Abstract
For humans to explore and colonize the universe, both engineering and physiological obstacles must be successfully addressed. A major physiological problem is that humans lose bone rapidly in microgravity. Understanding the underlying mechanisms for this bone loss is crucial for designing strategies to ameliorate these effects. Because bone physiology is entangled with other organ systems, and bone loss is a component of human adaptation to microgravity, strategies to reduce bone loss must also account for potential effects on other systems. Here, we consider the receptors involved in normal bone remodeling and how this regulation is altered in low-gravity environments. We examine how single cells, tissues and organs, and humans as a whole are affected by low gravity, and the role of receptors that have been implicated in responses leading to bone loss. These include receptors linking cells to the extracellular matrix and to each other, alterations in the extracellular matrix associated with changes in gravity, and changes in fluid distribution and fluid behavior due to lack of gravity that may have effects on receptor-based signaling shared by bone and other regulatory systems. Inflammatory responses associated with the environment in space, which include microgravity and radiation, can also potentially trigger bone loss.
Keywords:
osteoclast; osteoblast; osteocyte; RANKL; RANK; osteoprotegerin; sclerostin; Wnt; β-catenin; integrin 1. Introduction
Humans are adapted to life experienced in Earth’s gravity. It was noted in the earliest space flights that astronauts and cosmonauts lost bone rapidly [1,2,3,4]. The bone loss detected varied with the location and type of bone and was sufficient to be a major potential roadblock to human space travel [5]. The mechanisms underlying this response are under intense scrutiny in order to identify the best approaches to counteract the deleterious effects of low gravity on bone. Addressing these questions is vital for the future of space exploration.
Bone is continually remodeled [6]. This is important for maintaining bone strength, adapting to the forces applied, and repairing both micro- and macro-damage. It is also intimately linked to overall systemic physiology, particularly calcium and phosphate regulation. Bone remodeling can, usefully, be considered to be the province of three specialized cell types, although, as we will see, various other cell types and systemic regulation systems impinge on the basic bone remodeling cycle (Figure 1). Below we describe the cells that are central to the bone remodeling process and the core mechanisms that regulate bone remodeling.
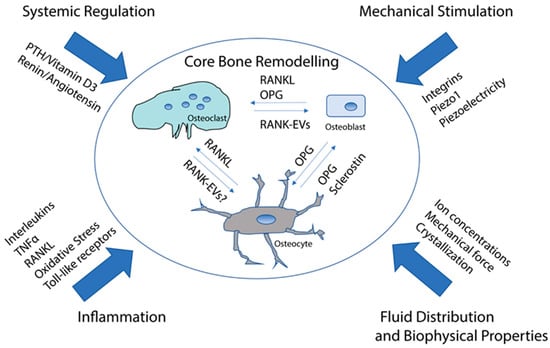
Figure 1.
Simplified overview of the regulation of bone remodeling. Osteocytes positively regulate osteoclast differentiation with RANKL and negatively regulate osteoclasts with OPG. Osteocytes regulate osteoblasts with sclerostin, which blocks Wnt/beta catenin signaling, which stimulates osteoblasts to mineralize, as well as with OPG, which binds to RANKL and would be expected to inhibit RANKL reverse signaling. Osteoclasts regulate osteoblasts with RANK-containing EVs, which stimulate mineralization. RANK-EVs may also regulate osteocytes. This regulation is modified by various types of signals that either promote or inhibit bone resorption/bone formation or both. Microgravity has direct or indirect effects on most or all of the regulation. See text for detailed discussion.
The current article examines bone loss in microgravity, focusing on receptors involved at various levels of regulation implicated in bone loss. We also direct the reader’s attention to an excellent short review of the literature, which includes a detailed analysis of methods for simulating microgravity [7]. A very recent review examines mechanotransduction pathways, including in microgravity, in greater detail than we provide [8].
We refer to microgravity simulations extensively in this review. In addition to Man, et al. [7], other articles are available for readers seeking a more detailed understanding of the strengths and weaknesses of various approaches to simulating microgravity [9,10].
2. Cells and Signaling Directly Involved in Bone Remodeling
2.1. Osteoclasts
Osteoclasts are cells of the hematopoietic lineage that are close relatives of dendritic cells [11]. They differentiate as the result of stimulation by a transmembrane protein, namely, the Receptor Activator of Nuclear Factor κB-Ligand (RANKL) [12,13]. RANKL (formerly known as osteoprotegerin-ligand, OPGL) is derived mostly from a second cell type, the osteocyte [14,15,16,17]. RANKL stimulates its receptor, Receptor Activator of Nuclear Factor κB (RANK), a transmembrane protein and member of the tumor necrosis factor (TNF) receptor superfamily that is present on the surface of osteoclast precursors and osteoclasts [18]. RANKL is a member of the TNF superfamily [13]. Like other members of the TNF superfamily, RANKL can be cleaved by exoproteases to release a soluble form [19].
RANKL binding to and stimulating RANK is required for osteoclast differentiation, activity, and survival [12]. RANKL is found in other types of cells, including T cells and B cells [13,20,21]. RANK is found in cells including dendritic cells and microglia [22,23]. RANKL and RANK are required for osteoclast formation. Knockout of these genes in mice leads to osteopetrosis because osteoclasts do not form [13]. Knockout mice also have alterations in immune cells and lack lymph nodes [13]. At a rudimentary level, osteoclast formation is based on the relative levels of RANKL to RANK. The more RANKL stimulation, the more osteoclasts and bone resorption, if all else is equal. This system is directly modulated by osteoprotegerin (OPG), also a member of the TNF receptor superfamily but lacking a transmembrane domain. OPG binds RANKL and competitively inhibits its binding to RANK [12,24].
Osteoclasts also require the stimulation of the colony-stimulating factor-1 receptor, c-Fms, although this is not as selective for the osteoclast lineage [25,26]. While the RANKL/RANK system directly regulates osteoclasts, c-Fms stimulation is required; however, it is not thought to be regulatory [26].
Osteoclasts carry other receptors, including receptors for various chemokines and cytokines, and also several toll-like receptors [27,28]. Stimulation of many of these receptors modifies the response of osteoclasts to RANKL. As examples, the stimulation of the TNF-alpha receptor and Toll-like receptor 4 synergize with RANKL, at least under some conditions, to increase the osteoclasts’ response to RANKL [29,30].
When osteoclasts contact bone under permissive conditions, they develop a complex and unique structure called the resorption complex [11]. The primary components of the resorption complex are the actin ring and the ruffled membrane (ruffled border) (Figure 2). The actin ring is composed of an organized assembly of individual structures called podosomes, which are built around microfilaments that are oriented perpendicular to the bone [31,32]. These podosomes undergo continuous polymerization at the cytosolic face of the plasma membrane, where they contact the bone and depolymerize in the cytosol [33,34]. Polymerization produces force pushing against the membrane using the connections with the larger cytoskeleton of the cell as anchorage [35]. The podosomes force the plasma membrane to conform to the bone surface, forming a tight “seal” with the bone [35]. Structures with similar or identical composition to podosomes (which are also referred to as invadopodia) occur in other cell types and are associated with the cell’s invasion through an unmineralized matrix [36,37]. For example, metastatic cancer cells utilize podosomes to escape from their site of origin [36,38]. When migrating through an unmineralized matrix, the podosomes function individually or in unstructured arrays. Osteoclasts, however, must essentially migrate through a mineralized matrix—the bone—and this requires a more elaborate and specialized usage of the podosomes [35]. They are arranged into a ring and press the membrane into the bone. This is resisted by focal adhesions mediated by integrins, notably, alphaV beta3 and alphaV beta5 [39,40,41], elsewhere on the osteoclast’s contact surface with the bone. These integrins also provide signals that are involved in the activation of the osteoclasts to resorb. The actin ring functionally segregates a region of the plasma membrane that becomes the ruffled border and an extracellular resorption compartment that is separate from the general extracellular milieu [42].
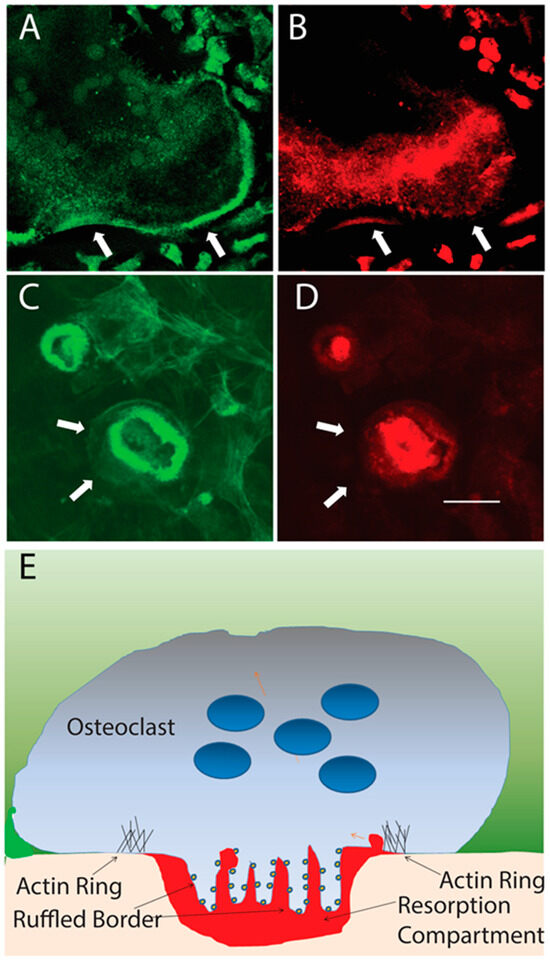
Figure 2.
