Advancing Viral Defense: Unravelling the Potential of Host-Directed Antivirals Against SARS-CoV-2
Abstract
1. Introduction
2. Virus Diversity and Replication Strategies
3. Viral-Host Interaction in Virus Infection: SARS-CoV-2
4. Current Antiviral Therapeutics
4.1. Viral-Directed Antiviral (VDA)
| Virus | Approved Antiviral Drug | Mode of Action | References |
|---|---|---|---|
| SARS-CoV-2 (COVID-19) | Paxlovid (Nirmatrelvir and Ritonavir) | Inhibits SARS-CoV-2 main protease (Mpro) | [47] |
| Veklury (Remdesivir) | Inhibits RNA-dependent RNA polymerase | [48] | |
| Lagevrio (Molnupiravir) | Introducing errors into viral RNA | [48] | |
| Bamlanivimab and Etesevimab | Monoclonal antibodies targeting spike protein | [49] | |
| Casirivimab and Imdevimab (Ronapreve) | Monoclonal antibodies targeting spike protein | [50] | |
| Sotrovimab ** | Monoclonal antibodies targeting spike protein | [51,52] | |
| Evusheld (tixagevimab and cligavimab) ** | Monoclonal antibodies targeting spike protein | [53] | |
| Influenza A virus (IAV) | Tamiflu (Oseltamivir) | Inhibit neuraminidase (NA) | [54,55] |
| Relenza (Zanamivir) | Inhibit neuraminidase (NA) | [54,55] | |
| Rapivab (Peramivir) | Inhibit neuraminidase (NA) | [54,55] | |
| Xofluza (Baloxavir Marboxil) | Inhibit cap-dependent endonuclease | [54,55] | |
| Amantadine ** | M2 ion channel inhibitors | [56,57] | |
| Rimantadine ** | M2 ion channel inhibitors | [56,57] |
4.2. Host-Directed Antivirals (HDA)
| Virus | Approved Host-Directed Drug | Proposed Antiviral Action | References |
|---|---|---|---|
| SARS-CoV-2 (COVID-19) | Imatinib |
| [76,77,78,79] |
| Clofazimine |
| [80,81] | |
| Herpes Simplex Virus (HSV) | Ouabain |
| [82,83,84] |
| Influenza A virus (IAV) | Verapamil |
| [85,86,87] |
5. Harnessing Host Proteins to Combat SARS-CoV-2
5.1. Kinases
5.2. Heat Shock Proteins (HSPs)
5.3. Lipid Metabolism Proteins in Viral Replication
5.4. Immunological Pathway Proteins
6. Future Prospects of Targeting Host Proteins Against SARS-CoV-2
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ICTV Current ICTV Taxonomy Release. 2024. Available online: https://ictv.global/taxonomy (accessed on 1 January 2025).
- Koonin, E.V.; Krupovic, M.; Agol, V.I. The Baltimore Classification of Viruses 50 Years Later: How Does It Stand in the Light of Virus Evolution? Microbiol. Mol. Biol. Rev. 2021, 85, 10–1128. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Lal, S.K. The Molecular Biology of SARS-CoV-2 and Its Evolving Variants. In Learning from the COVID-19 Pandemic; CRC Press: Boca Raton, FL, USA, 2023; pp. 67–82. ISBN 978-1-00-335890-9. [Google Scholar]
- Bhadoria, P.; Gupta, G.; Agarwal, A. Viral Pandemics in the Past Two Decades: An Overview. J. Fam. Med. Prim. Care 2021, 10, 2745–2750. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Chan, A.M.L.; Choo, W.S. 14-3-3 Family of Proteins: Biological Implications, Molecular Interactions, and Potential Intervention in Cancer, Virus and Neurodegeneration Disorders. J. Cell. Biochem. 2024, 125, e30624. [Google Scholar] [CrossRef]
- Zabidi, N.Z.; Liew, H.L.; Farouk, I.A.; Puniyamurti, A.; Yip, A.J.W.; Wijesinghe, V.N.; Low, Z.Y.; Tang, J.W.; Chow, V.T.K.; Lal, S.K. Evolution of SARS-CoV-2 Variants: Implications on Immune Escape, Vaccination, Therapeutic and Diagnostic Strategies. Viruses 2023, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- FDA Coronavirus (COVID-19). Drugs: FDA Approved or Authorized Drugs to Reduce the Risk of Hospitalization or Death for Patients with Mild to Moderate COVID-19 2023; FDA: Silver Spring, MD, USA, 2023.
- European Centre for Disease Prevention and Control. SARS-CoV-2 Variant Mutations Conferring Reduced Susceptibility to Antiviral Drugs and Monoclonal Antibodies: A Non Systematic Literature Review for Surveillance Purposes; Publications Office: Luxembourg, 2023.
