Abstract
Background/Objectives: The thiazolo [5,4-d]pyrimidine scaffold is a class of drugs known for its anticancer, antitumor, anti-inflammatory, and antimicrobial properties. In this study, the electrochemical properties of novel thiazolo [5,4-d]pyrimidine derivatives and their interactions with DNA were characterized for the first time using voltammetric methods. Determining the interactions of new drug candidate molecules with DNA is crucial for drug development studies and is the main objective of this research. Methods: Both molecules were immobilized on the surface of the electrodes by passive adsorption, and their electrochemical properties were determined by voltammetric methods through reduction currents. Their interactions with DNA were carried out in the solution phase and examined by the changes in the oxidation peak potential and current of the guanine base. Results: For both molecules, it was determined that the electrochemical reduction processes are diffusion-controlled and irreversible, with an equal number of protons and electrons being transferred during this process. The detection limits for TP-NB (4-chloro-N-(5-chlorothiazolo [5,4-d]pyrimidin-2-yl)-3-nitrobenzamide) and TP-PC (1-(2-(4-(4-carbamoylpiperidin-1-yl)-3-nitrobenzamido)thiazolo [5,4-d]pyrimidin-5-yl)piperidine-4-carboxamide) were determined to be 12 µg/mL and 16 µg/mL, respectively. As a result of the interaction between both molecules with DNA, the guanine oxidation current decreased. It was found that TP-NB could act as an intercalator, while TP-PC could affect DNA electrostatically, both showing toxic effects on DNA. Conclusions: An electrochemical method was developed for the rapid, cost-effective, and sensitive detection of both molecules and their DNA interactions. Both compounds exhibited notable affinity towards DNA, as evidenced by significant changes in oxidation peak currents, shifts in peak potentials, and calculated toxicity values. These findings suggest their potential use as DNA-interacting drugs, such as anticancer and antimicrobial agents. Our study offers a quick, cost-effective, and reliable electrochemical approach for the evaluation of drug–DNA interactions.
1. Introduction
Thiazolopyrimidine scaffolds are essential pharmacophores found in compounds exhibiting a wide range of biological activities. These fused heterocyclic ring systems closely resemble purine isosteres at first glance. Due to their structural similarity to adenine, guanine, and their derivatives—such as adenosine, guanosine, cAMP (cyclic adenosine monophosphate), cGMP (cyclic guanosine monophosphate), and other related biomolecules—medicinal chemists have extensively explored and utilized thiazolo [4,5-d]pyrimidine scaffolds in the design of novel therapeutics [1].
These derivatives have been reported to exhibit a variety of biological activities. Notably, they demonstrate antitumor [2], anticancer [3], antibacterial [4], antiviral including HIV [1], anti-inflammatory and analgesic [5], antinociceptive [6], and corticotropin-releasing factor receptor antagonist effects [7]. Their potent activity against various cancer types, including breast, lung, and colon cancer, along with their antitumoral effects observed in multiple cell lines [8], suggests that these compounds may exert their effects through interactions with DNA. In this study, we investigated the electrochemical properties and DNA interactions of two newly synthesized thiazolo [5,4-d]pyrimidine derivatives. For clarity and conciseness, we refer to 4-chloro-N-(5-chlorothiazolo [5,4-d]pyrimidin-2-yl)-3-nitrobenzamide as TP-NB and 1-(2-(4-(4-carbamoylpiperidin-1-yl)-3-nitrobenzamido)thiazolo [5,4-d]pyrimidin-5-yl)piperidine-4-carboxamide as TP-PC throughout the manuscript. The piperidine-4-carboxamide substituent, known for its activity against various biological targets, was introduced into the TP-NB structure to improve binding affinity and pharmacokinetic properties, such as solubility and metabolic stability. Its functional groups, capable of acting as hydrogen bond donors and acceptors, were expected to enhance interactions with key residues at the target site [9]. The chemical structures of TP-NB and TP-PC, along with their synthesis steps, are presented in Figure 1.
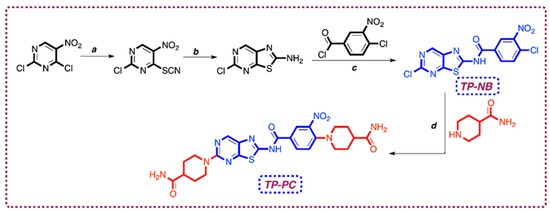
Figure 1.
Reagents and conditions of synthesis pathways (a) KSCN, in AcA (acetic acid), ice bath, 2 h, 90%; (b) Fe, in AcA, reflux, 2 h, 45%; (c) 4-chloro-3-nitrobenzoyl chloride, DMAP, in pyridine, ice bath, rt, overnight, 35%; (d) piperidine-4-carboxamide, TEA, in DMF (N,N-dimethylformamide), 90 °C 6 h, 18%.
The interaction between a drug candidate molecule and DNA plays a crucial role in drug development, as it provides valuable insights into the potential mechanisms of action of the drug. Understanding these interactions can aid in optimizing drug efficacy, improving target specificity, and minimizing off-target effects, ultimately contributing to the development of more effective therapeutic agents [10]. Drugs interact with DNA through three primary mechanisms: (1) by influencing transcription factors and polymerases; (2) through RNA binding to DNA, forming triple-helix structures or DNA-RNA hybrids that affect transcription; and (3) by small aromatic ligands binding directly to the DNA double helix. Small molecules interact with DNA via electrostatic attractions, intercalation between base pairs, and binding to the minor or major grooves of the DNA, each of which can significantly impact gene expression and cellular function [11]. Drugs bind to DNA through two primary pathways: covalent and non-covalent interactions. Covalent binding is irreversible, leading to permanent inhibition of DNA processes and ultimately causing cell death. In contrast, non-covalent interactions are weaker and reversible, allowing for dynamic drug–DNA interactions.
Examples of non-covalent binding include intercalation (where molecules insert between base pairs), groove binding (where molecules attach to the minor or major grooves of the DNA helix), and electrostatic interactions (where positively charged molecules associate with the negatively charged phosphate backbone of DNA) [12]. In intercalation, molecules insert between DNA base pairs without forming covalent bonds or disrupting hydrogen bonds, causing stabilization, elongation, stiffening, and unwinding of the double helix. Electrostatic interactions, though weaker, involve positively charged molecules associating with DNA’s negatively charged phosphate backbone, influencing binding affinity and specificity of DNA-targeting drugs [13]. In groove binding, molecules attach to the minor or major groove via hydrogen bonding and van der Waals forces. While generally non-cytotoxic, they can induce DNA damage if facilitating covalent attachment or free radical-dependent double-strand breaks. These interactions can also modulate gene expression and DNA–protein interactions by affecting transcription factor accessibility [14]. Several techniques are used to analyze drug–DNA interactions, including UV–visible, Raman, IR, and NMR spectroscopies [12]; high-performance liquid chromatography (HPLC) [15]; molecular modelling [16]; and surface plasmon resonance [17]. These techniques have certain drawbacks, including being time-consuming, requiring expensive equipment for analysis, exhibiting low sensitivity in some cases, and necessitating well-trained personnel for operation. In addition to these traditional methods, electrochemical methods such as voltammetry, amperometry, and electrochemical impedance spectroscopy (EIS) are widely used to study drug–DNA interactions. Electrochemical methods offer several advantages over conventional techniques, including faster response times, the ability to work with smaller sample volumes, portability, simpler procedures, and high sensitivity and specificity. These features make electrochemical approaches highly attractive for rapid and cost-effective drug–DNA interaction studies [18].