Resting and resorbing osteoclasts. (A,B). Inactive osteoclast on coverslip stained with phalloidin to detect actin filaments (green) and anti-E subunit of V-ATPase (red). V-ATPase is present in vesicles in the cytosol. Actin filaments are spread through the cytosol and an actin belt is located at the periphery of the cell (arrows). (C,D.) Resorbing osteoclasts on a bone slice. The actin ring is not at the periphery of the cell (arrows). Outside the actin ring attachments are made between the cells and the bone with integrins and bone matrix. These resist the force produced by the actin ring pushing the membrane into the bone. V-ATPase is in the plasma membrane (ruffled border) which is bounded by the actin ring. A second smaller resorbing osteoclast is on the upper left of (C,D). Scale bar = 100 microns in (A,B) and 20 microns in (C–E). Schematic of a vertical section through a resorbing osteoclast. The flesh color is bone, red is the acidified resorption compartment. V-ATPases (small dark blue circles) stud the ruffled border. Light blue is the osteoclast, dark blue is nuclei, and green is the extracellular milieu.
The specialized ruffled border that forms is packed with an enzyme called the vacuolar H+-ATPase (V-ATPase) [43]. V-ATPases are found in all cells and are essential housekeeping enzymes tasked with acidifying lysosomes, endosomes, and Golgi compartments [44]. Normally, V-ATPase is present at low levels. V-ATPases are composed of at least 16 different proteins organized into a complex with 29 subunits [45,46]. Like the ATP synthase (F-ATPase) in mitochondria, a close relative, V-ATPases are rotary motors [44]. Unlike F-ATPases, which use a proton gradient to drive ATP synthesis, V-ATPases utilize ATP hydrolysis to pump protons against an electrochemical gradient. The V-ATPase, normally present at low levels, is hugely (two orders of magnitude at least) upregulated in osteoclasts, and two subunits of the V-ATPase have osteoclast-selective isoforms expressed [44,47,48].
V-ATPases in the ruffle membrane pump large numbers of protons into the sealed resorption compartment to maintain the pH at 5.0 or lower [42,43]. This is required to solubilize bone minerals and provide a suitable environment for the activity of cathepsin K, an acid cysteine proteinase that is secreted by the osteoclast into the resorption compartment [49]. To prevent alkalization of the osteoclast cytosol, protons are generated through carbonic anhydrase II, which converts carbon dioxide from mitochondrial metabolism to protons and bicarbonate [50]. Glycolysis, which is coupled directly to V-ATPases through aldolase, also generates protons as ATP [51,52].
The pumping of protons across the plasma membrane creates membrane potential. Unless that potential is dissipated, the V-ATPase is unable to lower the pH below about 6.0, which is not sufficient to resorb bone efficiently. A voltage-gated chloride channel, CLC-7, and its subunit, OSTM1, respond to the increasing voltage generated by the V-ATPases activity by allowing chloride ions to escape, neutralizing the membrane potential [53,54]. The chloride ions are replenished in the cell through a chloride/bicarbonate exchanger, which also releases the bicarbonate generated by carbonic anhydrase as it produces protons. The validity of this scheme is well supported by mutations in the osteoclast-selective a3 subunit of V-ATPase, in CLC-7, OSTM-1, and in carbonic anhydrase 2, which all lead to defective osteoclastic bone resorption and osteopetrosis [53,54,55,56].
As would be expected for an enzyme of central importance to bone remodeling, direct links to osteoporosis and bone loss in microgravity have been reported. The regulatory subunit ATP6V1H has been tied to osteoporosis, first through single nucleotide polymorphism analysis of osteoporotic patients, and then by knocking out an allele in a mouse model, which showed an osteoporotic phenotype [57]. A mouse hind limb unloading model, which is one method for simulating microgravity, showed that the gene was associated with bone loss in unloaded bone [58].
Osteoclasts contain beta1, beta3, and beta5 integrins [59,60,61]. Alpha2 beta1 is the most common beta1 integrin [62]. Beta3 and beta5 integrins are paired with alphaV [63,64,65]. Since alpha2 beta1 binds native collagen and alphaV beta3 and alphaV beta5 bind arginine–glycine–aspartic acid (RGD) sequences that are cryptic in native collagen but exposed when collagen is denatured by the actions of interstitial collagenase or structural damage, it has been suggested that integrin switching is a major controller of osteoclast activation [66]. For example, the action of interstitial collagenase was shown to stimulate osteoclast activation through denaturing type I collagen and exposing cryptic RGD sequences [67,68,69].
Both Beta3 and Beta5 integrins have been shown to be involved in regulating osteoclastic activity in vitro and in mice [40,41]. Disruption of Beta3 and Beta5 leads to different consequences with respect to osteoclast maturation, activity, overall bone remodeling, and sensitivity to reduction in estrogen in female mice. Glanzmann’s Disease, which has a bone phenotype, is caused by specific mutations in Beta3 integrin [70]. As we will see in Section 5.1, the integrin signaling pathway has been implicated in low-gravity bone loss [58,71,72,73,74,75,76,77]; however, the presence of these specific integrins, and others that use similar downstream signaling pathways in various cell types, and the multifarious effects microgravity has on humans, make it difficult to specifically identify the contributions of different cells and integrins to the loss of bone.
Because bone is lost, it is clear that at least some osteoclast activity is present in microgravity, and resorption by osteoclasts is not adequately coupled to new bone formation. Changes in osteoclast activity, if any, could be the result of increased activity of osteoclasts, or increased numbers of osteoclasts.
A direct method of testing whether osteoclasts are affected by microgravity was performed by testing the ability of recombinant RANKL-stimulated Raw 264.7 osteoclast-like cells capacity to differentiate and resorb osteologic discs (glass-discs covered with a proprietary hydroxyapatite mineral) which serve as a convenient substrate for osteoclasts [78]. RAW 264.7 cells comprise a mouse monocyte/macrophage cell line that does not require CSF-1 [79]. When they are at the correct starting density and are stimulated with excess recombinant RANKL, these cells differentiate into cells that morphologically and biochemically resemble osteoclasts. When tested in space flight, these cells were more effective at resorbing osteologic discs than identical cells at Earth gravity. This suggests that, under the same level of stimulation, osteoclasts may resorb more efficiently in microgravity. However, this system is an imperfect model for bone resorption. We have used Raw 264.7 cells extensively in our research; however, unlike primary osteoclasts, we have not detected V-ATPase-packed ruffled membranes. Nor, in our hands, do they form pits on bone slices.
It has been reported that rats and mice in space have the same or more osteoclasts in microgravity compared with littermates on Earth, which suggests that regulatory signals leading to osteoclast differentiation and survival may be enhanced in microgravity [80]. Although data are not definitive, osteoclast numbers and activity are probably similar or higher in microgravity compared to Earth [80], which helps account for observed bone loss.
2.2. Osteoblasts
Osteoblasts are mesenchymal lineage cells specialized to form bone [81]. Osteoblasts express RANKL and for many years it was thought that this RANKL was primarily responsible for stimulating osteoclasts to resorb bone [82]. They also produce OPG, which binds RANKL and competitively inhibits the binding between RANKL and RANK [82]. This suggested that osteoblasts might be central controllers of bone remodeling by modulating both the stimulation of bone resorption positively and negatively and forming bone to replace bone that was removed. This was thought to be controlled by osteocytes, which modulated remodeling indirectly. However, twin studies in 2011 provided evidence that the RANKL that stimulates osteoclasts is mostly derived from osteocytes [15,16]. This is discussed below in more detail. Thus, the central regulatory system controlling bone remodeling is more complex than originally thought, and the roles of osteoclasts and osteoblasts, and especially osteocytes, still require further clarification.
Osteoblasts respond to a variety of cytokines and hormones. Key among these is parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 (vitamin D3) [83,84]. These hormones link bone remodeling to systemic calcium and phosphate regulation. Crucial to osteoblast differentiation are low-density lipoprotein receptor-related protein (LRP)5 and LRP6 [85,86,87]. These receptors signal through the Wnt/Beta catenin pathway to promote bone formation [88]. Mutations in LRP5 and LRP6 lead to very high bone density in humans [89,90,91]. An important regulator of Wnt/beta catenin regulation in osteoblasts is sclerostin, which is produced by osteocytes and is discussed in greater detail below [92].
Osteoblasts go through a series of differentiation steps prior to becoming bone-forming osteoblasts [93]. Like osteoclasts, an important cell adhesion molecule receptor that is involved in regulation is alphaV beta3 integrin. Osteoblasts also express Beta1 integrins [94,95]. An interesting recent finding is that a secreted growth factor called Nell-1, which synergizes with Wnt/beta catenin signaling to promote bone formation, acts through binding to Beta1 integrins [96,97]. This is a target for therapy to prevent bone loss in microgravity. Various other factors regulate osteoblast differentiation including hormones and various cytokines [98,99,100,101] (Figure 3A). Elements of the renin/angiotensin signaling network (RAS) also regulate osteoblasts [102,103]. Both osteoblasts and osteoclasts carry the angiotensin 2 receptor. Evidence suggests that local RAS is important for regulating bone remodeling, but systemic RAS may also play a role. This is of interest as systemic RAS responds to the low gravity environment [104,105].
