Abstract
Current treatment options for Alzheimer’s disease target neurotransmitters following the disease onset, and they offer limited efficacy without slowing down the disease progression. There has been an increasing concern in recent years targeting the histamine H3 receptor (H3R) in treating cognitive disorders, including dementia. Preclinical studies have shown that antagonists of H3R or inverse agonists enhance the cognitive function in animal models with dementia by increasing the release of neurotransmitters associated with learning and memory. This review employed a systematic literature search across databases including PubMed, Scopus, Google Scholar, and ClinicalTrials.gov, selecting peer-reviewed studies. The results of this study illustrate the complex landscape of research on H3R modulators in dementia, highlighting both promising findings and ongoing challenges in translating preclinical discoveries into effective clinical interventions. Knowing the role of H3R in dementia and developing novel pharmacological interventions targeting these receptors represent a promising avenue for future research, leading to the development of new treatments for this devastating condition.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative and incapacitating condition marked by the gradual deterioration of cognitive abilities, specifically the impairment of memory and diminished reasoning. This is the primary cause of cognitive decline in elderly individuals, affecting many people globally. The disease, named after the German neurologist Alois Alzheimer, who initially documented the condition in 1906 [1], primarily impacts the brain by causing the buildup of atypical protein deposits like beta-amyloid plaques and tau tangles, and the death of nerve cells. These atypical formations interfere with the regular transmission of signals between neurons, leading to the progressive decline of cognitive functions like memory, language, problem-solving, and daily activities [2].
The precise etiology of AD remains elusive; however, advancing age, genetic predisposition, and lifestyle factors are considered potential determinants in its pathogenesis. The disease exhibits individual variability in its progression, typically commencing with mild symptoms that gradually deteriorate over time [2]. AD not only impacts the individuals who are diagnosed but also imposes considerable emotional and psychological strains on their families and caregivers.
Despite thorough investigations, there is presently no remedy for AD as the available treatment options only mitigate symptoms to improve the quality of life of the patients. Thus, current scientific endeavors are concentrated on comprehending the fundamental mechanisms of the disease, creating early diagnostic assays, and investigating potential therapeutic interventions.
2. Drug Targets for the Treatment of AD
Ongoing research into drug targets for AD is advancing, with an exploration of the multiple potential targets. It is crucial to acknowledge that drug development is an intricate procedure, and numerous potential treatments undergo a thorough examination in preclinical and clinical trials before being released in the market. Scientists are constantly investigating new methods, and our knowledge of the underlying causes of AD is developing. The primary drug targets that are being studied for AD are summarized below.
3. Beta-Amyloid Plaque and Its Role in AD
Beta-amyloid (Aβ) is a protein that creates deposits in the brain of people with AD. Scientists have been investigating medications that specifically target Aβ to decrease its production or improve its removal. Nevertheless, clinical trials focused on Aβ have yielded inconclusive outcomes, thus, its importance in this disease continues to be actively studied.
The posttranslational modification of amyloid precursor protein (APP) involves cleavage at α- or β-side enzymes, and the processing of the C-terminal fragment produced by gamma secretase. If the cleavage is from the α-secretase product, it results in a non-toxic protein fragment (p3), but the products of the cleavages by the Beta site cleavage enzyme (BACE) and the Gamma secretase yield 38-43 amino acid fibrillogenic Aβ, though Aβ42 is very prone to deposition in the core neurotic plaques [3]. To reduce Aβ production, efforts have been made to target drugs that can activate alpha secretase [4,5] or inhibit beta and gamma secretase [6,7,8]. The degradation of the toxic form of Aβ is also a target for dementia management, as Aβ degradation enzymes are low in human [9] and animal [10] models of AD, and the endogenous pathways to achieve this include neutral endopeptidase (neprilysin) [11,12], metalloproteinase, endothelin-converting enzyme, and angiotensin-converting enzyme.
Studies have shown that the constitutive monomer form of Aβ or the fibrillar network form aggregated in plaques is not the synaptotoxic form [13,14], but rather the oligomerization of monomeric Aβ into dimer, trimer, and other higher molecular mass combinations at the aggregation stage. Thus, oligomeric inhibition is a top target for the prevention of AD. Some agents that are at different stages of clinical trial for the prevention of the oligomerization of the Aβ monomer include grape-derived polyphenols [15], curcumin, and Omega-3 fatty acids [16].
4. Involvement of Tau Protein in AD
Intracellular neurofibrillary tangle, which is a condensed form of the cytoskeletal structure consisting of hyperphosphorylated helical paired filaments of microtubules associated with Tau protein, is the second pathological marker of AD. While Tau phosphorylation is important for its functioning, the hyperphosphorylated Tau no longer binds the microtubule but rather aggregates into paired helical filaments [17], ultimately leading to microtubule instability and disruption of axonal transport. Various kinases, including glycogen synthase kinase 3 beta (GSK-3β) and cyclin-dependent kinase 5, which are involved in tau hyperphosphorylation, have been identified and are also therapeutic targets. Agents like lithium and valproate inhibit GSK-3β to stabilize tau. Protein phosphatases, which dephosphorylate tau, are also an important target for the inhibition of hyperphosphorylation [18,19].
5. Cholinergic System and Its Effects in AD
The inhibition of cholinergic function leads to attention deficit, while the facilitation of cholinergic transmission improves it [20,21]. The major role of the cholinergic system in the learning process [22] and memory [23] has been established, as endogenous acetylcholine modulates the acquisition [24], encoding [25], consolidation [26], reconsolidation [27], extinction [28], and retrieval of memory [29]. In fact, memory loss in AD patients is associated with cholinergic neuron degeneration from the nucleus basalis of Meynert [30,31,32]. There is evidence showing that cholinergic system disruption impairs memory, attention, and learning [33,34]. Some examples of drugs that target the cholinergic system are acetylcholinesterase (AChE) inhibitors and neuronal nicotinic receptors’ agonists [35,36,37].
6. Serotonergic Pathway Modulation in AD
The expression of serotonin receptors in the brain areas important for memory and learning, and their decline in AD and dementia, have been well-established [38,39]. Moreover, the beneficial effect of selective agonists and antagonists of serotonin receptors on cognition in animal [40] and human [41,42] models has been reported.
7. Inflammation and Its Effects in AD
Neuroinflammation is thought to contribute to the advancement of AD. Researchers are currently studying the anti-inflammatory medications and substances that specifically target inflammatory pathways as potential treatments for AD. Some drugs involved in the regulation of inflammation that are under different clinical trial phases for AD are Masitinib [43,44], NE3107 [45,46], Semaglutide [47], AL002 [48], Bacillus Calmette-Guerin [49], Baricitinib [50,51], Canakinumab [52], Daratumumab [53,54,55], Lenalidomide [56], Montelukast [57,58], Pepinemab [59], Proleukin [60,61], Rapamycin [62,63], Sargramostim [64,65], Senicapoc [66], TB006 [67], the Tdap vaccine [68,69], Valacyclovir [70], XPro1595 [71], CpG1018 [72], Emtricitabine [73], IBC-Ab002 [74,75], Salsalate [76,77], and VT301 [78].
8. Neuroprotective Factors as Regulators of AD
Certain studies focus on investigating factors that enhance the resilience of nerve cells, shielding them from harm and boosting their longevity. This encompasses neurotrophic factors and other molecules that have the potential to augment neuronal resilience, enhancing synaptic plasticity, or producing neuroprotective effects. Some of the drugs at different phases of clinical trial for neuroprotective effects in AD include AGB101 [79,80], Blarcamesine [81], Fosgonimeton [82,83,84], Simufilam [85,86,87], Tertomotide [88,89], AL001 [90,91,92], Bryostatin1 [93,94], CY6463 [95], Dalzanemdor [96], Edonerpic [97], Elayta [98], EX039 [99], ExPlas [100], MW150 [101], Neflamapimod [102,103], and Centella asiatica [104].
