Role of Glucocorticoid Receptor in Triple-Negative Breast Cancer
Abstract
1. Introduction
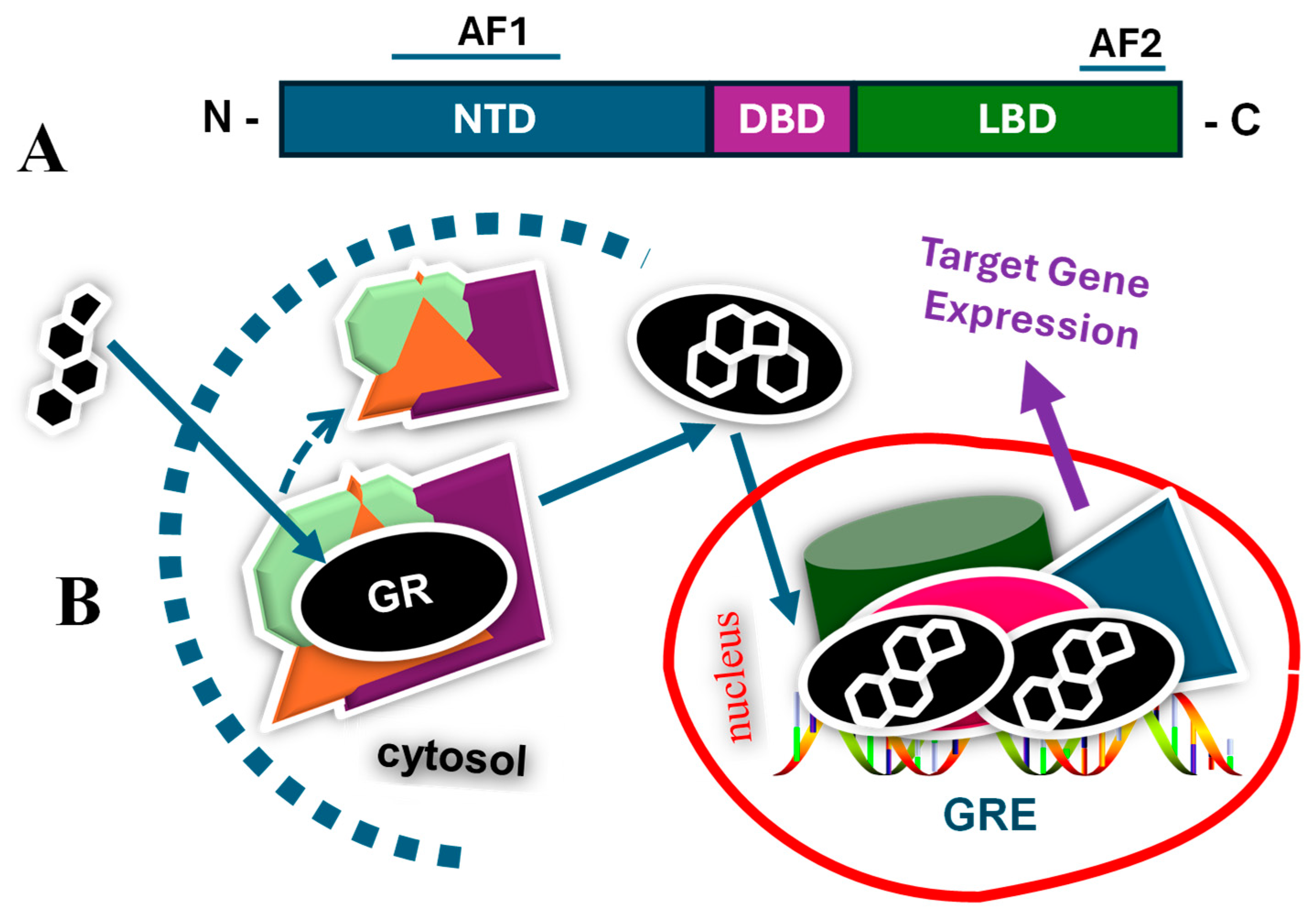
2. Glucocorticoids and Glucocorticoid Receptor
3. Type of Breast Cancers and Targeted Therapies
4. Triple-Negative Breast Cancer and Glucocorticoid Receptor
5. Summary and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar]
- Geyer, F.C.; Pareja, F.; Weigelt, B.; Rakha, E.; Ellis, I.O.; Schnitt, S.J.; Reis-Filho, J.S. The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am. J. Pathol. 2017, 187, 2139–2151. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer–expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar]
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer—An Overview. Hereditary Genet. 2013, 2013 (Suppl. S2), 001. [Google Scholar]
- Buschmann, D.; Gonzalez, R.; Kirchner, B.; Mazzone, C.; Pfaffl, M.W.; Schelling, G.; Steinlein, O.; Reithmair, M. Glucocorticoid receptor overexpression slightly shifts microRNA expression patterns in triple-negative breast cancer. Int. J. Oncol. 2018, 52, 1765–1776. [Google Scholar] [CrossRef]
- Mitre-Aguilar, I.B.; Moreno-Mitre, D.; Melendez-Zajgla, J.; Maldonado, V.; Jacobo-Herrera, N.J.; Ramirez-Gonzalez, V.; Mendoza-Almanza, G. The Role of Glucocorticoids in Breast Cancer Therapy. Curr. Oncol. 2022, 30, 298–314. [Google Scholar] [CrossRef]
- Dowsett, M.; Smith, I.E.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Griffith, C.; Boeddinghaus, I.; Salter, J.; Detre, S.; Hills, M.; et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin. Cancer Res. 2006, 12, 1024s–1030s. [Google Scholar]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar]
- Cleator, S.; Heller, W.; Coombes, R.C. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007, 8, 235–244. [Google Scholar]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar]
- Liedtke, C.; Rody, A. Neoadjuvant therapy for patients with triple negative breast cancer (TNBC). Rev. Recent Clin. Trials. 2017, 12, 73–80. [Google Scholar]
- Bhattacharya, R.; Banerjee, K.; Mukherjee, N.; Sen, M.; Mukhopadhyay, A. From molecular insight to therapeutic strategy: The holistic approach for treating triple negative breast cancer. Pathol. Res. Pract. 2017, 213, 177–182. [Google Scholar] [PubMed]
- Székely, B.; Silber, A.L.; Pusztai, L. New Therapeutic Strategies for Triple-Negative Breast Cancer. Oncology 2017, 31, 130–137. [Google Scholar]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar]
- Li, Z.; Dong, J.; Zou, T.; Du, C.; Li, S.; Chen, C.; Liu, R.; Wang, K. Dexamethasone induces docetaxel and cisplatin resistance partially through up-regulating Krüppel-like factor 5 in triple-negative breast cancer. Oncotarget 2017, 8, 11555–11565. [Google Scholar]
