Design of Hetero-Dinuclear Metallic Complexes as Potential Metal-Based Drugs With a Zinc Metal Center in a Square-Pyramidal Structure
Abstract
1. Introduction
2. Hetero-Dinuclear Metallic Complexes with Zinc(II) in a Square-Pyramidal Structure
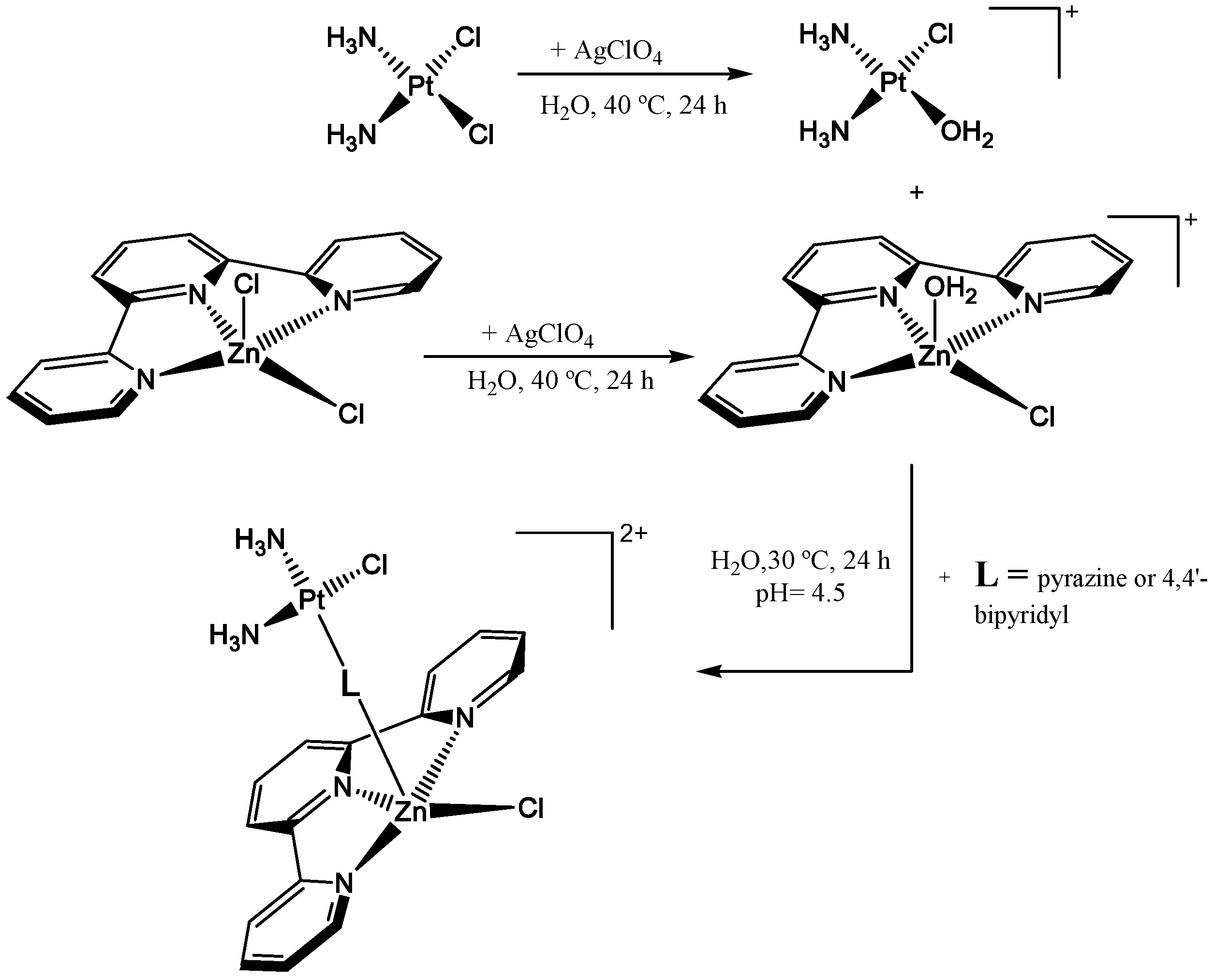
2.1. Hetero-Dinuclear Metallic Complexes with General Formula Zn(II)-L-Cu(II)
2.2. Hetero-Dinuclear Metallic Complexes with General Formula Pt(II)-L-Zn(II)
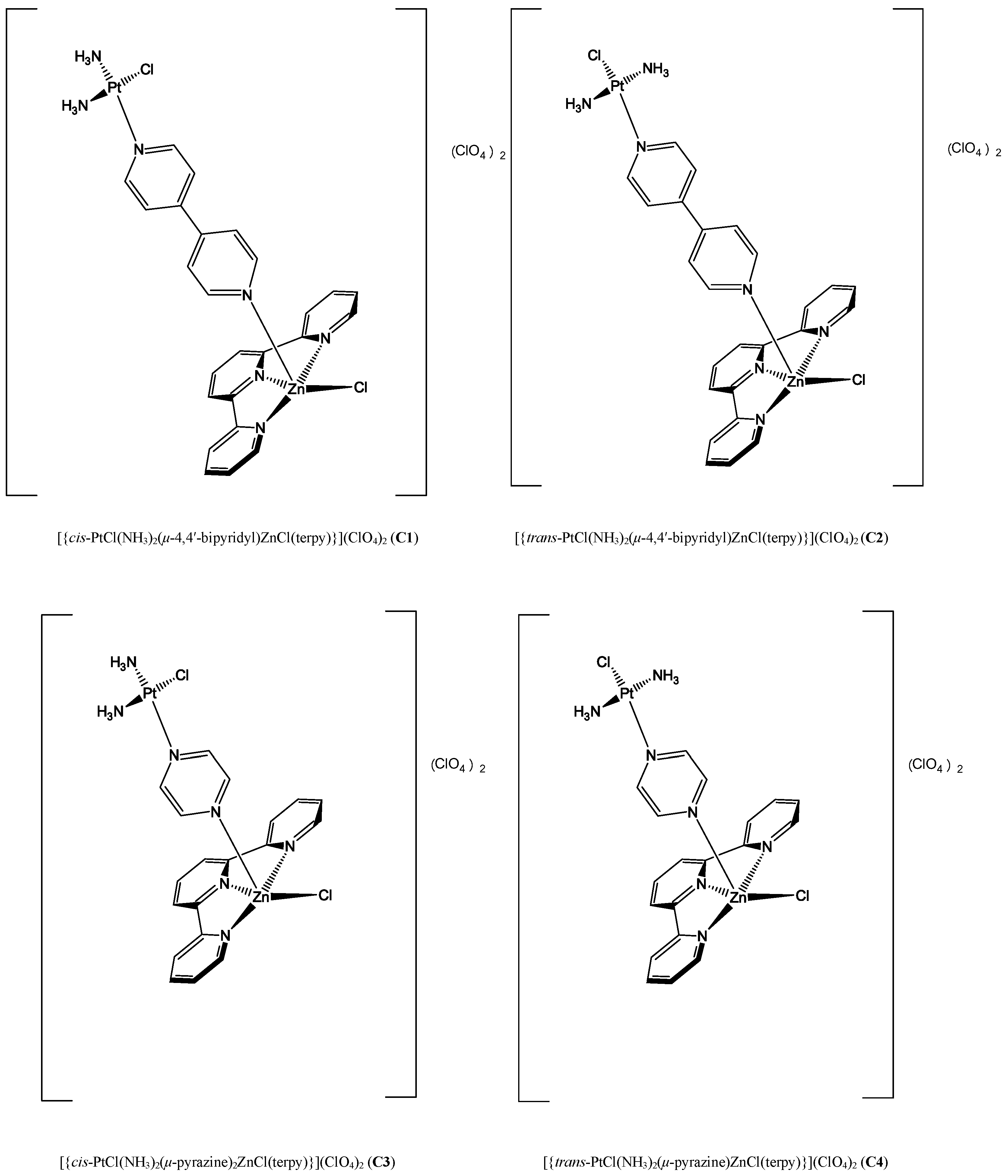
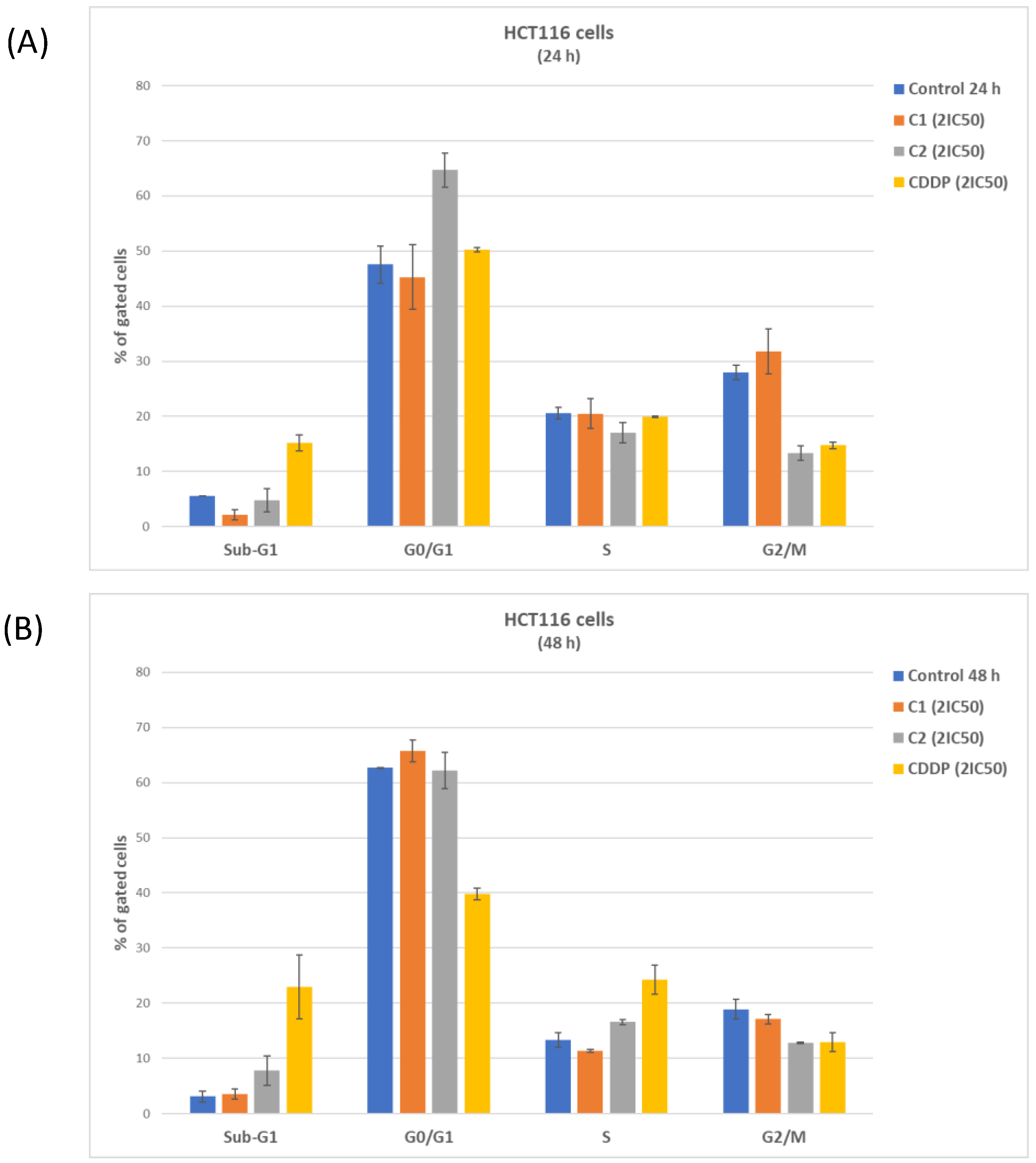
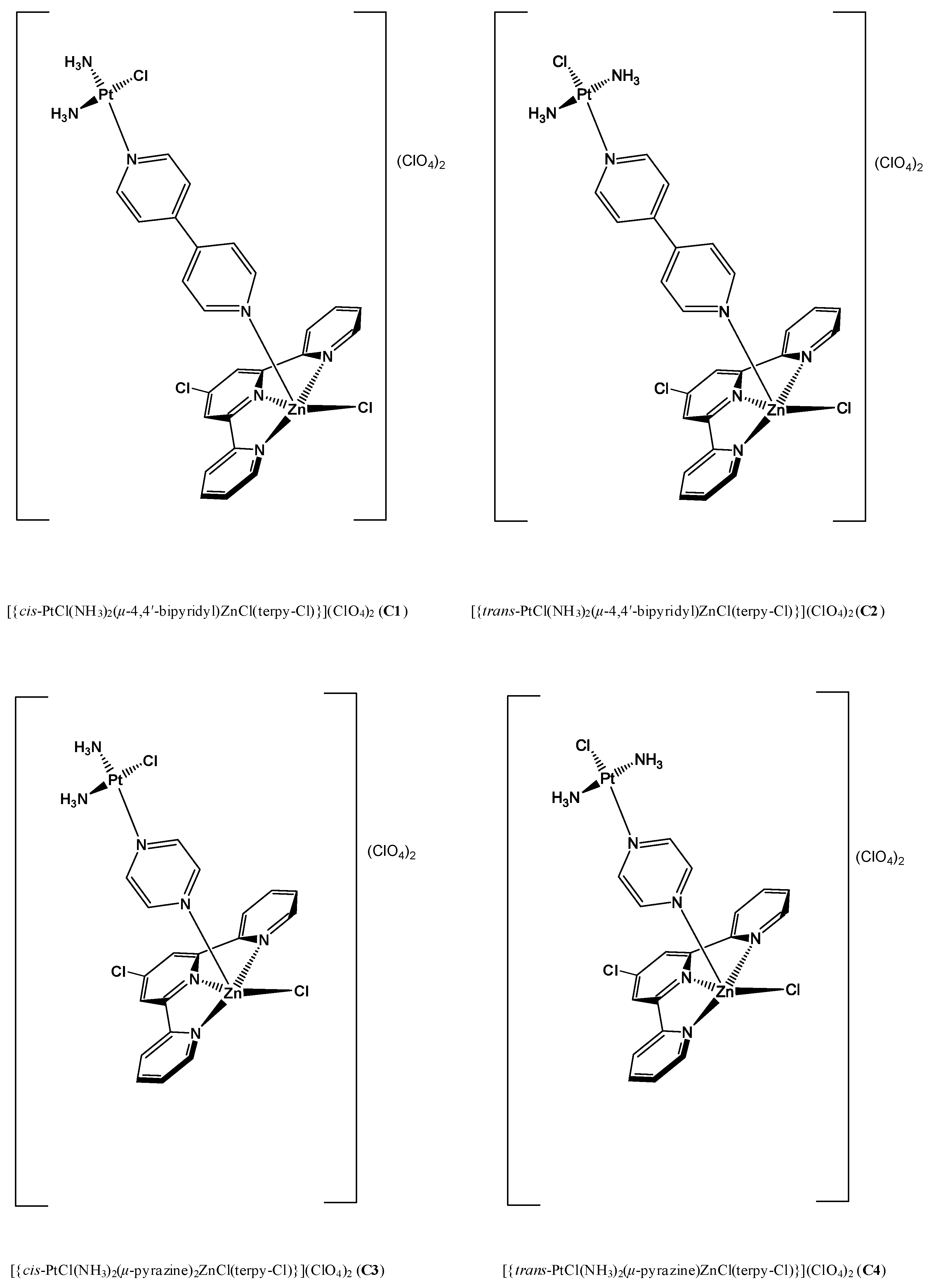
2.2.1. Hetero-Dinuclear Metallic Complexes [{cis-PtCl(NH3)2(μ-4,4′-bipyridyl)ZnCl(terpy)}](ClO4)2 (C1), [{trans-PtCl(NH3)2(μ-4,4′-bipyridyl)ZnCl(terpy)}](ClO4)2 (C2), [{cis-PtCl(NH3)2(μ-pyrazine)ZnCl(terpy)}](ClO4)2 (C3) and [{trans-PtCl(NH3)2(μ-pyrazine) ZnCl(terpy)}](ClO4)2 (C4) and Their Biological Activity
2.2.2. Hetero-Dinuclear Metallic Complexes [{cis-PtCl(NH3)2(μ-4,4′-bipyridyl)ZnCl(terpy-Cl)}](ClO4)2 (C1a), [{trans-PtCl(NH3)2(μ-4,4′-bipyridyl)ZnCl(terpy)}](ClO4)2 (C2a), [{cis-PtCl(NH3)2(μ-pyrazine)ZnCl(terpy-Cl)}](ClO4)2 (C3a) and [{trans-PtCl(NH3)2(μ-pyrazine)ZnCl(terpy-Cl)}](ClO4)2 (C4a) and Their Biological Activity
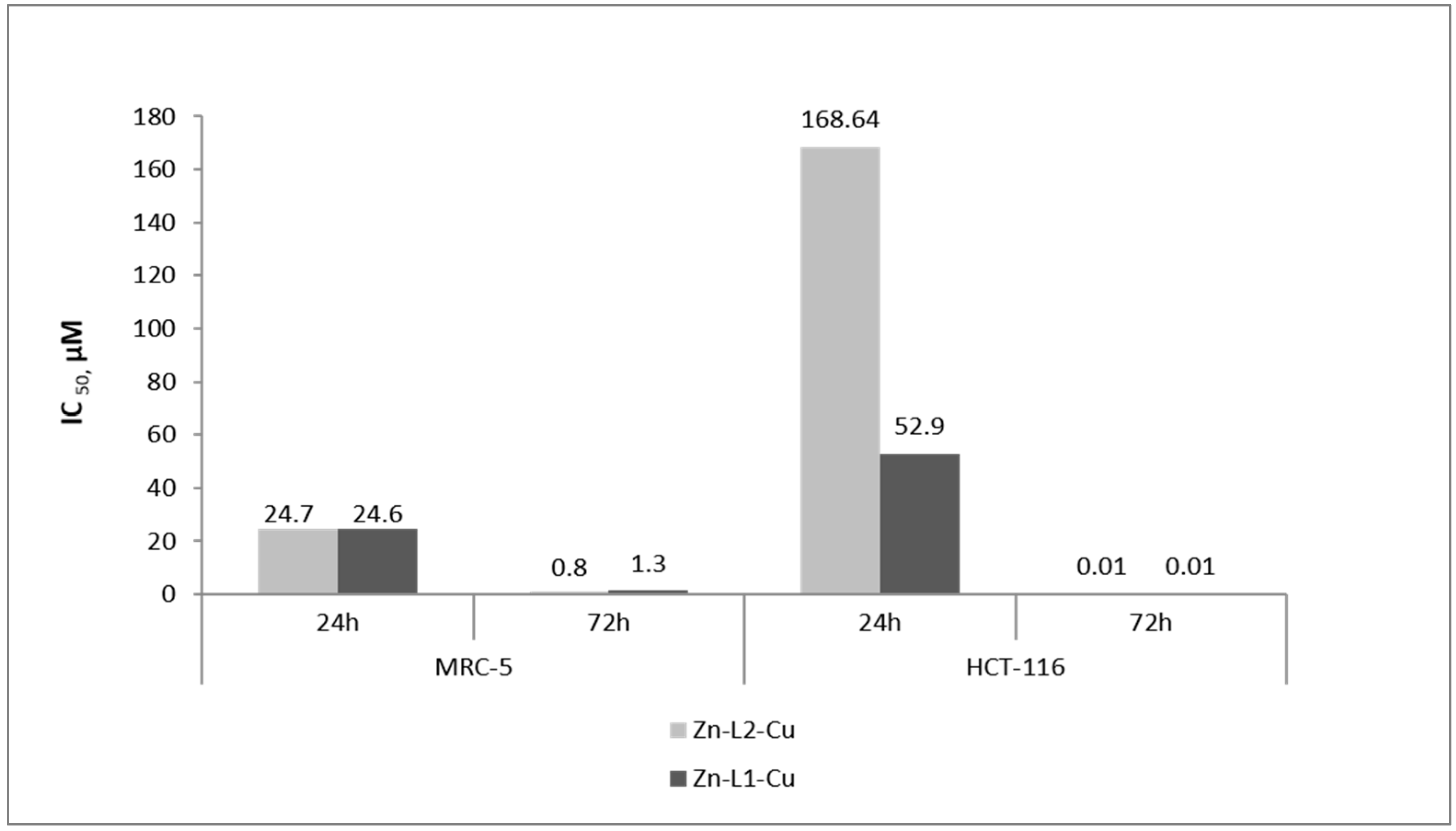
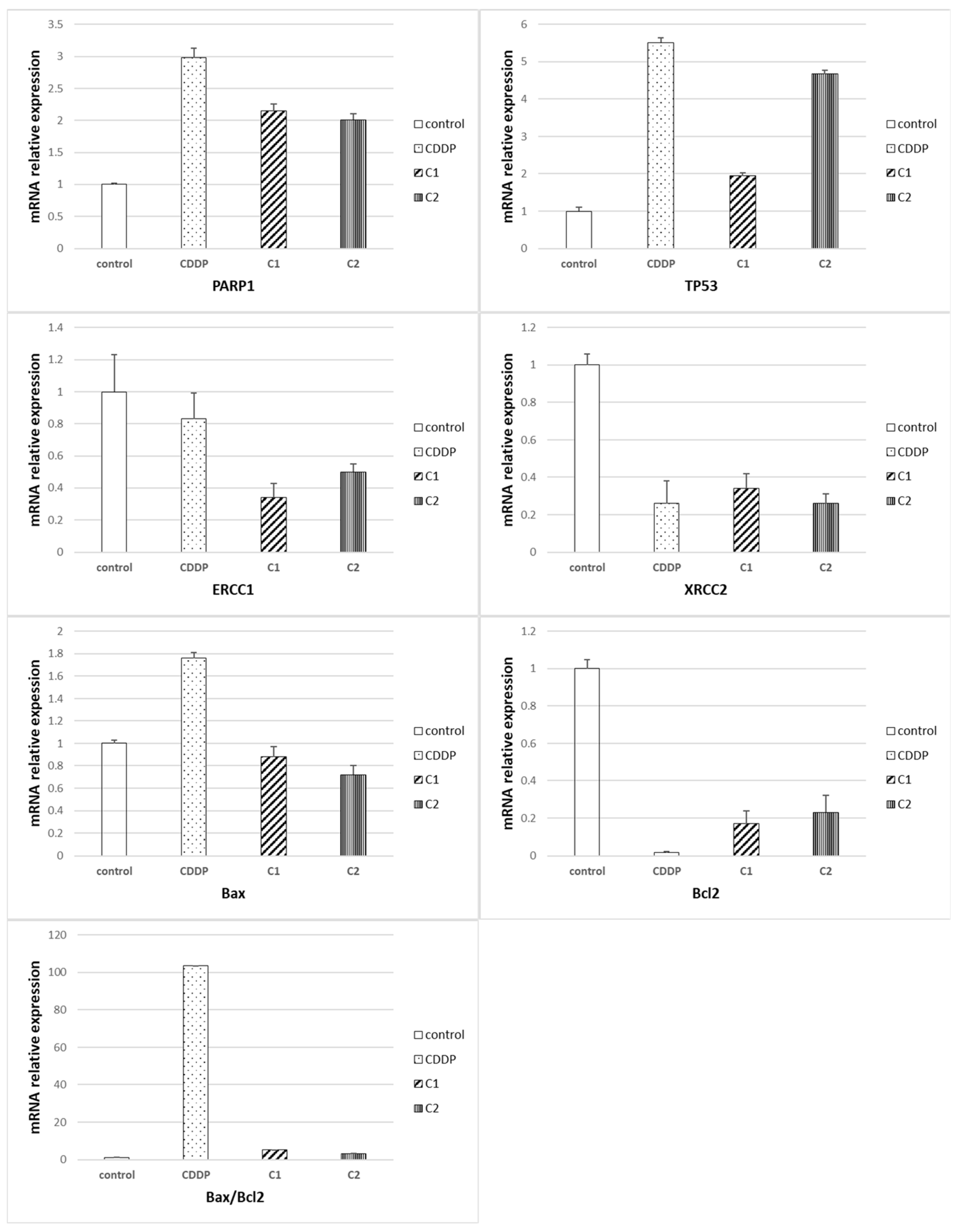
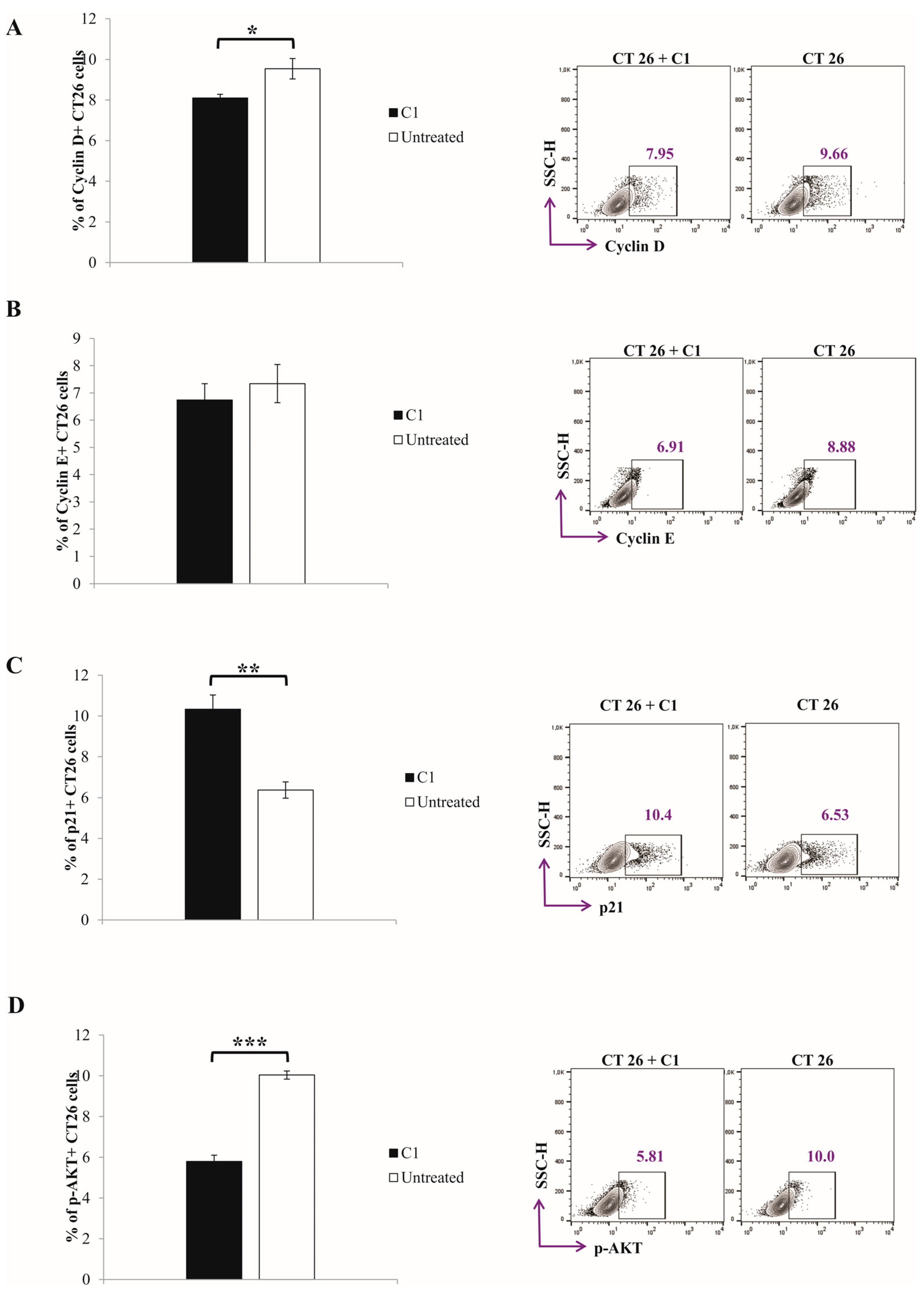