9. Neurogenesis in AD
Agents that promote neurogenesis are among those that are in the pipeline for the treatment of AD. An example is allopregnanolone, an allosteric modulator of inhibitory gamma-aminobutyric acid-A receptors, which reduces the deposition of Aβ and enhances memory and learning [105]. Another example is sovateltide, an Endothelin-B receptor antagonist that promotes the differentiation of neuronal progenitors for the production of mature neuronal cells. This agent exhibits anti-apoptotic and antioxidant properties while also enhancing mitochondrial functions [106].
10. Role of the Genetic Factor in AD
Persons carrying apolipoprotein E Ɛ 4 are at high risk of developing AD at an earlier age, as it leads to increased Aβ deposition in the brain by regulating the passage of Aβ from the blood to the brain. Some drugs target the apolipoprotein E Ɛ 4 gene carrier (APOE4), being a very influential risk factor after the age of an individual and the most vital genetic factor for the development of AD. This protein has a strong interaction with Aβ, thereby reducing the amyloid brain accumulation age and elevating the total Aβ burden in gene carriers [107]. Furthermore, APOE4 exacerbates Tau neurofibrillary tangle-related blood–brain barrier disruption, neurodegeneration, microglial responses, astroglial activity, and neuroinflammation [108]. Some therapies targeting this marker are hydroxypropyl-beta-cyclodextrin [109], LX 1001, and obicetrapib [110].
11. Oxidative Stress and Its Role in AD
Shreds of evidence have shown that the enhancement of antioxidant status and attenuation of oxidative stress can reduce, prevent, or treat AD, as the foods rich in polyphenols, antioxidants, and polyunsaturated fatty acids reduce AD risk [111]. Drugs like hydralazine [112] and edaravone [113], among others, are at various clinical trial stages for the management of AD.
12. Others
Other mediators and pathways are targeted for drug development in the management of AD. Some of these are drugs that regulate vascular factors (telmisartan, perindopril), circadian rhythm (piromelatine) [114], and epigenetics (lamivudine) [115].
13. Lifestyle and Supportive Interventions
Non-pharmacological interventions are essential for effectively managing the symptoms of AD. These factors encompass cognitive stimulation, physical activity, and a nutritious diet. Establishing a nurturing and organized setting can additionally improve the general welfare of individuals with AD.
14. Clinical Trials and Research on Histamine Receptors in AD Treatment
Ongoing research is being conducted to formulate novel treatments and therapies for AD. Clinical trials provide the opportunity to access experimental medications or interventions that are currently undergoing testing to determine their efficacy. The AD research is constantly evolving, with continuous endeavors to discover novel therapeutic targets and create more efficient interventions. It is recommended that individuals suffering from AD, their families, and their caregivers remain updated on the most recent research discoveries and treatment choices by consulting with healthcare professionals and reliable sources.
15. Histamine
Histamine is a neurotransmitter that performs various physiological functions in the body through four receptor subunits, including the G-protein coupled H1R, H2R, H3R, and H4R. Various agonists and antagonists were developed for these receptors [116]. Allergic indications like asthma, rhinitis, conjunctivitis, and atopic dermatitis are treated with H1R antagonists [117]. The activation of H2R stimulates the gastric secretion while H1R and H2R mediate the opposing pharmacological and physiological effects on lungs and heart [118]. H3R is a presynaptic autoreceptor that inhibits the production and release of histamine in histaminergic neurons [119], while H4R is expressed in the immune cells. Evidence has shown that both H3R and H4R have homology, and some H3R agonists and antagonists equally bind to H4R [120]. A recent nationwide cohort study in Taiwan showed that the use of H1R antagonists is linked with increased dementia risk [121].
H3R is encoded on chromosome 20 and is displayed in many regions of the brain, like the cerebral cortex, CNS basal ganglia, and hypothalamus, all of which play some important roles in cognition [122]. It binds to the Gi protein to negatively control the intracellular second messenger cAMP formation by inhibition of adenylyl cyclase [123], an effect that is blocked by pre-treatment with pertussis toxin [124]. Histamine is known to downregulate the acetylcholine-induced calcium signaling of the muscarinic receptor via H3R-mediated mechanisms [125]. It also activates PLA2 and inhibits Na+/H+ exchanger activity [126]. Previous reports show that H3R in transfected SK-N-MC cells and primary cultures of cortical neurons activates the Akt/GSK-3b axis through phosphoinositol-3-kinase (PI3K) via a PTX-sensitive G i/o-protein-dependent, but Src and epidermal growth factor receptor (EGFR)-independent pathway [127] Also, treatment of transfected COS-7 and CHO cells with an agonist resulted in the rapid activation of ERK1/2, even though the underlying molecular mechanisms regulating the H3R-mediated ERK1/2 activation remain largely unknown [126,128]. It is also reported in HEK293 cells that, upon exposure to agonists, activated H3R evoked ERK1/2 phosphorylation via PLC/PKC-, PLDs, and MMP/EGFR transactivation-dependent pathways, and that the Gβγ subunit, as dissociated from the activated Gi/o protein, plays a central role in the regulation of H3R-mediated ERK1/2 activation.
It is also known that H3R antagonists can stimulate histamine, dopamine, acetylcholine, and norepinephrine, all of which are involved in some specific cognitive aspects, making H3R antagonists important drug targets to improve cognition in dementia patients. Furthermore, H3R inverse agonists can increase histaminergic neuron activity by inhibiting the H3R-mediated suppression of histamine release in the brain, making them a target for AD treatment drug development.
Although there are recent studies that have reported the involvement of histamine in neurodegenerative diseases, such as dementia, the connection between the two is intricate, and our comprehension is still incomplete. Below are several key factors concerning the potential role of histamine in dementia progression [129,130,131].
16. Role of Histamine in the Pathogenesis of Dementia
Histamine interacts with histamine receptors, specifically H1R, H2R, H3R, and H4R (Figure 1 and Figure 2; Table 1). Cognitive deficits occur when histamine is unable to bind to the receptors. The H3R reduces histamine release in the brain, resulting in AD. Inverse agonists of H3R are crucial in counteracting the effects of histamine-induced AD [132,133].
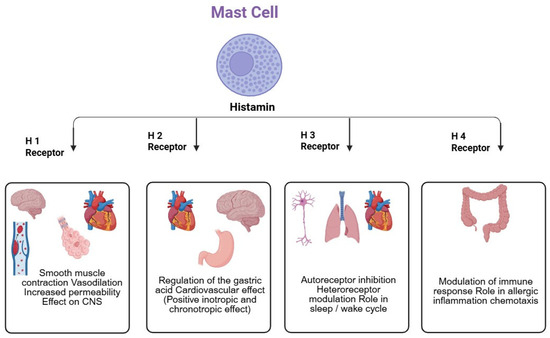
Figure 1.
Overview of Histamine Regulation.
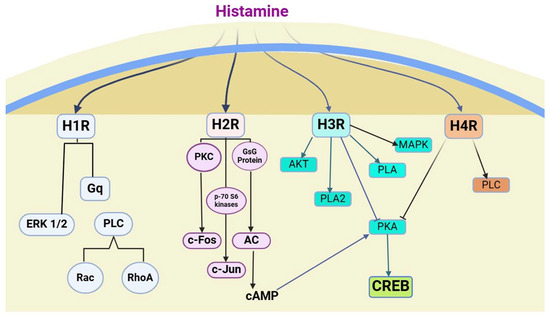
Figure 2.
Histamine receptors—Signaling pathways.

Table 1.
Characteristics of Histamine Receptors.
Antagonists of H3R stimulate the production of histamine, ACh, and other neurotransmitters, thereby enhancing cognitive function. Histamine plays a role in both short- and long-term cognitive processes [134]. Recent studies indicate that the deterioration of histamine neurons is the causative factor in the development of AD. The presence of histamine in the brain improves cognitive function and memory, although the specific mechanism by which it does so remains unclear [135]. The histaminergic neuron system in the brain regulates various roles, i.e., homeostasis, learning, arousal memory, and circadian rhythms. Furthermore, certain studies explained that histamine plays a part in regulating specific behavioral tasks, although the underlying mechanism remains unclear [136]. To treat AD, various pre-clinical methods have been developed to specifically target H3R [137,138].
