The Binding and Effects of Boron-Containing Compounds on G Protein-Coupled Receptors: A Scoping Review
Abstract
1. Introduction
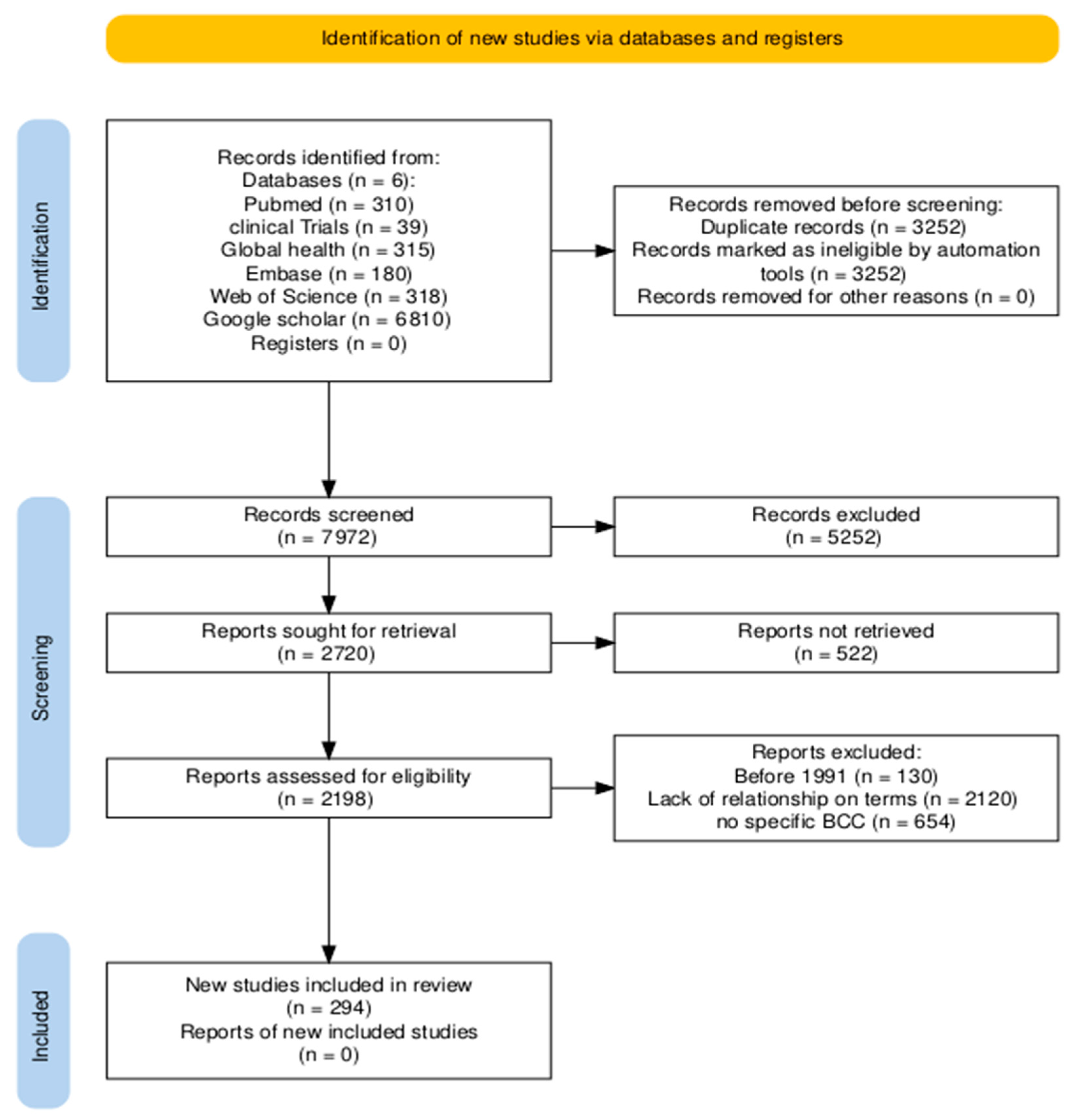
2. Materials and Methods
3. Results
3.1. Experimental Evidence of Effects of BCCs on Family A GPCRs
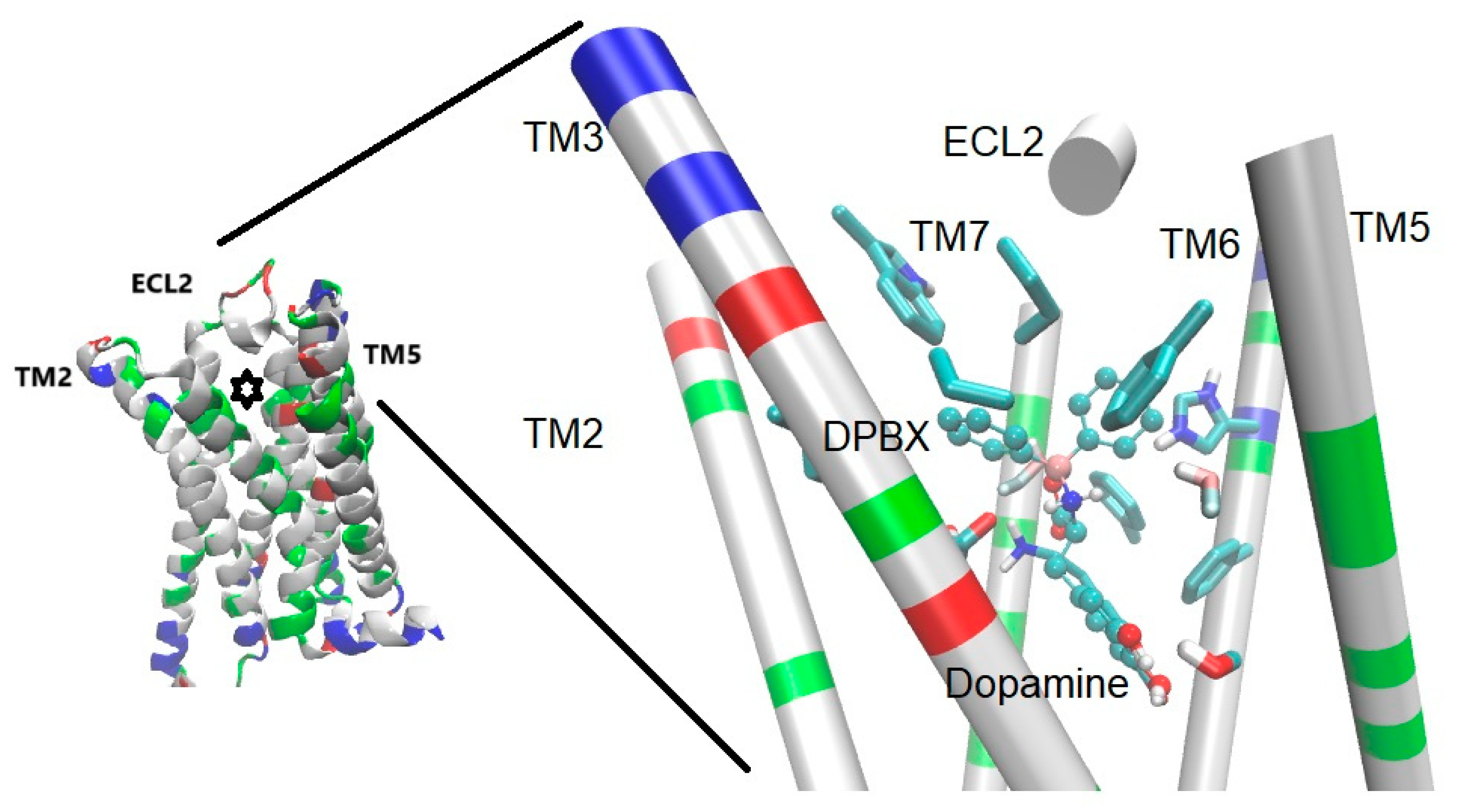
3.1.1. Catecholamine (Dopamine and Adrenergic) Receptors
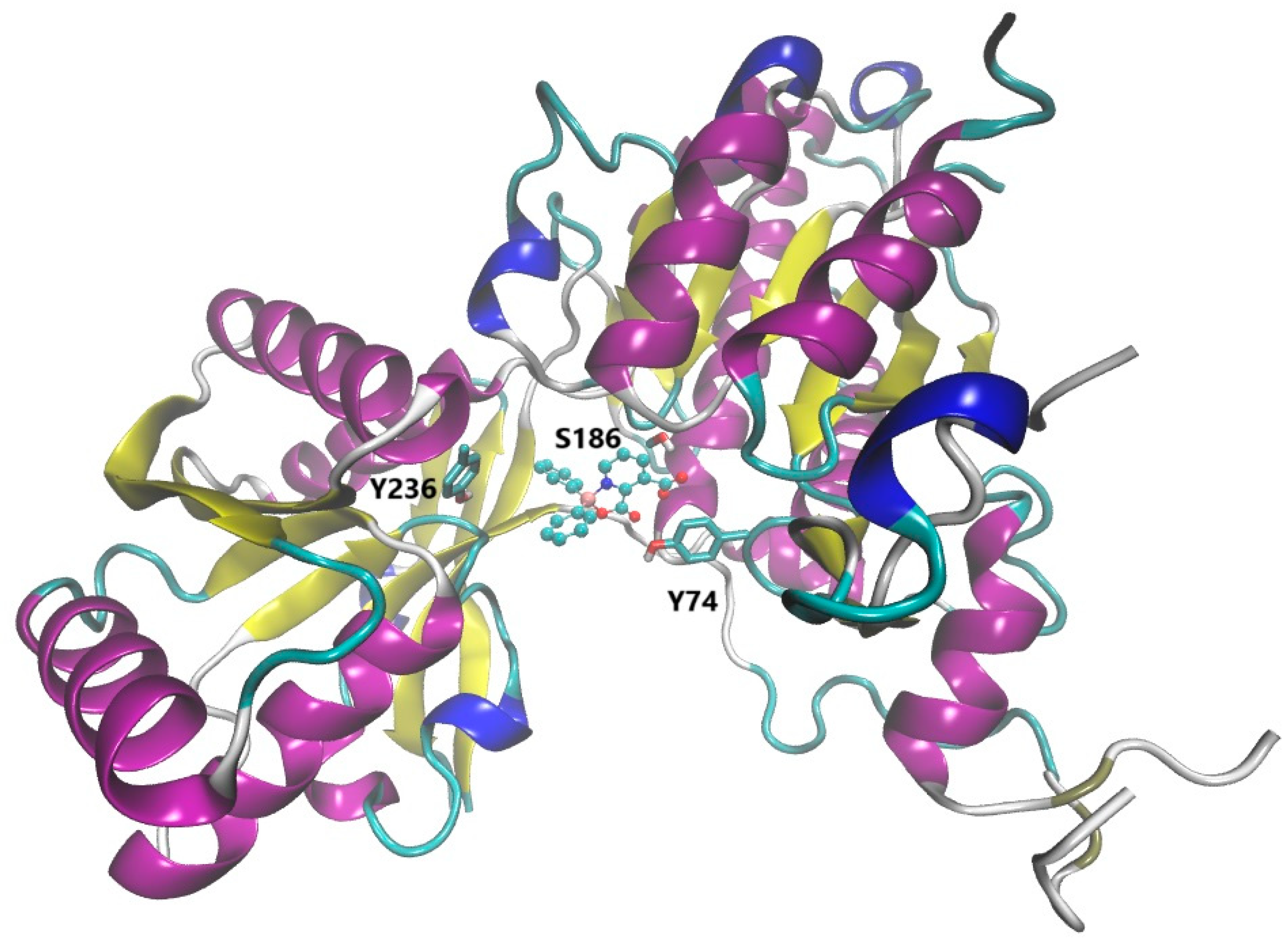
3.1.2. Other Class A GPCRs
3.2. Effects of BCCs on Family B and Family C GPCRs
3.2.1. Interaction and Effects of BCCs on Family B GPCRs
3.2.2. Effects of BCCs on Family C GPCRs
3.3. Theoretical Assays Supporting Interactions of BCCs with GPCRs
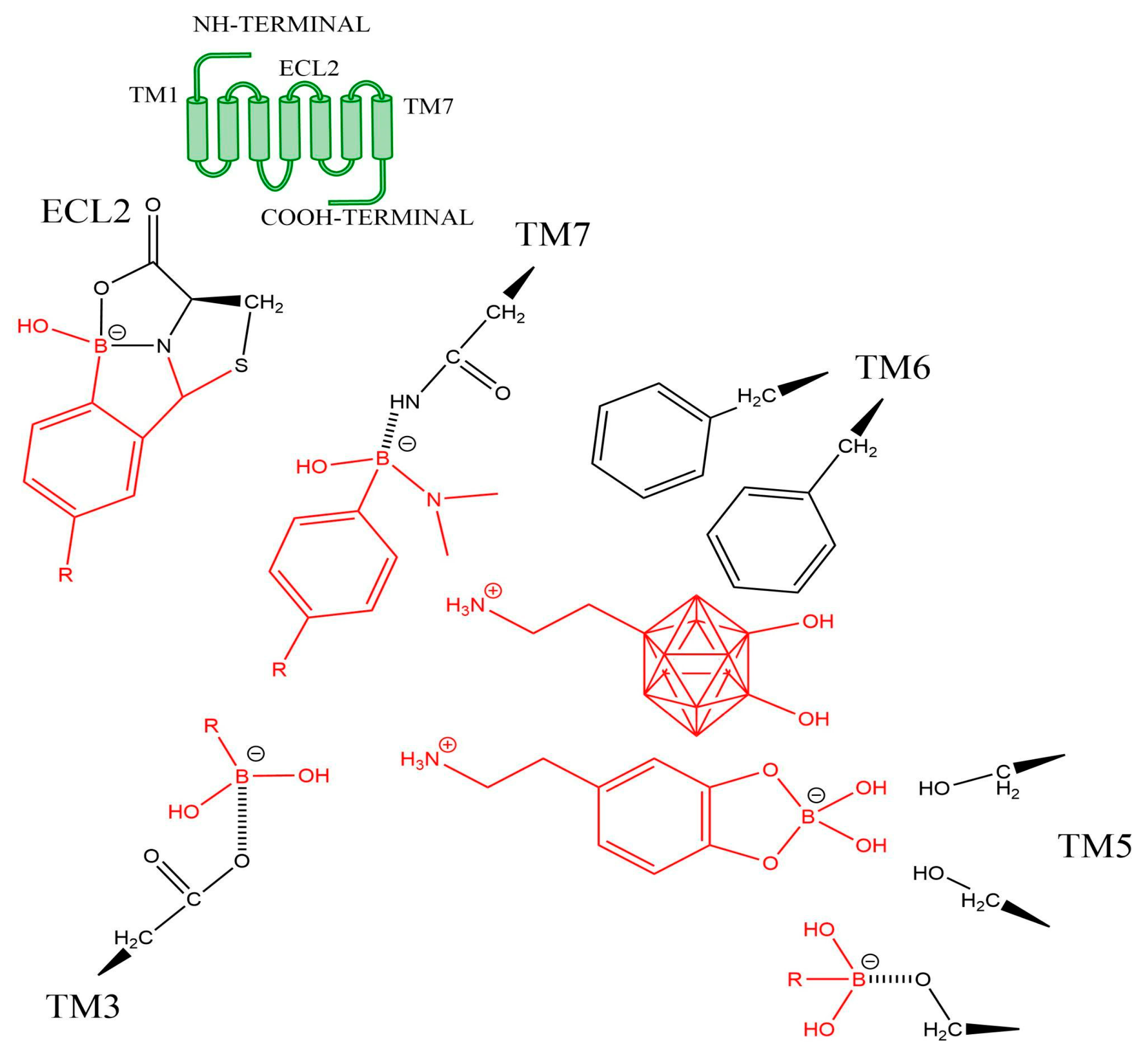
3.4. Role of Moieties Including Boron Atoms in Interactions with GPCRs
3.5. Clinical Trials and Effects of GPCRs in Humans
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron chemicals in drug discovery and development: Synthesis and medicinal perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Cordova-Chávez, R.I.; Farfan-García, E.D.; Kabalka, G. Boron-containing compounds as labels, drugs, and theranostic agents for diabetes and its complications. World J. Diabetes 2024, 15, 1060. [Google Scholar] [CrossRef]
- Barrón-González, M.; Montes-Aparicio, A.V.; Cuevas-Galindo, M.E.; Orozco-Suárez, S.; Barrientos, R.; Alatorre, A.; Querejeta, E.; Trujillo-Ferrara, J.G.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Boron-containing compounds on neurons: Actions and potential applications for treating neurodegenerative diseases. J. Inorg. Biochem. 2023, 238, 112027. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tripathi, N.M.; Bandyopadhyay, A. The modern role of boron as a ‘magic element’ in biomedical science: Chemistry perspective. Chem. Commun. 2021, 57, 13629–13640. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, A.; Ocampo-Néstor, A.L.; Das, B.C.; Farfán-García, E.D.; Bello, M.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Interactions of a boron-containing levodopa derivative on D 2 dopamine receptor and its effects in a Parkinson disease model. J. Biol. Inorg. Chem. 2022, 27, 121–131. [Google Scholar] [CrossRef]
- Kooistra, A.J.; Mordalski, S.; Pándy-Szekeres, G.; Esguerra, M.; Mamyrbekov, A.; Munk, C.; Keserű, G.M.; Gloriam, D.E. GPCRdb in 2021: Integrating GPCR sequence, structure and function. Nucleic Acids Res. 2021, 49, D335–D343. [Google Scholar] [CrossRef]
- Daly, C.J.; Milligan, C.M.; Milligan, G.; Mackenzie, J.F.; McGrath, J.C. Cellular localization and pharmacological characterization of functioning alpha-1 adrenoceptors by fluorescent ligand binding and image analysis reveals identical binding properties of clustered and diffuse populations of receptors. J. Pharmacol. Exp. Ther. 1998, 286, 984–990. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Mackenzie, J.F.; Daly, C.J. Pharmacological implications of cellular localization of alpha1-adrenoceptors in native smooth muscle cells. J. Auton. Pharmacol. 1999, 19, 303–310. [Google Scholar] [CrossRef]