In this study, the electrochemical properties of novel thiazolo [5,4-d]pyrimidine derivatives were characterized for the first time, and their interactions with DNA were electrochemically investigated to elucidate their mechanisms of action. Initially, the electrochemical profiles of these drug candidates were analyzed using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Key parameters, including pH, scan rate, immobilization time, and drug concentration, were optimized to achieve highly sensitive detection of these molecules under ideal conditions. Following the optimizations, drug–DNA interactions were examined to explore their potential antimicrobial mechanisms. Previous studies have shown that one of these new drug candidate molecules exhibits antileishmanial activity [19]. However, the role of its interaction with DNA in the emergence of these antimicrobial effects has never been investigated. Therefore, determining the effects and binding properties of drug candidate molecules on DNA is crucial for identifying their potential mechanism of action and assessing their possible applications. After interaction in the solution phase, changes in the oxidation peak potential and current of guanine in the DNA structure were monitored. Additionally, the toxicity effect (S%) of the drug candidates on DNA was calculated. In this way, both the characterization of various electrochemical properties of new drug candidate molecules for further studies and their effects and binding properties on DNA, which may be the source of many potential therapeutic effects, were determined quickly, easily, cheaply, and with high sensitivity by the electrochemical method we developed. Determination of the potential mechanisms of action on DNA is very important in revealing many new areas of use, especially in addition to supporting the areas where these molecules are currently thought to be used.
2. Results and Discussion
2.1. Electrochemical Profiling of TP-NB and TP-PC
The electrochemical redox signals of TP-NB and TP-PC were first scanned in the oxidation (from 0 to +1.5 V) and reduction (from 0 to −1.0 V) directions using the protocol described in the Materials and Methods section. In addition, after deciding on the signal to be used in the study, buffer solutions with different pHs were used in the preparation of TP-NB and TP-PC solutions, and the highest signal was also examined. The results are presented in Figure 2. In addition, ACB (acetate buffer, pH: 4.8), which was used in the preparation of TP-NB and TP-PC solutions, was used for the blank solutions. In this way, oxidation and/or reduction signals that could originate from the blank solutions and potentially interfere with the signals of these solutions were identified.
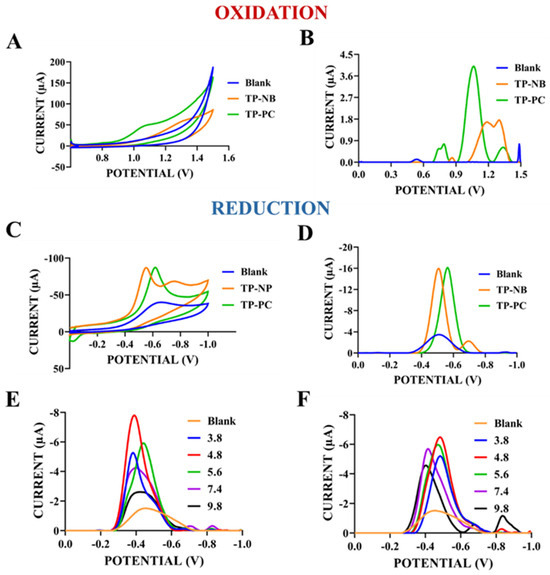
Figure 2.
CV (A) and DPV (B) of blank (ACB (pH: 4.8)), TP-NB, and TP-PC in the reduction range (0.0 V to −1.0 V). CV (C) and DPV (D) of blank, TP-NB, and TP-PC in the oxidation range (0.0 V to +1.5 V). DPV, in the range 0.0 V to −1.0 V, of reduction current TP-NB (E) and TP-PC (F) prepared with various buffer solutions with different pHs.
As shown in Figure 2A (CV) and Figure 2B (DPV), TP-NB exhibited a distinct reduction peak around −0.5 V, while TP-PC showed a similar peak around −0.6 V. In contrast, oxidation peak currents in CV scans (Figure 2C) were weak for both molecules. However, in DPV (Figure 2D), which is more sensitive, TP-PC showed a stronger oxidation peak at +1.1 V compared to CV scans, whereas TP-NB displayed a weaker oxidation peak at +1.2 V. But these oxidation peak currents were weaker than these reduction peak currents. For this reason, reduction signals were primarily analyzed due to their higher intensity, sharper shape, and smoother profiles compared to the flat oxidation signals. Based on molecular structure and the literature, the reduction currents observed at similar potentials for both molecules were likely due to the reduction of their common amide groups to amines [20]. As seen in Figure 2E,F, the reduction peak currents obtained for TP-NB and TP-PC prepared with ACB at pH 4.8 were higher than the reduction peak currents obtained for TP-NB and TP-PC prepared at other pHs. For this reason, ACB with a pH of 4.8 was selected and used for the preparation of solutions of both TP-NB and TP-PC in the rest of the study. After determining the redox currents and optimum preparing solution, the optimization and characterization of TP-NB and TP-PC were carried out, and the results are presented in Figure 3. In this section, as well as throughout the remainder of the study, measurements were conducted using blank solutions composed of ACB (pH: 4.8), as previously described. The peak currents observed at potentials corresponding to those of TP-NB and TP-PC in the blank solutions were subtracted from the peak currents obtained for the respective compounds. Based on these corrected values, the graphs were plotted (with the exception of the voltammograms).
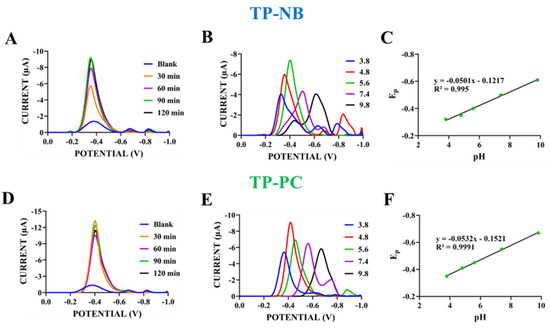
Figure 3.
DPVs of TP-NB with different passive adsorption times (30–120 min) (A) and with various supporting electrolytes with different pHs (ACB with pHs of 3.8, 4.8, and 5.6; PBS (phosphate buffered saline) with a pH of 7.4; and BBS (borate buffer) with a pH of 9.8) and (B) scanning from 0.0 V to −1.0 V at 100 mV/s. The plot of reduction peak potential (Ep) of TP-NB (C) vs. pHs of different supporting electrolytes (n = 3). DPVs of TP-PC with different passive adsorption times (30–120 min) (D) and with various supporting electrolytes with different pHs (ACB with pHs of 3.8, 4.8, and 5.6; PBS with a pH of 7.4; and BBS with a pH of 9.8) and (E) scanning from 0.0 V to −1.0 V at 100 mV/s. The plot of reduction peak potential (Ep) of TP-PC (F) vs. pHs of different supporting electrolytes (n = 3).
After determining the redox currents, their optimization and characterization were carried out. First, the immobilization of TP-NB and TP-PC onto the electrode surface was optimized. Electrodes that were activated via applied +1.4 V for 30 s were immersed in solutions of both molecules at a concentration of 500 µg/mL for 30–120 min, allowing for passive adsorption, and then DPV measurements were done (Figure 3A,D). In Figure 3A, when the passive adsorption time for TP-NB was increased from 30 to 90 min, the reduction peak currents observed around −0.35 V increased, indicating more TP-NB immobilization on the electrode surface. In this case, unlike Figure 2, the measurements were not conducted directly in the TP-NB solution; instead, they were performed in a buffer solution using electrodes pre-coated with TP-NB. This resulted in a slight shift in the peak potential, from −0.5 V to −0.35 V.