- Regan Anderson, T.M.; Ma, S.H.; Raj, G.V.; Cidlowski, J.A.; Helle, T.M.; Knutson, T.P.; Krutilina, R.I.; Seagroves, T.N.; Lange, C.A. Breast Tumor Kinase (Brk/PTK6) Is Induced by HIF, Glucocorticoid Receptor, and PELP1-Mediated Stress Signaling in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 1653–1663. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, X.; Wu, D.; Sunkel, B.; Ye, Z.; Huang, J.; Liu, Z.; Clinton, S.K.; Jin, V.X.; Wang, Q. Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat. Commun. 2015, 6, 8323. [Google Scholar] [CrossRef]
- Frei, E., 3rd; Karon, M.; Levin, R.H.; Freireich, E.J.; Taylor, R.J.; Hananian, J.; Selawry, O.; Holland, J.F.; Hoogstraten, B.; Wolman, I.J.; et al. The effectiveness of combinations of ntileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood 1965, 26, 642–656. [Google Scholar]
- Schmidt, S.; Rainer, J.; Ploner, C.; Presul, E.; Riml, S.; Kofler, R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: Molecular mechanisms and clinical relevance. Cell Death Differ. 2004, 11 (Suppl. S1), S45–S55. [Google Scholar]
- Zhang, C.; Wenger, T.; Mattern, J.; Ilea, S.; Frey, C.; Gutwein, P.; Altevogt, P.; Bodenmüller, W.; Gassler, N.; Schnabel, P.A.; et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol. Ther. 2007, 6, 278–287. [Google Scholar] [CrossRef]
- Rutz, H.P. Effects of corticosteroid use on treatment of solid tumours. Lancet 2002, 360, 1969–1970. [Google Scholar]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor negative breast cancer. Cancer Res. 2011, 71, 6360–6370. [Google Scholar] [PubMed]
- Pang, D.; Kocherginsky, M.; Krausz, T.; Kim, S.Y.; Conzen, S.D. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol. Ther. 2006, 5, 933–940. [Google Scholar] [PubMed]
- McEwan, I.J.; Kumar, R. Historical overview of nuclear hormone receptor structure. In Nuclear Receptors: From Structure to the Clinic; Springer Publishers: New York, NY, USA, 2015; Chapter 1; pp. 1–14. [Google Scholar]
- Kumar, R.; Johnson, B.H.; Thompson, E.B. Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essay Biochem. 2004, 40, 27–39. [Google Scholar]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar]
- Hawkins, U.A.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.M.; Gomez-Sanchez, C.E. The ubiquitous mineralocorticoid receptor: Clinical implications. Curr. Hypertens. Rep. 2012, 14, 573–580. [Google Scholar]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2014, 22, 20–32. [Google Scholar]
- Bosscher, K.; Haegeman, G. Minireview: Latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 2008, 23, 281–291. [Google Scholar]
- Donatti, T.; Koch, V.; Takayama, L.; Pereira, R. Effects of glucocorticoids on growth and bone mineralization. J. Pediatria. 2011, 87, 4–12. [Google Scholar]