- McGrath, J.C.; Daly, C.J. Use of fluorescent ligands and receptors to visualize adrenergic receptors. In The Adrenergic Receptors: In the 21st Century; Humana Press: Totowa, NJ, USA, 2006; pp. 151–172. [Google Scholar]
- Baker, J.G.; Hall, I.P.; Hill, S.J. Pharmacology and direct visualisation of BODIPY-TMR-CGP: A long-acting fluorescent β2-adrenoceptor agonist. Br. J. Pharmacol. 2003, 139, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ursúa, M.A.; Valencia-Hernández, I.; Arellano-Mendoza, M.G.; Correa-Basurto, J.; Trujillo-Ferrara, J.G. Synthesis, pharmacological and in silico evaluation of 1-(4-di-hydroxy-3,5-dioxa-4-borabicyclo[4.4.0]deca-7,9,11-trien-9-yl)-2-(tert-butylamino)ethanol, a compound designed to act as a β2 adrenoceptor agonist. Eur. J. Med. Chem. 2009, 44, 2840–2846. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Bello, M.; Hernández-Martínez, C.F.; Santillán-Torres, I.; Guerrero-Ramírez, R.; Correa-Basurto, J.; Arias-Montaño, J.-A.; Trujillo-Ferrara, J.G. Cell-based assays and molecular dynamics analysis of a boron-containing agonist with different profiles of binding to human and guinea pig beta2 adrenoceptors. Eur. Biophys. J. 2019, 48, 83–97. [Google Scholar] [CrossRef]
- Louie, A.S.; Vasdev, N.; Valliant, J.F. Preparation, characterization, and screening of a high affinity organometallic probe for α-adrenergic receptors. J. Med. Chem. 2011, 54, 3360–3367. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef]
- Wang, C.; Jin, E.; Deng, J.; Pei, Y.; Ren, M.; Hu, Q.; Gu, Y.; Li, S. GPR30 mediated effects of boron on rat spleen lymphocyte proliferation, apoptosis, and immune function. Food Chem. Toxicol. 2020, 146, 111838. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Koebberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. J. Pept. Sci. 2018, 24, e3119. [Google Scholar] [CrossRef]
- Worm, D.J.; Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. Selective neuropeptide Y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. J. Med. Chem. 2019, 63, 2358–2371. [Google Scholar] [CrossRef] [PubMed]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.-G.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A selective carborane-functionalized gastrin-releasing peptide receptor agonist as boron delivery agent for boron neutron capture therapy. J. Org. Chem. 2019, 85, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Mendive-Tapia, L.; Miret-Casals, L.; Barth, N.D.; Wang, J.; de Bray, A.; Beltramo, M.; Robert, V.; Ampe, C.; Hodson, D.J.; Madder, A.; et al. Acid-Resistant BODIPY Amino Acids for Peptide-Based Fluorescence Imaging of GPR54 Receptors in Pancreatic Islets. Angew. Chem. 2023, 135, e202302688. [Google Scholar] [CrossRef]