- Yang, J.; Hong, W.; Shi, H.; Wang, Z.; He, C.; Lei, H.; Yan, H.; Alu, A.; Ao, D.; Chen, Z.; et al. A Recombinant Protein Vaccine Induces Protective Immunity against SARS-CoV-2 JN.1 and XBB-Lineage Subvariants. Sig. Transduct. Target. Ther. 2025, 10, 58. [Google Scholar] [CrossRef]
- Shi, H.; Sun, J.; Zeng, Y.; Wang, X.; Liu, S.; Zhang, L.; Shao, E. Immune Escape of SARS-CoV-2 Variants to Therapeutic Monoclonal Antibodies: A System Review and Meta-Analysis. Virol. J. 2023, 20, 266. [Google Scholar] [CrossRef]
- Lopez, U.M.; Hasan, M.M.; Havranek, B.; Islam, S.M. SARS-CoV-2 Resistance to Small Molecule Inhibitors. Curr. Clin. Micro. Rpt. 2024, 11, 127–139. [Google Scholar] [CrossRef]
- Ip, J.D.; Wing-Ho Chu, A.; Chan, W.-M.; Cheuk-Ying Leung, R.; Umer Abdullah, S.M.; Sun, Y.; To, K.K.-W. Global Prevalence of SARS-CoV-2 3CL Protease Mutations Associated with Nirmatrelvir or Ensitrelvir Resistance. eBioMedicine 2023, 91, 104559. [Google Scholar] [CrossRef]
- Mahmoudabadi, G.; Phillips, R. A Comprehensive and Quantitative Exploration of Thousands of Viral Genomes. eLife 2018, 7, e31955. [Google Scholar] [CrossRef]
- Robinson, M.; Schor, S.; Barouch-Bentov, R.; Einav, S. Viral Journeys on the Intracellular Highways. Cell. Mol. Life Sci. 2018, 75, 3693–3714. [Google Scholar] [CrossRef]
- Ryu, W.-S. Virus Life Cycle. In Molecular Virology of Human Pathogenic Viruses; Elsevier: Amsterdam, The Netherlands, 2017; pp. 31–45. ISBN 978-0-12-800838-6. [Google Scholar]
- Rampersad, S.; Tennant, P. Replication and Expression Strategies of Viruses. In Viruses; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–82. ISBN 978-0-12-811257-1. [Google Scholar]
- Louten, J. Virus Replication. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–70. ISBN 978-0-12-800947-5. [Google Scholar]
- Weber, F.; Wagner, V.; Rasmussen, S.B.; Hartmann, R.; Paludan, S.R. Double-Stranded RNA Is Produced by Positive-Strand RNA Viruses and DNA Viruses but Not in Detectable Amounts by Negative-Strand RNA Viruses. J. Virol. 2006, 80, 5059–5064. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Sharma, A.; Lal, S.K. SARS Coronavirus Outbreaks Past and Present—A Comparative Analysis of SARS-CoV-2 and Its Predecessors. Virus Genes 2021, 57, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Payne, S. Introduction to RNA Viruses. In Viruses; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–105. ISBN 978-0-12-803109-4. [Google Scholar]
- Hughes, S.H. Reverse Transcription of Retroviruses and LTR Retrotransposons. Microbiol. Spectr. 2015, 3, 1051–1077. [Google Scholar] [CrossRef]
- Wei, L.; Ploss, A. Mechanism of Hepatitis B Virus cccDNA Formation. Viruses 2021, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chang, J.; Rijnbrand, R.; Lam, A.M.; Sofia, M.J.; Cuconati, A.; Guo, J.-T. Pregenomic RNA Launch Hepatitis B Virus Replication System Facilitates the Mechanistic Study of Antiviral Agents and Drug-Resistant Variants on Covalently Closed Circular DNA Synthesis. J. Virol. 2022, 96, e01150-22. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Olivares, C.; Martínez-Alvarado, E.; Rivera-Toledo, E. Persistence of RNA Viruses in the Respiratory Tract: An Overview. Viral Immunol. 2023, 36, 3–12. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An Overview of Viral Structure and Host Response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Subissi, L.; Imbert, I.; Ferron, F.; Collet, A.; Coutard, B.; Decroly, E.; Canard, B. SARS-CoV ORF1b-Encoded Nonstructural Proteins 12–16: Replicative Enzymes as Antiviral Targets. Antivir. Res. 2014, 101, 122–130. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Tam, D.; Lorenzo-Leal, A.C.; Hernández, L.R.; Bach, H. Targeting SARS-CoV-2 Non-Structural Proteins. Int. J. Mol. Sci. 2023, 24, 13002. [Google Scholar] [CrossRef]
- Low, Z.Y.; Zabidi, N.Z.; Yip, A.J.W.; Puniyamurti, A.; Chow, V.T.K.; Lal, S.K. SARS-CoV-2 Non-Structural Proteins and Their Roles in Host Immune Evasion. Viruses 2022, 14, 1991. [Google Scholar] [CrossRef] [PubMed]