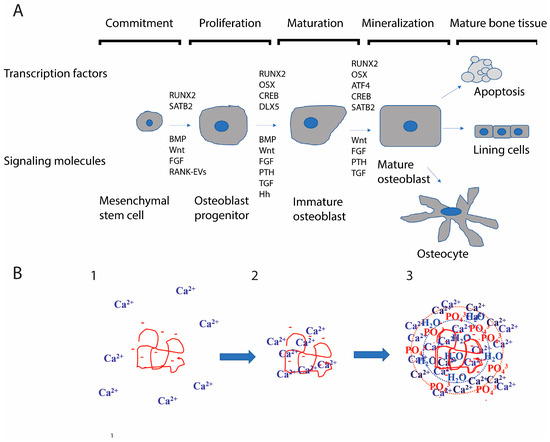
Figure 3.
Osteoblast differentiation and mechanism by which osteoblasts mineralize bone: (A) Osteoblasts differentiate from multipotent mesenchymal stem cells through a number of steps. The schematic shows transcription factors that are crucial and signaling molecules involved. Note that while Wnt/β-catenin is vital for osteoblast differentiation, various other factors contribute to differentiation. Each differentiation stage contributes differently to signaling that occurs in the local bone microenvironment. Osteocytes, the final differentiation stage of the osteoblast and the most abundant cells in bone modulate the differentiation of the immature osteoblasts at every stage. (B) PILP mineralization mechanism: 1. A protein with a stretch of acidic amino acids and calcium ions. 2. The acidic region recruits calcium ions. 3. A larger shell of calcium ions, phosphate ions, and water; the PILP droplet develops. This infiltrates the collagen matrix and then forms a nano-crystal within the matrix. Altered fluid dynamics may affect this crystallization process. It is known that larger and more symmetrical crystals are formed in microgravity. Subtle alterations in PILP crystallization (for example, the formation of slightly larger crystals) could have profound consequences for the quality of bone.
Osteoblasts also respond to piezoelectricity, which emanates from the response of bone to forces applied [106,107,108]. Voltage-gated channels on osteoblasts have been shown to respond to piezoelectric stimulation. This is, therefore, potentially impacted in low gravity. As we will see, osteocytes, the terminal differentiation state of the osteoblasts, which are the most abundant cells in bone and form a network within the bone, may be the primary direct target of low gravity-induced changes in piezoelectricity [108].
Bone formation by osteoblasts is still not fully understood. It seems to involve a process called polymer-induced liquid precursor (PILP) [109,110]. Bone is composed of tiny, nanometer-scale crystals of hydroxyapatite deposited within a matrix that is primarily composed of type I collagen [110]. This produces strength and resistance to catastrophic fractures. Bone-associated proteins like osteopontin and bone sialoprotein have long stretches of acidic amino acids and can also be heavily phosphorylated [111,112,113]. The PILP process suggests that these acidic proteins gather a shell of calcium ions that resist crystal formation until they infiltrate into the dense collagen matrix [110,114] (Figure 3B). The PILP droplet then allows the tiny nanocrystals to form within the organic scaffold.
Does low gravity affect the PILP process, and might this have something to do with bone loss? Direct tests have not been performed; however, it is of interest, as both reduced bone [115,116,117] and increased risk of kidney stones [118,119], also formed by PILP, are risks for humans in space. The latter presumably arises in large measure due to increased systemic calcium but is also associated with PILP-induced crystallization [120]. Altered fluid dynamics, as described in a subsequent section, could affect the efficiency of the PILP process. Although the growth of crystals, in general, is enhanced because of density differences, fluid flows and sedimentation are reduced [121,122,123]; moreover, it is not known how this affects the crystallization of minerals in bone. It has been reported that bone maturation is disrupted in microgravity [124]. Moreover, changes in circulating calcium, whatever the mechanism, will have effects on PTH and vitamin D3 signaling, which, in turn, will affect bone remodeling, the uptake of calcium in the intestines, and the loss of calcium in urine [125]. In humans, microgravity causes the net release of calcium from the bones, leading to the suppression of parathyroid hormone (PTH) and a decrease in the levels of circulating vitamin D3. As a result, calcium absorption in humans is reduced [126,127].
2.3. Osteocytes
Osteocytes are the most abundant cells in bone; yet, until recently, they have been the least understood [128,129]. They are a further differentiation stage of osteoblasts and become encased in bone, existing in a lacuna that permeates the bone [128]. The appearance and organization of osteocytes in bone resembles a neural network, and it has been long hypothesized that they sense changes in the bone, for example, force applied, and regulate the actions of osteoclasts and osteoblasts in order to respond [130]. Osteocytes consist of a cell body, and long dendrite-like processes extending into small lacunas establishing communication networks within the bone. It has been reported that osteocytes undergo apoptosis within three days of exposure to microgravity during spaceflight, resulting in a higher number of unfilled lacunae [131,132].
The idea that osteocytes sense changes in the bone and regulate responses has proven true, but only in the past two decades have some of the underlying mechanisms been uncovered. As mentioned earlier, osteocytes are known to control bone remodeling. First, most (90%) of bone resorption is stimulated using RANKL from osteocytes [15,16,17]. This holds true for normal bone remodeling and also induced bone remodeling stimulated using periodontal disease or orthodontic force application [133,134,135]. The pharmaceutical denosumab (Prolia, Xgeva), which is a humanized monoclonal that binds RANKL and prevents RANKL-RANK binding in a manner conceptually similar to OPG, is thus presumably acting to inhibit osteoclast formation primarily by binding RANKL from osteocytes [136]. Denosumab is being tested to prevent bone loss in microgravity [137].
Second, sclerostin from osteocytes controls osteoblast differentiation by blocking Wnt/Beta catenin signaling through LRP5 and LRP6 [92,128] (Figure 4). The pharmaceutical romosozumab (Evinity) is a humanized monoclonal antibody that binds sclerostin and prevents its interaction with LRP5/6, counteracting the effects of sclerostin. This allows osteoblasts to progress freely toward the differentiation state where mineralization occurs [138,139]. Interestingly, osteocytes also require Wnt/beta catenin signaling and may also be a target of sclerostin [140]. The knockout of beta-catenin specifically on osteocytes mostly blocked the accrual of bone mass and led to early death in mice [141]. Osteoclasts were overabundant, and OPG was expressed at low levels. Thus, osteocytes may be an important source of regulatory OPG downstream of WNT/beta catenin signaling.
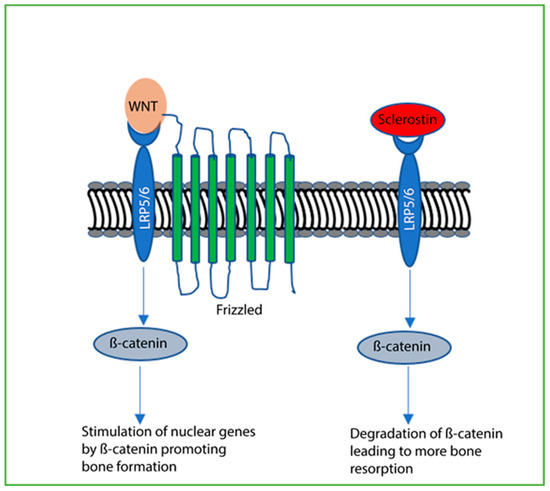
Figure 4.
Sclerostin blocks Wnt/β-catenin signaling. When Wnt binds LRP5 or LRP6, Frizzled is recruited and β-catenin stimulates nuclear genes that, ultimately, promote bone formation. When sclerostin binds LRP5 or LRP6 in place of Wnt, Frizzled is not recruited, and β-catenin is degraded. Bone formation is blocked and increased bone resorption occurs.
Osteocytes, like osteoblasts and osteoclasts, contain both Beta1 and Beta3 integrins [142,143,144,145,146]. Beta1 integrins are paired with alpha2, alpha3, alpha4, and alpha5, while beta3 integrin is mostly paired with alphaV. Recent results have shown that the knockout of integrin beta3 in osteocytes resulted in reduced bone formation [143]. Knockout of beta 1 integrin in osteocytes also reduced bone formation and impaired mechanotransduction [145].
Exposure to microgravity during spaceflight has been discovered to hinder the process of osteoblast differentiation into osteocytes. This results in the underdevelopment of Golgi complexes in osteocytes, which are responsible for secreting matrix proteins. As a consequence, there is a delay in the mineralization of the bone matrix [132].
Osteocytes have been shown to be affected by weightlessness [132]. In mice, osteocytes are triggered to undergo apoptosis via microgravity [147]. Mechanical stimulation, integrin, Src activity, and ERKs protect osteocytes from apoptosis [148]. Studies have implicated beta1 integrin as contributing to the protection against apoptosis in osteocytes [144].
2.4. RANKL and RANK in Extracellular Vesicles
Until recently, it was thought that the key set of interactions in bone biology was RANKL, either on the surface of osteoblasts or solubilized by an exoprotease, interacting with RANK on the surface of osteoclast lineage cells. Soluble OPG, then, would modulate that interaction by serving as a decoy receptor for RANKL. However, in the last decade, it has emerged that both RANKL and RANK are packaged into extracellular vesicles (exosomes or microvesicles), tiny 30–150-nm vesicles released from cells (Figure 5) [149,150]. RANKL in extracellular vesicles can stimulate RANK to trigger osteoclast differentiation and activity [151,152,153]. This may represent a solution to the conundrum of how RANKL (a transmembrane protein) from osteocytes could stimulate most osteoclast differentiation [129]. It is not clear that osteocytes are ever in contact with osteoclasts. Extracellular vesicles from osteocytes, like sclerostin, which is a soluble cytokine, could, in principle, migrate through lacuna to activate osteoclasts, although this has yet to be confirmed.
