Bioelectric Membrane Potential and Breast Cancer: Advances in Neuroreceptor Pharmacology for Targeted Therapeutic Strategies
Abstract
1. Introduction
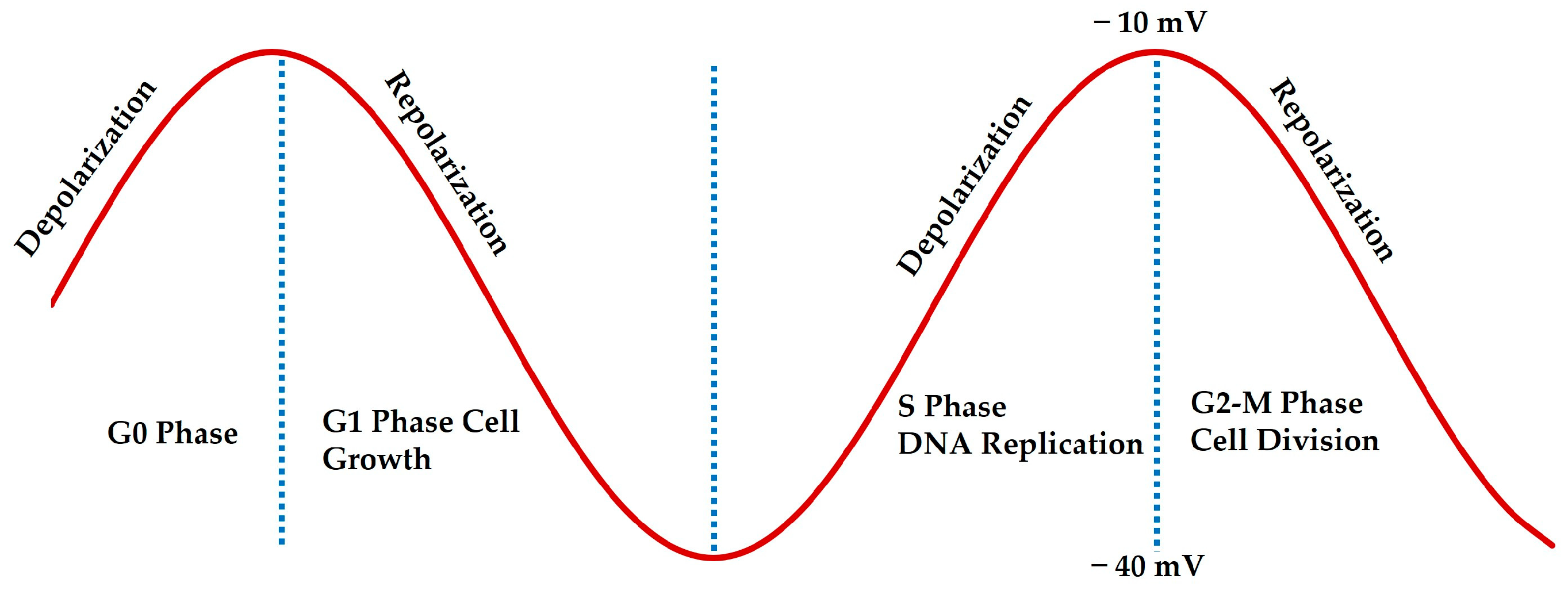
2. Bioelectric Membrane Potential in Breast Cancer
3. Neuroreceptors, Neurotransmitters, Membrane Potential, and Breast Cancer
4. Neuroreceptor Pharmacology and Therapeutic Advances
5. Future Directions and Challenges
- Identifying bioelectric signatures for different breast cancer subtypes: Identifying bioelectric signatures in breast cancer subtypes can be improved using voltage-sensitive dyes for membrane potential monitoring, patch-clamp techniques for ion channel activity, and single-cell transcriptomics to link gene expression with bioelectric signaling. Combining these with imaging tools like fluorescence microscopy or optogenetics helps reveal bioelectricity’s role in tumor behavior, offering insights into new therapeutic targets for personalized treatment strategies, and ultimately supporting personalized treatment strategies for different breast cancer subtypes.
