The Role of a Glucal-Based Molecule in the Reduction of Pancreatic Adenocarcinoma—An In Vitro and In Silico Approach
Abstract
1. Introduction
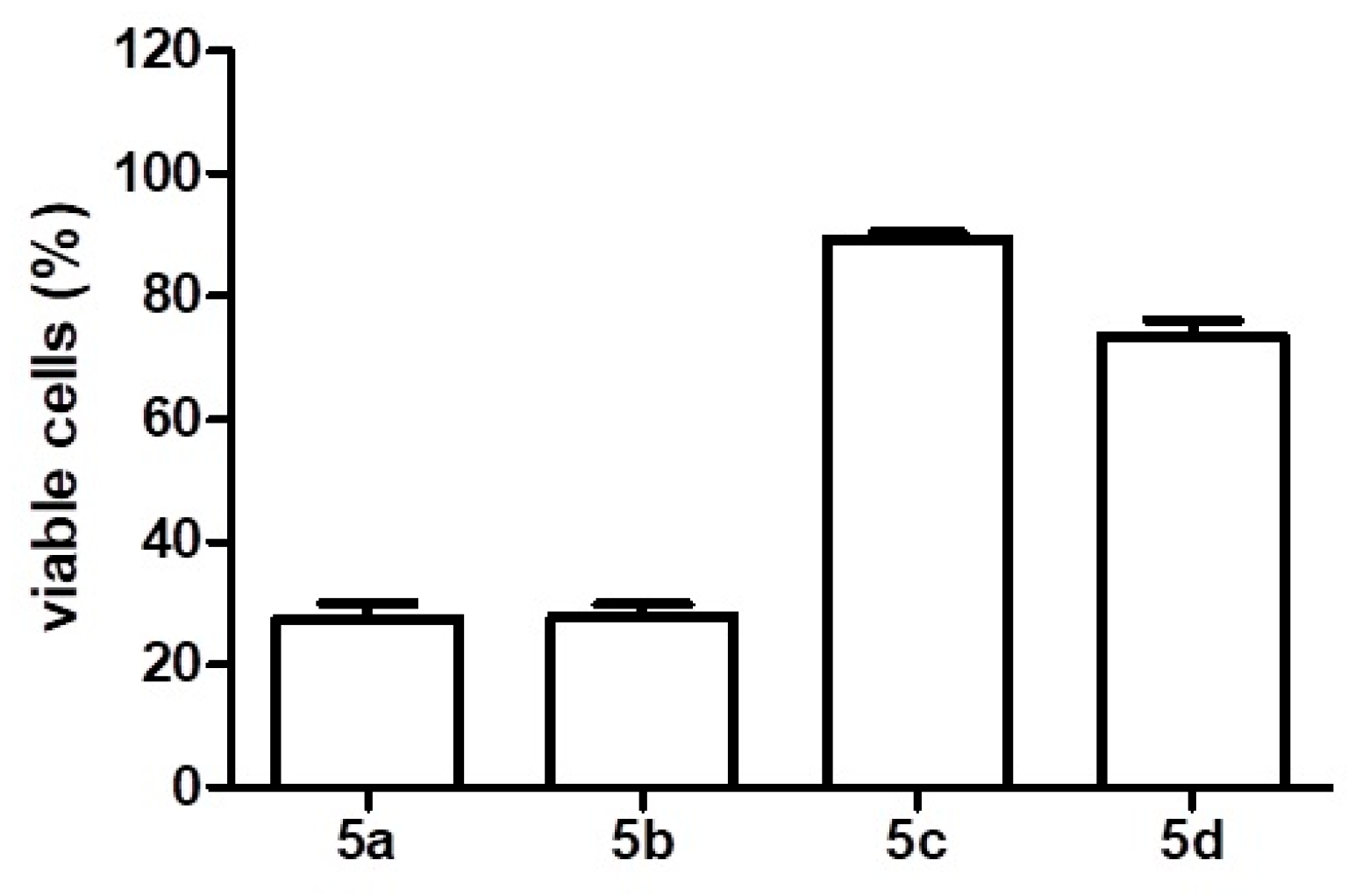
2. Results
3. Discussion
4. Materials and Methods
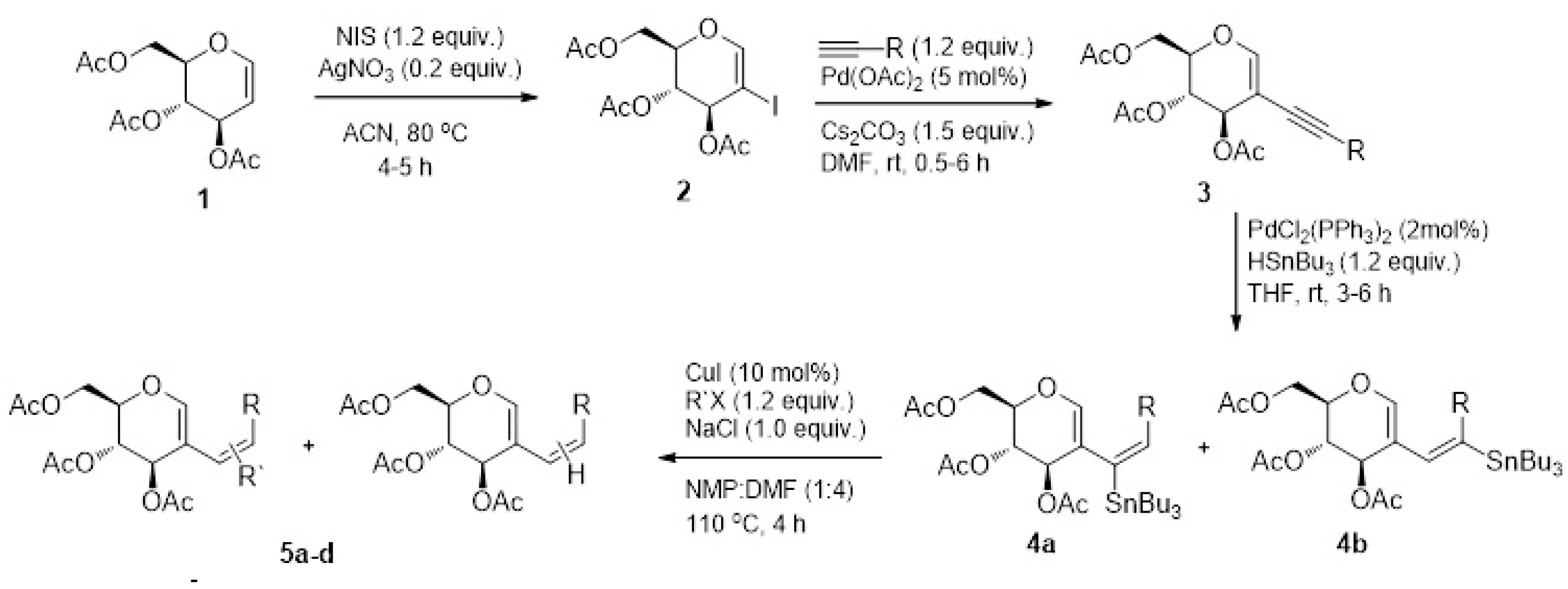
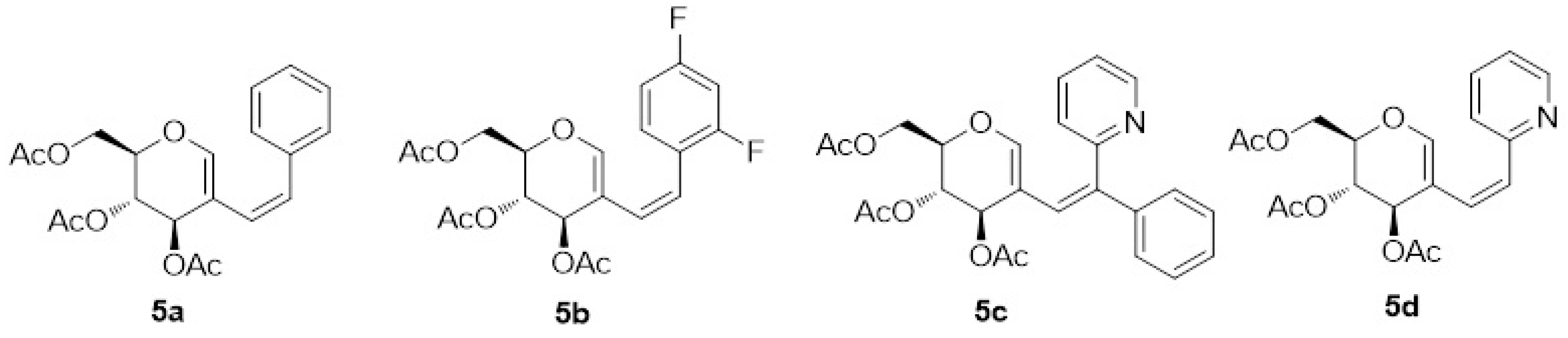
4.1. Synthesis
4.2. Cell Viability Assay
4.3. Target Prediction
4.4. Molecular Docking
4.5. ADMET
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- New Global Cancer Data: GLOBOCAN. Available online: https://gco.iarc.fr/en (accessed on 1 July 2024).
- Goess, R.; Friess, H. A Look at the Progress of Treating Pancreatic Cancer over the Past 20 Years. Expert Rev. Anticancer Ther. 2018, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Polireddy, K.; Chen, Q. Cancer of the Pancreas: Molecular Pathways and Current Advancement in Treatment. J. Cancer 2016, 7, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Brody, J.R.; Abrams, R.A.; Lewis, N.L.; Yeo, C.J. Cancer of the Pancreas. In DeVita, Hellman and Rosenberg’s Cancer: Principles and Practice of Oncology; Wolters Kluwer Health: Philadelphia, PA, USA, 2015. [Google Scholar]
- Iacobuzio-Donahue, C.A.; Velculescu, V.E.; Wolfgang, C.L.; Hruban, R.H. Genetic Basis of Pancreas Cancer Development and Progression: Insights from Whole-Exome and Whole-Genome Sequencing. Clin. Cancer Res. 2012, 18, 4257–4265. [Google Scholar] [CrossRef]
- Murthy, D.; Attri, K.S.; Singh, P.K. Phosphoinositide 3-Kinase Signaling Pathway in Pancreatic Ductal Adenocarcinoma Progression, Pathogenesis, and Therapeutics. Front. Physiol. 2018, 9, 335. [Google Scholar] [CrossRef]
- Bernard, V.; Fleming, J.; Maitra, A. Molecular and Genetic Basis of Pancreatic Carcinogenesis. Surg. Oncol. Clin. N. Am. 2016, 25, 227–238. [Google Scholar] [CrossRef]