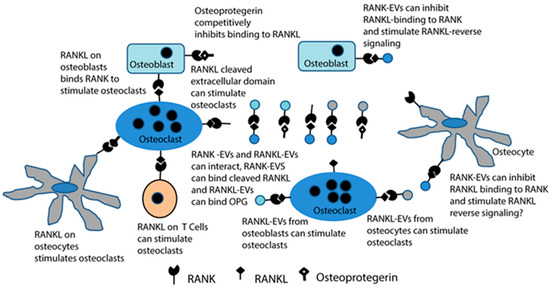
Figure 5.
Extracellular vesicles containing RANKL or RANK add increased complexity to the RANKL/RANK/osteoprotegerin signaling network that is at the core of bone biology. Until recently, RANKL stimulation of RANK to stimulate osteoclast formation and bone resorption by osteoclasts, and the ability of osteoprotegerin to bind RANKL and competitively inhibit this signaling, were considered the primary features of this network. Now, it is known that osteocytes contribute most of the RANKL to stimulate osteoclasts, either directly or by producing RANKL-EVs. RANKL-EVs can stimulate osteoclast formation and bone resorption through RANK stimulation, and RANK-EVs bind to RANKL on osteoblasts to stimulate RANKL reverse signaling and bone formation. The latter serves to couple bone resorption and bone formation. It is also possible that RANK-EVs can bind RANKL or RANKL-EVs and competitively inhibit their stimulation of RANK on osteoclasts. RANKL-EVs could serve as competitive inhibitors of RANK-EV’s stimulation of RANKL reverse signaling.
RANK in extracellular vesicles from osteoclasts binds to RANKL to stimulate a RANKL reverse signaling pathway that pushes the osteoblastic lineage cells toward maturation [154,155]. RANKL on osteoblasts, which are also typically separated from osteoclasts physically, would then be viewed primarily as receptors rather than ligands. It is not known if RANKL on osteocytes also responds to RANK-containing EVs [154].
In the context of microgravity, this raises the question of whether the release of extracellular vesicles changes in low gravity. If so, this may influence bone remodeling outside of other considerations. There have been several studies of extracellular vesicle release in simulated or real microgravity from various cell types, mostly tumor cells [156,157,158]. These studies suggest that there are significant changes in the number of extracellular vesicles shed as well as in the overall composition of the extracellular vesicles in microgravity. It is plausible that changes in the shedding of extracellular vesicles, simply due to microgravity, may contribute to, and perhaps even be a primary factor in, bone loss in low gravity. For example, changes in the biophysics of fluid behavior in microgravity may be sufficient to alter the rate of release and the composition of regulatory extracellular vesicles released by bone cells. This hypothesis warrants further testing.
3. Patterns of Human Bone Loss in Low Gravity
It is well known that humans lose bone on space missions, as much as 1–2% of bone mass per month in weight-bearing bones [159]. However, this bone loss does not occur equally throughout the body. There is, in fact, a significant gain in bone density in the skull [160], only small losses in the upper limbs, and greater losses in bone in the pelvic region and lower limbs [161,162,163,164,165]. This may be partially attributable to mechanical loading. However, there are a number of findings that are not consistent with Frost’s mechanostat hypothesis [166,167,168]. The mechanostat is a useful concept for explaining how mechanical loading influences the mass (amount of bone) and architecture (bone’s arrangement). The underlying idea is that, in the absence of disease, bone adapts to prevent fracturing in response to normal activity. Elements of the response to spaceflight that are not consistent with the mechanostat hypothesis include the fact that exercise does not fully protect against bone loss. This conclusion is based on a metanalysis from 2020 [159]. The bone gain in the skull [160], which is mechanically neutral, is not consistent with the mechanostat hypothesis. There is also no reduction in increased bone resorption over time, inconsistent with the mechanostat hypothesis, which predicts that, after unloading, the bone should adapt and resorption levels should fall [168].
Other factors have also been considered and tested that might underly bone loss in microgravity. These include calcium homeostasis [169], stress [170,171], altered metabolism [172,173], immune responses [174,175,176,177], systemic oxidative stress [178,179], changes in fluid distribution and composition [180,181,182,183], and radiation [184,185]. An examination of these potential regulators, which are discussed in more detail in subsequent sections, does not provide a clear picture. There is still room for new ideas, like altered extracellular vesicle release due to low gravity. Indeed, it is possible that there are significant missing pieces to our current understanding.
Data from long-term space missions suggest that bone loss moderates over time [159]. Bone loss during the longest missions was no worse than missions that were half as long. This observation, however, may be influenced by different exercise programs, the taking of anti-osteoporotic medications, different nutrition, and the genetic characteristics of the people involved [159]. Upon returning to normal gravity most, but not all, of the lost bone is recovered, although this takes several years [186]. Unfortunately, recovery of strength at some weight-bearing sites does not adequately occur [186,187].
Finally, it is worth noting that the loss of bone is variable, suggesting that some people are better suited for long-term missions in low gravity than others [159]. Recent studies of muscle and bone loss in mice in simulated microgravity suggest a genetic basis for differences [188,189], consistent with the idea that bone loss in microgravity may be influenced by genes. In the future, astronaut selection may, in part, depend on genetic characteristics that limit pathological responses to low gravity and other adverse, but unavoidable, elements (radiation for example) of space flight [190].
4. Changes in Fluid Distribution and Circulation in Low Gravity
Gravity produces forces that profoundly affect fluids on Earth [191,192]. Since human beings are typically 55–70% water [193], it is not surprising that low gravity experienced by space travelers affects their cardiovascular system, lymphatic system, interstitial fluid distribution, and even the fluid distribution inside cells.
Upon entering a low-gravity environment, the shape of the heart changes, and stroke volume and cardiac output increase [182,194,195]. The heart does not have to work as hard to pump blood, and, over time, cardiac atrophy occurs. Blood volume decreases. This is regulated by atrial natriuretic peptide (ANP), its receptor, natriuretic peptide receptor-A (NPR-A), and through changes in signaling through the renin/angiotensin/aldosterone system [104]. Microgravity-induced changes in the cardiovascular system also trigger increases in oxidative stress and inflammatory biomarkers [194]. All of these changes could include crosstalk with bone remodeling regulation, as bone cells have receptors and are controlled by many of the same signals involved in cardiovascular and immune regulation [27].
In addition to profound changes in the cardiovascular circulation, the lymphatic system is also altered by low gravity [196,197,198]. The lymphatic system is responsible for the disposal of excess fluid, cells, and metabolic waste. It also contributes to immune vigilance and in this role serves the innate immune system [174]. Severe problems with the lymphatic system manifest as edema. In particular, space flight is associated with cephalad fluid shifts and facial and intracranial edemas, termed spaceflight-associated neuro-ocular syndrome [199].
At the cellular level, changes in fluid distribution may lead to cytoskeletal filament reorganizations [77,181,200]. Since the cytoskeleton is linked to cell polarity, cell adhesions, and signaling associated with cell–cell and cell–matrix adhesions, as well as from ligand receptors, such nanoscale alterations could represent major players in the changes that trigger microgravity-induced bone loss [77,201,202,203].
5. The Effect of Microgravity on Cell–Cell and Cell–Extracellular Matrix Interactions
5.1. Low Gravity Effects on the Matrisome
The matrisome consists of core components, collagens, proteoglycans and extracellular matrix glycoproteins, and other components of the extracellular matrix (ECM) including collagenases and other kinases and phosphatases that modify the matrix, as well as secreted factors associated with the extracellular matrix [204]. The matrisome interacts with cells through transmembrane receptors, most commonly integrins [205], which stimulate changes in the cell’s behavior, including cytoskeletal reorganizations, the stimulation of signaling pathways, and changes in gene expression [206].
The matrisome has been hypothesized to be a gravity sensor [74]. There are various potential mechanisms. Changes in matrix protein expression could result in the altered physical support of cells and altered signaling to the cells. There are many examples of altered matrix protein expression in microgravity studies [72,73,207,208]. In bone, changes in acidic matrix proteins like osteocalcin and osteopontin could alter mineralization rates and quality due to their key role in the PILP process described earlier [110]. The expression of proteinases and phosphatases could trigger remodeling of the ECM and affect signals leading to bone remodeling [209,210]. For example, increased collagenase expression could produce signals that stimulate increased activation of osteoclasts [68]. All of these changes in matrisome protein expression have been observed either in space flight or in various low-gravity-simulating model systems [74].
The matrix also produces piezoelectricity in response to force application. This is primarily due to the deformation of collagen fibers [107]. The accumulated charge has been shown to direct osteoblasts to specific locations and stimulate their activity [106,107,211,212]. Microgravity would be expected to alter piezoelectric stimulation.
Mechanical force applied to a cell’s membrane, for example, when adhered to the matrisome when the matrisome is deformed by mechanical force, stimulates the mechanical sensor Piezo1 [213,214]. The force causes Piezo1 to open and allow an influx of Ca2+ ions, which trigger signal transduction pathways that regulate differentiation, development, and bone formation by osteoblasts.
The sensing mechanisms described above could deliver signals either directly due to forces (or lack thereof) or could respond to the redistribution of fluid that occurs in low gravity. The second idea is attractive given the increase in bone density in the skull, where there is increased pressure compared with Earth’s gravity [159,215].