- Fermin, G. Host Range, Host–Virus Interactions, and Virus Transmission. In Viruses; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–134. ISBN 978-0-12-811257-1. [Google Scholar]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV–Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.J.; Lacerda, S.M.S.N.; Figueiredo, A.F.A.; Wnuk, N.T.; Brener, M.R.G.; Andrade, L.M.; Campolina-Silva, G.H.; Kauffmann-Zeh, A.; Pacifico, L.G.G.; Versiani, A.F.; et al. High SARS-CoV-2 Tropism and Activation of Immune Cells in the Testes of Non-Vaccinated Deceased COVID-19 Patients. BMC Biol. 2023, 21, 36. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- FDA. Influenza (Flu) Antiviral Drugs and Related Information: FDA Approved Drugs for Influenza; FDA: Silver Spring, MD, USA, 2022.
- Amarelle, L.; Lecuona, E.; Sznajder, J.I. Anti-Influenza Treatment: Drugs Currently Used and Under Development. Arch. Bronconeumol. 2017, 53, 19–26. [Google Scholar] [CrossRef]
- Piperi, E.; Papadopoulou, E.; Georgaki, M.; Dovrat, S.; Bar Illan, M.; Nikitakis, N.G.; Yarom, N. Management of Oral Herpes Simplex Virus Infections: The Problem of Resistance. A narrative review. Oral. Dis. 2024, 30, 877–894. [Google Scholar] [CrossRef]
- Frejborg, F.; Kalke, K.; Hukkanen, V. Current Landscape in Antiviral Drug Development against Herpes Simplex Virus Infections. Smart Med. 2022, 1, e20220004. [Google Scholar] [CrossRef]
- Lim, H.; Baek, A.; Kim, J.; Kim, M.S.; Liu, J.; Nam, K.-Y.; Yoon, J.; No, K.T. Hot Spot Profiles of SARS-CoV-2 and Human ACE2 Receptor Protein Protein Interaction Obtained by Density Functional Tight Binding Fragment Molecular Orbital Method. Sci. Rep. 2020, 10, 16862. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Kuroda, M.; Armbrust, T.; Theiler, J.; Balaram, A.; Moreno, G.K.; Accola, M.A.; Iwatsuki-Horimoto, K.; Valdez, R.; Stoneman, E.; et al. Characterization of the SARS-CoV-2 B.1.621 (Mu) Variant. Sci. Transl. Med. 2022, 14, eabm4908. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 Humoral Immunity Induces Convergent Omicron RBD Evolution. Nature 2022, 614, 521–529. [Google Scholar] [CrossRef]
- Shaheen, N.; Mohamed, A.; Soliman, Y.; Abdelwahab, O.A.; Diab, R.A.; Desouki, M.T.; Rababah, A.A.; Khaity, A.; Hefnawy, M.T.; Swed, S.; et al. Could the New BA.2.75 Sub-Variant Lead to Another COVID-19 Wave in the World?—Correspondence. Int. J. Surg. 2022, 105, 106861. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Zhou, H.; Dcosta, B.M.; Samanovic, M.I.; Chivukula, V.; Herati, R.S.; Hubbard, S.R.; Mulligan, M.J.; Landau, N.R. Increased Resistance of SARS-CoV-2 Omicron Variant to Neutralization by Vaccine-Elicited and Therapeutic Antibodies. eBioMedicine 2022, 78, 103944. [Google Scholar] [CrossRef]
- Mader, A.-L.; Tydykov, L.; Glück, V.; Bertok, M.; Weidlich, T.; Gottwald, C.; Stefl, A.; Vogel, M.; Plentz, A.; Köstler, J.; et al. Omicron’s Binding to Sotrovimab, Casirivimab, Imdevimab, CR3022, and Sera from Previously Infected or Vaccinated Individuals. iScience 2022, 25, 104076. [Google Scholar] [CrossRef]
- Bege, M.; Borbás, A. The Design, Synthesis and Mechanism of Action of Paxlovid, a Protease Inhibitor Drug Combination for the Treatment of COVID-19. Pharmaceutics 2024, 16, 217. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir Remain Active against SARS-CoV-2 Omicron and Other Variants of Concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef]
- Chen, P.; Behre, G.; Hebert, C.; Kumar, P.; Farmer Macpherson, L.; Graham-Clarke, P.L.; De La Torre, I.; Nichols, R.M.; Hufford, M.M.; Patel, D.R.; et al. Bamlanivimab and Etesevimab Improve Symptoms and Associated Outcomes in Ambulatory Patients at Increased Risk for Severe Coronavirus Disease 2019: Results From the Placebo-Controlled Double-Blind Phase 3 BLAZE-1 Trial. Open Forum Infect. Dis. 2022, 9, ofac172. [Google Scholar] [CrossRef]
- Miyashita, N.; Nakamori, Y.; Ogata, M.; Fukuda, N.; Yamura, A.; Ishiura, Y. Clinical Efficacy of Casirivimab-Imdevimab Antibody Combination Treatment in Patients with COVID-19 Delta Variant. J. Infect. Chemother. 2022, 28, 1344–1346. [Google Scholar] [CrossRef]
- Heo, Y.-A. Sotrovimab: First Approval. Drugs 2022, 82, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Butzer, S.K.; Habbig, S.; Mehler, K.; Haumann, S.; Meyer, M.; Jung, N.; Oberthür, A. Use of Sotrovimab in 14 Children with COVID-19: A Single-Center Experience. Pediatr. Infect. Dis. J. 2023, 42, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Announces Evusheld Is Not Currently Authorized for Emergency Use in the U.S.; FDA: Silver Spring, MD, USA, 2023.