The development of AD is attributed to the induction of neurotoxicity by Aβ, 1-42. However, this neurotoxicity can be mitigated by histamine acting on histamine receptors (H2 and H3) [139,140]. Using H2 receptor agonists leads to a gradual reduction in AD. HIR-KO mice exhibit cognitive symptoms because of alterations in the brain levels of AChE and dopamine [141]. The involvement of H1R and H2R in cognitive function is substantiated by the presence of cognitive deficits resulting from null mutations in the genes encoding these receptors. Both H1R and H2R act like excitatory neurotransmitters, whereas H3 functions differently as an inhibitory neurotransmitter and acts as an autoreceptor and heteroreceptor. Interactions between histaminergic, peptidergic, and aminergic systems can regulate homeostatic functions like sleep-wake cycles, cognition, and synaptic plasticity [142,143,144].
Histamine receptors are involved in regulating the functional activity of dendritic cell subsets. The H2R antagonist characterizes the specific mechanisms of a histamine-induced decrease of CD1a (+) DCs, IL6, and IL10 increased production, upregulation of chemokines, expression of C5aR1 through the CD1a (−) and DC subset, and increased migration of activated DC subsets, which are stimulated by the secretion of MMP-9 and MMP-12 enzymes [145]. Store-operated calcium entry (SOCE) is the main mechanism by which DCs (dendritic cells) allow Ca2+ ions to enter. DCs that have been primed with histamine can initiate the Th2 immune response by interacting with several types of histamine receptors. Histamine-activated DCs trigger the release of calcium ions (Ca2+) from their intracellular reservoirs. Histamine elevates IL-10 levels while decreasing the IL-12p70 levels that are produced by DCs. Pretreating DCs with H1R antagonists, SOC blockers, and H4R antagonists can prevent the histamine-induced Th2 polarization of T-helper cells in the mixed responses of lymphocytes. Recent research indicates that SOCE is crucial in the Th(2) response and histamine-induced maturation of DCs through the activation of both H1R and H4R [146]. Research has demonstrated that young individuals who produce elevated levels of IL-2 and IFN-γ possess a specific type of T-cell memory called beta (1-42)-specific Th1-type T-cell memory. There is evidence indicating that as individuals age, there is a decline in the production of IFN-γ and IL-2, while there is a noticeable increase in the release of regulatory IL-10 by CD4(+) T-cells. However, despite the absence of an effector cytokine, individuals with AD can still generate IL-10 [147]. The proinflammatory cytokine IL-32 can activate nuclear factor κB and p38 mitogen-activated protein kinase (p38MAPK) pathways. IL-32 can induce histamine synthesis in human-derived core blood mast cells (HDCBMCs; Figure 1). Therefore, it can be demonstrated that IL-32 is specific to a particular species and functions in fully developed human mast cells (LAD 2 cells) [148]. Research has demonstrated that IL-32 plays a role in controlling neuroinflammatory responses in various neuronal diseases, including AD [149] (Figure 3).
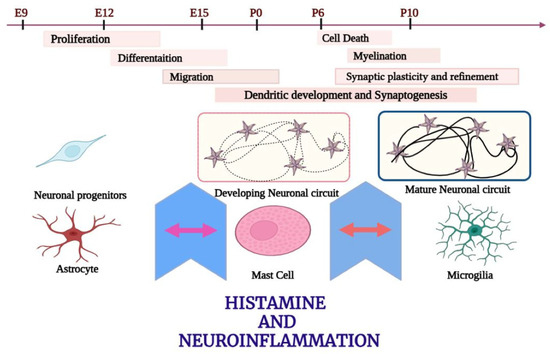
Figure 3.
Role of histamine in neuronal development.
17. Recently Developed Drugs for the Treatment of Dementia by Blocking H3 Receptors
Despite the well-established role of histamine in memory, the stimulation of post-synaptic H1R or H2R is not an acceptable therapeutic target to enhance memory in AD because of their associated detrimental peripheral actions in the gastrointestinal and cardiovascular systems. Thus, the presynaptic H3R is considered the most suitable therapeutic target to enhance histaminergic signaling to boost memory with little peripheral side effects, as it is mostly expressed in the mammalian brain areas involved in cognitive processes and arousal, like the hippocampus, cerebral cortex, hypothalamus, and basal ganglia [150]. The activation of H3 autoreceptors leads to the inhibition of histamine synthesis and release from histaminergic neurons, while the activation of H3 heteroreceptors leads to the inhibition of release of other neurotransmitters such as acetylcholine, noradrenaline, dopamine, and 5-HT from nonhistaminergic neurons [151]. Conversely, a blockade of H3 receptors with selective antagonists can increase the release of neurotransmitters involved in cognitive processes [152].
The H3R is expressed in some brain regions involved in cognition, sleep, and homeostatic modulation, including the CNS, hypothalamus, cerebral cortex, and basal ganglia [122]. It affects various signaling pathways, such as the activation of PLA2, Akt, and the MAPK, G(i/o)-dependent inhibition of adenylyl cyclase, and the inhibition of both Na+/H+ exchanger and K+-induced Ca2+ mobilization [126]. In type 1 cells of rats, H3R is responsible for the histamine attenuation of calcium signaling induced by muscarinic cholinergic receptors [153]. Generally, H3Rs serve as H3 autoreceptors modulating the synthesis and release of the central.
17.1. Thioperamide
Thioperamide (Figure 4) is a highly potent and specific antagonist of imidazole. It was primarily developed to improve wakefulness and address issues related to learning and memory. According to recent research, thioperamide, despite its hepatotoxicity, has been found to have a significant impact on patients with circadian rhythm disorders and Parkinson’s disorder (Figure 4) [154]. It can cause a strong activation of histamine release and convert histamine to N-tele-methylhistamine, competitively suppressing the conversion of N-tele-methylhistamine to N-tele-methylimidazoleacetic acid in the brains of humans and monkeys due to monoamine oxidase B [155].
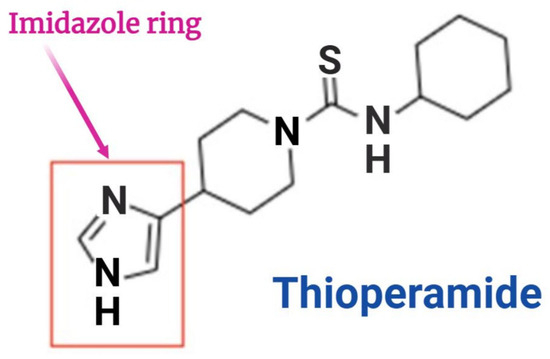
Figure 4.
Structure of Thioperamide.
17.2. Pitolisant
Pitolisant (also called BF2.649 or tiprolisant) with full name [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, Hydrochloride] is a high-affinity, competitive antagonist, and potent inverse agonist of the H3R [156] that has been approved by regulatory agencies in the United States and Europe. Its high oral bioavailability allows easy access to the brain. It undergoes metabolism by the enzyme CYP4A in the gastrointestinal tract. It is employed in managing narcolepsy to sustain wakefulness during the daytime. Headache, anxiety, and QT prolongation have been documented as adverse effects in clinical trials [157,158]. Wakix is a proprietary name for a product that has been commercially available since March 2016. The dosage is available in tablets of 4.5 mg and 18 mg. Pitolisant increases working memory using two-trial object recognition tasks [156] and fear conditioning tests.
- GSK189254
The compound GSK189254, a high-affinity H3R antagonist, has shown therapeutic potential for AD in both rats and humans [159]. It potently inhibits cortical ex vivo H3 receptor binding, consistent with good CNS penetration and H3 receptor occupancy, and increased cortical neuronal activation [160]. A microdialysis study with GSK189254 showed increases in acetylcholine, noradrenaline, and dopamine release in the cortex, consistent with the blockade of H3 heteroreceptors. In addition, GSK189254 potently blocked H3 agonist-induced dipsogenia, consistent with the functional blockade of H3 receptors in vivo. It was also shown to reverse the amnesia induced by cholinergic antagonist scopolamine. Furthermore, it had behavioral effects, where it reduced platform escape latency in aged rat water maze tests, improved task recall in probe trials, improved recognition and memory in object recognition and novelty detection tests, and improved attention reversal learning and attentional set shifting [150].