- Sharma, N.; Barbon, S.M.; Lalonde, T.; Maar, R.R.; Milne, M.; Gilroy, J.B.; Luyt, L.G. The development of peptide–boron difluoride formazanate conjugates as fluorescence imaging agents. RSC Adv. 2020, 10, 18970–18977. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.D.; Neale, C.; Gomes, G.-N.W.; Li, Y.; Malik, A.; Pandey, A.; Orazietti, A.P.; Wang, X.; Ye, L.; Prosser, R.S.; et al. Ligand modulation of the conformational dynamics of the A2A adenosine receptor revealed by single-molecule fluorescence. Sci. Rep. 2021, 11, 5910. [Google Scholar] [CrossRef]
- Bednarska-Szczepaniak, K.; Mieczkowski, A.; Kierozalska, A.; Saftić, D.P.; Głąbała, K.; Przygodzki, T.; Stańczyk, L.; Karolczak, K.; Watała, C.; Rao, H.; et al. Synthesis and evaluation of adenosine derivatives as A1, A2A, A2B and A3 adenosine receptor ligands containing boron clusters as phenyl isosteres and selective A3 agonists. Eur. J. Med. Chem. 2021, 223, 113607. [Google Scholar] [CrossRef]
- Barrón-González, M.; Rivera-Antonio, A.M.; Jarillo-Luna, R.A.; Santiago-Quintana, J.M.; Levaro-Loquio, D.; Pérez-Capistran, T.; Guerra-Araiza, C.H.; Soriano-Ursúa, M.A.; Farfán-García, E.D. Borolatonin limits cognitive deficit and neuron loss while increasing proBDNF in ovariectomised rats. Fundam. Clin. Pharmacol. 2024, 38, 730–741. [Google Scholar] [CrossRef]
- Hollenstein, K.; de Graaf, C.; Bortolato, A.; Wang, M.-W.; Marshall, F.H.; Stevens, R.C. Insights into the structure of class B GPCRs. Trends Pharmacol. Sci. 2014, 35, 12–22. [Google Scholar] [CrossRef]
- Karageorgos, V.; Venihaki, M.; Sakellaris, S.; ePardalos, M.; Kontakis, G.; Matsoukas, M.-T.; Gravanis, A.; Margioris, A.; Liapakis, G. Current understanding of the structure and function of family B GPCRs to design novel drugs. Hormones 2018, 17, 45–59. [Google Scholar] [CrossRef]
- Ishihara, T.; Nakamura, S.; Kaziro, Y.; Takahashi, T.; Takahashi, K.; Nagata, S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991, 10, 1635–1641. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Madrigal, M.; Roberts, R.W.; Benzer, S. The Drosophila G protein-coupled receptor, Methuselah, exhibits a promiscuous response to peptides. Protein Sci. 2009, 18, 2203–2208. [Google Scholar] [CrossRef]
- Ahn, J.M.; Han, S.Y.; Murage, E.; Beinborn, M. Rational Design of Peptidomimetics for Class B GPCRs: Potent Non-Peptide GLP-1 Receptor Agonists. In Peptides for Youth. Advances in Experimental Medicine and Biology; Valle, S.D., Escher, E., Lubell, W.D., Eds.; Springer: New York, NY, USA, 2009; Volume 611, pp. 125–126. [Google Scholar] [CrossRef]