- Eichberg, J.; Maiworm, E.; Oberpaul, M.; Czudai-Matwich, V.; Lüddecke, T.; Vilcinskas, A.; Hardes, K. Antiviral Potential of Natural Resources against Influenza Virus Infections. Viruses 2022, 14, 2452. [Google Scholar] [CrossRef] [PubMed]
- FDA Treating Flu with Antiviral Drugs; FDA: Silver Spring, MD, USA, 2024.
- Batool, S.; Chokkakula, S.; Song, M.-S. Influenza Treatment: Limitations of Antiviral Therapy and Advantages of Drug Combination Therapy. Microorganisms 2023, 11, 183. [Google Scholar] [CrossRef]
- Li, Y.; Huo, S.; Yin, Z.; Tian, Z.; Huang, F.; Liu, P.; Liu, Y.; Yu, F. Retracted and republished from: “The current state of research on influenza antiviral drug development: Drugs in clinical trial and licensed drugs”. mBio 2024, 15, e00175-24. [Google Scholar] [CrossRef]
- Chitalia, V.C.; Munawar, A.H. A Painful Lesson from the COVID-19 Pandemic: The Need for Broad-Spectrum, Host-Directed Antivirals. J. Transl. Med. 2020, 18, 390. [Google Scholar] [CrossRef]
- Jans, D.A.; Wagstaff, K.M. Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal? Cells 2020, 9, 2100. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.-W.; Einav, S. Preparing for the next Viral Threat with Broad-Spectrum Antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Martin, D.E.; Tripp, R.A. Host-Directed Antiviral Therapeutics and the NIAID Research Agenda: An Underexplored Frontier. Front. Virol. 2024, 4, 1458112. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Sig. Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Fensterl, V.; Sen, G.C. Interferons and Viral Infections. BioFactors 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bruder Costa, J.; Dufeu-Duchesne, T.; Leroy, V.; Bertucci, I.; Bouvier-Alias, M.; Pouget, N.; Brevot-Lutton, O.; Bourliere, M.; Zoulim, F.; Plumas, J.; et al. Pegylated Interferon α-2a Triggers NK-Cell Functionality and Specific T-Cell Responses in Patients with Chronic HBV Infection without HBsAg Seroconversion. PLoS ONE 2016, 11, e0158297. [Google Scholar] [CrossRef]
- Liu, N.; Liu, B.; Zhang, L.; Li, H.; Chen, Z.; Luo, A.; Chen, M.; Peng, M.; Yin, W.; Ren, H.; et al. Recovery of Circulating CD56dim NK Cells and the Balance of Th17/Treg after Nucleoside Analog Therapy in Patients with Chronic Hepatitis B and Low Levels of HBsAg. Int. Immunopharmacol. 2018, 62, 59–66. [Google Scholar] [CrossRef]
- Silva, M.; Poo, J.; Wagner, F.; Jackson, M.; Cutler, D.; Grace, M.; Bordens, R.; Cullen, C.; Harvey, J.; Laughlin, M. A Randomised Trial to Compare the Pharmacokinetic, Pharmacodynamic, and Antiviral Effects of Peginterferon Alfa-2b and Peginterferon Alfa-2a in Patients with Chronic Hepatitis C (COMPARE). J. Hepatol. 2006, 45, 204–213. [Google Scholar] [CrossRef]
- Jaeckel, E.; Cornberg, M.; Wedemeyer, H.; Santantonio, T.; Mayer, J.; Zankel, M.; Pastore, G.; Dietrich, M.; Trautwein, C.; Manns, M.P. Treatment of Acute Hepatitis C with Interferon Alfa-2b. N. Engl. J. Med. 2001, 345, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, J.; Gao, H.; Liu, C.; Zhan, P.; Liu, X. Broad-Spectrum Antiviral Strategy: Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Eur. J. Med. Chem. 2024, 265, 116069. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhu, Y.; Li, J.; Chen, Z.; Han, G.W.; Kufareva, I.; Li, T.; Ma, L.; Fenalti, G.; Li, J.; et al. Structure of the CCR5 Chemokine Receptor–HIV Entry Inhibitor Maraviroc Complex. Science 2013, 341, 1387–1390. [Google Scholar] [CrossRef]
- Choi, W.-T.; An, J. Biology and Clinical Relevance of Chemokines and Chemokine Receptors CXCR4 and CCR5 in Human Diseases. Exp. Biol. Med. 2011, 236, 637–647. [Google Scholar] [CrossRef]
- Kim, M.B.; Giesler, K.E.; Tahirovic, Y.A.; Truax, V.M.; Liotta, D.C.; Wilson, L.J. CCR5 Receptor Antagonists in Preclinical to Phase II Clinical Development for Treatment of HIV. Expert. Opin. Investig. Drugs 2016, 25, 1377–1392. [Google Scholar] [CrossRef]