- E177
E177 [1-(6-(naphthalen-2-yloxy)hexyl)azepane hydrogen oxalate] is a non-imidazole-based H3R antagonist E177, with high antagonist affinity (Ki = 69.40 nM) and high in vitro selectivity. It was shown to mitigate dizocilpine-induced cognitive impairments via the modulation of histaminergic neurotransmission [161].
- SAR110894
SAR110894 has been shown to prevent episodic memory deficits induced by scopolamine in rats or by the central infusion of the toxic amyloid fragment Aβ (25–35) in the object recognition test in mice. Its acute treatment improves cognition in several animal models displaying cognitive deficits relevant to those found in patients with AD [162]. Chronic administration of SAR110894 in a transgenic mouse model of tauopathy prevents cognitive deficits and inhibits tau pathology [162].
- ABT-239
ABT-239 stimulates biochemical signaling that improves cognitive performance and attenuates tau hyperphosphorylation [160].
- E169
E169 [1-(6-(naphthalen-1-yloxy)hexyl)azepane] is a newly developed, highly potent and selective non-imidazole H3R antagonist with high affinity [161]. Studies have reported the ADMET properties, in silico docking to human H3R, and in vivo memory-enhancing effects of E169 [161]. This agent has a high dG bind value of −108.24 kcal/mol and occupies the H3R binding pocket like the resolved complex ligand PF03654746, establishing the interaction of a crucial histamine H3R antagonist/inverse agonist like salt bridge and/or hydrogen bond formation between protonated amine nitrogen and ASP1143.32 [Levoin et al., 2008], and west-end stabilization with caging the aromatic sidechains of Y1153.33, F3987.39, and Y3746.51 [162]. The east-end naphthalene substituent of E169 occupied the space fenced by aromatic features of TYR189 (ECL2) on the top, and TYR912.61 and TYR942.64 on the sides that also stabilized the structure through Π–Π stacking interactions (Figure 5). A recent study with mice showed that E169 ameliorated MK801-induced reduction in the short- and long-term memory and the disturbance in neurochemicals, including PI3K, Akt, and GSK-3β in the hippocampus [162], suggesting it as a candidate for H3R in the treatment of AD.
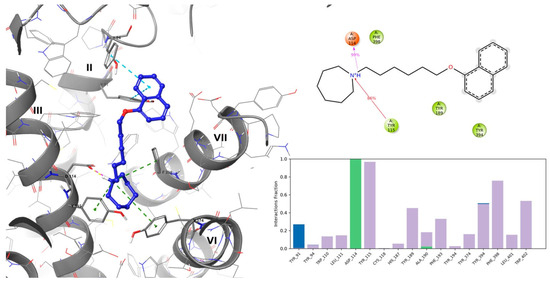
Figure 5.
(Left panel): Putative binding mode of E169 (left) in the histamine H3R binding site. Yellow dashed lines denote hydrogen bonds, magenta denotes salt bridges, green denotes cation-Π interactions, and blue denotes Π-ΠΠ interactions, while Roman numbers denote respective TMs. (Right panel): MD stimulation of ligand-protein contact summary (top), and contacts histogram (bottom: green—H-bond; violet—hydrophobic contact; blue—water bridges).
18. Conclusions
Neuropharmacology has a lot of potential as H3R modulators are being used to treat dementia after being experimentally developed. Investigating these modulators has yielded valuable understandings of the intricate mechanisms that underlie dementia and has unveiled fresh prospects for therapeutic interventions. The bench-to-bedside approach prioritizes the smooth conversion of scientific findings into tangible implementations for patient treatment.
The combined endeavors of researchers, clinicians, and pharmaceutical developers are instrumental in creating groundbreaking solutions that could revolutionize dementia treatment in the future. As research and clinical trials progress, our understanding of H3 receptor modulators will improve, leading to a better understanding of their role in the comprehensive care and management of dementia patients.
19. Future Prospective
While H3R modulators seem to be promising potential therapeutic agents for dementia, further research and development efforts are needed to realize their full clinical potential and impact on patient outcomes. Collaboration between academia, industry, regulatory agencies, and patient advocacy groups will be essential in advancing this field and addressing the growing burden of dementia worldwide.
Author Contributions
N.B., S.P.N.B., and H.O.: Conceptualized, designed, and coordinated the work, writing-original draft preparation; V.M.D.P., G.S., H.O., and V.S.S.: Manuscript review, revision, and reference work; S.N. and P.B.D.: Manuscript review and revision; S.P.N.B., A.I.A., J.O.C.E., and H.O.: Coordination work, figure generation, writing-review and editing, manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no any external funding.
Data Availability Statement
Data used in this study are available within the manuscript.
Conflicts of Interest
All authors report that there was no conflict of interest in this work.
Abbreviations
Aβ: Amyloid-beta protein; AC: adenylyl cyclase; AD: Alzheimer’s Disease; AKT: protein kinase B; APOE4: Apolipoprotein E Ɛ 4 gene carrier; APP: Amyloid Precursor Protein, BACE: Beta site cleavage enzyme; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding protein; DC: Dendritic cells; ERK, extracellular signal-regulated kinase; H1R, histamine receptor subtype 1; H2R, histamine receptor subtype 2; H3R, histamine receptor subtype 3; H4R, histamine receptor subtype 4; HDCBMC: human-derived core blood mast cells; MAPK, mitogen-activated protein kinase; p38MAPK: p38 mitogen activated protein kinase; PKA: protein kinase A; PKC: protein kinase C; PLA2: phospholipase A2; PLC: Phospholipase C; Rac, Ras-related C3 botulinum toxin substrate; RhoA, Ras homolog family member A; SOCE: Store-Operated Calcium Entry.
References
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s Disease. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Harris, J.R., Ed.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; Volume 65, pp. 329–352. ISBN 978-94-007-5415-7. Available online: https://link.springer.com/10.1007/978-94-007-5416-4_14 (accessed on 15 March 2025).