- Nussinovitch, U.; de Carvalho, J.F.; Pereira, R.M.; Shoenfeld, Y. Glucocorticoids and the cardiovascular system: State of the art. Curr. Pharm. Des. 2010, 16, 3574–3585. [Google Scholar]
- Cruz-Topete, D.; Myers, P.H.; Foley, J.F.; Willis, M.S.; Cidlowski, J.A. Corticosteroids are essential for maintaining cardiovascular function in male mice. Endocrinology 2016, 157, 2759–2771. [Google Scholar] [PubMed]
- Farrell, C.; O’Keane, V. Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res. 2016, 242, 349–356. [Google Scholar]
- Joëls, M. Impact of glucocorticoids on brain function: Relevance for mood disorders. Psychoneuroendocrinology 2011, 36, 406–414. [Google Scholar]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014, 87, 3–6. [Google Scholar]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and reproduction: Traffic control on the road to reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [PubMed]
- Fowden, A.L.; Forehead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure and functions relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar]
- Khan, S.H.; Ling, J.; Kumar, R. TBP binding-induced folding of the glucocorticoid receptor AF1 domain facilitates its interaction with steroid receptor coactivator-1. PLoS ONE 2011, 6, e21939. [Google Scholar]
- Simons, S.S.; Kumar, R. Variable responses by steroid receptors: Intrinsically disordered AF1 is the key. Mol. Cell. Endo. 2013, 376, 81–84. [Google Scholar]
- Simons, S.S.; Edwards, D.P.; Kumar, R. Dynamic Structures of Nuclear Hormone Receptors: New Promises and Challenges. Mol. Endocrinol. 2014, 28, 173–182. [Google Scholar] [PubMed]
- Kumar, R.; McEwan, I.J. Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation. Endocr. Rev. 2012, 33, 271–299. [Google Scholar] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. On the trail of the glucocorticoid receptor: Into the nucleus and back. Traffic 2011, 13, 364–374. [Google Scholar] [PubMed]
- Menon, G.; Alkabban, F.M.; Ferguson, T. Breast Cancer. [Updated 2024 Feb 25]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Roulot, A.; Héquet, D.; Guinebretière, J.M.; Vincent-Salomon, A.; Lerebours, F.; Dubot, C.; Rouzier, R. Tumoral heterogeneity of breast cancer. Ann. Biol. Clin. 2016, 74, 653–660. [Google Scholar]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar]
- Wu, Y.T.; Xu, Z.; Zhang, K.; Wu, J.S.; Li, X.; Arshad, B.; Li, Y.C.; Wang, Z.L.; Li, H.Y.; Wu, K.N.; et al. Efficacy and cardiac safety of the concurrent use of trastuzumab and anthracycline-based neoadjuvant chemotherapy for HER2-positive breast cancer: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2018, 14, 1789–1797. [Google Scholar]