- Tan, J.; Grouleff, J.J.; Jitkova, Y.; Diaz, D.B.; Griffith, E.C.; Shao, W.; Bogdanchikova, A.F.; Poda, G.; Schimmer, A.D.; Lee, R.E.; et al. De Novo Design of Boron-Based Peptidomimetics as Potent Inhibitors of Human ClpP in the Presence of Human ClpX. J. Med. Chem. 2019, 62, 6377–6390. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Meng, F.; Jensen, K.; McDaniel, K.; Rahal, K.; Onori, P.; Gaudio, E.; Alpini, G.; Glaser, S.S. The physiological roles of secretin and its receptor. Ann. Transl. Med. 2013, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Parkinson, C.; Stevens, E.C.; Trainer, P.J. Growth hormone receptor antagonists: Discovery, development, and use in patients with acromegaly. Endocr. Rev. 2002, 23, 623–646. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ursúa, M.A.; Arias-Montaño, J.A.; Correa-Basurto, J.; Hernández-Martínez, C.F.; López-Cabrera, Y.; Castillo-Hernández, M.C.; Padilla-Martínez, I.I.; Trujillo-Ferrara, J.G. Insights on the role of boron containing moieties in the design of new potent and efficient agonists targeting the β2 adrenoceptor. Bioorg. Med. Chem. Lett. 2015, 25, 820–825. [Google Scholar] [CrossRef]
- Tian, Y.; Fang, M.; Lin, Q. Intracellular bioorthogonal labeling of glucagon receptor via tetrazine ligation. Bioorg. Med. Chem. Lett. 2021, 43, 116256. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.A.; Berry-Kravis, E.; Czech, C.; Hagerman, R.J.; Hessl, D.; Wong, C.Y.; Rabbia, M.; Deptula, D.; John, A.; Kinch, R.; et al. Effect of the mGluR5-NAM Basimglurant on Behavior in Adolescents and Adults with Fragile X Syndrome in a Randomized, Double-Blind, Placebo-Controlled Trial: FragXis Phase 2 Results. Neuropsychopharmacology 2018, 43, 503–512. [Google Scholar] [CrossRef]
- Urwyler, S. Allosteric modulation of family C G-protein-coupled receptors: From molecular insights to therapeutic perspectives. Pharmacol. Rev. 2011, 63, 59–126. [Google Scholar] [CrossRef]
- Nicoletti, F.; Di Menna, L.; Iacovelli, L.; Orlando, R.; Zuena, A.R.; Conn, P.J.; Dogra, S.; Joffe, M.E. GPCR interactions involving metabotropic glutamate receptors and their relevance to the pathophysiology and treatment of CNS disorders. Neuropharmacology 2023, 235, 109569. [Google Scholar] [CrossRef]
- Cuevas-Galindo, M.E.; Rubio-Velázquez, B.A.; Jarillo-Luna, R.A.; Padilla-Martínez, I.I.; Soriano-Ursúa, M.A.; Trujillo-Ferrara, J.G. Synthesis, In Silico, In Vivo, and Ex Vivo Evaluation of a Boron-Containing Quinolinate Derivative with Presumptive Action on mGluRs. Inorganics 2023, 11, 94. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Chen, X.; Huang, L.; Li, J.; You, Z.; Huang, Q.; Ren, S.; He, K.; Schibli, R.; et al. Metabotropic glutamate receptor 5 (mGluR5) is associated with neurodegeneration and amyloid deposition in Alzheimer’s disease: A [18F]PSS232 PET/MRI study. Alzheimer’s Res. Ther. 2024, 16, 9. [Google Scholar] [CrossRef]