2.3. Comparative Cytotoxic Studies of Newly Synthesized Mononuclear zinc(II) and Hetero-Dinuclear Platinum(II)/Zinc(II) Complexes toward Colorectal Cancer Cells
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lippert, B. Cisplatin Chemistry and Biochemistry of Leading Anticancer Drugs; Wiley-VCH: Zürich, Switzerland, 1999. [Google Scholar]
- Wang, D.; Lippard, S.J. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, S.; Reedijk, J. Targeting Platinum Anti-Tumour Drugs: Overview of Strategies Employed to Reduce Systemic Toxicity. Coord. Chem. Rev. 2005, 249, 2845–2853. [Google Scholar] [CrossRef]
- Wenzel, M.; Bigaeva, E.; Richard, P.; Le Gendre, P.; Picquet, M.; Casini, A.; Bodio, E. New Heteronuclear Gold(I)–Platinum(II) Complexes with Cytotoxic Properties: Are Two Metals Better than One? J. Inorg. Biochem. 2014, 141, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, J.; Yang, P.; Huang, B.; Li, R.; Ye, R. Synthesis, Characterization and Antitumor Mechanism Investigation of Heterometallic Ru(II)-Re(I) Complexes. Front. Chem. 2022, 10, 890925. [Google Scholar] [CrossRef]
- Ma, D.-L.; Wu, C.; Cheng, S.-S.; Lee, F.-W.; Han, Q.-B.; Leung, C.-H. Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies. Int. J. Mol. Sci. 2019, 20, 341. [Google Scholar] [CrossRef]