At 120 min, the reduction peak current (8.97 ± 0.36 µA) was very close to that obtained after 90 min of immobilization (9.21 ± 0.32 µA). This suggests that saturation was achieved within 90 min, and therefore, this duration was selected for TP-NB detection in the rest of the study. For TP-PC, the reduction peak current (12.43 ± 1.40 µA) observed around −0.4 V after 30 min of immobilization was higher than those recorded at longer immobilization times (60–120 min) (Figure 3D). Therefore, 30 min was sufficient for electrode surface saturation with TP-PC, and this duration was selected for TP-PC detection in the rest of the study. The effects of pHs of supporting electrolytes on the reduction peak potential and current of TP-NB and TP-PC were also investigated. In this phase, DPV measurements were taken in supporting electrolytes with different pH values (ACB with pHs of 3.8, 4.8, and 5.6; PBS with a pH of 7.4; and BBS with a pH of 9.8) for both TP-NB and TP-PC, prepared with ACB (pH: 4.8) (Figure 3B,C,E,F).
As shown in Figure 3B and 3E, the effect of the pH of the support electrolyte on the reduction peak potential and peak currents of TP-NB and TP-PC, respectively, was investigated. As seen in Figure 3B, as the pH increased, the reduction peak potential of TP-NB shifted towards more negative values. This indicates that protons were involved in the reduction of TP-NB [21]. When the relationship between the cathodic peak potential of TP-NB and pH was examined, the equation of Epc = −0.0501 pH—0.1217 R2 = 0.995 was obtained (Figure 3C). The slope of this equation with a value of 50.1 mV/pH was close to the theoretical value of 59 mV/pH [22]. When the literature was examined, this showed that the number of protons and electrons transferred in the electrochemical reduction of TP-NB was equal [23]. For TP-PC, as in TP-NB, it was observed that the reduction peak potential shifted towards more negative values as pH increased (Figure 3E). This also indicates that protons were involved in the reduction of TP-PC. As done for TP-NB, when the relationship between the cathodic peak potential of TP-NB and pH was examined, the equation of Epc = −0.0532 pH—0.1521 R2 = 0.9991 was obtained (Figure 3F). The slope of this equation with a value of 53.2 mV/pH was close to the theoretical value of 59 mV/pH. This indicates that similar to TP-NB, the number of protons and electrons transferred in the electrochemical reduction of TP-PC was equal [23].
After determining the effect of pH on the electrochemical behavior of both drug candidate molecules, the effects of scan rate on the reduction peak potential and current were investigate, and the results are presented in Figure 4. In this scan rate study, TP-NB and TP-PC solutions prepared with ACB (pH: 4.8) at a concentration of 500 µg/mL were placed into the electrochemical measurement cell, and CV measurements were conducted at different scan rates (10–150 mV/s) (Figure 4). Based on the graphs, the equations in Table 1 were obtained.
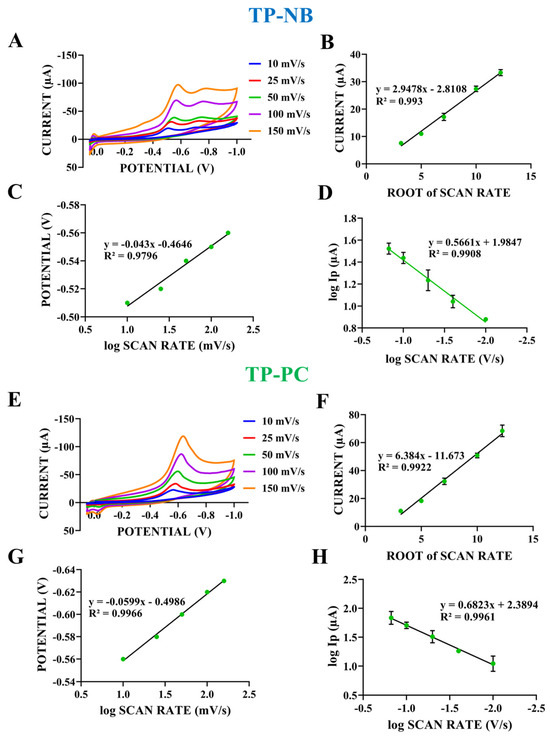
Figure 4.
(A) CV of the TP-NB taken at various scan rates (10–150 mV/s) in the potential range of 0.0 V to −1.0 V. The plots of the relationships of the reduction peak current of TP-NB (B) vs. the square root of the scan rate, the reduction peak potential of TP-NB (C) vs. the logarithm of the scan rate, and the logarithm of the reduction peak current of TP-NB (D) vs. the logarithm of the scan rate (n = 5). (E) CV of the TP-PC taken at various scan rates (10–150 mV/s) in the potential range of 0.0 V to −1.0 V. The plots of the relationships of the reduction peak current of TP-PC (F) vs. the square root of the scan rate, the reduction peak potential of TP-PC (G) vs. the logarithm of the scan rate, and the logarithm of the reduction peak current of TP-PC (H) vs. the logarithm of the scan rate (n = 5).

Table 1.
Some equations obtained for TP-NB and TP-PC as a result of CV measurements taken at different scan rates (ν corresponds to the scan rate (in mV/s), Ip to the peak current, Epc to the cathodic peak potential, and log to the decimal logarithm).
As seen in Figure 4A, the reduction peak currents obtained for TP-NB increased as the scan rate increased. A linear relationship between the square root of the scan rate and the reduction peak current of TP-NB was observed (Figure 4B). As seen in Equation (1) in Table 1, the increase in the reduction peak current as the square root of the scan rate increased together with the high correlation coefficient, indicating that a diffusion-controlled process took place when the literature was examined [24]. To determine whether the electrochemical process of TP-NB is reversible, the relationship between the logarithm of the scan rate and the obtained peak potential was examined (Figure 4C). The linear relationship between the peak potential and the logarithm of the scan rate indicates that the electrochemical process of TP-NB occurs irreversibly (Equation (2)) [25]. This occurs because the electrochemical reaction rate is controlled by the diffusion of reactants to the electrode surface. As the scan rate increases, the diffusion process falls behind the reaction, resulting in an irreversible process [26]. To confirm that a diffusion-controlled process occurs on the electrode surface for TP-NB, the relationship between the logarithm of the scan rate and the logarithm of the reduction peak current was also examined (Figure 4D). The slope of Equation (3), which was 0.56, was close to the theoretical value of 0.5 for a diffusion-controlled ideal reaction, confirming that a diffusion-controlled process occurs for TP-NB [27]. As seen in Figure 4E, the reduction peak currents obtained for TP-PC increased as the scan rate increased, like in TP-NB. A linear relationship between the square root of the scan rate and the reduction peak current of TP-PC was observed (Figure 4F). As observed in Equation (4), the increase in the reduction peak current with the square root of the scan rate, along with the high correlation coefficient, indicates that a diffusion-controlled process occurred for TP-PC, like in TP-NB, as supported by the literature [24]. As done for TP-NB, the relationship between the reduction peak potential and the logarithm of the scan rate was examined for TP-PC to determine whether the electrochemical process is reversible (Figure 4G). Here, the linear relationship between the reduction peak potential and the logarithm of the scan rate indicates that the electrochemical process was also irreversible for TP-PC (Equation (5)).
As done for TP-NB, the relationship between the logarithm of the scan rate and the logarithm of the resulting reduction current was examined for TP-PC to confirm that a diffusion-controlled process occurred on the electrode surface (Figure 4H). The slope of Equation (6), which was 0.68, was close to the theoretical value of 0.5 for a diffusion-controlled ideal reaction, confirming that a diffusion-controlled process occurred for TP-PC.
After characterizing various electrochemical properties of both molecules by examining parameters such as pH and scan rate, concentration studies were conducted. For this purpose, electrodes that were activated via applied +1.4 V for 30 s were immersed in TP-NB and TP-PC solutions prepared using ACB (pH: 4.8) at concentrations ranging from 10 to 500 µg/mL. After an immobilization period of 90 min for TP-NB and 30 min for TP-PC, DPV measurements were done (Figure 5).