- von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Żurawska, K.; Stokowy, M.; Kapica, P.; Olesiejuk, M.; Kudelko, A.; Papaj, K.; Skonieczna, M.; Szeja, W.; Walczak, K.; Kasprzycka, A. Synthesis and Preliminary Anticancer Activity Assessment of N-Glycosides of 2-Amino-1,3,4-Thiadiazoles. Molecules 2021, 26, 7245. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Mauro, M.; Cascioferro, S. Recent Developments of 1,3,4-Thiadiazole Compounds as Anticancer Agents. Pharmaceuticals 2025, 18, 580. [Google Scholar] [CrossRef]
- Sahu, S.K.; Prabhakar, P.K.; Vyas, M. Therapeutical potential of natural products in treatment of pancreatic cancer: A review. Mol. Biol. Rep. 2025, 52, 179. [Google Scholar] [CrossRef]
- Calheiros, J.; Silva, R.; Barbosa, F.; Morais, J.; Moura, S.R.; Almeida, S.; Fiorini, E.; Mulhovo, S.; Aguiar, T.Q.; Wang, T.; et al. A first-in-class inhibitor of homologous recombination DNA repair counteracts tumour growth, metastasis and therapeutic resistance in pancreatic cancer. J. Exp. Clin. Cancer Res. 2025, 44, 129. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sikander, M.; Malik, S.; Tripathi, M.K.; Hafeez, B.B.; Yallapu, M.M.; Chauhan, S.C.; Khan, S.; Jaggi, M. Steviol Represses Glucose Metabolism and Translation Initiation in Pancreatic Cancer Cells. Biomedicines 2021, 9, 1814. [Google Scholar] [CrossRef]
- Lindholm, H.; Ejeskär, K.; Szekeres, F. Digitoxin Affects Metabolism, ROS Production and Proliferation in Pancreatic Cancer Cells Differently Depending on the Cell Phenotype. Int. J. Mol. Sci. 2022, 23, 8237. [Google Scholar] [CrossRef]