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. 2011, 32, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Fisher, A.; Pittel, Z.; Haring, R.; Bar-Ner, N.; Kliger-Spatz, M.; Natan, N.; Egozi, I.; Sonego, H.; Marcovitch, I.; Brandeis, R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: Implications in future therapy. J. Mol. Neurosci. 2003, 20, 349–356. [Google Scholar] [CrossRef]
- Caccamo, A.; Oddo, S.; Billings, L.M.; Green, K.N.; Martinez-Coria, H.; Fisher, A.; LaFerla, F.M. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 2006, 49, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bolon, B.; Kahn, S.; Bennett, B.D.; Babu-Khan, S.; Denis, P.; Fan, W.; Kha, H.; Zhang, J.; Gong, Y.; et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001, 4, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.B.; Frigon, N.L.; Games, D.; Hu, K.; et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kumaragurubaran, N.; Hong, L.; Kulkarni, S.S.; Xu, X.; Chang, W.; Weerasena, V.; Turner, R.; Koelsch, G.; Bilcer, G.; et al. Design, synthesis, and X-ray structure of potent memapsin 2 (beta-secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J. Med. Chem. 2007, 50, 2399–2407. [Google Scholar] [CrossRef]
- Yasojima, K.; McGeer, E.G.; McGeer, P.L. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001, 919, 115–121. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Turner, A.J.; Tanzawa, K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997, 11, 355–364. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E.; Kawashima-Morishima, M.; Lee, H.J.; Hama, E.; Sekine-Aizawa, Y.; et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef]
- Cheng, I.H.; Scearce-Levie, K.; Legleiter, J.; Palop, J.J.; Gerstein, H.; Bien-Ly, N.; Puoliväli, J.; Lesné, S.; Ashe, K.H.; Muchowski, P.J.; et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J. Biol. Chem. 2007, 282, 23818–23828. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008, 283, 32176–32187. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- Lee, V.M.; Trojanowski, J.Q. The disordered neuronal cytoskeleton in Alzheimer’s disease. Curr. Opin. Neurobiol. 1992, 2, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Vogelsberg-Ragaglia, V.; Schuck, T.; Trojanowski, J.Q.; Lee, V.M. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp. Neurol. 2001, 168, 402–412. [Google Scholar] [CrossRef]
- Sontag, E.; Luangpirom, A.; Hladik, C.; Mudrak, I.; Ogris, E.; Speciale, S.; White, C.L. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J. Neuropathol. Exp. Neurol. 2004, 63, 287–301. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G.; Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 2013, 203, 361–364. [Google Scholar] [CrossRef]
- Bracco, L.; Bessi, V.; Padiglioni, S.; Marini, S.; Pepeu, G. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer’s disease patients. J. Alzheimers Dis. 2014, 40, 737–742. [Google Scholar] [CrossRef]
- Miranda, M.I.; Bermúdez-Rattoni, F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc. Natl. Acad. Sci. USA 1999, 96, 6478–6482. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A.; Honig, W.; Raaijmakers, W.G.M. Effects of intra-hippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology 1992, 109, 373–376. [Google Scholar] [CrossRef]
- Winters, B.D.; Bussey, T.J. Removal of cholinergic input to perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur. J. Neurosci. 2005, 21, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Power, A. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 2003, 80, 178–193. [Google Scholar] [CrossRef]
- Boccia, M.M.; Acosta, G.B.; Blake, M.G.; Baratti, C.M. Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: Effects of i.c.v. injections of hemicholinium-3. Neuroscience 2004, 124, 735–741. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Baratti, C.M.; McGaugh, J.L. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol. Learn. Mem. 2009, 91, 93–97. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Acosta, G.B.; Baratti, C.M. Atropine, an anticholinergic drug, impairs memory retrieval of a high consolidated avoidance response in mice. Neurosci. Lett. 2003, 345, 97–100. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Whitehouse, P.; Price, D.; Struble, R.; Clark, A.; Coyle, J.; DeLong, M.R. Alzheimer’s Disease and Senile Dementia: Loss of Neurons in the Basal Forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Ducatenzeiler, A.; Ribeiro-da-Silva, A.; Duff, K.; Bennett, D.A.; Cuello, A.C. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol. Aging 2006, 27, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T. On Neurodegenerative Diseases, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Rogers, J.L.; Kesner, R.P. Cholinergic Modulation of the Hippocampus During Encoding and Retrieval of Tone/Shock-Induced Fear Conditioning. Learn. Mem. 2004, 11, 102–107. [Google Scholar] [CrossRef]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Egawa, G.; Doi, H.; Miyachi, Y.; Kabashima, K. Histamine modulates the responsiveness of keratinocytes to IL-17 and TNF-α through the H1-receptor. J. Dermatol. Sci. 2011, 61, 79–81. [Google Scholar] [CrossRef]
- Tagawa, M.; Kano, M.; Okamura, N.; Higuchi, M.; Matsuda, M.; Mizuki, Y.; Arai, H.; Fujii, T.; Komemushi, S.; Itoh, M.; et al. Differential cognitive effects of ebastine and (+)-chlorpheniramine in healthy subjects: Correlation between cognitive impairment and plasma drug concentration. Br. J. Clin. Pharmacol. 2002, 53, 296–304. [Google Scholar] [CrossRef]
- Verdurand, M.; Bérod, A.; Le Bars, D.; Zimmer, L. Effects of amyloid-β peptides on the serotoninergic 5-HT1A receptors in the rat hippocampus. Neurobiol. Aging 2011, 32, 103–114. [Google Scholar] [CrossRef]
- Elliott, M.S.J.; Ballard, C.G.; Kalaria, R.N.; Perry, R.; Hortobágyi, T.; Francis, P.T. Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain J. Neurol. 2009, 132, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa, V.; Duchatelle, P.; Boulouard, M.; Dauphin, F. Selective 5-HT6 receptor blockade improves spatial recognition memory and reverses age-related deficits in spatial recognition memory in the mouse. Neuropsychopharmacology 2009, 34, 488–500. [Google Scholar] [CrossRef]
- Upton, N.; Chuang, T.T.; Hunter, A.J.; Virley, D.J. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurother. J. Am. Soc. Exp. Neurother. 2008, 5, 458–469. [Google Scholar] [CrossRef]
- Patat, A.; Parks, V.; Raje, S.; Plotka, A.; Chassard, D.; Le Coz, F. Safety, tolerability, pharmacokinetics and pharmacodynamics of ascending single and multiple doses of lecozotan in healthy young and elderly subjects. Br. J. Clin. Pharmacol. 2009, 67, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Lanni, C.; Racchi, M.; Govoni, S. Targeting dementias through cancer kinases inhibition. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12044. [Google Scholar] [CrossRef]
- Li, T.; Martin, E.; Abada, Y.; Boucher, C.; Cès, A.; Youssef, I.; Fenaux, G.; Forand, Y.; Legrand, A.; Nachiket, N.; et al. Effects of Chronic Masitinib Treatment in APPswe/PSEN1dE9 Transgenic Mice Modeling Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1339–1345. [Google Scholar] [CrossRef]
- Reading, C.L.; Ahlem, C.N.; Murphy, M.F. NM101 Phase III Study of NE3107 in Alzheimer’s Disease: Rationale, Design and Therapeutic Modulation of Neuroinflammation and Insulin Resistance. Neurodegener. Dis. Manag. 2021, 11, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Balzano, T.; Esteban-García, N.; Blesa, J. Neuroinflammation, immune response and α-synuclein pathology: How animal models are helping us to connect dots. Expert Opin. Drug Discov. 2023, 18, 13–23. [Google Scholar] [CrossRef]