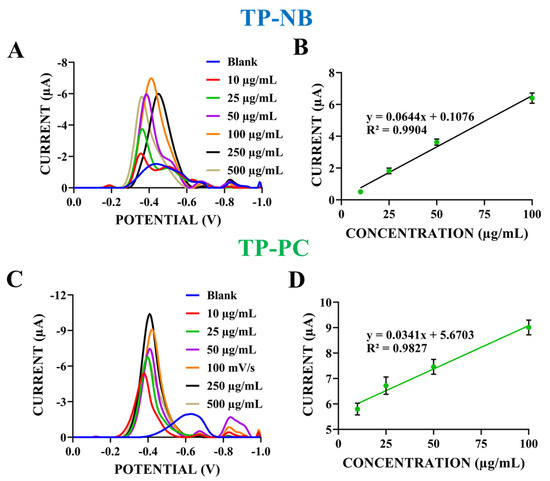
Figure 5.
Differential pulse voltammograms of TP-NB (A) and TP-PC (C) prepared at concentrations of 10–500 µg/mL in ACB (pH: 4.8) within the potential range of 0.0 V to −1.0 V. Calibration plot of the reduction peak current vs. different concentrations of TP-NB (B) and TP-PC (D) (n = 5).
As shown in Figure 5A, the reduction peak current increased as the TP-NB concentration rose from 10 µg/mL to 100 µg/mL. Figure 5B illustrates a linear relationship between concentration and current within the 10–100 µg/mL range for TP-NB. The limit of detection (LOD) and limit of quantification (LOQ) were calculated using the formulas (3 × sd)/slope and (10 × sd)/slope, respectively, where sd represents the standard deviation from regression analysis for a signal-to-noise ratio of 3 [28]. Based on these calculations, the LOD and LOQ for TP-NB detection were determined as 12 µg/mL and 40 µg/mL, respectively. At higher concentrations (250 and 500 µg/mL), the reduction peak currents were lower than those at 100 µg/mL (Figure 5A), indicating surface saturation at 100 µg/mL TP-NB. Therefore, 100 µg/mL was selected as the TP-NB concentration for subsequent experiments.
For TP-PC, the reduction peak current increased as the concentration rose from 10 µg/mL to 250 µg/mL (Figure 5C). Figure 5D presents the calibration graph and equation, demonstrating a linear relationship between TP-PC concentration and reduction current. Based on the calibration graph, the LOD and LOQ for TP-PC detection were determined as 16 µg/mL and 54 µg/mL, respectively.
As shown in Figure 5C, the peak current at 500 µg/mL TP-PC was lower than that at 250 µg/mL, indicating electrode surface saturation at 250 µg/mL. Therefore, 250 µg/mL was selected for TP-PC in subsequent experiments.
2.2. Interaction Between Drug Candidates and DNA
Before examining the interaction of TP-NB and TP-PC with dsDNA (double stranded DNA), studies were conducted to determine the dsDNA concentration to be used. In our previous studies, ACB buffer solution with a pH of 4.8 was optimized as the buffer for dsDNA preparation, and the immobilization time was set to 1 h [29]. dsDNA was prepared at concentrations ranging from 50 to 750 µg/mL and immobilized on the electrode surface via passive adsorption for 1 h. After a single wash with ACB (pH: 4.8) to remove unbound dsDNA, DPV measurements were performed in ACB (pH: 4.8) between 0.4 and 1.4 V. The oxidation peak currents of electroactive bases in the dsDNA structure were analyzed, with guanine appearing around +1.0 V and adenine around +1.2 V (Figure 6). A review of the literature on the electrochemical detection of DNA indicates that DNA can be directly oxidized on solid electrodes. [30]. It has been reported that all nucleobase species undergo irreversible oxidation on carbon electrodes, both as free molecules and as components of nucleosides and nucleotides [31,32]. When using bare carbon electrodes, DNA molecules have been reported to produce two well-distinguishable oxidation peaks at approximately 0.9 V and 1.2 V (vs. Ag/AgCl), corresponding to the oxidation of guanine and adenine residues, respectively [33,34]. It has been reported that guanine undergoes oxidation through a process involving a total of four electrons and four protons, leading to the formation of 8-oxoguanine. This compound can further undergo reversible oxidation via a two-electron, two-proton process. These oxidation products are rapidly hydrolyzed. Therefore, the observed oxidation peak current for guanine has been attributed to its conversion into 8-oxoguanine [35,36]. It has also been reported that adenine undergoes oxidation to form 2,8-oxoadenine through a three-step process involving a total of six electrons and six protons. The observed oxidation current for adenine is attributed to this transformation [37]. Therefore, the direct electrochemical detection of DNA is often based on the well-defined oxidation peaks of guanine and adenine bases.
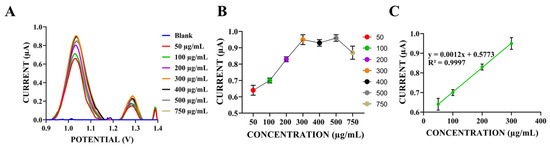
Figure 6.
(A) Differential pulse voltammograms of the dsDNA-coated electrodes prepared at concentrations of 50–100 µg/mL in ACB (pH: 4.8) at a scan rate of 100 mV/s within the potential range of 0.8 V to 1.4 V in ACB (pH: 4.8). (B) Histogram of the relationship between oxidation peak currents of guanine and different concentrations of dsDNA. (C) Calibration plot of the oxidation peak currents of guanine vs. different concentrations of dsDNA (n = 5).
When analyzing the guanine oxidation peak currents around +1.0 V, the peak current gradually increased as the dsDNA concentration rose from 50 µg/mL to 300 µg/mL. However, at concentrations above 300 µg/mL, the peak currents remained similar to or lower than those observed at 300 µg/mL (Figure 6A,B). Therefore, 300 µg/mL was selected as the dsDNA concentration for subsequent experiments.
Based on the calibration graph and equation obtained from the guanine oxidation current in Figure 6C, the limit of detection (LOD) and limit of quantification (LOQ) for dsDNA detection were calculated as 7 µg/mL and 23 µg/mL, respectively. This LOD value is consistent with our previous electrochemical studies on DNA detection [29]. One limitation of our study is that the lowest analyte concentrations detected fell below the range of the standard curve shown in Figure 6C. While the calibration curve demonstrated high linearity (R2 > 0.99) across the tested concentrations, we acknowledge that linearity at very low concentrations, particularly near the LOD, should ideally be confirmed by including additional lower concentration standards. Due to practical constraints, we were not able to include these points in the current study. Therefore, the reported LOD values should be interpreted with this limitation in mind.
After optimizing the dsDNA concentration, the interaction between the drug candidate molecules and dsDNA was examined. As reported in the literature, drug–DNA interactions have long been studied using electrochemical methods [38]. In these studies, voltammetric methods are frequently used to assess whether a drug or drug candidate molecule affects DNA and its binding properties by analyzing changes in the oxidation peak potential and peak current of guanine and adenine bases in the DNA structure [39]. To indicate the extent of the interaction, the percentage change (typically a decrease) in the oxidation peak current of guanine and/or adenine induced by the molecules is reported [40]. To determine the interaction mode, shifts in the oxidation peak potentials of guanine or adenine bases before and after interaction with drug molecules are analyzed. If the peak potential shifts toward a more negative value after interaction, it indicates that the interaction occurs electrostatically [41]. Conversely, if the peak potential shifts toward a more positive value, the interaction is attributed to intercalation [42]. Intercalation positions the drug molecules perpendicularly to the helical axis, stabilizing the adduct through interactions with DNA bases. While intercalators prefer (guanine–citosine) GC-rich regions, they lack sequence specificity. Although primary and secondary DNA structures remain unchanged, intercalation alters the tertiary structure, leading to helix bending, DNA stiffening, and elongation [43]. In electrostatic interactions, positively charged drug molecules interact with the negatively charged phosphate groups of DNA [44]. DPV is one of the most commonly used voltammetric techniques in drug–DNA interaction studies. In DPV, fixed-magnitude pulses superimposed on a linear potential ramp are applied to the working electrode just before the end of the drop. The first current is instrumentally subtracted from the second, and the resulting current difference is plotted against the applied potential. This differential pulse process effectively corrects the charging background current, enhancing signal resolution [45].