- Yamaya, M.; Shimotai, Y.; Hatachi, Y.; Lusamba Kalonji, N.; Tando, Y.; Kitajima, Y.; Matsuo, K.; Kubo, H.; Nagatomi, R.; Hongo, S.; et al. The Serine Protease Inhibitor Camostat Inhibits Influenza Virus Replication and Cytokine Production in Primary Cultures of Human Tracheal Epithelial Cells. Pulm. Pharmacol. Ther. 2015, 33, 66–74. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Arora, P.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; et al. Camostat Mesylate Inhibits SARS-CoV-2 Activation by TMPRSS2-Related Proteases and Its Metabolite GBPA Exerts Antiviral Activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsuyama, S.; Li, X.; Takeda, M.; Kawaguchi, Y.; Inoue, J.; Matsuda, Z. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob. Agents Chemother. 2016, 60, 6532–6539. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.; Herring, S.; Hsiang, T.-Y.; Ianevski, A.; Biering, S.B.; Xu, S.; Hoffmann, M.; Pöhlmann, S.; Gale, M.; Aittokallio, T.; et al. Combinations of Host- and Virus-Targeting Antiviral Drugs Confer Synergistic Suppression of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e03331-22. [Google Scholar] [CrossRef]
- Sacha, T. Imatinib in Chronic Myeloid Leukemia: An Overview. Mediterr. J. Hematol. Infect. Dis. 2013, 6, e2014007. [Google Scholar] [CrossRef] [PubMed]
- Strobelt, R.; Adler, J.; Paran, N.; Yahalom-Ronen, Y.; Melamed, S.; Politi, B.; Shulman, Z.; Schmiedel, D.; Shaul, Y. Imatinib Inhibits SARS-CoV-2 Infection by an off-Target-Mechanism. Sci. Rep. 2022, 12, 5758. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, S.; Park, T.; Hwang, H.; Park, S.; Kim, J.; Pyun, J.; Lee, M. Reduction of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variant Infection by Blocking the Epidermal Growth Factor Receptor (EGFR) Pathway. Microbiol. Spectr. 2024, 12, e01583-24. [Google Scholar] [CrossRef]
- Razzaq, A.; Disoma, C.; Zhou, Y.; Tao, S.; Chen, Z.; Liu, S.; Zheng, R.; Zhang, Y.; Liao, Y.; Chen, X.; et al. Targeting Epidermal Growth Factor Receptor Signalling Pathway: A Promising Therapeutic Option for COVID-19. Rev. Med. Virol. 2024, 34, e2500. [Google Scholar] [CrossRef]
- Stadler, J.A.M.; Maartens, G.; Meintjes, G.; Wasserman, S. Clofazimine for the Treatment of Tuberculosis. Front. Pharmacol. 2023, 14, 1100488. [Google Scholar] [CrossRef]
- Yuan, S.; Yin, X.; Meng, X.; Chan, J.F.-W.; Ye, Z.-W.; Riva, L.; Pache, L.; Chan, C.C.-Y.; Lai, P.-M.; Chan, C.C.-S.; et al. Clofazimine Broadly Inhibits Coronaviruses Including SARS-CoV-2. Nature 2021, 593, 418–423. [Google Scholar] [CrossRef]
- Ebert, S.N.; Subramanian, D.; Shtrom, S.S.; Chung, I.K.; Parris, D.S.; Muller, M.T. Association between the P170 Form of Human Topoisomerase II and Progeny Viral DNA in Cells Infected with Herpes Simplex Virus Type 1. J. Virol. 1994, 68, 1010–1020. [Google Scholar] [CrossRef]
- Dodson, A.W.; Taylor, T.J.; Knipe, D.M.; Coen, D.M. Inhibitors of the Sodium Potassium ATPase That Impair Herpes Simplex Virus Replication Identified via a Chemical Screening Approach. Virology 2007, 366, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Boff, L.; Schneider, N.F.Z.; Munkert, J.; Ottoni, F.M.; Ramos, G.S.; Kreis, W.; Braga, F.C.; Alves, R.J.; De Pádua, R.M.; Simões, C.M.O. Elucidation of the Mechanism of Anti-Herpes Action of Two Novel Semisynthetic Cardenolide Derivatives. Arch. Virol. 2020, 165, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Aberer, F.; Eckstein, M.L.; Haupt, S.; Erlmann, M.P.; Moser, O. Verapamil and Its Role in Diabetes. Diabetology 2022, 3, 393–406. [Google Scholar] [CrossRef]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2019, 9, 94. [Google Scholar] [CrossRef]
- Jayaseelan, V.P.; Paramasivam, A. Repurposing Calcium Channel Blockers as Antiviral Drugs. J. Cell Commun. Signal. 2020, 14, 467–468. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Kawahata, I.; Fukunaga, K. Protein Kinases and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 5574. [Google Scholar] [CrossRef]
- Rice, A.P. Cyclin-Dependent Kinases as Therapeutic Targets for HIV-1 Infection. Expert Opin. Ther. Targets 2016, 20, 1453–1461. [Google Scholar] [CrossRef]
- Diehl, N.; Schaal, H. Make Yourself at Home: Viral Hijacking of the PI3K/Akt Signaling Pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Sig. Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Chowdhury, I.; Dashi, G.; Keskitalo, S. CMGC Kinases in Health and Cancer. Cancers 2023, 15, 3838. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Thakur, S.S. SARS-CoV-2 Infection Triggers Phosphorylation: Potential Target for Anti-COVID-19 Therapeutics. Front. Immunol. 2022, 13, 829474. [Google Scholar] [CrossRef]
- Bouhaddou, M.; Memon, D.; Meyer, B.; White, K.M.; Rezelj, V.V.; Correa Marrero, M.; Polacco, B.J.; Melnyk, J.E.; Ulferts, S.; Kaake, R.M.; et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell 2020, 182, 685–712.e19. [Google Scholar] [CrossRef]