5.2. Evidence for Integrin-Mediated Response to Low Gravity
Soon after their discovery, integrins were postulated to be elements of the mechanisms that lead to microgravity-induced bone loss. As discussed above, bone cells are known to contain and be regulated by specific integrins. In 1995, it was reported that the integrin LFA1 (alpha L beta 2 integrin) alters their distribution in space flight [216]. Studies have subsequently shown that osteoblasts in low gravity or low gravity simulation lead to reductions in cell adhesions through Beta 1 integrins, and this results in changes in the extracellular matrix, decreased integrin expression, changes in the cell cycle, and changes in focal adhesions [71,73,217]. Alterations in cell adhesion, integrin expression, as well as the downstream signaling of integrins, have been described in various types of cells as the result of microgravity [58,73,74,76,77,218,219].
Based on evidence that integrin responses are involved in bone loss associated with microgravity, studies using simulated microgravity indicated that several pathways regulated by integrins, through focal adhesion kinase (FAK), mTORC1, AMPK, and ERK1/2, are affected by low gravity in an osteoblast-like cell line [220]. Microgravity was then shown to downregulate FAK, Wnt/Beta catenin, and signaling downstream of Wnt/beta catenin. As a consequence, several markers of osteoblast differentiation and bone formation were decreased. In a hind limb unloading model, bone loss was observed, and this was prevented by using an activator of FAK [220]. Although this pathway may contribute to bone loss in low gravity, FAK is crucial for many cell types under many conditions, and unless an FAK-targeting treatment could be focused on osteoblasts, this does not appear to provide a route for preventing bone loss in low gravity.
5.3. Cell–Cell Adhesions and Tight Junctions in Low Gravity
There is considerable evidence that in addition to cell–ECM adhesions, cell–cell adhesions are also perturbed by low gravity [77]. One area where that is important is in the behavior of cancer cells. Reduction in cell–cell adhesion may make metastatic cancers more probable in low gravity [76,221,222,223]. This is combined with higher radiation, which could lead to an increased appearance of cancer—a serious problem on long missions [76,224]. Such perturbations might affect the behavior of one or more of the types of bone cells [225].
Low gravity simulation and spaceflight have been shown to negatively impact the blood–brain barrier. Proteomics showed alterations in the expression of cell–cell adhesion molecules and Zonula occludins-1, a key component of tight junctions [226].
Endothelial leaks are also observed in astronauts, and together with alterations in the heart and reductions in Mg2+ due to malabsorption, lead to dehydration, and all promote increased oxidative stress, which is linked to bone loss in osteoporosis [77,227,228].
A crucial organ affected by low gravity is the kidney, where urine flow is attenuated [229,230,231]. The parathyroid/kidney axis, sensing using the calcium-sensing receptor, regulation through parathyroid hormone and vitamin D3, the role of the kidney in maintaining water balance, and the homeostasis of calcium and other vital ions in the blood represents an extraordinarily complex system [232]. The kidney, along with bone and the intestines, are vital components of the regulatory system that maintains circulating calcium levels within the very tight tolerances necessary for life, and this is regulated by the PTH/vitamin D3 regulatory axis [233]. A recent meta-analysis found that PTH levels decrease in microgravity [159]. This reduction also results in a reduction in vitamin D3, which prevents the effective absorption of calcium from the intestines, leading to increased bone resorption to mobilize calcium stored in the bone [159]. Although this represents a satisfying and straightforward explanation for bone loss during space flight, it does not account for the differential effects on bone depending on location, including increasing bone density in the skull. It is also worth noting that there are variations in the PTH/vitamin D3 findings; therefore, all studies do not agree well with the conclusions of the meta-analysis.
In principle, the regulation of the PTH/vitamin D3 regulatory axis could also be achieved at the receptor level. The parathyroid hormone type 1 receptor (PTH1R) is a class B1 G protein-coupled receptor [234]. It plays several critical roles in bone turnover and calcium homeostasis. The vitamin D receptor (VDR) is a member of the nuclear receptor family and is found throughout the body [235]. However, these receptors are more difficult to study in space than the hormones, and, to date, space or microgravity studies on these crucial receptors are lacking.
The renin/angiotensin/aldosterone system is an important regulator associated with kidney function [236]. It has been studied on several space flights; however, data are somewhat conflicting [237]. In general, it seems there is a sharp decrease in renin and aldosterone levels during the first 24 hours of flight, and then a rebound to preflight levels or above. Since osteoclasts and osteoblasts have receptors for angiotensin II, these fluctuations could directly affect bone remodeling [102].
6. Summary
Bone loss during space flight is a major physiological problem that must be satisfactorily addressed in order to permit long space missions. The core regulations of bone remodeling take place through RANKL/RANK/OPG and LRP5/6/WNT/beta catenin signaling. Data suggest that at least five different layers of regulation may influence core regulation leading to bone loss. Each of these has a specific set of receptors. First, reductions in PTH/vitamin D3 and reduced stimulation of their receptors, PTH1R and VDR, are involved in lower calcium uptake from the intestines, which leads to increased bone resorption, which is required to mobilize calcium to maintain systemic calcium homeostasis. Second, fluid redistribution in microgravity may contribute to changes in forces leading to bone density increases in the skull and a reduction in bone density in load-bearing bones. Piezo1 is a potential mediator of this response. Third, a reduction in direct mechanical force on bone may trigger a loss of density on load-bearing bones. This may be mediated by mechanical signals, and changes in piezoelectricity generation due to reduced mechanical forces in microgravity may play a role. Fourth, perturbation in cytoskeletal components and associated matrisome may cause various local and systemic problems affecting bone cells directly or indirectly through inflammatory or stress responses. These may crosstalk with bone regulation. Finally, changes in fluid flow characteristics at the micro- and nano-level may have effects on cellular function, for example, through the disruption of cytoskeletal processes and interactions with the matrisome, changing the rate of EV shedding or EV composition, or altering the PILP process, which provides a likely mechanism for bone formation.
It is clear that microgravity stresses various organ systems in humans, which is not surprising given that our physiology is adapted to Earth’s gravity. It is remarkable that humans adapt as well as they do to microgravity. Bone is integrated into overall physiology because of its crucial role in systemic calcium regulation (Figure 6). Systemic calcium must be maintained within the narrow tolerances required for human survival. While it is important to prevent excess bone loss, it is also crucial to consider that bone loss in microgravity is a component of a more general physiologic adaptation to microgravity that involves multiple organ systems. Care must be taken that efforts to limit bone loss (for example through antiresorptives) do not provoke catastrophic failures in other systems. In preparation for long space voyages, to Mars or beyond, it is imperative that a better understanding of the interconnected physiologic adaptations that occur to adapt to microgravity, and then again, to readapt to Earth’s (or another planet’s) gravity, is achieved. Such knowledge should allow obvious problems like bone loss to be ameliorated without deleterious off-target effects on other organ systems.
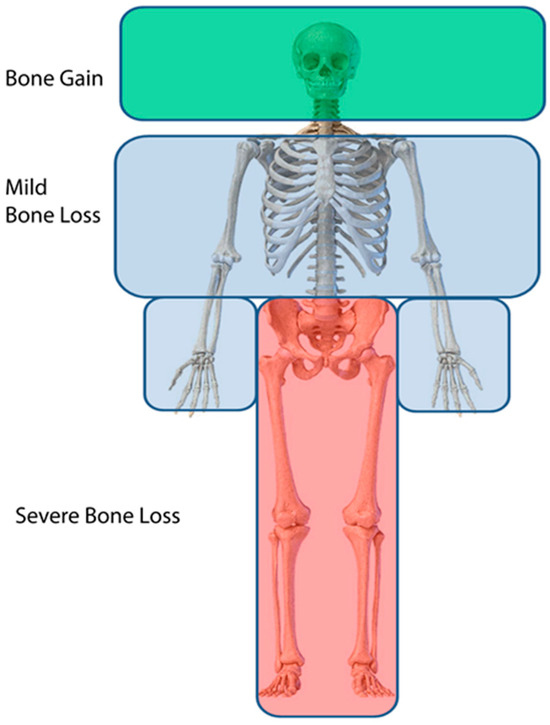
Figure 6.
Pattern of bone loss in humans subjected to microgravity. Green indicates bone gain, light blue denotes modest bone loss, and red indicates severe bone loss. The intensity of this pattern varies significantly between individuals.
In Earth gravity, it is well established that susceptibility to bone loss and osteoporotic fractures is genetically linked [238,239,240,241]. Genetic links have also been identified for susceptibility to the medicine-related osteonecrosis of the jaw (MRONJ), a pathology that occurs when people are treated with antiresorptives, including bisphosphonates and denosumab [242]. Both of these have been advanced for use during space flight to reduce bone loss [127]. Empirically, there are wide variations in the amount of bone lost by astronauts and cosmonauts during space missions [243]. This may be the result of differential activity in space and different regimens of training prior to space flight. For example, initial research suggests that markers of bone turnover and exercise history predict bone loss in microgravity [190]. Moreover, like on Earth, these may reflect genetic differences. Direct evidence suggests that this is the case with respect to muscle wasting and bone loss in mice [188,189], which have served as a useful model for human musculoskeletal disorders. Currently, astronaut and cosmonaut selection includes numerous characteristics that are genetically linked, including height, correctable vision, allergies, migraines, and colorblindness [244]. In preparation for long space voyages, the identification and selection of astronauts who naturally best resist bone loss, muscle wasting, and other deleterious consequences of microgravity may be of paramount importance. As a deeper understanding of bone loss in space develops, personalized medicine approaches can also be developed to better enable spacefaring by people who are less able to adapt to microgravity [245,246].