First, interaction studies between TP-NB and dsDNA were conducted using the optimized concentrations of 100 µg/mL TP-NB and 300 µg/mL dsDNA. Stock solutions of TP-NB (prepared in DMF) and dsDNA (prepared in TE (tris-EDTA) buffer) were diluted with ACB (pH: 4.8) and mixed to achieve final concentrations of 100 µg/mL TP-NB and 300 µg/mL dsDNA. A 100 µL aliquot of this mixture was transferred into tubes, and electrodes were immersed. The samples were stirred at 650 rpm for 1 h on a thermal shaker. After incubation, DPV measurements were performed to monitor changes in guanine oxidation peak currents and potential. The same procedure was repeated with varying TP-NB concentrations (25–125 µg/mL) while maintaining a fixed dsDNA concentration (300 µg/mL), and DPV measurements were recorded. Figure 7 presents the differential pulse voltammograms and histogram plots obtained from these measurements.
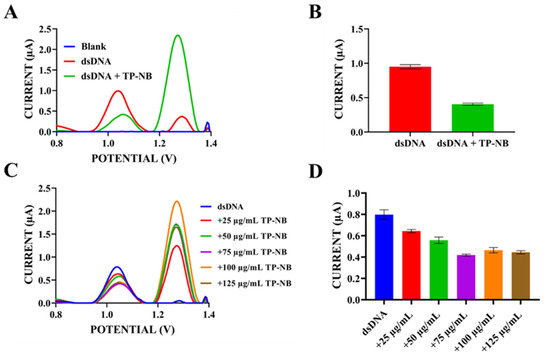
Figure 7.
(A) DPV after the interaction between TP-NB and dsDNA in ACB (pH: 4.8) at a scan rate of 100 mV/s within the potential range of 0.8 V to 1.4 V. (B) Histogram showing the change in the average guanine oxidation currents as a result of the interaction between TP-NB and dsDNA. (C) Differential pulse voltammogram after the interaction between different concentrations (25–125 µg/mL) of TP-NB and dsDNA, taken in ACB (pH: 4.8) at a scan rate of 100 mV/s within the potential range of 0.8 V to 1.4 V. (D) Histogram showing the change in the average guanine oxidation current as a result of the interaction between different concentrations (25–125 µg/mL) of TP-NB and dsDNA.
As shown in Figure 7A,B, a significant decrease in guanine oxidation peak current was observed upon the interaction of 100 µg/mL TP-NB with 300 µg/mL dsDNA. While the average guanine oxidation peak current was 0.95 ± 0.06 µA before interaction, this average peak current was 0.41 ± 0.03 µA after interaction with TP-NB. This revealed a significant decrease of 56.8% in the percentage of the guanine peak current change. As seen in Figure 7A, guanine oxidation peak potentials shifted towards a more positive value after interaction with TP-NB (+1.02 V → +1.05 V). This suggests that TP-NB could be act on dsDNA structure through intercalation [46]. Although a shift in redox potential was observed and interpreted as evidence supporting intercalation, we acknowledge that the magnitude of this shift was relatively small. Statistical analysis to confirm that this shift was significantly different from baseline variability was not conducted in the current study. Therefore, the interpretation of intercalation based on this shift should be viewed with caution. Since it was determined in the previous steps that TP-NB has an oxidation peak current around +1.2 V and adenine base has an oxidation peak current around +1.2 V and these two peaks could potentially merge, adenine peak currents were not examined in the evaluation of the interaction. Based on guanine signals obtained from Figure 7A, TP-NB’s toxicity effect (S) on DNA was computed according to Equation (7) [47]:
S = (Sa/Sb) × 100%
- S: Percentage of the guanine peak current change;
- Sa: The magnitude of the guanine current upon interaction with drug candidate;
- Sb: The magnitude of the guanine current before interaction with drug candidate.
The percentage change in the guanine peak currents (S) was calculated to assess the interaction between TP-NB and dsDNA. Here, Sa represents the height of the guanine peak current after interaction with TP-NB, while Sb denotes the height of the guanine peak current before the interaction. Generally, an S value above 85 indicates non-toxicity. A value between 50 and 85 suggests moderate toxicity, while a value below 50 is classified as toxic [48]. Utilizing this equation, the S value of TP-NB on dsDNA was 43.16%. This indicates that TP-NB could be toxic to DNA [29,49].
Additionally, to demonstrate the dose-dependent effects of TP-NB on DNA, dsDNA at a fixed concentration of 300 µg/mL was exposed to TP-NB within the concentration range of 25–125 µg/mL. As can be seen in Figure 7C,D, the guanine peak current decreased in proportion to the concentration as TP-NB was added at increasing concentrations from 25 µg/mL to 75 µg/mL, whereas the guanine peak current did not decrease further at concentrations higher than 75 µg/mL. TP-NB exhibited the highest activity at a dose of 75 µg/mL. Next, interaction studies between TP-PC and dsDNA were conducted using optimized concentrations of 250 µg/mL TP-PC and 300 µg/mL dsDNA. Stock solutions of TP-PC in DMF and dsDNA in TE buffer were diluted with ACB (pH: 4.8), yielding final concentrations of 250 µg/mL TP-PC and 300 µg/mL dsDNA. A 100 µL aliquot of the mixture was transferred to tubes, and electrodes were immersed while stirring at 650 rpm for 1 h on a thermal shaker. DPV measurements were then performed to monitor changes in guanine oxidation peak current and potential. Additionally, interactions were examined by varying TP-PC concentrations at a fixed dsDNA concentration, followed by DPV measurements. Figure 8 presents the resulting differential pulse voltammograms and histogram plots.
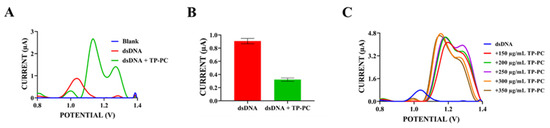
Figure 8.
(A) DPV after the interaction between TP-PC and dsDNA, taken in ACB (pH: 4.8) at a scan rate of 100 mV/s within the potential range of 0.8 V to 1.4 V. (B) Histogram showing the change in the average guanine oxidation current as a result of the interaction between TP-PC and dsDNA. (C) Differential pulse voltammogram after the interaction between different concentrations (150–350 µg/mL) of TP-PC and dsDNA, taken in ACB (pH: 4.8) at a scan rate of 100 mV/s within the potential range of 0.8 V to 1.4 V.
As shown in Figure 8A,B, a significant decrease in guanine oxidation peak current was observed upon the interaction of 250 µg/mL TP-PC with 300 µg/mL dsDNA. While the average guanine oxidation peak current was 0.91 ± 0.08 µA before interaction, this average peak current was 0.32 ± 0.05 µA after interaction with TP-PC. This unveils a significant decrease of 64.8% in the percentage of the guanine peak current change. As seen in Figure 8A, guanine oxidation peak potentials shifted towards a more negative value after interaction with TP-PC (+1.03 V → +0.99 V), unlike TP-NB. This suggests that TP-PC could interact with dsDNA electrostatically [50].