- Developing ion channel modulators targeting cancer cells while minimizing off-target effects: Ion channel modulators show promise as breast cancer therapies by regulating cell functions such as proliferation and migration. For instance, Nav1.7 sodium channels contribute to metastasis, with inhibitors like tetrodotoxin being studied to limit cancer spread. TRPM7, involved in calcium and magnesium influx, is linked to poor prognosis, and inhibitors like NS8593 may slow tumor growth. Additionally, TPCs (TPC1 and TPC2) regulate calcium signaling and tumor progression, with modulators potentially reducing cell proliferation. While preclinical results are promising, further research is needed to evaluate their clinical effectiveness.
- Exploring the combination of bioelectric therapies with conventional treatments in clinical trials: Combining bioelectric therapies with conventional treatments like chemotherapy or immunotherapy is gaining attention in clinical trials. This approach aims to enhance treatment efficacy, overcome drug resistance, and minimize side effects. For example, ion channel modulators could sensitize cancer cells to chemotherapy, while bioelectric stimulation may boost immune response during immunotherapy. Ongoing trials are exploring how these combinations could provide synergistic benefits, leading to more effective and personalized cancer treatments.
- Advanced Imaging: New fluorescent probes and imaging techniques are needed to measure membrane potential and ion channel activity in real time within live cells and tissues [94].
- Integrative Multi-Omics: Combining genomics, proteomics, and electrophysiology can provide comprehensive models of membrane potential modulation by neuroreceptors and ion channels [95].
- Personalized Medicine: Understanding individual responses to agonists and antagonists can help design personalized therapeutic strategies [96].
- Animal Models and Clinical Trials: Translating laboratory findings into animal models and clinical trials is essential for testing the efficacy of bioelectric-based therapies [97].
- Digital Twin and Computational Electrophysiology: Digital twin models, which replicate patients or biological systems, offer promising tools to address drug resistance in cancer by integrating clinical and multi-omics data to simulate disease progression and treatment outcomes. Initiatives like the PRIMUS project and NIH-funded INCEPTION have utilized digital twins to predict resistance mechanisms in cancers such as pancreatic, breast, and lung cancer. Combining these models with CRISPR screening enables genome-wide identification of resistance-related genes, like ABC transporters or MYC, and simulates their impact on tumor behavior. This approach refines therapeutic strategies, suggests combination therapies, and accelerates drug development by reducing preclinical costs while exploring immune escape mechanisms, paving the way for personalized cancer treatments. Digital twin models offer customized virtual representations of patients, enabling predictive modeling to optimize treatment strategies [98].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Sondel, P.M. Immunocytokines for cancer treatment: Past, present and future. Curr. Opin. Immunol. 2016, 40, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Qayoom, H. Introduction to breast cancer. In Therapeutic Potential of Cell Cycle Kinases in Breast Cancer; Springer Nature: Singapore, 2023; pp. 1–22. [Google Scholar]