Author Contributions
Conception of the manuscript (E.F.M. and A.A.P.). Literature review (E.F.M., A.A.P. and L.S.H.). Draft of the manuscript (L.S.H.). Reviewing and editing (E.F.M. and A.A.P.). All authors have read and agreed to the published version of the manuscript.
Funding
EFM and AAP were funded by R-Crio Células-Tronco (Campinas, Brazil), ANADEM (Brasilia, Brazil), and the KSCIA International Space Academy while they were writing this manuscript. EFM was also funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Research Productivity Fellowship to Elizabeth Ferreira Martinez, Grant/Award Number: #307169/2021-9.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mack, P.B.; Lacuance, P.A.; Vose, G.P.; Vogt, F.B. Bone Demineralization of Foot and Hand of Gemini-Titan Iv, V and Vii Astronauts during Orbital Flight. Am. J. Roentgenol. 1967, 100, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Vose, G.P. Review of Roentgenographic Bone Demineralization Studies of the Gemini Space Flights. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1974, 121, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Chappard, D.; Palle, S.; Bakulin, A.V.; Novikov, V.E.; Alexandre, C. Trabecular Bone Remodeling after Seven Days of Weightlessness Exposure (Biocosmos 1667). Am. J. Physiol. 1988, 255 Pt 2, R243–R247. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehaillia, M.; Alexandre, C. Effects of Long-Term Microgravity Exposure on Cancellous and Cortical Weight-Bearing Bones of Cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Van Loon, J.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards Human Exploration of Space: The Theseus Review Series on Muscle and Bone Research Priorities. npj Microgravity 2017, 3, 8. [Google Scholar] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Graham, T.; Squires-Donelly, G.; Laslett, A.L. The Effects of Microgravity on Bone Structure and Function. npj Microgravity 2022, 8, 9. [Google Scholar]
- Liu, Z.; Wang, Q.; Zhang, J.; Qi, S.; Duan, Y.; Li, C. The Mechanotransduction Signaling Pathways in the Regulation of Osteogenesis. Int. J. Mol. Sci. 2023, 24, 14326. [Google Scholar] [CrossRef] [PubMed]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-Based Facilities for Simulation of Microgravity: Organism-Specific Recommendations for Their Use, and Recommended Terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Nishimura, Y. Technology Using Simulated Microgravity. Regen. Ther. 2023, 24, 318–323. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand Is a Cytokine That Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Kong, Y.-Y.; Yoshida, H.; Sarosi, I.; Tan, H.-L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-Dos-Santos, A.J.; Van, G.; Itie, A.; et al. Opgl Is a Key Regulator of Osteoclastogenesis, Lymphocyte Development and Lymph-Node Organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Xiong, J.; O’Brien, C.A. Osteocyte Rankl: New Insights into the Control of Bone Remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-Embedded Cells Control Osteoclast Formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for Osteocyte Regulation of Bone Homeostasis through Rankl Expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. Bone: Osteocyte Rankl in Bone Homeostasis: A Paradigm Shift? Nat. Rev. Rheumatol. 2011, 7, 619. [Google Scholar] [CrossRef]
- Kong, Y.-Y.; Penninger, J. Molecular Control of Bone Remodeling and Osteoporosis. Exp. Gerontol. 2000, 35, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Hikita, A.; Yana, I.; Wakeyama, H.; Nakamura, M.; Kadono, Y.; Oshima, Y.; Nakamura, K.; Seiki, M.; Tanaka, S. Negative Regulation of Osteoclastogenesis by Ectodomain Shedding of Receptor Activator of Nf-Kappab Ligand. J. Biol. Chem. 2006, 281, 36846–36855. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Boyle, W.J.; Penninger, J.M. Osteoprotegerin Ligand: A Common Link between Osteoclastogenesis, Lymph Node Formation and Lymphocyte Development. Immunol. Cell. Biol. 1999, 77, 188–193. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T Cells Regulate Bone Loss and Joint Destruction in Adjuvant Arthritis through Osteoprotegerin Ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A Homologue of the Tnf Receptor and Its Ligand Enhance T-Cell Growth and Dendritic-Cell Function. Nature 1997, 390, 175–179. [Google Scholar] [CrossRef]
- Serrano, E.M.; Ricofort, R.D.; Zuo, J.; Ochotny, N.; Manolson, M.F.; Holliday, L.S. Regulation of Vacuolar H(+)-Atpase in Microglia by Rankl. Biochem. Biophys. Res. Commun. 2009, 389, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Sims, N.A. Rankl/Opg; Critical Role in Bone Physiology. Rev. Endocr. Metab. Disord. 2015, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Faccio, R.; Takeshita, S.; Zallone, A.; Ross, F.P.; Teitelbaum, S.L. C-Fms and the Alphavbeta3 Integrin Collaborate during Osteoclast Differentiation. J. Clin. Investig. 2003, 111, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Ohneda, O.; Arai, F.; Iwamoto, K.; Okada, S.; Takagi, K.; Anderson, D.M.; Suda, T. Bifurcation of Osteoclasts and Dendritic Cells from Common Progenitors. Blood 2001, 98, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [PubMed]
- Jiang, J.; Zuo, J.; Hurst, I.; Holliday, L. The Synergistic Effect of Peptidoglycan and Lipopolysaccaride on Osteoclast Formation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Abu-Amer, Y.; Nelson, C.A.; Fremont, D.H.; Ross, F.P.; Teitelbaum, S.L. Tumour Necrosis Factor Superfamily Cytokines and the Pathogenesis of Inflammatory Osteolysis. Ann. Rheum. Dis. 2002, 61, ii82–ii83. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. Tnf-Alpha Induces Osteoclastogenesis by Direct Stimulation of Macrophages Exposed to Permissive Levels of Rank Ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- King, G.J.; Holtrop, M.E. Actin-Like Filaments in Bone Cells of Cultured Mouse Calvaria as Demonstrated by Binding to Heavy Meromyosin. J. Cell Biol. 1975, 66, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.; Tuukkanen, J.; Hentunen, T.; Järvelin, K.; Väänänen, K. Organization of Osteoclast Microfilaments during the Attachment to Bone Surface In Vitro. J. Bone Miner. Res. 1989, 4, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Destaing, O.; Saltel, F.; Géminard, J.-C.; Jurdic, P.; Bard, F. Podosomes Display Actin Turnover and Dynamic Self-Organization in Osteoclasts Expressing Actin-Green Fluorescent Protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-Mediated Actin Dynamics in Resorbing Osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Jurdic, P.; Saltel, F.; Chabadel, A.; Destaing, O. Podosome and Sealing Zone: Specificity of the Osteoclast Model. Eur. J. Cell Biol. 2005, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Seano, G.; Primo, L. Podosomes and Invadopodia: Tools to Breach Vascular Basement Membrane. Cell Cycle 2015, 14, 1370–1374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Génot, E.; Gligorijevic, B. Invadosomes in Their Natural Habitat. Eur. J. Cell Biol. 2014, 93, 367–379. [Google Scholar] [PubMed]
- Destaing, O.; Petropoulos, C.; Albiges-Rizo, C. Coupling between Acto-Adhesive Machinery and Ecm Degradation in Invadosomes. Cell Adhes. Migr. 2014, 8, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. The Osteoclast and Its Unique Cytoskeleton. Ann. N. Y. Acad. Sci. 2011, 1240, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Yao, W.; Nakamura, M.C.; Humphrey, M.B.; Kimmel, D.; Huang, X.; Sheppard, D.; Ross, F.P.; Teitelbaum, S.L. Mice Lacking the Integrin Beta5 Subunit Have Accelerated Osteoclast Maturation and Increased Activity in the Estrogen-Deficient State. J. Bone Miner. Res. 2005, 20, 58–66. [Google Scholar]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice Lacking Beta3 Integrins Are Osteosclerotic Because of Dysfunctional Osteoclasts. J. Clin. Investig. 2000, 105, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Baron, R. Molecular Mechanisms of Bone Resorption by the Osteoclast. Anat. Rec. 1989, 224, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic Bone Resorption by a Polarized Vacuolar Proton Pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Collins, M.P.; Forgac, M. Regulation and Function of V-Atpases in Physiology and Disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.M.; Wu, D.; Bueler, S.A.; Robinson, C.V.; Rubinstein, J.L. Structure of V-Atpase from the Mammalian Brain. Science 2020, 367, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Long, T.; Hassan, A.; Wang, J.; Sun, Y.; Xie, X.-S.; Li, X. Cryo-Em Structures of Intact V-Atpase from Bovine Brain. Nat. Commun. 2020, 11, 3921. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Holliday, L.S.; Krits, I.; Gluck, S.L. Vacuolar H+-Atpase Activity and Expression in Mouse Bone Marrow Cultures. J. Bone Miner. Res. 1999, 14, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Toyomura, T.; Oka, T.; Yamaguchi, C.; Wada, Y.; Futai, M. Three Subunit a Isoforms of Mouse Vacuolar H(+)-Atpase. Preferential Expression of the A3 Isoform during Osteoclast Differentiation. J. Biol. Chem. 2000, 275, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Okamoto, K.; Wang, B.B.; Biroc, S. Human Cathepsin O2, a Matrix Protein-Degrading Cysteine Protease Expressed in Osteoclasts. Functional Expression of Human Cathepsin O2 in Spodoptera Frugiperda and Characterization of the Enzyme. J. Biol. Chem. 1996, 271, 2126–2132. [Google Scholar] [CrossRef]
