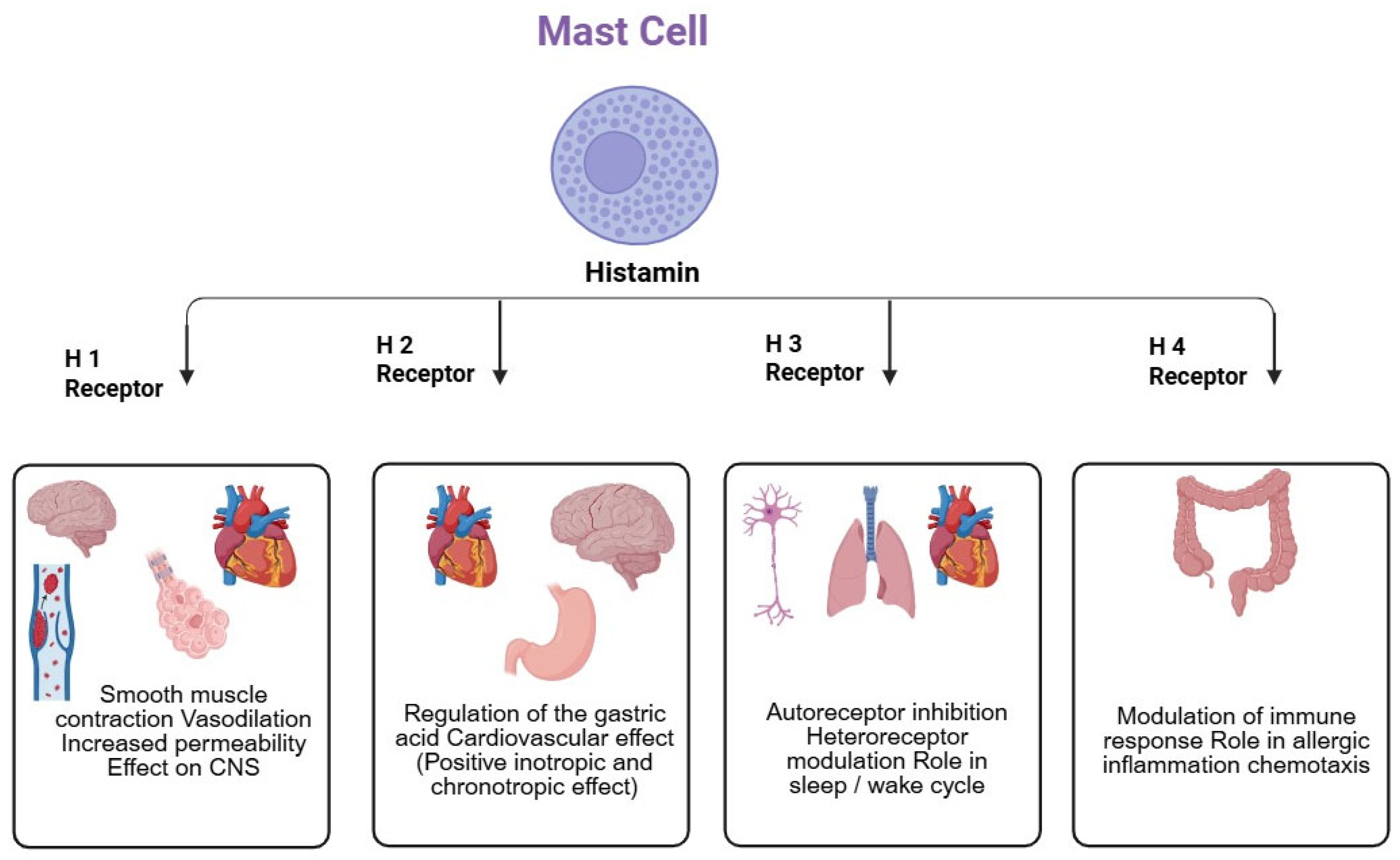


| S.No | Type | Location | Function | Binding Affinity to Histamine (pKi) | Signaling Pathway (Figure 2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Central | Peripheral | ||||||
| 1 | H1R | Forebrain, cerebral cortex, Hippocampus, and Thalamus | Heart and smooth muscles | Decreasing blood pressure, inflammatory response, and increased wakefulness | 4.2 | Phospholipase C (PLC) | [132] |
| 2 | H2R | Substantia Nigra, raphe nuclei, Hippocampus, and basal ganglia | Intestinal smooth muscles, heart, and lungs | Regulation of hormone release, fluid balance, excitation, relaxation of airway smooth muscles, blood pressure regulation, and gastric acid regulation | 4.3 | Protein kinase C (PKC) activation | [132] |
| 3 | H3R | Cerebral cortex, basal ganglia, and hypothalamus | Lung, cardiovascular system (CVS), and intestine | Histamine release, regulation, and stimulation | 8.0 | Inhibition of protein kinase A (PKA), activation of Phospholipase 2, mitogen-activated protein kinase (MAPK) | [133] |
| 4 | H4R | Cerebellum and Hippocampus | Hematopoietic cells | Modulation of the Immune system | 7.8 | PKA Inhibition, PLC and MAPK activation | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandaru, N.; Bukke, S.P.N.; Pedapati, V.M.D.; Sowjanaya, G.; Suggu, V.S.; Nalla, S.; Dudhe, P.B.; Ezeonwumelu, J.O.C.; Alagbonsi, A.I.; Onohuean, H. Pathophysiology of Alzheimer’s Disease: Focus on H3 Receptor Modulators and Their Implications. Drugs Drug Candidates 2025, 4, 22. https://doi.org/10.3390/ddc4020022
Bandaru N, Bukke SPN, Pedapati VMD, Sowjanaya G, Suggu VS, Nalla S, Dudhe PB, Ezeonwumelu JOC, Alagbonsi AI, Onohuean H. Pathophysiology of Alzheimer’s Disease: Focus on H3 Receptor Modulators and Their Implications. Drugs and Drug Candidates. 2025; 4(2):22. https://doi.org/10.3390/ddc4020022
Chicago/Turabian StyleBandaru, Nagaraju, Sarad Pawar Naik Bukke, Veera Mani Deepika Pedapati, Gurugubelli Sowjanaya, Vangmai Swaroopa Suggu, Swathi Nalla, Prashik Bhimrao Dudhe, Joseph Obiezu Chukwujekwu Ezeonwumelu, Abdullateef Isiaka Alagbonsi, and Hope Onohuean. 2025. "Pathophysiology of Alzheimer’s Disease: Focus on H3 Receptor Modulators and Their Implications" Drugs and Drug Candidates 4, no. 2: 22. https://doi.org/10.3390/ddc4020022
APA StyleBandaru, N., Bukke, S. P. N., Pedapati, V. M. D., Sowjanaya, G., Suggu, V. S., Nalla, S., Dudhe, P. B., Ezeonwumelu, J. O. C., Alagbonsi, A. I., & Onohuean, H. (2025). Pathophysiology of Alzheimer’s Disease: Focus on H3 Receptor Modulators and Their Implications. Drugs and Drug Candidates, 4(2), 22. https://doi.org/10.3390/ddc4020022