- Lanyi, M. Malignant and Benign Lobular and Ductal Lesions with Perifocal Reactions. In Mammography: Diagnosis and Pathological Analysis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 145–212. [Google Scholar]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Mahapatra, C.; Kumar, R. Biophysical Mechanisms of Vaginal Smooth Muscle Contraction: The Role of the Membrane Potential and Ion Channels. Pathophysiology 2024, 31, 225–243. [Google Scholar] [CrossRef]
- An, Q.; Yue, G.; Yang, X.; Lou, J.; Shan, W.; Ding, J.; Jin, Z.; Hu, Y.; Du, Q.; Liao, Q.; et al. Pathophysiological role of purinergic P2X receptors in digestive system diseases. Front. Physiol. 2021, 12, 781069. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. Rep. 2009, 5, 231–246. [Google Scholar] [CrossRef]
- Déliot, N.; Constantin, B. Plasma membrane calcium channels in cancer: Alterations and consequences for cell proliferation and migration. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2512–2522. [Google Scholar] [CrossRef]
- Rao, V.R.; Perez-Neut, M.; Kaja, S.; Gentile, S. Voltage-gated ion channels in cancer cell proliferation. Cancers 2015, 7, 849–875. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [PubMed]
- Lauder, J.M. Neurotransmitters as growth regulatory signals: Role of receptors and second messengers. Trends Neurosci. 1993, 16, 233–240. [Google Scholar] [PubMed]
- Hnasko, T.S.; Edwards, R.H. Neurotransmitter corelease: Mechanism and physiological role. Annu. Rev. Physiol. 2012, 74, 225–243. [Google Scholar]
- Ständer, S.; Luger, T.A. Neuroreceptors and neuromediators. In Pruritus; Springer: London, UK, 2010; pp. 7–15. [Google Scholar]
- Rahmann, H. Calcium-ganglioside interactions and modulation of neuronal functions. In Current Aspects of the Neurosciences; Springer: London, UK, 1992; Volume 4, pp. 87–125. [Google Scholar]
- Brown, A.G. The Postsynaptic Neuron I: Actions of Neurotransmitters. In Nerve Cells and Nervous Systems: An Introduction to Neuroscience; Springer: London, UK, 2001; pp. 87–100. [Google Scholar]
- Mahaut-Smith, M.P.; Taylor, K.A.; Evans, R.J. Calcium Signalling through ligand-gated ion channels such as P2X1 receptors in the platelet and other non-excitable cells. In Calcium Entry Pathways in Non-Excitable Cells; Springer: Cham, Switzerland, 2016; pp. 305–329. [Google Scholar]
- Moreddu, R. Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine. Adv. Sci. 2024, 11, 2304110. [Google Scholar]
- Marino, A.A.; Iliev, I.G.; Schwalke, M.A.; Gonzalez, E.; Marler, K.C.; Flanagan, C.A. Association between cell membrane potential and breast cancer. Tumor Biol. 1994, 15, 82–89. [Google Scholar]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar]
- Dai, J. The Continuous Relative Deficiency of Intracellular Potassium Is a Core Mechanism for the Occurrence and Metastasis of Tumor Cancer Cells. Nat. Sci. 2022, 14, 492–496. [Google Scholar]
- Robinson, A.J.; Jain, A.; Sherman, H.G.; Hague, R.J.M.; Rahman, R.; Sanjuan-Alberte, P.; Rawson, F.J. Toward hijacking bioelectricity in cancer to develop new bioelectronic medicine. Adv. Ther. 2021, 4, 2000248. [Google Scholar]
- Sheth, M.; Esfandiari, L. Bioelectric dysregulation in cancer initiation, promotion, and progression. Front. Oncol. 2022, 12, 846917. [Google Scholar]
- Wang, Z. Cell cycle progression and synchronization: An overview. In Cell-Cycle Synchronization: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 3–23. [Google Scholar]
- Wang, S.; Melkoumian, Z.; Woodfork, K.A.; Cather, C.; Davidson, A.G.; Wonderlin, W.F.; Strobl, J.S. Evidence for an early G1 ionic event necessary for cell cycle progression and survival in the MCF-7 human breast carcinoma cell line. J. Cell. Physiol. 1998, 176, 456–464. [Google Scholar]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [PubMed]