- Ma, L.; Ma, R.; Wang, Z.; Yiu, S.-M.; Zhu, G. Heterodinuclear Pt(IV)–Ru(II) Anticancer Prodrugs to Combat Both Drug Resistance and Tumor Metastasis. ChemComm 2016, 52, 10735–10738. [Google Scholar] [CrossRef]
- Soldatović, T.V.; Selimović, E.; Milivojević, N.; Jovanović, M.; Šmit, B. Novel Heteronuclear Pt (II)-L-Zn (II) Complexes: Synthesis, Interactions with Biomolecules, Cytotoxic Properties. Two Metals Give Promising Antitumor Activity? Appl. Organomet. Chem. 2020, 34, e5864. [Google Scholar] [CrossRef]
- Soldatović, T.V.; Šmit, B.; Mrkalić, E.M.; Matić, S.L.j.; Jelić, R.M.; Serafinović, M.Ć.; Gligorijević, N.; Čavić, M.; Aranđelović, S.; Grgurić-Šipka, S. Exploring Heterometallic Bridged Pt(II)-Zn(II) Complexes as Potential Antitumor Agents. J. Inorg. Biochem. 2023, 240, 112100. [Google Scholar] [CrossRef]
- Halilagić, A.; Selimovića, E.; Katanić Stanković, J.S.; Srećković, N.; Virijević, K.; Živanović, M.N.; Šmit, B.; Soldatović, T.V. Novel Heterometallic Zn(II)-L-Cu(II) Somplexes: Studies of the Nucleophilic Substitution Reactions, Antimicrobial, Redox and Cytotoxic Activity. J. Coord. Chem. 2022, 75, 472–492. [Google Scholar] [CrossRef]
- Serezlić, M.K.; Hasić, R.; Ašanin, D.; Šmit, B.; Matić, S.L.; Serafinović, M.Ć.; Nikodijević, D.; Jovankić, J.; Grgurić-Šipka, S.; Soldatović, T.V. Heterometallic Bridged Pt(II)-Zn(II) Complexes: Influence of the Substituent in 4′-position in Inert Terpy Ligand on Antigenotoxicity, Potential Antitumor Activity and Mechanism of Interactions of the Complexes with Biomolecules, Appl. Organomet. Chem. 2024, 38, e7413. [Google Scholar] [CrossRef]