- Rana, A.K.; Rahmatkar, S.N.; Kumar, A.; Singh, D. Glycogen Synthase Kinase-3: A Putative Target to Combat Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pandemic. Cytokine Growth Factor. Rev. 2021, 58, 92–101. [Google Scholar] [CrossRef]
- Higgins, C.A.; Nilsson-Payant, B.E.; Bonaventure, B.; Kurland, A.P.; Ye, C.; Yaron, T.M.; Johnson, J.L.; Adhikary, P.; Golynker, I.; Panis, M.; et al. SARS-CoV-2 Hijacks P38β/MAPK11 to Promote Virus Replication. mBio 2023, 14, e01007-23. [Google Scholar] [CrossRef]
- Guo, S.; Lei, X.; Chang, Y.; Zhao, J.; Wang, J.; Dong, X.; Liu, Q.; Zhang, Z.; Wang, L.; Yi, D.; et al. SARS-CoV-2 Hijacks Cellular Kinase CDK2 to Promote Viral RNA Synthesis. Sig. Transduct. Target. Ther. 2022, 7, 400. [Google Scholar] [CrossRef]
- Jain, S.; Rego, S.; Park, S.; Liu, Y.; Parn, S.; Savsani, K.; Perlin, D.S.; Dakshanamurthy, S. RNASeq Profiling of COVID19-infected Patients Identified an EIF2AK2 Inhibitor as a Potent SARS-CoV-2 Antiviral. Clin. Transl. Med. 2022, 12, e1098. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Yeh, S.-H.; Tsay, Y.-G.; Shieh, Y.-H.; Kao, C.-L.; Chen, Y.-S.; Wang, S.-H.; Kuo, T.-J.; Chen, D.-S.; Chen, P.-J. Glycogen Synthase Kinase-3 Regulates the Phosphorylation of Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Protein and Viral Replication. J. Biol. Chem. 2009, 284, 5229–5239. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.M.; Grimes, K.V. P38 MAPK Inhibition: A Promising Therapeutic Approach for COVID-19. J. Mol. Cell. Cardiol. 2020, 144, 63–65. [Google Scholar] [CrossRef]
- Dubey, A.; Prajapati, K.S.; Swamy, M.; Pachauri, V. Heat Shock Proteins: A Therapeutic Target Worth to Consider. Vet. World 2015, 8, 46–51. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Wang, D.; Chen, Z. Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions. Genes 2023, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef]
- Campanella, C.; Pace, A.; Caruso Bavisotto, C.; Marzullo, P.; Marino Gammazza, A.; Buscemi, S.; Palumbo Piccionello, A. Heat Shock Proteins in Alzheimer’s Disease: Role and Targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef]
- Seo, H.W.; Seo, J.P.; Jung, G. Heat Shock Protein 70 and Heat Shock Protein 90 Synergistically Increase Hepatitis B Viral Capsid Assembly. Biochem. Biophys. Res. Commun. 2018, 503, 2892–2898. [Google Scholar] [CrossRef]
- Joshi, P.; Garg, S.; Mani, S.; Shoaib, R.; Jakhar, K.; Almuqdadi, H.T.A.; Sonar, S.; Marothia, M.; Behl, A.; Biswas, S.; et al. Targeting Host Inducible-Heat Shock Protein 70 with PES-Cl Is a Promising Antiviral Strategy against SARS-CoV-2 Infection and Pathogenesis. Int. J. Biol. Macromol. 2024, 279, 135069. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, L.-D.; Zhang, F.; Liang, Q.-Z.; Jiao, Y.; Shi, F.-S.; He, B.; Xu, P.; Huang, Y.-W. Heat Shock Protein 90 Facilitates SARS-CoV-2 Structural Protein-Mediated Virion Assembly and Promotes Virus-Induced Pyroptosis. J. Biol. Chem. 2023, 299, 104668. [Google Scholar] [CrossRef]
- Navhaya, L.T.; Blessing, D.M.; Yamkela, M.; Godlo, S.; Makhoba, X.H. A Comprehensive Review of the Interaction between COVID-19 Spike Proteins with Mammalian Small and Major Heat Shock Proteins. Biomol. Concepts 2024, 15, 20220027. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Wei, R.; Zhou, B.; Li, S.; Zhong, D.; Li, B.; Qin, J.; Zhao, L.; Qin, L.; Hu, J.; Wang, J.; et al. Plasma Gp96 Is a Novel Predictive Biomarker for Severe COVID-19. Microbiol. Spectr. 2021, 9, e00597-21. [Google Scholar] [CrossRef]
- Stalin, A.; Saravana Kumar, P.; Senthamarai Kannan, B.; Saravanan, R.; Ignacimuthu, S.; Zou, Q. Potential Inhibition of SARS-CoV-2 Infection and Its Mutation with the Novel Geldanamycin Analogue: Ignaciomycin. Arab. J. Chem. 2024, 17, 105493. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Q.; Ma, L.; Zhao, J.; McIntosh, F.; Liu, Z.; Ding, S.; Lin, R.; Cen, S.; Finzi, A.; et al. Anthracyclines Inhibit SARS-CoV-2 Infection. Virus Res. 2023, 334, 199164. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Russell, V.S.; Tu, J.J.; Thomas, C.; Hughes, P.; Kelly, F.; Langel, S.N.; Steppe, J.; Palmer, S.M.; Haystead, T.; et al. Oral Hsp90 Inhibitor SNX-5422 Attenuates SARS-CoV-2 Replication and Dampens Inflammation in Airway Cells. iScience 2021, 24, 103412. [Google Scholar] [CrossRef]
