1. Introduction
Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes [
1]. According to the World Malaria Report, there were 241 million cases of malaria in 2020 with an estimated mortality of 627,000 compared to 227 million cases in 2019 leading to an increase of over 69,000 deaths which were due to disruptions during the COVID-19 pandemic and a recent change in WHO’s methodology for calculating malaria mortality [
1,
2]. Four African countries accounted for just over half of all malaria deaths worldwide: Nigeria (31.9%), the Democratic Republic of the Congo (13.2%), the United Republic of Tanzania (4.1%) and Mozambique (3.8%) [
1]. Children under 5 years of age accounted for about 80% of all malaria deaths in the African regions.
An estimated 50 million travelers visit malaria endemic areas annually and about 30,000 malaria cases in non-endemic industrialized countries are reported yearly. Imported malaria remains a public health problem associated with high fatality rates in European countries, with the United Kingdom, France, Italy and Germany accounting for about 70% [
3,
4,
5]. Malaria can be prevented via vaccine, personal protection and chemoprophylaxis. However, the malaria vaccine is not on the near horizon, despite the reports of new data [
6,
7]. Personal protection, albeit an important tool, is often not sufficient, thus chemoprophylaxis remains the principal means to prevent malaria [
8]. Prevention of malaria in travelers to endemic areas has been confined and is fully dependent on chemoprophylaxis [
5]. Although malaria chemoprophylaxis refers to all malaria species, it is important to note that there is a distinction between
falciparum malaria prophylaxis and the prophylaxis of the relapsing malaria species (
vivax and
ovale); thus,
falciparum prophylaxis use has been complicated due to emergence of drug resistant strains, and virtually high costs and adverse reactions to medications, and there are virtually no drugs available for
vivax prophylaxis, except primaquine [
5].
Traditionally, antimalarial drugs have been developed as agents for dual indications (treatment and prophylaxis). There are at least three prophylaxis strategies of administration that have been utilized; the most common strategy is the administration of casual or suppressive drugs at efficacious prophylaxis doses throughout the period of exposure to malaria which must be continuous [
9], a post-exposure PART regime which is required to prevent subsequent relapse of
P. vivax [
10] and an alternative approach called ‘fire and forget’ prophylaxis, or ‘pre-exposure prophylaxis’, in which travelers are given a single dose or short course regime of a long half-life drug at a treatment dose that will protect them throughout the duration of exposure [
11]. However, this approach is currently unproven in clinical practice and no drug for malaria prevention is adequate and effective in all respects [
12]. Thus, there is a need to search and develop new prophylactic antimalarial drugs.
Ochna kibbiensis (OK) belonging to the Ochnaceae family is a shrub or small tree found in tropical Africa from Guinea to southern and northern Nigeria with brilliant red calyx in fruit; the plant has been used in ethnomedicine to treat and/or manage wound infections, pain, inflammation and malaria [
3]; it is also used as a laxative, antiseptic, stimulant and febrifuge, among others [
13]. We have reported the antimicrobial [
14] and anti-proliferative [
15] activities of the plant. Previous phytochemical investigations on the leaf of the plant revealed the isolation of ochnaflavone as one of the major bioactive constituents of the ethyl-acetate fraction [
15]. In this paper, we report the prophylactic antimalarial properties of
Ochna kibbiensis leaves, isolation and characterization of two known steroids, stigmasterol and β-sitosterol and their effect against
Plasmodium falciparum Lactate Dehydrogenase (
pfLDH) in silico.
3. Discussion
Considering the widespread usage of medicinal plants as remedies by a large proportion (>80%) of the world population as complementary and alternative medicines with little idea about their toxicity [
18], it is very vital to conduct toxicity tests in order to evaluate the possible harmful side effects of these products. The increased use of these natural medicines is attributed to the fact that they are considered safe with little or no side effects compared to the orthodox medicines [
19]. Thus, OK was subjected to an acute toxicity test to evaluate its median lethal dose (LD
50), and the findings demonstrated that the plant is relatively safe and acceptable. These values recorded for the methanol extract and its fractions agreed with the values reported for a related Ochna species (
O. schweinfurthiana) at 5000 mg/kg orally [
20].