- Vučel, S.; Hasić, R.; Ašanin, D.P.; Šmit, B.; Caković, A.; Bogojeski, J.; Ćendić, S.M.; Simović, M.B.; Stojanović, B.; Pavlović, S.; et al. Modes of Interactions With DNA/HSA Biomolecules and Comparative Cytotoxic Studies of Newly Synthesized Mononuclear Zinc(II) and Heteronuclear Platinum(II)/Zinc(II) ComplexesToward Colorectal Cancer Cells, Int. J. Mol. Sci. 2024, 25, 3027. [Google Scholar] [CrossRef]
- Bertini, I.; Gray, H.B.; Stiefel, E.I.; Valentine, J.S. (Eds.) Biological Inorganic Chemistry. Structure and Reactivity; University Science Books: Sausalito, CA, USA, 2007. [Google Scholar]
- Alessio, E. Bioinorganic Medicinal Chemistry; Wiley-VCH Verlag GmbH & Co, KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Roat-Malone, R.M. Bioinorganic Chemistry: A Short Course, 3rd ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Kong, C.C.; Zhou, J.Z.; Yu, J.H.; Li, S.L. Crystal Structure of Di-chlorido-(2,2′:6′,2″-terpyridine-κ(3) N,N’,N’‘)Zinc: A Redeter-mination. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m382–m383. [Google Scholar] [CrossRef]