- Nørgaard, C.H.; Friedrich, S.; Hansen, C.T.; Gerds, T.; Ballard, C.; Møller, D.V.; Knudsen, L.B.; Kvist, K.; Zinman, B.; Holm, E.; et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Bercovier, H.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med. Hypotheses 2019, 123, 95–97. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Italiani, P.; Puxeddu, I.; Napoletano, S.; Scala, E.; Melillo, D.; Manocchio, S.; Angiolillo, A.; Migliorini, P.; Boraschi, D.; Vitale, E.; et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: New markers of disease progression? J. Neuroinflamm. 2018, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.; Privat, A.-L.; Bressac, L.; Toulorge, D. CD38 in Neurodegeneration and Neuroinflammation. Cells 2020, 9, 471. [Google Scholar] [CrossRef]
- Blacher, E.; Dadali, T.; Bespalko, A.; Haupenthal, V.J.; Grimm, M.O.W.; Hartmann, T.; Lund, F.E.; Stein, R.; Levy, A. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol. 2015, 78, 88–103. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Syed, Y.Y. Lenalidomide: A Review in Newly Diagnosed Multiple Myeloma as Maintenance Therapy After ASCT. Drugs 2017, 77, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mei, Z.L.; Wang, H.; Hu, M.; Long, Y.; Miao, M.X.; Li, N.; Hong, H. Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochem. Int. 2014, 75, 26–31. [Google Scholar] [CrossRef]
- Michael, J.; Zirknitzer, J.; Unger, M.S.; Poupardin, R.; Rieß, T.; Paiement, N.; Zerbe, H.; Hutter-Paier, B.; Reitsamer, H.; Aigner, L. The Leukotriene Receptor Antagonist Montelukast Attenuates Neuroinflammation and Affects Cognition in Transgenic 5xFAD Mice. Int. J. Mol. Sci. 2021, 22, 2782. [Google Scholar] [CrossRef] [PubMed]
- Feigin, A.; Evans, E.E.; Fisher, T.L.; Leonard, J.E.; Smith, E.S.; Reader, A.; Mishra, V.; Manber, R.; Walters, K.A.; Kowarski, L.; et al. Pepinemab antibody blockade of SEMA4D in early Huntington’s disease: A randomized, placebo-controlled, phase 2 trial. Nat. Med. 2022, 28, 2183–2193. [Google Scholar] [CrossRef]
- Alves, S.; Churlaud, G.; Audrain, M.; Michaelsen-Preusse, K.; Fol, R.; Souchet, B.; Braudeau, J.; Korte, M.; Klatzmann, D.; Cartier, N. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain J. Neurol. 2017, 140, 826–842. [Google Scholar] [CrossRef]
- Faridar, A.; Vasquez, M.; Thome, A.D.; Yin, Z.; Xuan, H.; Wang, J.H.; Wen, S.; Li, X.; Thonhoff, J.R.; Zhao, W.; et al. Ex vivo expanded human regulatory T cells modify neuroinflammation in a preclinical model of Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 144. [Google Scholar] [CrossRef]
- LiCausi, F.; Hartman, N.W. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, Z.; Li, Y.; Luo, M.; Luo, S.; He, G. Alteration of the Wnt/GSK3β/β-catenin signalling pathway by rapamycin ameliorates pathology in an Alzheimer’s disease model. Int. J. Mol. Med. 2019, 44, 313–323. [Google Scholar] [CrossRef]
- Kiyota, T.; Machhi, J.; Lu, Y.; Dyavarshetty, B.; Nemati, M.; Yokoyama, I.; Mosley, R.L.; Gendelman, H.E. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. J. Neuroimmunol. 2018, 319, 80–92. [Google Scholar] [CrossRef]
- Potter, H.; Woodcock, J.H.; Boyd, T.D.; Coughlan, C.M.; O’Shaughnessy, J.R.; Borges, M.T.; Thaker, A.A.; Raj, B.A.; Adamszuk, K.; Scott, D.; et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement. 2021, 7, e12158. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-W.; Lucente, J.D.; Nguyen, H.M.; Singh, V.; Singh, L.; Chavez, M.; Bushong, T.; Wulff, H.; Maezawa, I. Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; García-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennström, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef]
- Rubin, K.; Glazer, S. The pertussis hypothesis: Bordetella pertussis colonization in the pathogenesis of Alzheimer’s disease. Immunobiology 2017, 222, 228–240. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Jacobs, C.; Morley, J.E.; Hoft, D.F. Lower Risk for Dementia Following Adult Tetanus, Diphtheria, and Pertussis (Tdap) Vaccination. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1436–1443. [Google Scholar] [CrossRef]
- Weidung, B.; Hemmingsson, E.-S.; Olsson, J.; Sundström, T.; Blennow, K.; Zetterberg, H.; Ingelsson, M.; Elgh, F.; Lövheim, H. VALZ-Pilot: High-dose valacyclovir treatment in patients with early-stage Alzheimer’s disease. Alzheimers Dement. 2022, 8, e12264. [Google Scholar] [CrossRef]
- MacPherson, K.P.; Sompol, P.; Kannarkat, G.T.; Chang, J.; Sniffen, L.; Wildner, M.E.; Norris, C.M.; Tansey, M.G. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol. Dis. 2017, 102, 81–95. [Google Scholar] [CrossRef]
- Patel, A.G.; Nehete, P.N.; Krivoshik, S.R.; Pei, X.; Cho, E.L.; Nehete, B.P.; Ramani, M.D.; Shao, Y.; Williams, L.E.; Wisniewski, T.; et al. Innate immunity stimulation via CpG oligodeoxynucleotides ameliorates Alzheimer’s disease pathology in aged squirrel monkeys. Brain J. Neurol. 2021, 144, 2146–2165. [Google Scholar] [CrossRef]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Ghareghani, M.; Rivest, S. The Synergistic Potential of Combining PD-1/PD-L1 Immune Checkpoint Inhibitors with NOD2 Agonists in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2023, 24, 10905. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.A.; et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-K.; Vázquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732.e23. [Google Scholar] [CrossRef]
- Stym-Popper, G.; Matta, K.; Chaigneau, T.; Rupra, R.; Demetriou, A.; Fouquet, S.; Dansokho, C.; Toly-Ndour, C.; Dorothée, G. Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology. J. Neuroinflamm. 2023, 20, 64. [Google Scholar] [CrossRef]
- Bakker, A.; Albert, M.S.; Krauss, G.; Speck, C.L.; Gallagher, M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 2015, 7, 688–698. [Google Scholar] [CrossRef]
- Rosenzweig-Lipson, S.; Barton, R.; Gallagher, M.; Edgar, C.J.; Maruff, P.T.; Mohs, R. HOPE4MCI trial: First trial targeting reduction of hippocampal overactivity to treat mild cognitive impairment due to Alzheimer’s disease with AGB101: Human/Human trials: Other. Alzheimers Dement. 2020, 16, e045331. [Google Scholar] [CrossRef]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2014, 8, 463. [Google Scholar] [CrossRef]
- Hamasaki, H.; Honda, H.; Suzuki, S.O.; Hokama, M.; Kiyohara, Y.; Nakabeppu, Y.; Iwaki, T. Down-regulation of MET in hippocampal neurons of Alzheimer’s disease brains. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2014, 34, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Church, K.; Walker, W.; L’Hostis, P.; Viardot, G.; Danjou, P.; Hendrix, S.; Moebius, H.J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Positive Modulator of HGF/MET, Fosgonimeton, in Healthy Volunteers and Subjects with Alzheimer’s Disease: Randomized, Placebo-Controlled, Double-Blind, Phase I Clinical Trial. J. Alzheimers Dis. 2022, 86, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Reda, S.M.; Setti, S.E.; Taylor, R.W.; Berthiaume, A.-A.; Walker, W.E.; Wu, W.; Moebius, H.J.; Church, K.J. Fosgonimeton, a Novel Positive Modulator of the HGF/MET System, Promotes Neurotrophic and Procognitive Effects in Models of Dementia. Neurother. J. Am. Soc. Exp. Neurother. 2023, 20, 431–451. [Google Scholar] [CrossRef]
- Burns, L.H.; Pei, Z.; Wang, H.-Y. Targeting α7 nicotinic acetylcholine receptors and their protein interactions in Alzheimer’s disease drug development. Drug Dev. Res. 2023, 84, 1085–1095. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lee, K.-C.; Pei, Z.; Khan, A.; Bakshi, K.; Burns, L.H. PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer’s disease pathogenesis. Neurobiol. Aging 2017, 55, 99–114. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Pei, Z.; Lee, K.-C.; Lopez-Brignoni, E.; Nikolov, B.; Crowley, C.A.; Marsman, M.R.; Barbier, R.; Friedmann, N.; Burns, L.H. PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J. Prev. Alzheimers Dis. 2020, 7, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Yu, H.-J.; Kim, S.; Kim, G.; Choi, N.-Y.; Lee, E.-H.; Lee, Y.J.; Yoon, M.-Y.; Lee, K.-Y.; Koh, S.-H. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology 2016, 55, 131–141. [Google Scholar] [CrossRef]
- Koh, S.-H.; Kwon, H.S.; Choi, S.H.; Jeong, J.H.; Na, H.R.; Lee, C.N.; Yang, Y.; Lee, A.Y.; Lee, J.-H.; Park, K.W.; et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: A phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res. Ther. 2021, 13, 66. [Google Scholar] [CrossRef]