- Lorizate, M.; Krausslich, H.-G. Role of Lipids in Virus Replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef]
- Sumbria, D.; Berber, E.; Mathayan, M.; Rouse, B.T. Virus Infections and Host Metabolism—Can We Manage the Interactions? Front. Immunol. 2021, 11, 594963. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef]
- Zhang, Z.; He, G.; Filipowicz, N.A.; Randall, G.; Belov, G.A.; Kopek, B.G.; Wang, X. Host Lipids in Positive-Strand RNA Virus Genome Replication. Front. Microbiol. 2019, 10, 286. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Multifaceted Roles for Lipids in Viral Infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar] [CrossRef]
- Cosset, F.-L.; Denolly, S. Lipoprotein receptors: A little grease for enveloped viruses to open the lock? J. Biol. Chem. 2024, 300, 107849. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martínez-Mier, G.; Quistián-Galván, J.; Muñoz-Pérez, A.; Bernal-Dolores, V.; Del Ángel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef]
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef] [PubMed]
- Theken, K.N.; Tang, S.Y.; Sengupta, S.; FitzGerald, G.A. The Roles of Lipids in SARS-CoV-2 Viral Replication and the Host Immune Response. J. Lipid Res. 2021, 62, 100129. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-Scavenger Receptor B Type 1 Facilitates SARS-CoV-2 Entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef]
- Müller, C.; Hardt, M.; Schwudke, D.; Neuman, B.W.; Pleschka, S.; Ziebuhr, J. Inhibition of Cytosolic Phospholipase A2 α Impairs an Early Step of Coronavirus Replication in Cell Culture. J. Virol. 2018, 92, e01463-17. [Google Scholar] [CrossRef]
- Vijay, R.; Hua, X.; Meyerholz, D.K.; Miki, Y.; Yamamoto, K.; Gelb, M.; Murakami, M.; Perlman, S. Critical Role of Phospholipase A2 Group IID in Age-Related Susceptibility to Severe Acute Respiratory Syndrome–CoV Infection. J. Exp. Med. 2015, 212, 1851–1868. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Linoleic Acid Binds to SARS-CoV-2 RdRp and Represses Replication of Seasonal Human Coronavirus OC43. Sci. Rep. 2022, 12, 19114. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated ω-3 Fatty Acids Inhibit ACE2-Controlled SARS-CoV-2 Binding and Cellular Entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef]
- Fernández-Oliva, A.; Ortega-González, P.; Risco, C. Targeting Host Lipid Flows: Exploring New Antiviral and Antibiotic Strategies. Cell. Microbiol. 2019, 21, e12996. [Google Scholar] [CrossRef]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2021, 8, 618296. [Google Scholar] [CrossRef]
- Zhuang, X.-Y.; Zhang, Y.-H.; Xiao, A.-F.; Zhang, A.-H.; Fang, B.-S. Key Enzymes in Fatty Acid Synthesis Pathway for Bioactive Lipids Biosynthesis. Front. Nutr. 2022, 9, 851402. [Google Scholar] [CrossRef]
- Mekhail, K.; Lee, M.; Sugiyama, M.; Astori, A.; St-Germain, J.; Latreille, E.; Khosraviani, N.; Wei, K.; Li, Z.; Rini, J.; et al. FASN Inhibitor TVB-3166 Prevents S-Acylation of the Spike Protein of Human Coronaviruses. J. Lipid Res. 2022, 63, 100256. [Google Scholar] [CrossRef]
- Niort, K.; Dancourt, J.; Boedec, E.; Al Amir Dache, Z.; Lavieu, G.; Tareste, D. Cholesterol and Ceramide Facilitate Membrane Fusion Mediated by the Fusion Peptide of the SARS-CoV-2 Spike Protein. ACS Omega 2023, 8, 32729–32739. [Google Scholar] [CrossRef]
- Cesar-Silva, D.; Pereira-Dutra, F.S.; Giannini, A.L.M.; Maya-Monteiro, C.M.; De Almeida, C.J.G. Lipid Compartments and Lipid Metabolism as Therapeutic Targets against Coronavirus. Front. Immunol. 2023, 14, 1268854. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The Serotonin Reuptake Inhibitor Fluoxetine Inhibits SARS-CoV-2 in Human Lung Tissue. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef] [PubMed]
- Törnquist, K.; Asghar, M.Y.; Srinivasan, V.; Korhonen, L.; Lindholm, D. Sphingolipids as Modulators of SARS-CoV-2 Infection. Front. Cell Dev. Biol. 2021, 9, 689854. [Google Scholar] [CrossRef] [PubMed]
- Arish, M.; Husein, A.; Kashif, M.; Saleem, M.; Akhter, Y.; Rub, A. Sphingosine-1-Phosphate Signaling: Unraveling Its Role as a Drug Target against Infectious Diseases. Drug Discov. Today 2016, 21, 133–142. [Google Scholar] [CrossRef]