- Lehenkari, P.; Hentunen, T.A.; Laitala-Leinonen, T.; Tuukkanen, J.; Väänänen, H. Carbonic Anhydrase Ii Plays a Major Role in Osteoclast Differentiation and Bone Resorption by Effecting the Steady State Intracellular Ph and Ca2+. Exp. Cell Res. 1998, 242, 128–137. [Google Scholar] [CrossRef]
- Lu, M.; Holliday, L.S.; Zhang, L.; Dunn, W.A., Jr.; Gluck, S.L. Interaction between Aldolase and Vacuolar H+-Atpase: Evidence for Direct Coupling of Glycolysis to the Atp-Hydrolyzing Proton Pump. J. Biol. Chem. 2001, 276, 30407–30413. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Sautin, Y.Y.; Holliday, L.S.; Gluck, S.L. The Glycolytic Enzyme Aldolase Mediates Assembly, Expression, and Activity of Vacuolar H+-Atpase. J. Biol. Chem. 2004, 279, 8732–8739. [Google Scholar] [CrossRef] [PubMed]
- Kornak, U.; Kasper, D.; Bösl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the Clc-7 Chloride Channel Leads to Osteopetrosis in Mice and Man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.F.; Wartosch, L.; Jentsch, T.J.; Fuhrmann, J.C. Clc-7 Requires Ostm1 as a Beta-Subunit to Support Bone Resorption and Lysosomal Function. Nature 2006, 440, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; Orchard, P.J.; Sobacchi, C.; Giliani, S.; Abinun, M.; Mattsson, J.P.; Keeling, D.J.; Andersson, A.-K.; Wallbrandt, P.; Zecca, L.; et al. Defects in Tcirg1 Subunit of the Vacuolar Proton Pump Are Responsible for a Subset of Human Autosomal Recessive Osteopetrosis. Nat. Genet. 2000, 25, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sly, W.S.; Hewett-Emmett, D.; Whyte, M.P.; Yu, Y.S.; Tashian, R.E. Carbonic Anhydrase Ii Deficiency Identified as the Primary Defect in the Autosomal Recessive Syndrome of Osteopetrosis with Renal Tubular Acidosis and Cerebral Calcification. Proc. Natl. Acad. Sci. USA 1983, 80, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, J.; Zheng, X.; Wang, Z.; Zhang, Y.; Hao, Y.; Yang, T.; Deng, H. Deficiency of Atp6v1h Causes Bone Loss by Inhibiting Bone Resorption and Bone Formation through the Tgf-Beta1 Pathway. Theranostics 2016, 6, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Ma, Y.; Duan, X. Atp6v1h Deficiency Blocks Bone Loss in Simulated Microgravity Mice through the Fos-Jun-Src-Integrin Pathway. Int. J. Mol. Sci. 2024, 25, 637. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, M.H.; Nesbitt, S.A.; Horton, M.A. Integrins on Rat Osteoclasts: Characterization of Two Monoclonal Antibodies (F4 and F11) to Rat Beta 3. J. Bone Miner. Res. 1992, 7, 345–351. [Google Scholar] [CrossRef]
- Hughes, D.; Salter, D.; Dedhar, S.; Simpson, R. Simpson. Integrin Expression in Human Bone. J. Bone Miner. Res. 1993, 8, 527–533. [Google Scholar] [CrossRef]
- Nesbitt, S.; Nesbit, A.; Helfrich, M.; Horton, M. Biochemical Characterization of Human Osteoclast Integrins. Osteoclasts Express Alpha V Beta 3, Alpha 2 Beta 1, and Alpha V Beta 1 Integrins. J. Biol. Chem. 1993, 268, 16737–16745. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, M.H.; Nesbitt, S.A.; Lakkakorpi, P.T.; Barnes, M.J.; Bodary, S.C.; Shankar, G.; Mason, W.T.; Mendrick, D.L.; Vaananen, H.K.; Horton, M.A. Beta 1 Integrins and Osteoclast Function: Involvement in Collagen Recognition and Bone Resorption. Bone 1996, 19, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tachibana, I.; Miyado, K.; Kobayashi, M.; Miyazaki, T.; Funakoshi, T.; Kimura, H.; Yamane, H.; Saito, Y.; Goto, H.; et al. Tetraspanins Cd9 and Cd81 Function to Prevent the Fusion of Mononuclear Phagocytes. J. Cell Biol. 2003, 161, 945–956. [Google Scholar] [CrossRef]
- Kitazawa, S.; Ross, F.P.; McHugh, K.; Teitelbaum, S.L. Interleukin-4 Induces Expression of the Integrin Alpha V Beta 3 Via Transactivation of the Beta 3 Gene. J. Biol. Chem. 1995, 270, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Sago, K.; Teitelbaum, S.L.; Venstrom, K.; Reichardt, L.F.; Ross, F.P. The Integrin Alphavbeta5 Is Expressed on Avian Osteoclast Precursors and Regulated by Retinoic Acid. J. Bone Miner. Res. 1999, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.T.; Rodan, G.A. Integrin-Mediated Signaling in the Regulation of Osteoclast Adhesion and Activation. Front. Biosci. 1998, 3, d849–d864. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.; Welgus, H.; Hanna, J.; Lee, B.; Lu, M.; Jeffrey, J.; Gluck, S. Interstitial Collagenase Activity Stimulates the Formation of Actin Rings and Ruffled Membranes in Mouse Marrow Osteoclasts. Calcif. Tissue Int. 2003, 72, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Welgus, H.G.; Fliszar, C.J.; Veith, G.M.; Jeffrey, J.J.; Gluck, S.L. Initiation of Osteoclast Bone Resorption by Interstitial Collagenase. J. Biol. Chem. 1997, 272, 22053–22058. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Vakani, A.; Archer, L.; Dolce, C. Effects of Matrix Metalloproteinase Inhibitors on Bone Resorption and Orthodontic Tooth Movement. J. Dent. Res. 2003, 82, 687–691. [Google Scholar] [CrossRef]
- Feng, X.; Novack, D.V.; Faccio, R.; Ory, D.S.; Aya, K.; Boyer, M.I.; McHugh, K.P.; Ross, F.P.; Teitelbaum, S.L. A Glanzmann’s Mutation in Beta 3 Integrin Specifically Impairs Osteoclast Function. J. Clin. Investig. 2001, 107, 1137–1144. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Xu, J.; Zou, J. The Role of Integrin Family in Bone Metabolism and Tumor Bone Metastasis. Cell Death Discov. 2023, 9, 119. [Google Scholar] [PubMed]
- Andreeva, E.; Matveeva, D.; Zhidkova, O.; Zhivodernikov, I.; Kotov, O.; Buravkova, L. Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life 2022, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Meyers, V.E.; Zayzafoon, M.; Gonda, S.R.; Gathings, W.E.; McDonald, J.M. Modeled Microgravity Disrupts Collagen I/Integrin Signaling during Osteoblastic Differentiation of Human Mesenchymal Stem Cells. J. Cell. Biochem. 2004, 93, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.; Larina, I.; Andreeva, E.; Grigoriev, A. Microgravity Effects on the Matrisome. Cells 2021, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Guo, F.; Wu, F.; Xu, H.; Yang, C.; Li, J.; Liang, P.; Zhang, H.; Qu, L.; Tan, Y.; et al. Integrin Alphavbeta3 Mediates the Synergetic Regulation of Core-Binding Factor Alpha1 Transcriptional Activity by Gravity and Insulin-Like Growth Factor-1 through Phosphoinositide 3-Kinase Signaling. Bone 2014, 69, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.K. Implications of Microgravity-Induced Cell Signaling Alterations Upon Cancer Cell Growth, Invasiveness, Metastatic Potential, and Control by Host Immunity. Int. Rev. Cell Mol. Biol. 2021, 361, 107–164. [Google Scholar] [PubMed]
- Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of Microgravity on Osteoclast Bone Resorption and Osteoblast Cytoskeletal Organization and Adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hurst, I.R.; Zuo, J.; Jiang, J.; Holliday, L.S. Actin-Related Protein 2/3 Complex Is Required for Actin Ring Formation. J. Bone Miner. Res. 2004, 19, 499–506. [Google Scholar] [CrossRef]