- Vieira Veloso, R.; Shamim, A.; Lamarrey, Y.; Stefani, H.A.; Mozer Sciani, J. Antioxidant and Anti-Sickling Activity of Glucal-Based Triazoles Compounds—An in Vitro and in Silico Study. Bioorg. Chem. 2021, 109, 104709. [Google Scholar] [CrossRef]
- Shamim, A.; Barbeiro, C.S.; Ali, B.; Stefani, H.A. Synthesis of Stannyl-Substituted D-Glucal Derivatives via Palladium-Catalyzed Regioselective Hydrostannation and Their Synthetic Applications. ChemistrySelect 2016, 1, 5653–5659. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A Boiled-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT Signaling Pathway. J. Cell. Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Mace, T.A.; Shakya, R.; Elnaggar, O.; Wilson, K.; Komar, H.M.; Yang, J.; Pitarresi, J.R.; Young, G.S.; Ostrowski, M.C.; Ludwig, T.; et al. Single Agent BMS-911543 Jak2 Inhibitor Has Distinct Inhibitory Effects on STAT5 Signaling in Genetically Engineered Mice with Pancreatic Cancer. Oncotarget 2015, 6, 44509–44522. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, D.; Liu, Z.; Huang, X.; Liu, Q.; Kang, Y.; Chen, Z.; Guo, Y.; Zhu, H.; Sun, C. Combination of Gemcitabine and Erlotinib Inhibits Recurrent Pancreatic Cancer Growth in Mice via the JAK-STAT Pathway. Oncol. Rep. 2018, 39, 1081–1089. [Google Scholar] [CrossRef]
- Tang, S.-N.; Fu, J.; Shankar, S.; Srivastava, R.K. EGCG Enhances the Therapeutic Potential of Gemcitabine and CP690550 by Inhibiting STAT3 Signaling Pathway in Human Pancreatic Cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a Regulator of Cell Life and Death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Tessari, G.; Ferrara, C.; Poletti, A.; Dubrovich, A.; Corsini, A.; del Favero, G.; Naccarato, R. The Expression of Proto-Oncogene c-Jun in Human Pancreatic Cancer. Anticancer Res. 1999, 19, 863–867. [Google Scholar]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The Specificities of Protein Kinase Inhibitors: An Update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef]