- Hampel, H.; Lista, S.; Mango, D.; Nisticò, R.; Perry, G.; Avila, J.; Hernandez, F.; Geerts, H.; Vergallo, A. Alzheimer Precision Medicine Initiative (APMI) Lithium as a Treatment for Alzheimer’s Disease: The Systems Pharmacology Perspective. J. Alzheimers Dis. 2019, 69, 615–629. [Google Scholar] [CrossRef]
- Zhang, X.; Heng, X.; Li, T.; Li, L.; Yang, D.; Zhang, X.; Du, Y.; Doody, R.S.; Le, W. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J. Alzheimers Dis. 2011, 24, 739–749. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Appukuttan, A.T.; Moon, S.; Duce, J.A.; Volitakis, I.; Cherny, R.; Wood, S.J.; Greenough, M.; Berger, G.; et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol. Psychiatry 2017, 22, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-K.; Nelson, T.J.; Alkon, D.L. Towards universal therapeutics for memory disorders. Trends Pharmacol. Sci. 2015, 36, 384–394. [Google Scholar] [CrossRef]
- Farlow, M.R.; Thompson, R.E.; Wei, L.-J.; Tuchman, A.J.; Grenier, E.; Crockford, D.; Wilke, S.; Benison, J.; Alkon, D.L. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Assessing Safety, Tolerability, and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer’s Disease. J. Alzheimers Dis. 2019, 67, 555–570. [Google Scholar] [CrossRef]
- Correia, S.S.; Iyengar, R.R.; Germano, P.; Tang, K.; Bernier, S.G.; Schwartzkopf, C.D.; Tobin, J.; Lee, T.W.-H.; Liu, G.; Jacobson, S.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CY6463 Reveals its Therapeutic Potential in Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 656561. [Google Scholar] [CrossRef]
- Gamba, P.; Giannelli, S.; Staurenghi, E.; Testa, G.; Sottero, B.; Biasi, F.; Poli, G.; Leonarduzzi, G. The Controversial Role of 24-S-Hydroxycholesterol in Alzheimer’s Disease. Antioxidants 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Ono, K.; Matsumoto, J.; Yamada, M.; Nishijo, H. Effects of the neurotrophic agent T-817MA on oligomeric amyloid-β-induced deficits in long-term potentiation in the hippocampal CA1 subfield. Neurobiol. Aging 2014, 35, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Yuede, C.M.; LaBarbera, K.M.; Limegrover, C.S.; Rehak, C.; Yurko, R.; Waybright, L.; Look, G.; Rishton, G.; Safferstein, H.; et al. Preclinical and clinical biomarker studies of CT1812: A novel approach to Alzheimer’s disease modification. Alzheimers Dement. J. Alzheimers Assoc. 2021, 17, 1365–1382. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, P.-K.; Chang, Y.-C.; Chuo, L.-J.; Chen, Y.-S.; Tsai, G.E.; Lane, H.-Y. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biol. Psychiatry 2014, 75, 678–685. [Google Scholar] [CrossRef]
- Tari, A.R.; Berg, H.H.; Videm, V.; Bråthen, G.; White, L.R.; Røsbjørgen, R.N.; Scheffler, K.; Dalen, H.; Holte, E.; Haberg, A.K.; et al. Safety and efficacy of plasma transfusion from exercise-trained donors in patients with early Alzheimer’s disease: Protocol for the ExPlas study. BMJ Open 2022, 12, e056964. [Google Scholar] [CrossRef]
- Rutigliano, G.; Stazi, M.; Arancio, O.; Watterson, D.M.; Origlia, N. An isoform-selective p38α mitogen-activated protein kinase inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 70, 86–91. [Google Scholar] [CrossRef]
- Alam, J.J. Selective Brain-Targeted Antagonism of p38 MAPKα Reduces Hippocampal IL-1β Levels and Improves Morris Water Maze Performance in Aged Rats. J. Alzheimers Dis. 2015, 48, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Prins, N.; Lammertsma, A.; Yaqub, M.; Gouw, A.; Wink, A.M.; Chu, H.-M.; van Berckel, B.N.M.; Alam, J. An exploratory clinical study of p38α kinase inhibition in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2018, 5, 464–473. [Google Scholar] [CrossRef]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica—Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.M.; Irwin, R.W.; Yao, J.; Liu, L.; Brinton, R.D. Allopregnanolone promotes regeneration and reduces β-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE 2011, 6, e24293. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 3146. [Google Scholar] [CrossRef]
- Liu, C.-C.; Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Samant, N.P.; Gupta, G.L. Novel therapeutic strategies for Alzheimer’s disease targeting brain cholesterol homeostasis. Eur. J. Neurosci. 2021, 53, 673–686. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.-T.; Tan, L.; Wang, Y.-L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. BioMed Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.-S.; Yao, X.-Q.; Liu, Y.-H.; Wang, Q.-H.; Zeng, F.; Lu, J.-J.; Liu, J.; Zhu, C.; Shen, L.-L.; Liu, C.-H.; et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc. Natl. Acad. Sci. USA 2015, 112, 5225–5230. [Google Scholar] [CrossRef]
- He, P.; Ouyang, X.; Zhou, S.; Yin, W.; Tang, C.; Laudon, M.; Tian, S. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm. Behav. 2013, 64, 1–7. [Google Scholar] [CrossRef]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- Tiligada, E.; Kyriakidis, K.; Chazot, P.L.; Passani, M.B. Histamine Pharmacology and New CNS Drug Targets. CNS Neurosci. Ther. 2011, 17, 620–628. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M. Antihistamines and Mental Status. J. Clin. Psychopharmacol. 2016, 36, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S127–S135. [Google Scholar] [CrossRef]
- Leurs, R.; Bakker, R.A.; Timmerman, H.; De Esch, I.J.P. The histamine H3 receptor: From gene cloning to H3 receptor drugs. Nat. Rev. Drug Discov. 2005, 4, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, J.; Carruthers, N.; Thurmond, R. The Histamine H4 Receptor and Potential Therapeutic Uses for H4 Ligands. Mini-Rev. Med. Chem. 2004, 4, 993–1000. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chien, W.-C.; Chung, C.-H.; Lai, C.-Y.; Tzeng, N.-S. The Usage of Histamine Type 1 Receptor Antagonist and Risk of Dementia in the Elderly: A Nationwide Cohort Study. Front. Aging Neurosci. 2022, 14, 811494. [Google Scholar] [CrossRef]
- Gemkow, M.J.; Davenport, A.J.; Harich, S.; Ellenbroek, B.A.; Cesura, A.; Hallett, D. The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov. Today 2009, 14, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wellendorph, P.; Goodman, M.W.; Burstein, E.S.; Nash, N.R.; Brann, M.R.; Weiner, D.M. Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H(3) receptor. Neuropharmacology 2002, 42, 929–940. [Google Scholar] [CrossRef]
- Wieland, K.; Bongers, G.; Yamamoto, Y.; Hashimoto, T.; Yamatodani, A.; Menge, W.M.B.P.; Timmerman, H.; Lovenberg, T.W.; Leurs, R. Constitutive Activity of Histamine H3 Receptors Stably Expressed in SK-N-MC Cells: Display of Agonism and Inverse Agonism by H3 Antagonists. J. Pharmacol. Exp. Ther. 2001, 299, 908–914. [Google Scholar] [CrossRef]
- Thompson, C.M.; Troche, K.; Jordan, H.L.; Barr, B.L.; Wyatt, C.N. Evidence for functional, inhibitory, histamine H3 receptors in rat carotid body Type I cells. Neurosci. Lett. 2010, 471, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, G.; Bakker, R.A.; Leurs, R. Molecular aspects of the histamine H3 receptor. Biochem. Pharmacol. 2007, 73, 1195–1204. [Google Scholar] [CrossRef]
- Mariottini, C.; Scartabelli, T.; Bongers, G.; Arrigucci, S.; Nosi, D.; Leurs, R.; Chiarugi, A.; Blandina, P.; Pellegrini-Giampietro, D.E.; Beatrice Passani, M. Activation of the histaminergic H3 receptor induces phosphorylation of the Akt/GSK-3β pathway in cultured cortical neurons and protects against neurotoxic insults. J. Neurochem. 2009, 110, 1469–1478. [Google Scholar] [CrossRef]
- Lai, X.; Ye, L.; Liao, Y.; Jin, L.; Ma, Q.; Lu, B.; Sun, Y.; Shi, Y.; Zhou, N. Agonist-induced activation of histamine H3 receptor signals to extracellular signal-regulated kinases 1 and 2 through PKC-, PLD-, and EGFR-dependent mechanisms. J. Neurochem. 2016, 137, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Novoa, L.; Cacabelos, R. Histamine function in brain disorders. Behav. Brain Res. 2001, 124, 213–233. [Google Scholar] [CrossRef]