- Adrian, R.A.; Canales, D.; Arman, H.D. (4′-Chloro-2,2′:6′,2″-terpyridine-κ3N,N’,N″)bis(nitrato-κO)zinc(II). IUCrdata 2020, 5, 201344. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Muhammad, S.; Chen, X.; Akkurt, M.; Alshehri, A.M.; Din, S.U.; Ullah, H.; Al-Sehemi, A.G. Synthesis, Crystal Structure, and Nonlinear Optical Properties of Zn(II) Complex with 4,4′,4″-Tri-Tert-Butyl-2,2′:6′,2″-Terpyridine: A Dual Exploration. Russ. J. Inorg. Chem. 2020, 65, 368–377. [Google Scholar] [CrossRef]
- Lopachin, R.M.; Gavin, T.; Decaprio, A.; Barber, D.S. Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef]
- Quispe, A.; Zavala, C.; Rojas, J.; Posso, M.; Vaisberg, A. Efecto Citotóxico Selectivo in vitro de Muricin H(Acetogenina de Annona muricata) en Cultivos Celulares de Cáncer de Pulmón. Rev. Peru. Med. Exp. Salud Pública 2006, 23, 265–269. [Google Scholar]
- Garcia, S.V.; del Barrio, A.G.; Gaiten, Y.G.; Diaz, L.M. Evaluación Preliminar de la Actividad Antiviral del Extracto Acuoso de Phyllanthus orbicularis Frente al Virus HSV-1. Rev. Cubana Med. Trop. 2003, 55, 169–173. [Google Scholar]
- Hofmann, A.; Jaganyi, D.; Munro, O.Q.; Liehr, G.; van Eldik, R. Electronic Tuning of the Lability of Pt(II) Complexes through π-Acceptor Effects. Correlations between Thermodynamic, Kinetic, and Theoretical Parameters. Inorg. Chem. 2003, 42, 1688–1700. [Google Scholar] [CrossRef]