- Chandra, G.; Singh, D.V.; Mahato, G.K.; Patel, S. Fluorine-a small magic bullet atom in the drug development: Perspective to FDA approved and COVID-19 recommended drugs. Chem. Pap. 2023, 13, 4085–4106. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug. Deliv. Rev. 2001, 46, 3. [Google Scholar] [CrossRef]
- Shamim, A.; Vasconcelos, S.N.S.; Ali, B.; Madureira, L.S.; Zukerman-Schpector, J.; Stefani, H.A. Ligand and Copper Free Sonogashira Coupling to Achieve 2-Alkynyl d-Glucal Derivatives: Regioselective Electrophile Promoted Nucleophilic 5-Endo-Dig Cyclization. Tetrahedron Lett. 2015, 56, 5836–5842. [Google Scholar] [CrossRef]
- Betzer, J.-F.; Delaloge, F.; Muller, B.; Pancrazi, A.; Prunet, J. Radical Hydrostannylation, Pd(0)-Catalyzed Hydrostannylation, Stannylcupration of Propargyl Alcohols and Enynols: Regio- and Stereoselectivities. J. Org. Chem. 1997, 62, 7768–7780. [Google Scholar] [CrossRef]
- Dharuman, S.; Vankar, Y.D. N-Halosuccinimide/AgNO3-Efficient Reagent Systems for One-Step Synthesis of 2-Haloglycals from Glycals: Application in the Synthesis of 2C-Branched Sugars via Heck Coupling Reactions. Org. Lett. 2014, 16, 1172–1175. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a Protein-Small Molecule Docking Web Service Based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. AdmetSAR 2.0: Web-Service for Prediction and Optimization of Chemical ADMET Properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef] [PubMed]


| U251 | MCF-7 | OVCAR-3 | K562 | HCAT | |
|---|---|---|---|---|---|
| 5a | >1 | 0.9 | 0.7 | >1 | 0.7 |