- Dieci, M.V.; Vernaci, G.; Guarneri, V. Escalation and de-escalation in HER2 positive early breast cancer. Curr. Opin. Oncol. 2019, 31, 35–42. [Google Scholar]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.S.; Kumar, A.; Vishakha, B.T.; Jha, S.K.; Tang, H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer 2023, 22, 105. [Google Scholar]
- Husinka, L.; Koerner, P.H.; Miller, R.T.; Trombatt, W. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. J. Drug Assess. 2020, 10, 27–34. [Google Scholar]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer. 2023, 9, 7. [Google Scholar] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [PubMed]
- Ursin, G.; Bernstein, L.; Lord, S.J.; Karim, R.; Deapen, D.; Press, M.F.; Daling, J.R.; Norman, S.A.; Liff, J.M.; Marchbanks, P.A.; et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br. J. Cancer. 2005, 93, 364–371. [Google Scholar]
- Hirko, K.A.; Rocque, G.; Reasor, E.; Taye, A.; Daly, A.; Cutress, R.I.; Copson, E.R.; Lee, D.W.; Lee, K.H.; Im, S.A.; et al. The impact of race and ethnicity in breast cancer-disparities and implications for precision oncology. BMC Med. 2022, 20, 72. [Google Scholar]
- Hurson, A.M.; Ahearn, T.U.; Koka, H.; Jenkins, B.D.; Harris, A.R.; Roberts, S.; Fan, S.; Franklin, J.; Butera, G.; Keeman, R.; et al. Risk factors for breast cancer subtypes by race and ethnicity: A scoping review. JNCI J. Natl. Cancer Inst. 2024, 116, 1992–2002. [Google Scholar]
- Fillon, M. The association between menopausal hormone therapy and breast cancer remains unsettled. CA Cancer J. Clin. 2024, 74, 210–212. [Google Scholar]
- Leehy, K.A.; Regan Anderson, T.M.; Daniel, A.R.; Lange, C.A.; Ostrander, J.H. Modifications to glucocorticoid and progesterone receptors alter cell fate in breast cancer. J. Mol. Endocrinol. 2016, 56, R99–R114. [Google Scholar]
- Wu, W.; Chaudhuri, S.; Brickley, D.R.; Pang, D.; Karrison, T.; Conzen, S.D. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004, 64, 1757–1764. [Google Scholar]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar]
- Conway, M.E.; McDaniel, J.M.; Graham, J.M.; Guillen, K.P.; Oliver, P.G.; Parker, S.L.; Yue, P.; Turkson, J.; Buchsbaum, D.J.; Welm, B.E.; et al. STAT3 and GR Cooperate to Drive Gene Expression and Growth of Basal-Like Triple-Negative Breast Cancer. Cancer Res. 2020, 80, 4355–4370. [Google Scholar]
- Noureddine, L.M.; Trédan, O.; Hussein, N.; Badran, B.; Le Romancer, M.; Poulard, C. Glucocorticoid Receptor: A Multifaceted Actor in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 4446. [Google Scholar] [CrossRef]