- Fnu, G.; Weber, G.F. Alterations of ion homeostasis in cancer metastasis: Implications for treatment. Front. Oncol. 2021, 11, 765329. [Google Scholar]
- Garbern, J.C.; Lee, R.T. Mitochondria and metabolic transitions in cardiomyocytes: Lessons from development for stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2021, 12, 177. [Google Scholar]
- Iorio, J.; Petroni, G.; Duranti, C.; Lastraioli, E. Potassium and sodium channels and the Warburg effect: Biophysical regulation of cancer metabolism. Bioelectricity 2019, 1, 188–200. [Google Scholar]
- Wang, L.; Zhou, P.; Craig, R.W.; Lu, L. Protection from cell death by mcl-1 is mediated by membrane hyperpolarization induced by K+ channel activation. J. Membr. Biol. 1999, 172, 113–120. [Google Scholar]
- Tajbakhsh, A.; Pasdar, A.; Rezaee, M.; Fazeli, M.; Soleimanpour, S.; Hassanian, S.M.; FarshchiyanYazdi, Z.; Rad, T.Y.; Ferns, G.A.; Avan, A. The current status and perspectives regarding the clinical implication of intracellular calcium in breast cancer. J. Cell. Physiol. 2018, 233, 5623–5641. [Google Scholar]
- Quicke, P.; Sun, Y.; Arias-Garcia, M.; Beykou, M.; Acker, C.D.; Djamgoz, M.B.A.; Bakal, C.; Foust, A.J. Voltage imaging reveals the dynamic electrical signatures of human breast cancer cells. Commun. Biol. 2022, 5, 1178. [Google Scholar]
- Berzingi, S.; Newman, M.; Yu, H.-G. Altering bioelectricity on inhibition of human breast cancer cells. Cancer Cell Int. 2016, 16, 72. [Google Scholar] [PubMed]
- Stevens, E.B.; Stephens, G.J. Ion channels as targets in drug discovery: Outlook and perspectives. In Ion Channels as Targets in Drug Discovery; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–34. [Google Scholar]
- Dugas, H.; Penney, C.; Dugas, H.; Penney, C. Bioorganic chemistry of the amino acids. In Bioorganic Chemistry: A Chemical Approach to Enzyme Action; Springer: New York, NY, USA, 1981; pp. 13–92. [Google Scholar]
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 2021, 265, 118826. [Google Scholar]
- Malinak, D.; Korabecny, J.; Soukup, O.; Gorecki, L.; Nepovimova, E.; Psotka, M.; Dolezal, R.; Nguyen, T.D.; Mezeiova, E.; Musilek, K.; et al. A review of the synthesis of quaternary acetylcholinesterase reactivators. Curr. Org. Chem. 2018, 22, 1619–1648. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Nantel, F.; Bouvier, M. Receptor regulation. New Compr. Biochem. 1993, 24, 99–109. [Google Scholar]
- Lemoine, D.; Jiang, R.; Taly, A.; Chataigneau, T.; Specht, A.; Grutter, T. Ligand-gated ion channels: New insights into neurological disorders and ligand recognition. Chem. Rev. 2012, 112, 6285–6318. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.; Brain, K.L.; Manchanda, R. A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PLoS ONE 2018, 13, e0200712. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Ostrom, R.S. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell. Mol. Neurobiol. 2003, 23, 305–314. [Google Scholar] [CrossRef]
- Striessnig, J.; Ortner, N.J. Ca2+ channel blockers. In Encyclopedia of Molecular Pharmacology; Springer International Publishing: Cham, Switzerland, 2022; pp. 375–383. [Google Scholar]
- Di Resta, C.; Becchetti, A. Introduction to ion channels. In Integrins and Ion Channels: Molecular Complexes and Signaling; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; pp. 9–21. [Google Scholar]