- Appleton, T.G.; Bailey, A.J.; Barnham, K.J.; Hall, J.R. Aspects of the Solution Chemistry of Trans-diammineplatinum(II) complexes. Inorg. Chem. 1992, 31, 3077–3082. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Jiang, J.; Liang, X.; Huang, L.; Huang, G.; Chen, H.; Pan, L.; Ma, Z. Zinc(II) Terpyridine Complexes: Substituent Effect on Photoluminescence, Antiproliferative Activity, and DNA Interaction. Molecules 2019, 24, 4519. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Zhao, Y.; Zheng, W.; Zeng, W.; Luo, Q.; Wang, Z.; Wu, K.; Du, J.; Wang, F. Platinum(II) Terpyridine Anticancer Complexes Possessing Multiple Mode of DNA Interaction and EGFR Inhibiting Activity. Front. Chem. 2020, 8, 210. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, Z.-F.; Wang, S.-L.; Luo, D.-M.; Zou, B.-Q.; Yao, P.-F.; Tan, M.-X.; Liang, H. In Vitro and in Vivo Antitumor Activities of Three Novel Binuclear Platinum(II) Complexes with 4′-Substituted-2,2′:6′,2″-Terpyridine Ligands. Eur. J. Med. Chem. 2019, 170, 195–202. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e4. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor P21cip1/Waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef]




| Complex | pKa1 | pKa2 |
|---|---|---|
| C1 | 3.99 ± 0.06 | 6.97 ± 0.01 |
| C2 | 3.91 ± 0.02 | 7.12 ± 0.05 |
| C3 | 3.47 ± 0.03 | 5.19 ± 0.02 |