- Wulff, B.S.; Hastrup, S.; Rimvall, K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur. J. Pharmacol. 2002, 453, 33–41. [Google Scholar] [CrossRef]
- Zarrindast, M.-R.; Eidi, M.; Eidi, A.; Oryan, S. Effects of histamine and opioid systems on memory retention of passive avoidance learning in rats. Eur. J. Pharmacol. 2002, 452, 193–197. [Google Scholar] [CrossRef]
- Higuchi, M.; Yanai, K.; Okamura, N.; Meguro, K.; Arai, H.; Itoh, M.; Iwata, R.; Ido, T.; Watanabe, T.; Sasaki, H. Histamine H1 receptors in patients with Alzheimer’s disease assessed by positron emission tomography. Neuroscience 2000, 99, 721–729. [Google Scholar] [CrossRef]
- Motawaj, M.; Burban, A.; Davenas, E.; Gbahou, F.; Faucard, R.; Morisset, S.; Arrang, J.-M. Le système histaminergique : Une cible pour de nouveaux traitements des deficits cognitifs. Therapies 2010, 65, 415–422. [Google Scholar] [CrossRef]
- Brioni, J.D.; Esbenshade, T.A.; Garrison, T.R.; Bitner, S.R.; Cowart, M.D. Discovery of Histamine H 3 Antagonists for the Treatment of Cognitive Disorders and Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2011, 336, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Motawaj, M.; Peoc’h, K.; Callebert, J.; Arrang, J.-M. CSF Levels of the Histamine Metabolite tele-Methylhistamine are only Slightly Decreased in Alzheimer’s Disease. J. Alzheimers Dis. 2010, 22, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Da Silva, W.C.; Benetti, F.; Bonini, J.S. Histaminergic Mechanisms for Modulation of Memory Systems. Neural Plast. 2011, 2011, 328602. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Roberts, J.C.; Lee, J.; Chen, C.P.L.-H.; Brown, S.H.; Roman, S.; Lai, M.K.P. Characterization of histamine H3 receptors in Alzheimer’s Disease brain and amyloid over-expressing TASTPM mice. Br. J. Pharmacol. 2009, 157, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chazot, P.L. Therapeutic potential of histamine H3 receptor antagonists in dementias. Drug News Perspect. 2010, 23, 99. [Google Scholar] [CrossRef]
- Breitner, J. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol. Aging 1995, 16, 523–530. [Google Scholar] [CrossRef]
- Fu, Q.-L.; Dai, H.-B.; Shen, Y.; Chen, Z. Reversing effect of histamine on neurotoxicity induced by beta-amyloid1-42. J. Zhejiang Univ. Med. Sci. 2007, 36, 146–149. [Google Scholar] [CrossRef]
- Dere, E.; Zlomuzica, A.; Viggiano, D.; Ruocco, L.A.; Watanabe, T.; Sadile, A.G.; Huston, J.P.; De Souza-Silva, M.A. Episodic-like and procedural memory impairments in histamine H1 Receptor knockout mice coincide with changes in acetylcholine esterase activity in the hippocampus and dopamine turnover in the cerebellum. Neuroscience 2008, 157, 532–541. [Google Scholar] [CrossRef]
- Dai, H.; Kaneko, K.; Kato, H.; Fujii, S.; Jing, Y.; Xu, A.; Sakurai, E.; Kato, M.; Okamura, N.; Kuramasu, A.; et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci. Res. 2007, 57, 306–313. [Google Scholar] [CrossRef]
- Bandaru, N.; Komavari, C.; Gorla, U.S.; Koteswarao, G.; Kulandaivelu, U.; Ankarao, A. Neuroprotective effect of Conessinin on Elevated oxidative stress induced Alzheimers’disease in rats. Res. J. Pharm. Technol. 2020, 13, 2703. [Google Scholar] [CrossRef]
- Simon, T.; Gogolák, P.; Kis-Tóth, K.; Jelinek, I.; László, V.; Rajnavölgyi, É. Histamine modulates multiple functional activities of monocyte-derived dendritic cell subsets via histamine receptor 2. Int. Immunol. 2012, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Gao, Y.; Yang, J.; Zou, J.; Guo, W. Potential role of store-operated Ca2+ entry in Th2 response induced by histamine in human monocyte-derived dendritic cells. Int. Immunopharmacol. 2012, 12, 358–367. [Google Scholar] [CrossRef]
- Loewenbrueck, K.F.; Tigno-Aranjuez, J.T.; Boehm, B.O.; Lehmann, P.V.; Tary-Lehmann, M. Th1 responses to beta-amyloid in young humans convert to regulatory IL-10 responses in Down syndrome and Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Felaco, P.; Castellani, M.L.; De Lutiis, M.A.; Felaco, M.; Pandolfi, F.; Salini, V.; De Amicis, D.; Vecchiet, J.; Tete, S.; Ciampoli, C.; et al. IL-32: A newly-discovered proinflammatory cytokine. J. Biol. Regul. Homeost. Agents 2009, 23, 141–147. [Google Scholar]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Fox, G.B.; Esbenshade, T.A.; Pan, J.B.; Radek, R.J.; Krueger, K.M.; Yao, B.B.; Browman, K.E.; Buckley, M.J.; Ballard, M.E.; Komater, V.A.; et al. Pharmacological Properties of ABT-239 [4-(2-{2-[(2 R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological Characterization and Broad Preclinical Efficacy in Cognition and Schizophrenia of a Potent and Selective Histamine H3 Receptor Antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 176–190. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, S.H.; Joo, S.H.; Kim, S.-H.; Shin, C.Y. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochem. Biophys. Res. Commun. 2010, 402, 48–53. [Google Scholar] [CrossRef]
- Sakurai, E.; Sakurai, E.; Tanaka, Y.; Watanabe, T.; Singh Jossan, S.; Oreland, L. Effects of histamine H3-receptor ligands on brain monoamine oxidase in various mammalian species. Brain Res. 2001, 906, 180–183. [Google Scholar] [CrossRef]
- Ligneau, X.; Perrin, D.; Landais, L.; Camelin, J.-C.; Calmels, T.P.G.; Berrebi-Bertrand, I.; Lecomte, J.-M.; Parmentier, R.; Anaclet, C.; Lin, J.-S.; et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, Hydrochloride], a Nonimidazole Inverse Agonist/Antagonist at the Human Histamine H3 Receptor: Preclinical Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. The histamine H3 receptor: From discovery to clinical trials with pitolisant. Br. J. Pharmacol. 2011, 163, 713–721. [Google Scholar] [CrossRef]
- Kollb-Sielecka, M.; Demolis, P.; Emmerich, J.; Markey, G.; Salmonson, T.; Haas, M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Michel, A.D.; Kilpatrick, G.J. In vivo occupancy of histamine H3 receptors by thioperamide and (R)-α-methylhistamine measured using histamine turnover and an ex vivo labeling technique. Biochem. Pharmacol. 1992, 44, 1261–1267. [Google Scholar] [CrossRef]
- Alachkar, A.; Khan, N.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Histamine H3 receptor antagonist E177 attenuates amnesia induced by dizocilpine without modulation of anxiety-like behaviors in rats. Neuropsychiatr. Dis. Treat. 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Griebel, G.; Pichat, P.; Pruniaux, M.-P.; Beeské, S.; Lopez-Grancha, M.; Genet, E.; Terranova, J.-P.; Castro, A.; Sánchez, J.A.; Black, M.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays procognitive effects in rodents. Pharmacol. Biochem. Behav. 2012, 102, 203–214. [Google Scholar] [CrossRef]
- Delay-Goyet, P.; Blanchard, V.; Schussler, N.; Lopez-Grancha, M.; Ménager, J.; Mary, V.; Sultan, E.; Buzy, A.; Guillemot, J.; Stemmelin, J.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays disease-modifying activity in a transgenic mouse model of tauopathy. Alzheimers Dement. Transl. Res. Clin. Interv. 2016, 2, 267–280. [Google Scholar] [CrossRef]
- Passani, M.B.; Blandina, P. Cognitive implications for H3 and 5-HT3 receptor modulation ofcortical cholinergic function: A parallel story. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 725. [Google Scholar] [CrossRef] [PubMed]
- Bitner, R.S.; Markosyan, S.; Nikkel, A.L.; Brioni, J.D. In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer’s disease. Neuropharmacology 2011, 60, 460–466. [Google Scholar] [CrossRef]
- Łażewska, D.; Kaleta, M.; Hagenow, S.; Mogilski, S.; Latacz, G.; Karcz, T.; Lubelska, A.; Honkisz, E.; Handzlik, J.; Reiner, D.; et al. Novel naphthyloxy derivatives—Potent histamine H3 receptor ligands. Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2018, 26, 2573–2585. [Google Scholar] [CrossRef]
- Abdalla, S.; Eissa, N.; Jayaprakash, P.; Beiram, R.; Kuder, K.J.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice. Int. J. Mol. Sci. 2023, 24, 12719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).