- Mohammed, S.; Bindu, A.; Viswanathan, A.; Harikumar, K.B. Sphingosine 1-Phosphate Signaling during Infection and Immunity. Prog. Progress. Lipid Res. 2023, 92, 101251. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Shen, S.; Wen, C.; Xing, Z.; Shi, Y. Inhibition of Autophagy and Chemokine Induction by Sphingosine 1-Phosphate Receptor 1 through NF-κB Signaling in Human Pulmonary Endothelial Cells Infected with Influenza A Viruses. PLoS ONE 2018, 13, e0205344. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xiao, E.; Han, Q.; Wang, L. Sphingosine-1-phosphate Related Signalling Pathways Manipulating Virus Replication. Rev. Med. Virol. 2023, 33, e2415. [Google Scholar] [CrossRef]
- Edwards, M.J.; Becker, K.A.; Gripp, B.; Hoffmann, M.; Keitsch, S.; Wilker, B.; Soddemann, M.; Gulbins, A.; Carpinteiro, E.; Patel, S.H.; et al. Sphingosine Prevents Binding of SARS–CoV-2 Spike to Its Cellular Receptor ACE2. J. Biol. Chem. 2020, 295, 15174–15182. [Google Scholar] [CrossRef]
- Geiger, N.; Kersting, L.; Schlegel, J.; Stelz, L.; Fähr, S.; Diesendorf, V.; Roll, V.; Sostmann, M.; König, E.-M.; Reinhard, S.; et al. The Acid Ceramidase Is a SARS-CoV-2 Host Factor. Cells 2022, 11, 2532. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K. Toll-like Receptors in Innate Immunity. Int. Immunol. 2004, 17, 1–14. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Low, Z.Y.; Wen Yip, A.J.; Chow, V.T.K.; Lal, S.K. The Suppressor of Cytokine Signalling Family of Proteins and Their Potential Impact on COVID-19 Disease Progression. Rev. Med. Virol. 2022, 32, e2300. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Yang, M.-H.; Yu, K.; Lian, Z.-X.; Deng, S.-L. Toll-like Receptor (TLRs) Agonists and Antagonists for COVID-19 Treatments. Front. Pharmacol. 2022, 13, 989664. [Google Scholar] [CrossRef] [PubMed]
- Tugaeva, K.V.; Hawkins, D.E.D.P.; Smith, J.L.R.; Bayfield, O.W.; Ker, D.-S.; Sysoev, A.A.; Klychnikov, O.I.; Antson, A.A.; Sluchanko, N.N. The Mechanism of SARS-CoV-2 Nucleocapsid Protein Recognition by the Human 14-3-3 Proteins. J. Mol. Biol. 2021, 433, 166875. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the Art. Sig. Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Farouk, I.A.; Low, Z.Y.; Yip, A.J.W.; Lal, S.K. PABPC1: A Novel Emerging Target for Cancer Prognostics and Anti-Cancer Therapeutics. In Molecular Biomarkers for Cancer Diagnosis and Therapy; Sobti, R.C., Sugimura, H., Sobti, A., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 619–636. ISBN 978-981-9937-45-5. [Google Scholar]
- Lei, J.; Ma-Lauer, Y.; Han, Y.; Thoms, M.; Buschauer, R.; Jores, J.; Thiel, V.; Beckmann, R.; Deng, W.; Leonhardt, H.; et al. The SARS-unique Domain (SUD) of SARS-CoV and SARS-CoV-2 Interacts with Human Paip1 to Enhance Viral RNA Translation. EMBO J. 2021, 40, e102277. [Google Scholar] [CrossRef]
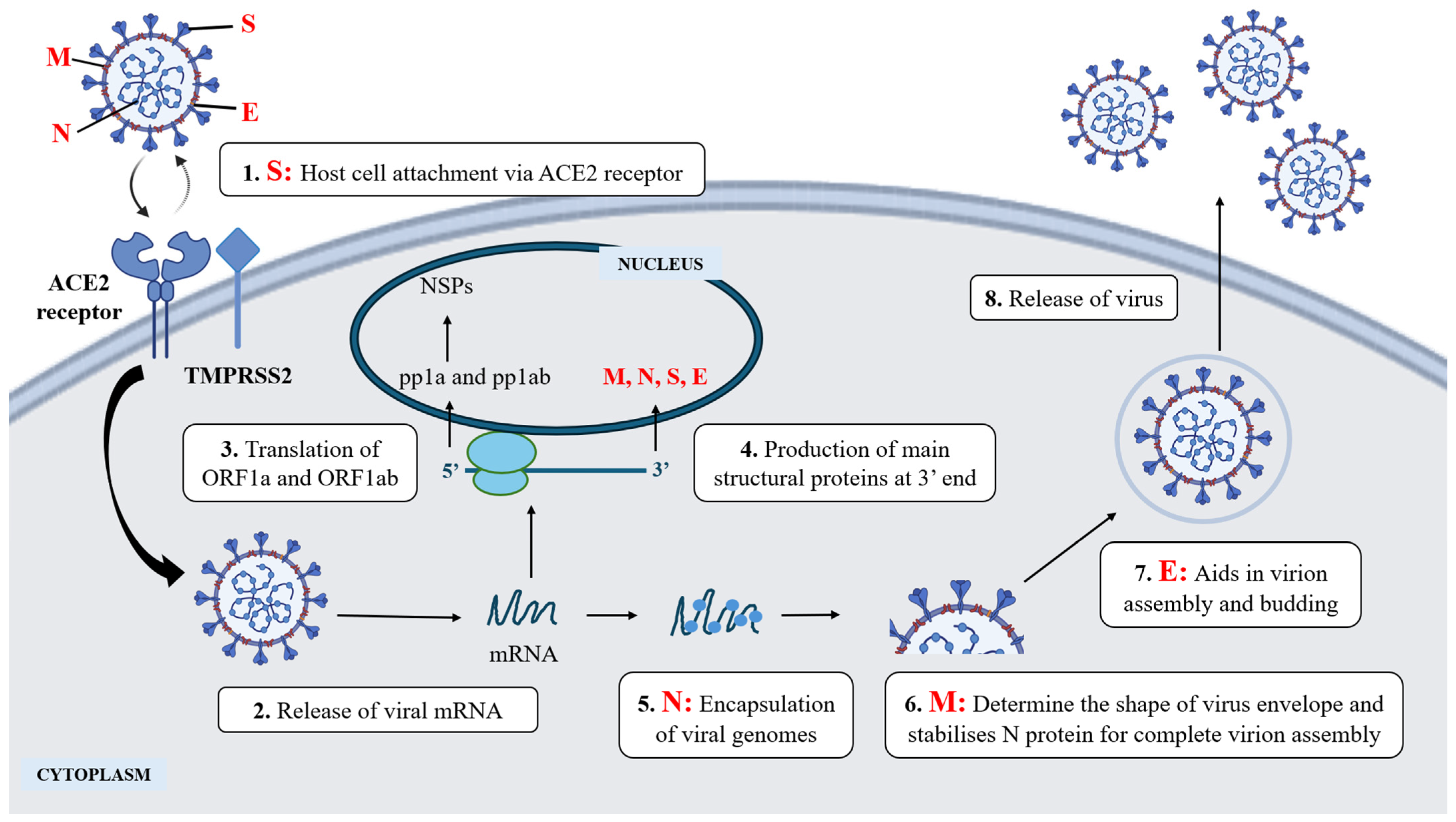
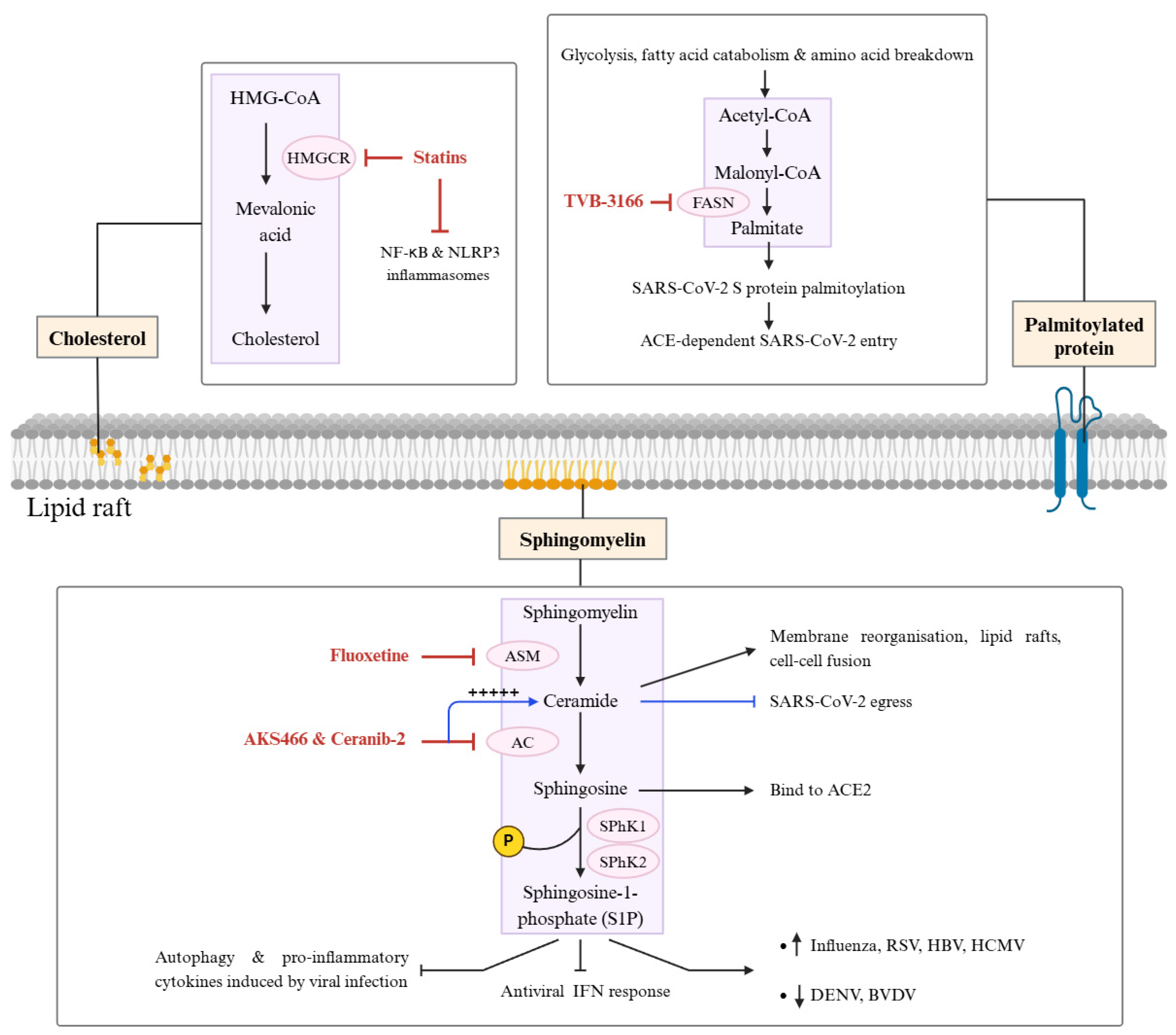

| Type of Viruses | Virus Class | Examples | Modes of Replication | References |
|---|---|---|---|---|
| DNA viruses | dsDNA | Herpesvirus |
| [16] |
| ssDNA | Parvovirus |
| [16] | |
| RNA viruses | dsRNA | Rotavirus, Bluetongue virus |
| [17,18] |
| +ssRNA | Coronavirus, Picornavirus, Flavivirus, Togavirus |
| [19,20] | |
| −ssRNA | Influenza virus, Rabies virus, Ebola virus, Measles virus, Mumps virus, Hantavirus |
| [20] | |
| Reverse-transcribing viruses/retroviruses | ssRNA-RT | HIV |
| [21] |
| dsDNA-RT | Hepadnavirus |
| [22,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, Z.Y.; Chin, S.W.; Syed Hassan, S.; Choo, W.S. Advancing Viral Defense: Unravelling the Potential of Host-Directed Antivirals Against SARS-CoV-2. Drugs Drug Candidates 2025, 4, 13. https://doi.org/10.3390/ddc4020013
Low ZY, Chin SW, Syed Hassan S, Choo WS. Advancing Viral Defense: Unravelling the Potential of Host-Directed Antivirals Against SARS-CoV-2. Drugs and Drug Candidates. 2025; 4(2):13. https://doi.org/10.3390/ddc4020013
Chicago/Turabian StyleLow, Zheng Yao, Siau Wui Chin, Sharifah Syed Hassan, and Wee Sim Choo. 2025. "Advancing Viral Defense: Unravelling the Potential of Host-Directed Antivirals Against SARS-CoV-2" Drugs and Drug Candidates 4, no. 2: 13. https://doi.org/10.3390/ddc4020013
APA StyleLow, Z. Y., Chin, S. W., Syed Hassan, S., & Choo, W. S. (2025). Advancing Viral Defense: Unravelling the Potential of Host-Directed Antivirals Against SARS-CoV-2. Drugs and Drug Candidates, 4(2), 13. https://doi.org/10.3390/ddc4020013