Since it was determined in the previous steps that TP-PC has an oxidation peak current around +1.1 V, adenine base has an oxidation peak current around +1.2 V, and these two peaks could potentially merge, adenine peak currents were not examined in the evaluation of the interaction like TP-NB. According to guanine signals obtained from Figure 8A, TP-PC’s toxicity effect (S%) on DNA was computed according to the Equation (7). With this equation, the S% value of TP-PC on dsDNA was 35.28%. This indicates that TP-NB could have toxic effects on DNA [29,49]. When S% values were analyzed, toxic effects of TP-PC on dsDNA were found to be higher than TP-NB. Thus, it was determined that TP-PC, formed by the piperidine carboxamide coupling of the TP-NB precursor compound, exhibited enhanced activity on DNA. Finally, to demonstrate the dose-dependent effects of TP-PC on DNA, dsDNA at a fixed concentration of 300 µg/mL was exposed to TP-PC within the concentration range of 150–350 µg/mL. As seen in Figure 8C, guanine peak current did not decrease in a concentration-proportional manner as TP-PC concentration increased. Due to the high effect of TP-PC on DNA, even low concentrations of TP-PC greatly reduced the guanine oxidation peak current. This suggests that it can be used in low doses in situations where its use is necessary due to its potential effects on DNA. One of the main advantages of using it in low doses is avoiding its adverse effects. Our results are related to the fact that potential anticancer activity is preliminary, based solely on structural analogy and electrochemical profile. We note that further studies, including cellular uptake and nuclear localization assays, as well as in vitro cytotoxicity experiments, are necessary to confirm any such activity [51].
3. Materials and Methods
3.1. Chemicals and Apparatus
Glacial acetic acid (CH3COOH) was purchased from Isolab Chemicals, while boric acid (H3BO3), sodium hydroxide (NaOH), sodium chloride (NaCl), dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4), and N,N-dimethylformamide (DMF) were obtained from Merck. Double-stranded DNA (dsDNA) from fish sperm and Tris-HCl were sourced from Sigma-Aldrich, and all solutions were prepared using ultrapure water. In this study, 0.5 M acetate buffers (ACB) (pH 3.8, 4.8, 5.6), 0.05 M phosphate buffer (pH 7.4), and 0.1 M borate buffer (pH 9.8), each containing 0.02 M NaCl, along with 0.05 M Tris-EDTA (TE) buffer (pH 8.0), were employed. A pencil graphite electrode (PGE), an Ag/AgCl reference electrode, and a platinum wire auxiliary electrode were utilized. To hold the 0.5 mm HB-grade pencil lead (Tombo, Japan), purchased from a local bookstore, a Rotring T 0.5 mm mechanical pencil (Hamburg, Germany) was used. The 10 mm graphite leads served for both measurement and immobilization steps. Electrochemical measurements were conducted using an AUTOLAB 204 FRA32M potentiostat/galvanostat/impedance analyzer controlled with NOVA 2.1 software. DPV measurements were conducted at a 100 mV/s scan rate with 0.5 s interval time in ACB (pH: 4.8) (step potential: 8 mV, modulation amplitude: 80 mV). CV measurements were conducted at a 50 mV/s scan rate with a 0.05 s interval time.
3.2. Synthesis of Thiazolo [5,4-d]pyrimidine Derivatives
In this study, we investigated the electrochemical properties and DNA interactions of two newly synthesized thiazolo [5,4-d]pyrimidine derivatives. TP-NB has been synthesized for the first time by our group and has been reported in the literature with its antileishmanial activity [19]. TP-PC, a bis(piperidine-4-carboxamide) derivative of TP-NB, is reported in the literature for the first time in this study.
3.3. Electrochemical Analysis of Drug Candidate Molecules and Their Interaction with DNA
Electrochemical Analysis: For electrode activation, +1.4 V was applied in ACB (pH: 4.8). Stock solutions of TP-NB and TP-PC were prepared with DMF and diluted in ACB (pH: 4.8) to the desired concentrations. In the first monitoring of the electrochemical redox signals of TP-NB and TP-PC, these drug solutions prepared with ACB at concentrations of 500 µg/mL were taken directly into the electrochemical measurement cell, and measurements were taken between 0.0 V and +1.5 V in the oxidation direction and between 0.0 V and −1.0 V in the reduction direction. For further optimization studies after the first signal scan, activated electrodes were incubated for passive adsorption and rinsed before analysis. DPV measurements were performed in ACB (pH: 4.8), while CV analysis involved direct immersion of electrodes in the respective solutions.
Interaction with DNA: Interactions between drug candidate molecules and dsDNA (double-stranded DNA) were studied in the solution phase. A stock dsDNA solution was prepared and diluted in ACB (pH: 4.8) to achieve the desired concentrations. The mixtures were incubated in a thermal shaker at 37 °C. After incubation, activated electrodes were immersed, followed by rinsing to remove non-adhered molecules [49].
4. Conclusions
In this study, the electrochemical behaviors and DNA interactions of two thiazolopyrimidine-based molecules, TP-NB and TP-PC, were investigated for the first time. TP-PC, synthesized with various substitutions to the core structure, and TP-NB, bearing the basic scaffold, were both electrochemically characterized. Both compounds exhibited oxidation and reduction peaks, with more prominent reduction signals leading to reduction-based detection. Their electrochemical processes were found to be diffusion-controlled and irreversible, involving equal numbers of electrons and protons. Determining such electrochemical properties—such as redox behavior, pH dependence, and transfer mechanisms—is highly valuable in the early stages of drug development, as these parameters can significantly influence drug activity, stability, and formulation strategies. In this context, the current findings provide a practical basis for evaluating the pharmacological potential of novel candidate drug molecules. The developed electrochemical method enabled sensitive detection with LOD values of 12 µg/mL for TP-NB and 16 µg/mL for TP-PC. The determination of these LOD and LOQ values in electrochemical methods developed for the detection of candidate drug molecules is essential for assessing the method’s sensitivity and reliability. These parameters ensure the accurate detection of the molecules even at trace concentrations, thereby playing a pivotal role in the development of potential pharmaceutical agents. Moreover, they are critical for the effective monitoring of therapeutic processes and the evaluation of drug efficacy. Interaction studies with DNA showed that both molecules significantly decreased the guanine oxidation signal, suggesting strong interactions. TP-NB could be interacting via intercalation, while TP-PC could be interacting with electrostatic interactions. TP-PC demonstrated a slightly stronger effect on DNA, indicating that structural modifications may enhance activity. These findings suggest that both molecules hold promise as anticancer agents and that the electrochemical approach used in this study provides a rapid and cost-effective strategy for evaluating drug–DNA interactions, offering valuable insights for early-stage drug development.