- Shpakov, A.O. Allosteric Regulation of G-Protein-Coupled Receptors: From Diversity of Molecular Mechanisms to Multiple Allosteric Sites and Their Ligands. Int. J. Mol. Sci. 2023, 24, 6187. [Google Scholar] [CrossRef]
- Budgett, R.F.; Bakker, G.; Sergeev, E.; Bennett, K.A.; Bradley, S.J. Targeting the Type 5 Metabotropic Glutamate Receptor: A Potential Therapeutic Strategy for Neurodegenerative Diseases? Front. Pharmacol. 2022, 13, 893422. [Google Scholar] [CrossRef]
- Kim, J.H.; Marton, J.; Ametamey, S.M.; Cumming, P. A review of molecular imaging of glutamate receptors. Molecules 2020, 25, 4749. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, B.; Xu, H.; He, M.; Deng, X.; Zhang, L.; Dang, Q.; Fan, J.; Guan, Y.; Peng, X.; et al. The chronological evolution of fluorescent GPCR probes for bioimaging. Coord. Chem. Rev. 2023, 480, 215040. [Google Scholar] [CrossRef]
- Soave, M.; Briddon, S.J.; Hill, S.J.; Stoddart, L.A. Fluorescent ligands: Bringing light to emerging GPCR paradigms. Br. J. Pharmacol. 2020, 177, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Dueñas, V.; Qian, M.; Argerich, J.; Amaral, C.; Risseeuw, M.D.; Van Calenbergh, S.; Ciruela, F. Design, synthesis and characterization of a new series of fluorescent metabotropic glutamate receptor type 5 negative allosteric modulators. Molecules 2020, 25, 1532. [Google Scholar] [CrossRef]
- Kampen, S.; Rodríguez, D.; Jørgensen, M.; Kruszyk-Kujawa, M.; Huang, X.; Collins, M., Jr.; Boyle, N.; Maurel, D.; Rudling, A.; Lebon, G.; et al. Structure-based discovery of negative allosteric modulators of the metabotropic glutamate receptor 5. ACS Chem. Biol. 2022, 17, 2744–2752. [Google Scholar] [CrossRef]
- Dogan, E.E. Computational bioactivity analysis and bioisosteric investigation of the approved breast cancer drugs proposed new design drug compounds: Increased bioactivity coming with silicon and boron. Lett. Drug Des. Discov. 2021, 18, 551–561. [Google Scholar] [CrossRef]
- Bello, M. Advances in theoretical studies on the design of single boron atom compounds. Curr. Pharm. Des. 2018, 24, 3466–3475. [Google Scholar] [CrossRef]
- Vincenzi, M.; Bednarska, K.; Leśnikowski, Z.J. Comparative Study of Carborane- and Phenyl-Modified Adenosine Derivatives as Ligands for the A2A and A3 Adenosine Receptors Based on a Rigid in Silico Docking and Radioligand Replacement Assay. Molecules 2018, 23, 1846. [Google Scholar] [CrossRef] [PubMed]
- Marfavi, A.; Kavianpour, P.; Rendina, L.M. Carboranes in drug discovery, chemical biology and molecular imaging. Nat. Rev. Chem. 2022, 6, 486–504. [Google Scholar] [CrossRef]
- Kok, Z.Y.; Stoddart, L.A.; Mistry, S.J.; Mocking, T.A.M.; Vischer, H.F.; Leurs, R.; Hill, S.J.; Mistry, S.N.; Kellam, B. Optimi-zation of Peptide Linker-Based Fluorescent Ligands for the Histamine H1 Receptor. J. Med. Chem. 2022, 65, 8258–8288. [Google Scholar] [CrossRef] [PubMed]