The methanol leaf extract of OK and its fractions exhibited significant (
p < 0.05) activity, which was comparable to pyrimethamine in the prophylaxis assay. DFL exhibited a very strong prophylactic antimalarial effect against
P. berghei. In a study by Mil-Homens et al. [
21], dichloromethane fraction was shown to exhibit the highest antimalarial activity compared to the other extracts and fractions studied. A decline in the prophylactic activity of MLE and HFL of OK at the medium dose (250 mg/kg) might be related to saturation at the active sites or the action of other endogenous substances in the body or activity on other body organs of the experimental animals [
20]. Even more so, increasing the dose of BFL will lead to a decrease in prophylactic effect as clearly indicated in this study.
The extracts of OK may act by inhibiting the multiplication of the parasites as well as direct cytotoxic effect on the parasites [
22] or by modulating the membrane properties of the erythrocytes there by preventing parasite invasion [
23]. Pyrimethamine acts by inhibiting the dihydrofolate reductase of plasmodia, thereby blocking the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication, which leads to a failure of nuclear division at the time of schizont formation in erythrocytes and liver [
24,
25,
26,
27]. Generally, prophylactic antimalarials work by either disrupting the initial development of malaria parasites in the liver (casual activity), suppressing the emergent asexual blood stages of parasite (suppressive activity) or preventing the relapses induced by the latent liver forms (hypnozoites) of relapsing
P. vivax and
P. ovale malaria (presumptive antirelapse therapy) [
10,
27,
28]. Thus,
O. kibbiensis might act via the same mechanism. In addition, a higher prophylactic effect exhibited by DFL of OK might also be linked to the presence of secondary metabolites such as steroids, triterpenes and flavonoids that were present in the fraction [
13].
Following the promising effects obtained in the prophylactic antimalarial effect using the repository test, the DFL of OK being the most active fraction was subjected to chromatographic studies which resulted in the isolation of a mixture of known steroids (
K4). The isolated compound(s) appeared as a white crystalline substance with an uncorrected melting point ranging from 136–137 °C and gave a positive reaction when tested with Liebermann–Burchard’s reagent, an indication of the presence of a steroid [
16,
29]. The
1H-NMR spectrum of K4 (600 Hz, in CDCl
3) revealed the presence of three olefinic resonances at
δH 5.28, 5.10 and 5.05, an oxy-methine proton at
δH 3.45 and six methyl signals at
δH 0.74, 1.00, 0.94, 0.85, 0.79 and 0.80, characteristic of a steroidal nucleus [
16]. The
13C-NMR and APT experiments of
K4 showed 29 carbon signals constituting four olefinic carbons at
δC 140.76, 121.72, 138.31 and 129.28 corresponding to C-5, C-6, C-22 and C-23, respectively [
30] and two angular methyl groups at
δC 12.05 and 21.20 corresponding to C-18 and C-19, respectively [
16,
31]. The signal at
δC 71.83 clearly indicated the presence of a β-hydroxyl group at C-3 typical of stigmasterol [
16,
30]. Additional peaks observed at
δC 33.95 and 26.08 which were assigned to C-22 and C-23 indicated that
K4 is a mixture of stigmasterol and β-sitosterol. Based on the spectral data of compound
K4, physicochemical tests and a direct comparison with existing data in the literature (
Table 3 and
Table 4) [
16,
30,
31,
32], compound
K4 was elucidated and confirmed as a mixture of two compounds stigmasterol and β-sitosterol (
Figure 3). Although the isolated compound(s) could not be tested for prophylactic antimalarial activity due to insufficient quantity, the compounds have been reported to possess antimalarial activity [
33,
34,
35]. Further bio-assay-guided isolation of the bioactive constituents responsible for the observed effect is presently ongoing in our lab.