| C4 | 2.82 ± 0.01 | 6.20 ± 0.05 |
| Agents | Half-Maximal Inhibitory Concentration IC50 (µM) | ||||
|---|---|---|---|---|---|
| HCT116 | LS-174 | MDA-MB-231 | A549 | MRC-5 | |
| C1 | 0.42 ± 0.15 | 45.74 ± 3.00 | 21.93 ± 4.2 | 42.88 ± 2.39 | 49.19 ± 4.18 |
| C2 | 3.08 ± 0.19 | >50 | 8.11 ± 1.97 | 10.27 ± 4.56 | 20.97 ± 5.51 |
| C3 | 0.51 ± 0.02 | 11.3 ± 2.17 | 9.52 ± 1.34 | 11.07 ± 3.67 | 9.29 ± 1.49 |
| C4 | 8.83 ± 0.64 | 40.14 ± 6.16 | 39.41 ± 7.82 | 33.96 ± 4.75 | >50 |
| CDDP | 28.7 ± 0.19 | 23.59 ± 4.36 | 8.97 ± 0.47 | 28.46 ± 0.37 | 14.60 ± 1.12 |
| Half-Maximal Inhibitory Concentration IC50 (µM) | ||||
|---|---|---|---|---|
| Agents | HCT-116 | SW-480 | ||
| 24 h | 72 h | 24 h | 72 h | |
| C1a | >200 | 43.20 ± 0.51 | >200 | >200 |
| C2a | >200 | 19.52 ± 0.78 | >200 | >200 |
| C3a | >200 | 73.31 ± 1.95 | >200 | >200 |
| C4a | >200 | >200 | >200 | >200 |
| cisplatin | 11.11 ± 0.13 | 5.33 ± 0.4 | 49.07 ± 0.41 | 8.13 ± 0.14 |
| Agents | IC50 ± SEM (μM) | |||
|---|---|---|---|---|
| CT26 | HCT 116 | SW480 | mMSCs | |
| C1 | 44.7 ± 58.73 | 88.23 ± 48.52 | 106.26 ± 126.08 | 56.72 ± 6.93 |
| C2 | 35.07 ± 39.65 | 26.57 ± 25.87 | 25.71 ± 2.93 | 10.1 ± 0.98 |
| C3 | 210.25 ± 86.98 | 181.35 ± 104.51 | 68.14 ± 5.21 | 76.24 ± 11.95 |
| C4 | 183.16 ± 72.37 | 89.43 ± 6.7 | 94.06 ± 9.89 | 60.34 ± 24.66 |
| C5 | 107.58 ± 79.89 | 151.95 ± 26.82 | 75.44 ± 21.18 | 40.17 ± 3.96 |