- Barrón-González, M.; Rosales-Hernández, M.C.; Abad-García, A.; Ocampo-Néstor, A.L.; Santiago-Quintana, J.M.; Pérez-Capistran, T.; Trujillo-Ferrara, J.G.; Padilla-Martínez, I.I.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat. Int. J. Mol. Sci. 2022, 23, 3229. [Google Scholar] [CrossRef]
- Zheng, M.; Kong, L.; Gao, J. Boron enabled bioconjugation chemistries. Chem. Soc. Rev. 2024, 53, 11888–11907. [Google Scholar] [CrossRef]
- Mafi, A.; Kim, S.-K.; Goddard, W.A. 3rd. The mechanism for ligand activation of the GPCR-G protein complex. Proc. Natl. Acad. Sci. USA 2022, 119, e2110085119. [Google Scholar] [CrossRef]
- Papasergi-Scott, M.M.; Pérez-Hernández, G.; Batebi, H.; Gao, Y.; Eskici, G.; Seven, A.B.; Panova, O.; Hilger, D.; Casiraghi, M.; He, F.; et al. Time-resolved cryo-EM of G-protein activation by a GPCR. Nature 2024, 629, 1182–1191. [Google Scholar] [CrossRef]
- Diaz, D.B.; Yudin, A.K. The versatility of boron in biological target engagement. Nat. Chem. 2017, 9, 731–742. [Google Scholar] [CrossRef]
- Coghi, P.; Li, J.; Hosmane, N.S.; Zhu, Y. Next generation of boron neutron capture therapy (BNCT) agents for cancer treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Zhang, C.; Lyons, J.A.; Holl, R.; Aragao, D.; Arlow, D.H.; Rasmussen, S.G.F.; Choi, H.-J.; DeVree, B.T.; Sunahara, R.K.; et al. Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 2011, 469, 236–240. [Google Scholar] [CrossRef]
- Peter, S.; Siragusa, L.; Thomas, M.; Palomba, T.; Cross, S.; O’bOyle, N.M.; Bajusz, D.; Ferenczy, G.G.; Keserű, G.M.; Bottegoni, G.; et al. Comparative Study of Allosteric GPCR Binding Sites and Their Ligandability Potential. J. Chem. Inf. Model. 2024, 64, 8176–8192. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.F.; Kabalka, G.W.; Fuhr, J.E. In Vitro Effects of Boron-Containing Compounds upon Glioblastoma Cells. Proc. Soc. Exp. Biol. Med. 1997, 216, 452–455. [Google Scholar] [CrossRef]
- Turkez, H.; Arslan, M.E.; Tatar, A.; Mardinoglu, A. Promising potential of boron compounds against Glioblastoma: In Vitro antioxidant, anti-inflammatory and anticancer studies. Neurochem. Int. 2021, 149, 105137. [Google Scholar] [CrossRef]
- Nguyen, P.; Doan, P.; Rimpilainen, T.; Mani, S.K.; Murugesan, A.; Yli-Harja, O.; Candeias, N.R.; Kandhavelu, M. Synthesis and preclinical validation of novel indole derivatives as a GPR17 agonist for glioblastoma treatment. J. Med. Chem. 2021, 64, 10908–10918. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Kauffman, M. Development of the Proteasome Inhibitor Velcade™ (Bortezomib). Cancer Investig. 2004, 22, 304–311. [Google Scholar] [CrossRef]