- Ubuka, T. Noradrenaline/adrenaline. In Handbook of Hormones; Academic Press: Cambridge, MA, USA, 2021; pp. 1041–1044. [Google Scholar]
- Silva, D.; Quintas, C.; Gonçalves, J.; Fresco, P. Contribution of adrenergic mechanisms for the stress-induced breast cancer carcinogenesis. J. Cell. Physiol. 2022, 237, 2107–2127. [Google Scholar] [CrossRef]
- Eng, J.W.-L.; Reed, C.B.; Kokolus, K.M.; Pitoniak, R.; Utley, A.; Bucsek, M.J.; Ma, W.W.; Repasky, E.A.; Hylander, B.L. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 2015, 6, 6426. [Google Scholar] [CrossRef]
- quốc Lu’o’ng, K.V.; Nguyễn, L.T.H. The roles of beta-adrenergic receptors in tumorigenesis and the possible use of beta-adrenergic blockers for cancer treatment: Possible genetic and cell-signaling mechanisms. Cancer Manag. Res. 2012, 4, 431–445. [Google Scholar]
- Strous, G.J.; Schantl, J.A. β-arrestin and Mdm2, unsuspected partners in signaling from the cell surface. Sci. STKE 2001, 2001, pe41. [Google Scholar] [CrossRef]
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front. Cell Dev. Biol. 2022, 10, 938289. [Google Scholar] [CrossRef]
- Conceição, F.; Sousa, D.M.; Paredes, J.; Lamghari, M. Sympathetic activity in breast cancer and metastasis: Partners in crime. Bone Res. 2021, 9, 9. [Google Scholar]
- Alicia Luthy, I.; Bruzzone, A.; Perez Pinero, C. Adrenergic action in breast cancer. Curr. Cancer Ther. Rev. 2012, 8, 90–99. [Google Scholar]
- Jayachandran, P.; Battaglin, F.; Strelez, C.; Lenz, A.; Algaze, S.; Soni, S.; Lo, J.H.; Yang, Y.; Millstein, J.; Zhang, W.; et al. Breast cancer and neurotransmitters: Emerging insights on mechanisms and therapeutic directions. Oncogene 2023, 42, 627–637. [Google Scholar] [PubMed]
- Johnston, S.R.D. The role of chemotherapy and targeted agents in patients with metastatic breast cancer. Eur. J. Cancer 2011, 47, S38–S47. [Google Scholar]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Propranolol as an anti-cancer agent. ecancermedicalscience 2016, 10, 680. [Google Scholar]
- Liu, D.; Yang, Z.; Wang, T.; Yang, Z.; Chen, H.; Hu, Y.; Hu, C.; Guo, L.; Deng, Q.; Liu, Y.; et al. β2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene 2016, 35, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Ji, N.N.; Duan, M.L.; Tong, J.H.; Xu, J.G.; Zhang, Y.M.; Wang, S.H. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3500–3506. [Google Scholar]
- Kim, M.H.; Oh, J.E.; Park, S.; Kim, J.H.; Lee, K.Y.; Bai, S.J.; Song, H.; Hwang, H.J.; Kim, D.W.; Yoo, Y.C. Tramadol use is associated with enhanced postoperative outcomes in breast cancer patients: A retrospective clinical study with in vitro confirmation. Br. J. Anaesth. 2019, 123, 865–876. [Google Scholar]
- Resende, R.R.; Adhikari, A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun. Signal. 2009, 7, 20. [Google Scholar] [CrossRef]
- Bertrand, D.; Wallace, T.L. A review of the cholinergic system and therapeutic approaches to treat brain disorders. In Behavioral Pharmacology of the Cholinergic System; Springer: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Khodabandeh, Z.; Valilo, M.; Velaei, K.; Tazehkand, A.P. The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy. Breast Cancer 2022, 29, 778–789. [Google Scholar] [CrossRef]
- Ochirbat, S.; Kan, T.-C.; Hsu, C.-C.; Huang, T.-H.; Chuang, K.-H.; Chen, M.; Cheng, C.-C.; Chang, C.-C.; Rahayu, S.; Chang, J. The angiogenic role of the alpha 9-nicotinic acetylcholine receptor in triple-negative breast cancers. Angiogenesis 2024, 27, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Español, A.; Salem, A.; Sanchez, Y.; Sales, M.E. Breast cancer: Muscarinic receptors as new targets for tumor therapy. World J. Clin. Oncol. 2021, 12, 404. [Google Scholar] [CrossRef]