- Skor, M.N.; Wonder, E.L.; Kocherginsky, M.; Goyal, A.; Hall, B.A.; Cai, Y.; Conzen, S.D. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin. Cancer Res. 2013, 19, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Ronfani, M.; Galasso, M.; Ventura, D.; Corsini, E.; Racchi, M. Cortisol-induced SRSF3 expression promotes GR splicing, RACK1 expression and breast cancer cells migration. Pharmacol. Res. 2019, 143, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Galasso, M.; Ronfani, M.; Serafini, M.M.; Lanni, C.; Corsini, E.; Racchi, M. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 2017, 120, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Huang, Y.C.; Chiu, P.Y.; Kuo, W.H.; Pan, Y.R.; Kuo, Y.T.; Wang, R.H.; Kao, Y.C.; Wang, Y.H.; Lin, Y.F.; et al. Combating breast cancer progression through combination therapy with hypomethylating agent and glucocorticoid. iScience. 2023, 26, 106597. [Google Scholar] [CrossRef]
- Diab, T.; AlKafaas, S.S.; Shalaby, T.I.; Hessien, M. Dexamethasone simulates the anticancer effect of nano-formulated paclitaxel in breast cancer cells. Bioorg. Chem. 2020, 99, 103792. [Google Scholar] [CrossRef]
- Dwyer, A.R.; Perez Kerkvliet, C.; Truong, T.H.; Hagen, K.M.; Krutilina, R.I.; Parke, D.N.; Oakley, R.H.; Liddle, C.; Cidlowski, J.A.; Seagroves, T.N.; et al. Glucocorticoid Receptors Drive Breast Cancer Cell Migration and Metabolic Reprogramming via PDK4. Endocrinology 2023, 164, bqad083. [Google Scholar] [CrossRef]
- Posani, S.H.; Gillis, N.E.; Lange, C.A. Glucocorticoid receptors orchestrate a convergence of host and cellular stress signals in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2024, 243, 106575. [Google Scholar] [CrossRef]
- Perez Kerkvliet, C.; Dwyer, A.R.; Diep, C.H.; Oakley, R.H.; Liddle, C.; Cidlowski, J.A.; Lange, C.A. Glucocorticoid receptors are required effectors of TGFβ1-induced p38 MAPK signaling to advanced cancer phenotypes in triple-negative breast cancer. Breast Cancer Res. 2020, 22, 39. [Google Scholar] [CrossRef]
- Agyeman, A.S.; Jun, W.J.; Proia, D.A.; Kim, C.R.; Skor, M.N.; Kocherginsky, M.; Conzen, S.D. Hsp90 Inhibition Results in Glucocorticoid Receptor Degradation in Association with Increased Sensitivity to Paclitaxel in Triple-Negative Breast Cancer. Horm. Cancer 2016, 7, 114–126. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R. Role of Glucocorticoid Receptor in Triple-Negative Breast Cancer. Receptors 2025, 4, 8. https://doi.org/10.3390/receptors4020008
Kumar R. Role of Glucocorticoid Receptor in Triple-Negative Breast Cancer. Receptors. 2025; 4(2):8. https://doi.org/10.3390/receptors4020008
Chicago/Turabian StyleKumar, Raj. 2025. "Role of Glucocorticoid Receptor in Triple-Negative Breast Cancer" Receptors 4, no. 2: 8. https://doi.org/10.3390/receptors4020008
APA StyleKumar, R. (2025). Role of Glucocorticoid Receptor in Triple-Negative Breast Cancer. Receptors, 4(2), 8. https://doi.org/10.3390/receptors4020008