Author Contributions
Conceptualization, H.O.K., H.İ. and S.N.T.; methodology, H.O.K., C.B. and S.N.T.; validation, H.O.K. and S.N.T.; formal analysis, H.O.K. and S.N.T.; investigation, H.O.K., C.B. and S.N.T.; resources, H.O.K., H.İ. and S.N.T.; data curation, H.O.K., H.İ. and S.N.T.; writing—original draft preparation, H.O.K., H.İ. and S.N.T.; writing—review and editing, S.N.T. and H.O.K.; visualization, H.O.K., H.İ., C.B. and S.N.T.; supervision, S.N.T.; project administration, S.N.T.; funding acquisition, C.B. and S.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific and Technological Research Council of Turkey (TUBİTAK), 2209-A University Students (Undergraduate Students) Research Projects Support Program, Project Number of 1919B012302051.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuppast, B.; Fahmy, H. Thiazolo[4,5-d]pyrimidines as a privileged scaffold in drug discovery. Eur. J. Med. Chem. 2016, 113, 198–213. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zhang, J.; Liu, X.-Q.; Geng, P.-F.; Ma, J.-L.; Wang, B.; Zhao, T.-Q.; Zhao, B.; Wei, H.-M.; Wang, C.; et al. Identification of thiazolo[5,4-d]pyrimidine derivatives as potent antiproliferative agents through the drug repurposing strategy. Eur. J. Med. Chem. 2017, 135, 204–212. [Google Scholar] [CrossRef]
- Fahmy, H.T.Y.; Rostom, S.A.F.; Saudi, M.N.; Zjawiony, J.K.; Robins, D.J. Synthesis andin vitro evaluation of the anticancer activity of novel fluorinated thiazolo[4, 5-d]pyrimidines. Arch. Pharm. 2003, 336, 216–225. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Deng, Y.; Yan, Z.; Liu, J.; Liu, Y.; Sun, L. Design and Synthesis of Novel Thiazolo[5,4- d ]pyrimidine Derivatives as Potential Angiogenesis Inhibitors. Chem. Biodivers. 2019, 16, e1900232. [Google Scholar] [CrossRef]
- Alam, O.; Khan, S.A.; Siddiqui, N.; Ahsan, W. Synthesis and pharmacological evaluation of newer thiazolo [3,2-a] pyrimidines for anti-inflammatory and antinociceptive activity. Med. Chem. Res. 2010, 19, 1245–1258. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vincenzi, F.; Betti, M.; Falsini, M.; Ravani, A.; Borea, P.A.; Colotta, V.; Varani, K. Design, Synthesis, and Pharmacological Characterization of 2-(2-Furanyl)thiazolo[5,4- d ]pyrimidine-5,7-diamine Derivatives: New Highly Potent A 2A Adenosine Receptor Inverse Agonists with Antinociceptive Activity. J. Med. Chem. 2016, 59, 10564–10576. [Google Scholar] [CrossRef]
- Kuppast, B.; Spyridaki, K.; Lynch, C.; Hu, Y.; Liapakis, G.; Davies, G.E.; Fahmy, H. Synthesis of New Thiazolo[4,5-d]pyrimidines as Corticotropin Releasing Factor Modulators. Med. Chem. 2014, 11, 50–59. [Google Scholar] [CrossRef][Green Version]
- Becan, L.; Wagner, E. Synthesis and anticancer evaluation of novel 3,5-diaryl-thiazolo[4,5-d] pyrimidin-2-one derivatives. Med. Chem. Res. 2013, 22, 2376–2384. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; He, J.; Li, J.; Xiong, M.; Su, H.; Li, M.; Hu, H.; Xu, Y. Structure-Based Design of Potent Peptidomimetic Inhibitors Covalently Targeting SARS-CoV-2 Papain-like Protease. Int. J. Mol. Sci. 2023, 24, 8633. [Google Scholar] [CrossRef]
- Selvaraj, C.; Singh, S.K. Computational and Experimental Binding Mechanism of DNA-drug Interactions. Curr. Pharm. Des. 2019, 24, 3739–3757. [Google Scholar] [CrossRef]
- Rauf, S.; Gooding, J.; Akhtar, K.; Ghauri, M.; Rahman, M.; Anwar, M.; Khalid, A. Electrochemical approach of anticancer drugs–DNA interaction. J. Pharm. Biomed. Anal. 2005, 37, 205–217. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Shahabadi, N.; Fili, S.M.; Kheirdoosh, F. Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2013, 128, 20–26. [Google Scholar] [CrossRef]
- Sánchez-González, Á.; Castro, T.G.; Melle-Franco, M.; Gil, A. From groove binding to intercalation: Unravelling the weak interactions and other factors modulating the modes of interaction between methylated phenanthroline-based drugs and duplex DNA. Phys. Chem. Chem. Phys. 2021, 23, 26680–26695. [Google Scholar] [CrossRef]
- Kuzpınar, E.; Al Faysal, A.; Şenel, P.; Erdoğan, T.; Gölcü, A. Quantification of mirtazapine in tablets via DNA binding mechanism; development of a new HPLC method. J. Chromatogr. B 2024, 1234, 124019. [Google Scholar] [CrossRef]
- Ponkarpagam, S.; Vennila, K.N.; Elango, K.P. Molecular spectroscopic and molecular simulation studies on the interaction of oral contraceptive drug Ormeloxifene with CT–DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 278, 121351. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Abdian, M.; Maleki, S.; Abbasgolizadeh, P.; Fathi, F. Intercalation of anticancer drug mitoxantrone into DNA: Studied by spectral and surface plasmon resonance methods. J. Mol. Struct. 2023, 1274, 134509. [Google Scholar] [CrossRef]
- Ramotowska, S.; Ciesielska, A.; Makowski, M. What Can Electrochemical Methods Offer in Determining DNA–Drug Interactions? Molecules 2021, 26, 3478. [Google Scholar] [CrossRef]
- Istanbullu, H.; Bayraktar, G.; Karakaya, G.; Akbaba, H.; Perk, N.E.; Cavus, I.; Podlipnik, C.; Yereli, K.; Ozbilgin, A.; Butuner, B.D.; et al. Design, synthesis, in vitro—In vivo biological evaluation of novel thiazolopyrimidine compounds as antileishmanial agent with PTR1 inhibition. Eur. J. Med. Chem. 2023, 247, 115049. [Google Scholar] [CrossRef]
- Okamoto, K.; Nagahara, S.; Imada, Y.; Narita, R.; Kitano, Y.; Chiba, K. Hydrosilane-Mediated Electrochemical Reduction of Amides. J. Org. Chem. 2021, 86, 15992–16000. [Google Scholar] [CrossRef]
- Aftab, S.; Kurbanoglu, S.; Ozcelikay, G.; Bakirhan, N.K.; Shah, A.; Ozkan, S.A. Carbon quantum dots co-catalyzed with multiwalled carbon nanotubes and silver nanoparticles modified nanosensor for the electrochemical assay of anti-HIV drug Rilpivirine. Sens. Actuators B Chem. 2019, 285, 571–583. [Google Scholar] [CrossRef]
- Kaya, S.; Demirkan, B.; Bakirhan, N.; Kuyuldar, E.; Kurbanoglu, S.; Ozkan, S.; Sen, F. Highly sensitive carbon-based nanohybrid sensor platform for determination of 5-hydroxytryptamine receptor agonist (Eletriptan). J. Pharm. Biomed. Anal. 2019, 174, 206–213. [Google Scholar] [CrossRef]
- Samanci, S.N.; Ozcelikay-Akyildiz, G.; Atici, E.B.; Ozkan, S.A. Electrochemical behaviour and determination of niraparib using glassy carbon and boron-doped diamond electrodes. Diam. Relat. Mater. 2025, 152, 111964. [Google Scholar] [CrossRef]
- Mohamed, M.A.; El-Gendy, D.M.; Ahmed, N.; Banks, C.E.; Allam, N.K. 3D spongy graphene-modified screen-printed sensors for the voltammetric determination of the narcotic drug codeine. Biosens. Bioelectron. 2018, 101, 90–95. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kurbanoglu, S.; Uslu, B.; Ozkan, S.A. An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples. Methods Protoc. 2022, 5, 90. [Google Scholar] [CrossRef]