- Stull, M.A.; Pai, V.; Vomachka, A.J.; Marshall, A.M.; Jacob, G.A.; Horseman, N.D. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc. Natl. Acad. Sci. USA 2007, 104, 16708–16713. [Google Scholar] [CrossRef]
- Olfati, Z.; Rigi, G.; Vaseghi, H.; Zamanzadeh, Z.; Sohrabi, M.; Hejazi, S.H. Evaluation of serotonin receptors (5HTR2A and 5HTR3A) mRNA expression changes in tumor of breast cancer patients. Med. J. Islam. Repub. Iran 2020, 34, 99. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef]
- Bala Bhaskar, S.; Manjuladevi, M. Drugs, Fluids and Cancer. In Textbook of Onco-Anesthesiology; Springer: Singapore, 2021; pp. 103–116. [Google Scholar]
- Chen, L.; Huang, S.; Wu, X.; He, W.; Song, M. Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities. Clin. Transl. Med. 2024, 14, e1750. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Antidepressants and antipsychotic agents as repurposable oncological drug candidates. Curr. Med. Chem. 2021, 28, 2137–2174. [Google Scholar] [CrossRef]
- Liby, K.; Neltner, B.; Mohamet, L.; Menchen, L.; Ben-Jonathan, N. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res. Treat. 2003, 79, 241–252. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Hu, L.-P.; Wang, X.; Li, J.; Zhang, Z.-G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Souteiro, P.; Karavitaki, N. Dopamine agonist resistant prolactinomas: Any alternative medical treatment? Pituitary 2020, 23, 27–37. [Google Scholar] [CrossRef]
- Feng, Z.; Xia, Y.; Gao, T.; Xu, F.; Lei, Q.; Peng, C.; Yang, Y.; Xue, Q.; Hu, X.; Wang, Q.; et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis. 2018, 9, 1006. [Google Scholar] [PubMed]
- Guo, S.; Gu, Y.; Qu, J.; Le, A. Bridging the metabolic parallels between neurological diseases and cancer. Heterog. Cancer Metab. 2021, 229. [Google Scholar] [CrossRef]
- Gumireddy, K.; Li, A.; Kossenkov, A.V.; Sakurai, M.; Yan, J.; Li, Y.; Xu, H.; Wang, J.; Zhang, P.J.; Zhang, L.; et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat. Commun. 2016, 7, 10715. [Google Scholar] [PubMed]
- Carnero, A.; Paramio, J.M. The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front. Oncol. 2014, 4, 252. [Google Scholar]
- Garib, V.; Lang, K.; Niggemann, B.; Zänker, K.S.; Brandt, L.; Dittmar, T. Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells. Eur. J. Anaesthesiol. 2005, 22, 609–615. [Google Scholar]
- Hardwick, M.; Fertikh, D.; Culty, M.; Li, H.; Vidic, B.; Papadopoulos, V. Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: Correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999, 59, 831–842. [Google Scholar]
- Marwah, H.; Pant, J.; Yadav, J.; Shah, K.; Dewangan, H.K. Biosensor Detection of COVID-19 in Lung Cancer: Hedgehog and Mucin Signaling Insights. Curr. Pharm. Des. 2023, 29, 3442–3457. [Google Scholar]
- Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Santoni, G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013, 13, 344–366. [Google Scholar]
- Medina, V.; Croci, M.; Crescenti, E.; Mohamad, N.; Sanchez-Jiménez, F.; Massari, N.; Nuñez, M.; Cricco, G.; Martin, G.; Bergoc, R.; et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol. Ther. 2008, 7, 28–35. [Google Scholar] [CrossRef]