| CDDP | 9.03 ± 3.55 | 14.7 ± 2.21 | 10.1 ± 3.98 | 7.32 ± 2.51 |
| Agents | Selectivity Index (IC50 mMSC/IC50) | ||
|---|---|---|---|
| CT26 | HCT 116 | SW480 | |
| C1 | 1.3 | 0.6 | 0.5 |
| C2 | 0.3 | 0.4 | 0.4 |
| C3 | 0.4 | 0.4 | 1.1 |
| C4 | 0.3 | 0.7 | 0.6 |
| C5 | 0.4 | 0.3 | 0.5 |
| CDDP | 0.8 | 0.5 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatović, T.V. Design of Hetero-Dinuclear Metallic Complexes as Potential Metal-Based Drugs With a Zinc Metal Center in a Square-Pyramidal Structure. Drugs Drug Candidates 2025, 4, 12. https://doi.org/10.3390/ddc4010012
Soldatović TV. Design of Hetero-Dinuclear Metallic Complexes as Potential Metal-Based Drugs With a Zinc Metal Center in a Square-Pyramidal Structure. Drugs and Drug Candidates. 2025; 4(1):12. https://doi.org/10.3390/ddc4010012
Chicago/Turabian StyleSoldatović, Tanja V. 2025. "Design of Hetero-Dinuclear Metallic Complexes as Potential Metal-Based Drugs With a Zinc Metal Center in a Square-Pyramidal Structure" Drugs and Drug Candidates 4, no. 1: 12. https://doi.org/10.3390/ddc4010012
APA StyleSoldatović, T. V. (2025). Design of Hetero-Dinuclear Metallic Complexes as Potential Metal-Based Drugs With a Zinc Metal Center in a Square-Pyramidal Structure. Drugs and Drug Candidates, 4(1), 12. https://doi.org/10.3390/ddc4010012