| 5b | >1 | >1 | >1 | >1 | >1 |
| Protein | PDB Code | Affinity (kcal/mol) | Inhibitor Site * |
|---|---|---|---|
| EGFR | 5GTY | −1.9 | No |
| KRAS | 7EWA | −7.25 | No |
| PI3K | 1E7V | 1.2 | Yes |
| MEK1 | 3DV3 | −7.77 | No |
| JNK1 | 2GMX | −8.17 | Yes |
| JAK1 | 4EHZ | −7.4 | No |
| JAK2 | 3IOK | −5.6 | No |
| JAK3 | 5TTS | −9.40 | Yes |
| STAT3 | 6NUQ | −6.77 | No |
| TYK2 | 4GJ2 | 4.9 | No |
| TAK1 | 5E7R | −7.72 | Yes |
| SMAD4 | 1YGS | −5.0 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcântara, P.; Siqueira, H.; Shamim, A.; Priolli, D.G.; Banagouro, K.C.Q.; Stefani, H.A.; Sciani, J.M. The Role of a Glucal-Based Molecule in the Reduction of Pancreatic Adenocarcinoma—An In Vitro and In Silico Approach. Drugs Drug Candidates 2025, 4, 21. https://doi.org/10.3390/ddc4020021
Alcântara P, Siqueira H, Shamim A, Priolli DG, Banagouro KCQ, Stefani HA, Sciani JM. The Role of a Glucal-Based Molecule in the Reduction of Pancreatic Adenocarcinoma—An In Vitro and In Silico Approach. Drugs and Drug Candidates. 2025; 4(2):21. https://doi.org/10.3390/ddc4020021
Chicago/Turabian StyleAlcântara, Pedro, Henrique Siqueira, Anwar Shamim, Denise Gonçalves Priolli, Karine C. Q. Banagouro, Hélio A. Stefani, and Juliana Mozer Sciani. 2025. "The Role of a Glucal-Based Molecule in the Reduction of Pancreatic Adenocarcinoma—An In Vitro and In Silico Approach" Drugs and Drug Candidates 4, no. 2: 21. https://doi.org/10.3390/ddc4020021
APA StyleAlcântara, P., Siqueira, H., Shamim, A., Priolli, D. G., Banagouro, K. C. Q., Stefani, H. A., & Sciani, J. M. (2025). The Role of a Glucal-Based Molecule in the Reduction of Pancreatic Adenocarcinoma—An In Vitro and In Silico Approach. Drugs and Drug Candidates, 4(2), 21. https://doi.org/10.3390/ddc4020021