- Sterle, H.A.; Nicoud, M.B.; Massari, N.A.; Delgado, M.A.T.; Ducloux, M.V.H.; Cremaschi, G.A.; Medina, V.A. Immunomodulatory role of histamine H4 receptor in breast cancer. Br. J. Cancer 2019, 120, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Liu, X.-B.; Wang, L.; Kang, F.-B. B7-H4 overexpression contributes to poor prognosis and drug-resistance in triple-negative breast cancer. Cancer Cell Int. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ospital, I.A.; Delgado, M.A.T.; Nicoud, M.B.; Corrêa, M.F.; Fernandes, G.A.B.; Andrade, I.W.; Lauretta, P.; Vivot, R.M.; Comba, M.B.; Zanardi, M.M.; et al. Therapeutic potential of LINS01 histamine H3 receptor antagonists as antineoplastic agents for triple negative breast cancer. Biomed. Pharmacother. 2024, 174, 116527. [Google Scholar] [CrossRef]
- Souazé, F.; Dupouy, S.; Viardot-Foucault, V.; Bruyneel, E.; Attoub, S.; Gespach, C.; Gompel, A.; Forgez, P. Expression of neurotensin and NT1 receptor in human breast cancer: A potential role in tumor progression. Cancer Res. 2006, 66, 6243–6249. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Kang, Q. Neuromodulation of bone: Role of different peptides and their interactions. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Pouya, F.D.; Rasmi, Y.; Asl, E.R. Role of neurotransmitters and neuropeptides in breast cancer metastasis. Biochem. Suppl. Ser. A Membr. Cell Biol. 2020, 14, 107–116. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophage in breast cancer. J. Cell. Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef]
- Arnoux, A.; Dupuis, L. Serotonin in Amyotrophic and the Lateral 5-HT2B Sclerosis Receptor. 5-HT2B Recept. Mol. Biol. Clin. Appl. 2021, 35, 367. [Google Scholar]
- Joffe, H.; Partridge, A.; Giobbie-Hurder, A.; Li, X.; Habin, K.; Goss, P.; Winer, E.; Garber, J. Augmentation of venlafaxine and selective serotonin reuptake inhibitors with zolpidem improves sleep and quality of life in breast cancer patients with hot flashes: A randomized, double-blind, placebo-controlled trial. Menopause 2010, 17, 908–916. [Google Scholar] [CrossRef]
- Przybylo, M.; Borowik, T.; Langner, M. Fluorescence techniques for determination of the membrane potentials in high throughput screening. J. Fluoresc. 2010, 20, 1139–1157. [Google Scholar] [CrossRef]
- Tetzlaff, S.K.; Reyhan, E.; Layer, N.; Bengtson, C.P.; Heuer, A.; Schroers, J.; Faymonville, A.J.; Langeroudi, A.P.; Drewa, N.; Keifert, E.; et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell 2024, 188, 390–411.e36. [Google Scholar] [PubMed]
- Yin, T.; Duan, J.; Xu, D.; Huang, M.; Yin, D. Precision individualized medication strategies and challenges for cardiovascular diseases. Precis. Medicat. 2024, 1, 7–15. [Google Scholar] [CrossRef]
- Velikic, G.; Maric, D.M.; Maric, D.L.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the stem cell niche in regenerative medicine: Innovative avenue to combat neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira El-Warrak, L.; de Farias, C.M. Could digital twins be the next revolution in healthcare? Eur. J. Public Health 2024, 35, 19–25. [Google Scholar]
- Tilan, J.; Kitlinska, J. Sympathetic neurotransmitters and tumor angiogenesis—Link between stress and cancer progression. J. Oncol. 2010, 2010, 539706. [Google Scholar]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar]
- Li, R.Q.; Zhao, X.H.; Zhu, Q.; Liu, T.; Hondermarck, H.; Thorne, R.F.; Zhang, X.D.; Gao, J.N. Exploring neurotransmitters and their receptors for breast cancer prevention and treatment. Theranostics 2023, 13, 1109. [Google Scholar]
- Singh, T.; Sharma, K.; Jena, L.; Kaur, P.; Singh, S.; Munshi, A. Mitochondrial bioenergetics of breast cancer. Mitochondrion 2024, 79, 101951. [Google Scholar]


| Neuroreceptor | Associated Neurotransmitters | Expression in Breast Cancer | Functional Role in Breast Cancer | Impact on Membrane Potential | References |