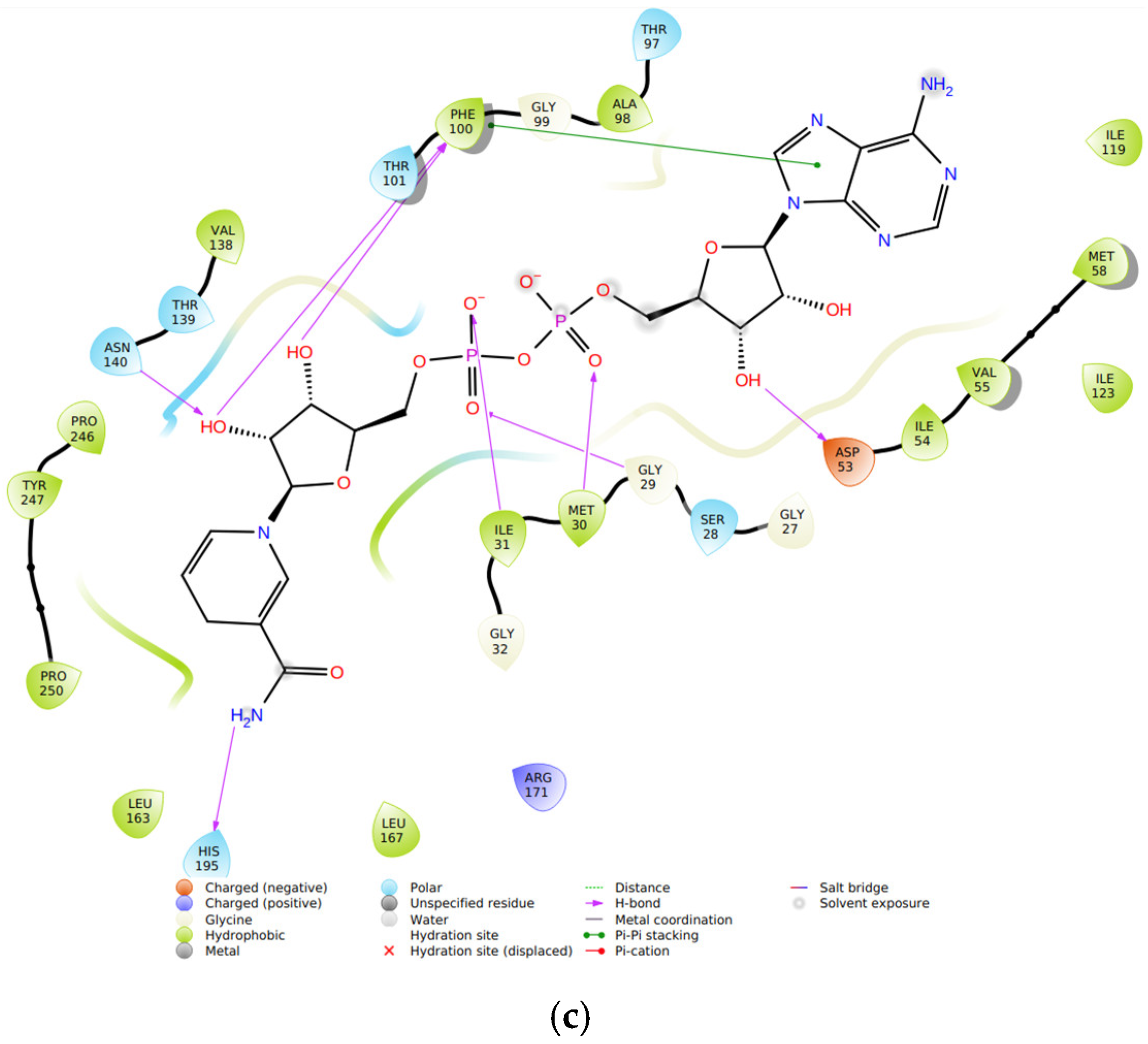
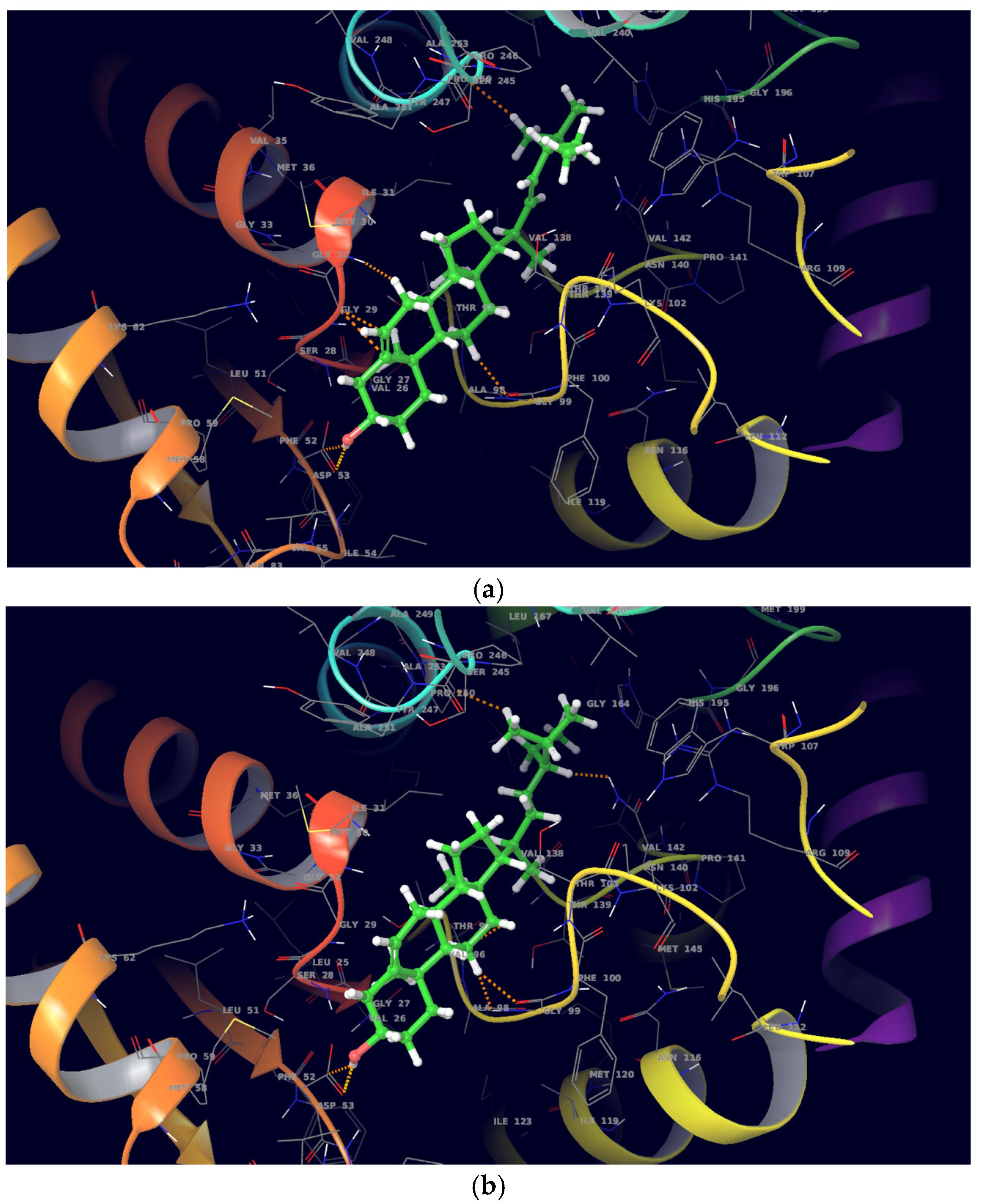
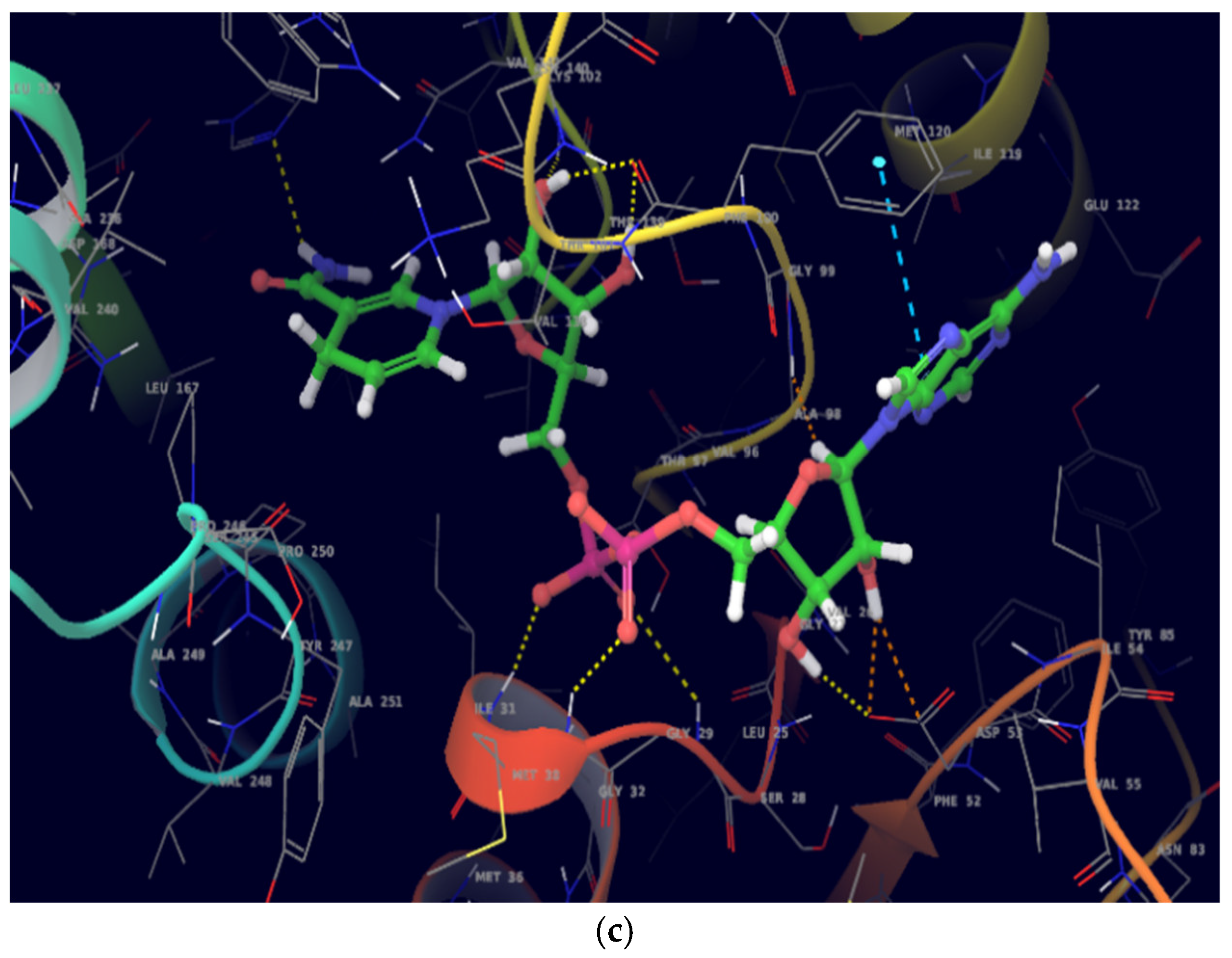
On molecular docking analysis, stigmasterol and β-sitosterol showed lower docking scores against
pfLDH compared to the co-crystallized ligand, NADH. Studies have shown that
pfLDH is composed of NADH-binding sites with amino acid residues such as Gly-27, Ser-28, Gly-29, Phe-52, Asp-53, Ile-54, Thr-97, Ala-98, Gly-99, Phe-100, Thr-139 and Asn-140, and the substrate-binding site constituting Lys-198, Met-199, Val-200, Leu-201, Glu-226, Phe-229, Asp-230, Val-233, Lys-314 and Glu-317 [
36]. In this study, stigmasterol and β-sitosterol interacted with clinically important amino acid residues similar to that of NADH; a similar study was reported by Read et al. [
37].
ADMET analysis indicated stigmasterol and β-sitosterol to be poorly soluble with low GI absorption and none of the compounds are either BBB permeant or Pgp substrate. Both compounds had one Lipinski violation for drug-likeness. None of the compounds violate the general rule of five or Lipinski’s rule for drug-likeness and both compounds are potential inhibitors of cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2D6 and CYP3A4); however, stigmasterol is a potential inhibitor of CYP2C9 and thus could lead to alteration of metabolism, which may lead to drug–drug interaction [
38]. Moderate toxicity was recorded for both compounds based on the predicted LD
50 value 890 mg/kg and toxicity class of 4. However, both compounds have a tendency to be immunotoxic with a percentage probability of 99%.