- Wiley, S.Z.; Sriram, K.; Salmerón, C.; Insel, P.A. GPR68: An emerging drug target in cancer. Int. J. Mol. Sci. 2019, 20, 559. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Wang, L.; Wang, W. Study on autonomic neuropathy of the digestive system caused by bortezomib in the treatment of multiple myeloma. Hematology 2023, 28, 2210907. [Google Scholar] [CrossRef]
- Yamamoto, S.; Egashira, N. Pathological mechanisms of bortezomib-induced peripheral neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef]
- Ghelardini, C.; Menicacci, C.; Cerretani, D.; Bianchi, E. Spinal administration of mGluR5 antagonist prevents the onset of bortezomib induced neuropathic pain in rat. Neuropharmacology 2014, 86, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Flinn, I.; Richardson, P.G.; Hari, P.; Callander, N.; Noga, S.J.; Stewart, A.K.; Turturro, F.; Rifkin, R.; Wolf, J.; et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012, 119, 4375–4382. [Google Scholar] [CrossRef]
- Kitzen, J.M.; Pergolizzi, J.V., Jr.; Tylor, R., Jr.; Raffa, R.B. Crisaborole and Apremilast: PDE4 Inhibitors with Similar Mechanism of Action, Different Indications for Management of Inflammatory Skin Conditions. Pharmacol. Pharm. 2018, 9, 357–381. [Google Scholar] [CrossRef][Green Version]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e6. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Fan, X.; Lai, Y.; Chen, J.; Peng, Y.; Peng, Y.; Xiang, L.; Ma, Y. Protease-Activated Receptor 2 in inflammatory skin disease: Current evidence and future perspectives. Front. Immunol. 2024, 15, 1448952. [Google Scholar] [CrossRef] [PubMed]
- Peña, S.M.; Oak, A.S.W.; Smith, A.M.; Mayo, T.T.; Elewski, B.E. Topical crisaborole is an efficacious steroid-sparing agent for treating mild-to-moderate seborrhoeic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, E809–E812. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Kirsner, R.S.; Margolis, D.J.; Tharp, M.; Myers, D.E.; Annis, K.; Graham, D.; Zang, C.; Vlahos, B.L.; Sanders, P. Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis: Results from a fully decentralized, randomized, proof-of-concept phase 2a study. J. Am. Acad. Dermatol. 2024, 90, 945–952. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z.-R.; Ding, H.-J.; Feng, J. Molecular and cellular mechanisms of itch sensation and the anti-itch drug targets. Acta Pharmacol. Sin. 2024, 46, 539–553. [Google Scholar] [CrossRef]
- Cunningham, C.C. Talabostat. Expert Opin. Investig. Drugs 2007, 16, 1459–1465. [Google Scholar] [CrossRef]
- Redman, B.G.; Ernstoff, M.S.; Gajewski, T.F.; Cunningham, C.; Lawson, D.H.; Gregoire, L.; Haltom, E.; Uprichard, M.J. Phase 2 trial of talabostat in stage IV melanoma. J. Clin. Oncol. 2005, 23 (Suppl. S16), 7570. [Google Scholar] [CrossRef]