|---|---|---|---|---|---|
| ß-adrenergic receptors | Norepinephrine and epinephrine | Overexpressed in breast cancer tissues | Promotes proliferation, migration, invasion, angiogenesis, and anti-apoptosis | Depolarization | [48,49,50] |
| Nicotinic (nAChRs) and muscarinic (mAChRs) receptors | Acetylcholine | α9-nAChR and α7-nAChR are highly expressed in triple-negative and advanced breast tumors; mAChRs are upregulated in breast tumors but absent in normal breast tissues | nAChRs enhance epithelial-to-mesenchymal transition, invasion, migration, and stemness; mAChRs inhibit tumor growth and promote anti-proliferative effects | Depolarization or Repolarization | [63,64,65] |
| 5-HT receptors | Serotonin | 5HTR2A and 5HTR3A are overexpressed in breast cancer tissues | Facilitates angiogenesis, proliferation, invasion, and autophagy | Depolarization | [67,68] |
| Dopamine receptors | Dopamine | Variable expression; some subtypes linked to tumor suppression while others promote progression | Modulates proliferation, invasion, and angiogenesis; influences prolactin secretion | Depolarization | [74,76] |
| GABA receptors | GABA | GABAA receptor α3 is overexpressed in breast cancer, particularly in invasive and metastatic cases | Promotes proliferation, migration, invasion, and activation of the AKT pathway | Repolarization | [78,79] |
| Histamine H4 receptor | Histamine | High expression correlates with better prognosis in triple-negative breast cancer | Reduces tumor growth, enhances apoptosis, and improves survival | Depolarization or repolarization | [84,85] |
| Neurotensin receptor (NTS-1) | Neurotensin | Overexpressed in approximately one-third of primary breast cancers | Promotes proliferation, invasion, migration, and resistance to apoptosis | Depolarization | [88,90] |
| Neuropeptide Y (NPY) receptors | Neuropeptide Y | Overexpressed in metastatic breast cancer tissues | Stimulates angiogenesis, proliferation, and metastasis | Depolarization | [89,90] |
| Neuropharmacological Identifier | Associated Neurotransmitters | Membrane Potential |
|---|---|---|
| NCT03108937, NCT04454515, NCT03109990, NCT02013492 | Norepinephrine and epinephrine | Depolarization |
| NCT01530373, NCT02312934 | Acetylcholine | Depolarization or repolarization |
| NCT02312934, NCT03122444, NCT00198250 | Serotonin | Depolarization |
| NCT01730729, NCT02312934, NCT02861859 | Dopamine | Depolarization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahapatra, C.; Gawad, J.; Bonde, C.; Palkar, M.B. Bioelectric Membrane Potential and Breast Cancer: Advances in Neuroreceptor Pharmacology for Targeted Therapeutic Strategies. Receptors 2025, 4, 9. https://doi.org/10.3390/receptors4020009
Mahapatra C, Gawad J, Bonde C, Palkar MB. Bioelectric Membrane Potential and Breast Cancer: Advances in Neuroreceptor Pharmacology for Targeted Therapeutic Strategies. Receptors. 2025; 4(2):9. https://doi.org/10.3390/receptors4020009
Chicago/Turabian StyleMahapatra, Chitaranjan, Jineetkumar Gawad, Chandrakant Bonde, and Mahesh B. Palkar. 2025. "Bioelectric Membrane Potential and Breast Cancer: Advances in Neuroreceptor Pharmacology for Targeted Therapeutic Strategies" Receptors 4, no. 2: 9. https://doi.org/10.3390/receptors4020009
APA StyleMahapatra, C., Gawad, J., Bonde, C., & Palkar, M. B. (2025). Bioelectric Membrane Potential and Breast Cancer: Advances in Neuroreceptor Pharmacology for Targeted Therapeutic Strategies. Receptors, 4(2), 9. https://doi.org/10.3390/receptors4020009