- Fekry, A.M.; Shehata, M.; Azab, S.M.; Walcarius, A. Voltammetric detection of caffeine in pharmacological and beverages samples based on simple nano- Co (II, III) oxide modified carbon paste electrode in aqueous and micellar media. Sens. Actuators B Chem. 2020, 302, 127172. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Kalanur, S.S.; Pollet, B.G.; Upadhyaya, K.P.; Ayachit, N.H.; Aminabhavi, T.M. Hf-Doped Tungsten Oxide Nanorods as Electrode Materials for Electrochemical Detection of Paracetamol and Salbutamol. ACS Appl. Nano Mater. 2022, 5, 1263–1275. [Google Scholar] [CrossRef]
- Koventhan, C.; Pandiyan, R.; Chen, S.-M.; Lo, A.-Y. Nickel molybdate/cobalt molybdate nanoflakes by one-pot synthesis approach for electrochemical detection of antipsychotic drug chlorpromazine in biological and environmental samples. J. Environ. Chem. Eng. 2023, 11, 110121. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Kaya, H.O.; Cetin, A.E. Electrochemical Detection of Linagliptin and its Interaction with DNA. Turk. J. Pharm. Sci. 2021, 18, 645–651. [Google Scholar] [CrossRef]
- Suprun, E.V.; Kutdusova, G.R.; Khmeleva, S.A.; Radko, S.P. Towards deeper understanding of DNA electrochemical oxidation on carbon electrodes. Electrochem. Commun. 2021, 124, 106947. [Google Scholar] [CrossRef]
- Špaček, J.; Fojta, M.; Wang, J. Electrochemical Reduction and Oxidation of Six Natural 2′-Deoxynucleosides at a Pyrolytic Graphite Electrode in the Presence or Absence of Ambient Oxygen. Electroanalysis 2019, 31, 2057–2066. [Google Scholar] [CrossRef]
- Hasoň, S.; Fojta, M.; Ostatná, V. Label-free electrochemical analysis of purine nucleotides and nucleobases at disposable carbon electrodes in microliter volumes. J. Electroanal. Chem. 2019, 847, 113252. [Google Scholar] [CrossRef]
- Vidláková, P.; Pivoňková, H.; Kejnovská, I.; Trnková, L.; Vorlíčková, M.; Fojta, M.; Havran, L. G-quadruplex-based structural transitions in 15-mer DNA oligonucleotides varying in lengths of internal oligo(dG) stretches detected by voltammetric techniques. Anal. Bioanal. Chem. 2015, 407, 5817–5826. [Google Scholar] [CrossRef]
- Stempkowska, I.; Ligaj, M.; Jasnowska, J.; Langer, J.; Filipiak, M. Electrochemical response of oligonucleotides on carbon paste electrode. Bioelectrochemistry 2007, 70, 488–494. [Google Scholar] [CrossRef]
- De-Los-Santos-Álvarez, N.; De-Los-Santos-Álvarez, P.; Lobo-Castañón, M.J.; López, R.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical oxidation of guanosine and adenosine: Two convergent pathways. Electrochem. Commun. 2007, 9, 1862–1866. [Google Scholar] [CrossRef]
- Li, Q.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical Oxidation of Guanine: Electrode Reaction Mechanism and Tailoring Carbon Electrode Surfaces To Switch between Adsorptive and Diffusional Responses. J. Phys. Chem. B 2010, 114, 7423–7428. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Batchelor-McAuley, C.; Barros, A.A.; Compton, R.G. Electrochemical Oxidation of Adenine: A Mixed Adsorption and Diffusion Response on an Edge-Plane Pyrolytic Graphite Electrode. J. Phys. Chem. C 2010, 114, 14213–14219. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Dogan-Topal, B.; Rodriguez, E.P.; Bozal-Palabiyik, B.; Ozkan, S.A.; Uslu, B. Advances in electrochemical DNA biosensors and their interaction mechanism with pharmaceuticals. J. Electroanal. Chem. 2016, 775, 8–26. [Google Scholar] [CrossRef]
- Koyuncu Zeybek, D.; Demir, B.; Zeybek, B.; Pekyardımcı, Ş. A sensitive electrochemical DNA biosensor for antineoplastic drug 5-fluorouracil based on glassy carbon electrode modified with poly(bromocresol purple). Talanta 2015, 144, 793–800. [Google Scholar] [CrossRef]
- Altay, C.; Eksin, E.; Congur, G.; Erdem, A. Electrochemical monitoring of the interaction between Temozolamide and nucleic acids by using disposable pencil graphite electrodes. Talanta 2015, 144, 809–815. [Google Scholar] [CrossRef]
- Bilge, S.; Dogan-Topal, B.; Taskin Tok, T.; Atici, E.B.; Sınağ, A.; Ozkan, S.A. Investigation of the interaction between anticancer drug ibrutinib and double-stranded DNA by electrochemical and molecular docking techniques. Microchem. J. 2022, 180, 107622. [Google Scholar] [CrossRef]
- Shakeel, M.; Butt, T.M.; Zubair, M.; Siddiqi, H.M.; Janjua, N.K.; Akhter, Z.; Yaqub, A.; Mahmood, S. Electrochemical investigations of DNA-Intercalation potency of bisnitrophenoxy compounds with different alkyl chain lengths. Heliyon 2020, 6, e04124. [Google Scholar] [CrossRef]
- Reinert, K.E. DNA-helix bending, stiffening and elongation on ligand binding; analysis for several DNA-drug systems, general viscometric DNA response and stereochemical implications. J. Biomol. Struct. Dyn. 1991, 9(2), 331–352. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Oliveira-Brett, A.M. In situ electrochemical evaluation of dsDNA interaction with the anticancer drug danusertib nitrenium radical product using the DNA-electrochemical biosensor. Bioelectrochemistry 2016, 107, 50–57. [Google Scholar] [CrossRef]
- De la Cruz Morales, K.; Alarcón-Angeles, G.; Merkoçi, A. Nanomaterial-based Sensors for the Study of DNA Interaction with Drugs. Electroanalysis 2019, 31, 1845–1867. [Google Scholar] [CrossRef]
- Bilge, S.; Topal, B.D.; Caglayan, M.G.; Unal, M.A.; Nazır, H.; Atici, E.B.; Sınağ, A.; Ozkan, S.A. Human hair rich in pyridinic nitrogen-base DNA biosensor for direct electrochemical monitoring of palbociclib-DNA interaction. Bioelectrochemistry 2022, 148, 108264. [Google Scholar] [CrossRef]
- Bagni, G.; Osella, D.; Sturchio, E.; Mascini, M. Deoxyribonucleic acid (DNA) biosensors for environmental risk assessment and drug studies. Anal. Chim. Acta 2006, 573–574, 81–89. [Google Scholar] [CrossRef]
- Muti, M.; Muti, M. Electrochemical monitoring of the interaction between anticancer drug and DNA in the presence of antioxidant. Talanta 2018, 178, 1033–1039. [Google Scholar] [CrossRef]
- Kaya, H.O.; Albayrak, G.; Isbilir, H.; Kurul, F.; Baykan, S.; Hartati, Y.W.; Topkaya, S.N. Electrochemical profiling of natural furanocoumarins: DNA interaction dynamics of oxypeucedanin and prantschimgin. ADMET DMPK 2024, 12, 319–334. [Google Scholar] [CrossRef]
- Keciba, A.; Bakirhan, N.K.; Gündüz, M.G.; Doulache, M.; Saidat, B.; Atici, E.B.; Ozkan, S.A. Electrochemical and theoretical investigations on the binding of anticancer drug olaparib to human serum albumin. J. Electrochem. Soc. 2024, 171, 066507. [Google Scholar] [CrossRef]
- Abd Elhameed, A.A.; El-Gohary, N.S.; El-Bendary, E.R.; Shaaban, M.I.; Bayomi, S.M. Synthesis and biological screening of new thiazolo[4,5-d]pyrimidine and dithiazolo[3,2-a:5′,4′-e]pyrimidinone derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Bioorganic. Chem. 2018, 81, 299–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).