- Eager, R.M.; Cunningham, C.C.; Senzer, N.; Richards, D.A.; Raju, R.N.; Jones, B.; Uprichard, M.; Nemunaitis, J. Phase II Trial of Talabostat and Docetaxel in Advanced Non-small Cell Lung Cancer. Clin. Oncol. 2009, 21, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Yang, P.W.; Sun, Y.; Song, S.L.; Chen, Z. Fibroblast activation protein-based theranostics in pancreatic cancer. Front. Oncol. 2022, 12, 969731. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L.; Tafelmeyer, P.; Golshayan, D. Regulation of Fibroblast Activation Protein-α Expression: Focus on Intracellular Protein Interactions. J. Med. Chem. 2021, 64, 14028–14045. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Ali, S.; Hoda, M. Current status of G-protein coupled receptors as potential targets against type 2 diabetes mellitus. Int. J. Biol. Macromol. 2018, 118, 2237–2244. [Google Scholar] [CrossRef]
- Johnson, K.M.S. Dutogliptin, a dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes mellitus. Curr. Opin. Investig. Drugs 2010, 11, 455–463. [Google Scholar]
- Li, J.; Klemm, K.; O’Farrell, A.M.; Guler, H.-P.; Cherrington, J.M.; Schwartz, S.; Boyea, T. Evaluation of the potential for pharmacokinetic and pharmacodynamic interactions between dutogliptin, a novel DPP4 inhibitor, and met-formin, in type 2 diabetic patients. Curr. Med Res. Opin. 2010, 26, 2003–2010. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-R.; Seng, D.-J.; Xu, Y.; Zhang, Y.-D.; Zhou, W.-J.; Jia, Y.-Y.; Song, J.; He, Z.-X.; Liu, H.-M.; Yuan, S. A comprehensive review of small molecule drugs approved by the FDA in 2023: Advances and prospects. Eur. J. Med. Chem. 2024, 276, 116706. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Quintana, J.M.; Barquet-Nieto, A.; Das, B.C.; Barrientos-López, R.; Rosalez, M.N.; Lopez-Mayorga, R.M.; Soriano-Ursúa, M.A. The Binding and Effects of Boron-Containing Compounds on G Protein-Coupled Receptors: A Scoping Review. Receptors 2025, 4, 15. https://doi.org/10.3390/receptors4030015
Santiago-Quintana JM, Barquet-Nieto A, Das BC, Barrientos-López R, Rosalez MN, Lopez-Mayorga RM, Soriano-Ursúa MA. The Binding and Effects of Boron-Containing Compounds on G Protein-Coupled Receptors: A Scoping Review. Receptors. 2025; 4(3):15. https://doi.org/10.3390/receptors4030015
Chicago/Turabian StyleSantiago-Quintana, José M., Alina Barquet-Nieto, Bhaskar C. Das, Rafael Barrientos-López, Melvin N. Rosalez, Ruth M. Lopez-Mayorga, and Marvin A. Soriano-Ursúa. 2025. "The Binding and Effects of Boron-Containing Compounds on G Protein-Coupled Receptors: A Scoping Review" Receptors 4, no. 3: 15. https://doi.org/10.3390/receptors4030015
APA StyleSantiago-Quintana, J. M., Barquet-Nieto, A., Das, B. C., Barrientos-López, R., Rosalez, M. N., Lopez-Mayorga, R. M., & Soriano-Ursúa, M. A. (2025). The Binding and Effects of Boron-Containing Compounds on G Protein-Coupled Receptors: A Scoping Review. Receptors, 4(3), 15. https://doi.org/10.3390/receptors4030015