- Fu, J.; Goldsmith, M.; Crooks, S.D.; Condon, S.F.; Morris, M.; Komarova, S.V. Bone Health in Spacefaring Rodents and Primates: Systematic Review and Meta-Analysis. Npj Microgravity 2021, 7, 19. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic. Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of Rankl/Rank/Opg in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Chan, A.S.M.; Clairfeuille, T.; Landao-Bassonga, E.; Kinna, G.; Ng, P.Y.; Loo, L.S.; Cheng, T.S.; Zheng, M.; Hong, W.; Teasdale, R.D.; et al. Sorting Nexin 27 Couples Pthr Trafficking to Retromer for Signal Regulation in Osteoblasts during Bone Growth. Mol. Biol. Cell 2016, 27, 1367–1382. [Google Scholar] [CrossRef]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Roberson, P.; Parfitt, A.M.; Manolagas, S.C. Increased Bone Formation by Prevention of Osteoblast Apoptosis with Parathyroid Hormone. J. Clin. Investig. 1999, 104, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Van Hul, W. The Genetics of Low-Density Lipoprotein Receptor-Related Protein 5 in Bone: A Story of Extremes. Endocrinology 2007, 148, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Plotkin, L.I.; Galli, C.; Goellner, J.J.; Gortazar, A.R.; Allen, M.R.; Robling, A.G.; Bouxsein, M.; Schipani, E.; Turner, C.H.; et al. Control of Bone Mass and Remodeling by Pth Receptor Signaling in Osteocytes. PLoS ONE 2008, 3, e2942. [Google Scholar]
- Kang, S. Low-Density Lipoprotein Receptor-Related Protein 6-Mediated Signaling Pathways and Associated Cardiovascular Diseases: Diagnostic and Therapeutic Opportunities. Hum. Genet. 2020, 139, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/Beta-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar]
- Van Wesenbeeck, L.; Cleiren, E.; Gram, J.; Beals, R.K.; Bénichou, O.; Scopelliti, D.; Key, L.; Renton, T.; Bartels, C.; Gong, Y.; et al. Six Novel Missense Mutations in the Ldl Receptor-Related Protein 5 (Lrp5) Gene in Different Conditions with an Increased Bone Density. Am. J. Hum. Genet. 2003, 72, 763–771. [Google Scholar] [CrossRef]
- Whyte, M.P.; McAlister, W.H.; Zhang, F.; Bijanki, V.N.; Nenninger, A.; Gottesman, G.S.; Lin, E.L.; Huskey, M.; Duan, S.; Dahir, K.; et al. New Explanation for Autosomal Dominant High Bone Mass: Mutation of Low-Density Lipoprotein Receptor-Related Protein 6. Bone 2019, 127, 228–243. [Google Scholar] [CrossRef]
- Whyte, M.P.; Mumm, S.; Baker, J.C.; Zhang, F.; Sedighi, H.; Duan, S.; Cundy, T. Lrp6 High Bone Mass Characterized in Two Generations Harboring a Unique Mutation of Low-Density Lipoprotein Receptor-Related Protein 6. JBMR Plus 2023, 7, e10717. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to Lrp5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive Development of the Rat Osteoblast Phenotype in Vitro: Reciprocal Relationships in Expression of Genes Associated with Osteoblast Proliferation and Differentiation during Formation of the Bone Extracellular Matrix. J. Cell. Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef]
- Hirai, F.; Nakayamada, S.; Okada, Y.; Saito, K.; Kurose, H.; Mogami, A.; Tanaka, Y. Small Gtpase Rho Signaling Is Involved in Beta1 Integrin-Mediated up-Regulation of Intercellular Adhesion Molecule 1 and Receptor Activator of Nuclear Factor Kappab Ligand on Osteoblasts and Osteoclast Maturation. Biochem. Biophys. Res. Commun. 2007, 356, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nakayamada, S.; Okada, Y.; Saito, K.; Tamura, M.; Tanaka, Y. Beta1 Integrin/Focal Adhesion Kinase-Mediated Signaling Induces Intercellular Adhesion Molecule 1 and Receptor Activator of Nuclear Factor Kappab Ligand on Osteoblasts and Osteoclast Maturation. J. Biol. Chem. 2003, 278, 45368–45374. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; James, A.W.; Chung, J.; Lee, K.; Zhang, J.B.; Ho, S.; Lee, K.S.; Kim, T.M.; Niimi, T.; Kuroda, S.; et al. Nell-1 Promotes Cell Adhesion and Differentiation via Integrinbeta1. J. Cell Biochem. 2012, 113, 3620–3628. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Shen, J.; Zhang, X.; Asatrian, G.; Goyal, R.; Kwak, J.H.; Jiang, L.; Bengs, B.; Culiat, C.T.; Turner, A.S.; et al. Nell-1 in the Treatment of Osteoporotic Bone Loss. Nat. Commun. 2015, 6, 7362. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast Programmed Cell Death (Apoptosis): Modulation by Growth Factors and Cytokines. J. Bone Miner. Res. 1998, 13, 793–802. [Google Scholar] [CrossRef] [PubMed]
- A Sims, N.; Martin, T.J. Coupling the Activities of Bone Formation and Resorption: A Multitude of Signals within the Basic Multicellular Unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Izu, Y.; Mizoguchi, F.; Kawamata, A.; Hayata, T.; Nakamoto, T.; Nakashima, K.; Inagami, T.; Ezura, Y.; Noda, M. Angiotensin Ii Type 2 Receptor Blockade Increases Bone Mass. J. Biol. Chem. 2008, 284, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Junior, C.M.; Santos, A.C.P.M.; Galvao, I.; Souto, G.R.; Mesquita, R.A.; Sa, M.A.; Ferreira, A.J. The Angiotensin Converting Enzyme 2/Angiotensin-(1-7)/Mas Receptor Axis as a Key Player in Alveolar Bone Remodeling. Bone 2019, 128, 115041. [Google Scholar] [CrossRef]
- Strollo, F. Hormonal Changes in Humans during Spaceflight. Adv. Space Biol. Med. 1999, 7, 99–129. [Google Scholar] [PubMed]
- Strollo, F.; Strollo, G.; More, M.; Bollanti, L.; Ciarmatori, A.; Longo, E.; Quintiliani, R.; Mambro, A.; Mangrossa, N.; Ferretti, C. Hormonal Adaptation to Real and Simulated Microgravity. J. Gravit. Physiol. 1998, 5, P89–P92. [Google Scholar] [PubMed]
- Bhaskar, N.; Kachappilly, M.C.; Bhushan, V.; Pandya, H.J.; Basu, B. Electrical Field Stimulated Modulation of Cell Fate of Pre-Osteoblasts on Pvdf/Bt/Mwcnt Based Electroactive Biomaterials. J. Biomed. Mater. Res. Part. A 2022, 111, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, P.; Wang, Z.; Zhang, J.; Ding, C.; Shang, P. Biomechanical and Biophysical Environment of Bone from the Macroscopic to the Pericellular and Molecular Level. J. Mech. Behav. Biomed. Mater. 2015, 50, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Kao, F.-C.; Chiu, P.-Y.; Tsai, T.-T.; Lin, Z.-H. The Application of Nanogenerators and Piezoelectricity in Osteogenesis. Technol. Adv. Mater. 2019, 20, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.-S.; Thula, T.T.; Gower, L.B. Development of Bone-Like Composites via the Polymer-Induced Liquid-Precursor (Pilp) Process. Part 1: Influence of Polymer Molecular Weight. Acta Biomater. 2010, 6, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J. Bone Structure and Formation: A New Perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Bouleftour, W.; Juignet, L.; Bouet, G.; Granito, R.N.; Vanden-Bossche, A.; Laroche, N.; Aubin, J.E.; Lafage-Proust, M.H.; Vico, L.; Malaval, L. The Role of the Sibling, Bone Sialoprotein in Skeletal Biology—Contribution of Mouse Experimental Genetics. Matrix Biol. 2016, 52–54, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Gordon, J.A.R.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone Sialoprotein Expression Enhances Osteoblast Differentiation and Matrix Mineralization in Vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef]
- Rodriguez, D.E.; Thula-Mata, T.; Toro, E.J.; Yeh, Y.-W.; Holt, C.; Holliday, L.S.; Gower, L.B. Multifunctional Role of Osteopontin in Directing Intrafibrillar Mineralization of Collagen and Activation of Osteoclasts. Acta Biomater. 2014, 10, 494–507. [Google Scholar] [CrossRef]
- Hu, L.-F.; Li, J.-B.; Qian, A.-R.; Wang, F.; Shang, P. Mineralization Initiation of Mc3t3-E1 Preosteoblast Is Suppressed under Simulated Microgravity Condition. Cell Biol. Int. 2014, 39, 364–372. [Google Scholar] [CrossRef]
- Klement, B.; Spooner, B. Mineralization and Growth of Cultured Embryonic Skeletal Tissue in Microgravity. Bone 1999, 24, 349–359. [Google Scholar] [CrossRef]
- Van Loon, J.J.; Bervoets, D.J.; Burger, E.H.; Dieudonne, S.C.; Hagen, J.W.; Semeins, C.M.; Doulabi, B.Z.; Veldhuijzen, J.P. Decreased Mineralization and Increased Calcium Release in Isolated Fetal Mouse Long Bones under near Weightlessness. J. Bone Miner. Res. 1995, 10, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Whitson, P.A.; Pietrzyk, R.A.; Sams, C.F. Space Flight and the Risk of Renal Stones. J. Gravit. Physiol. 1999, 6, P87–P88. [Google Scholar]
- Pietrzyk, R.A.; Jones, J.A.; Sams, C.F.; Whitson, P.A. Renal Stone Formation among Astronauts. Aviat. Space Environ. Med. 2007, 78, A9–A13. [Google Scholar] [PubMed]
- Amos, F.F.; Dai, L.; Kumar, R.; Khan, S.R.; Gower, L.B. Mechanism of Formation of Concentrically Laminated Spherules: Implication to Randall’s Plaque and Stone Formation. Urol. Res. 2008, 37, 11–17. [Google Scholar] [CrossRef]
- Yoshizaki, I.; Nakamura, H.; Fukuyama, S.; Komatsu, H.; Yoda, S. Scientific Approach to the Optimization of Protein Crystallization Conditions for Microgravity Experiments. Ann. N. Y. Acad. Sci. 2004, 1027, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, C.W., Jr.; Gerdts, C.; Johnson, M.D.; Webb, P. A Microfluidic, High throughput Protein Crystal Growth Method for Microgravity. PLoS ONE 2013, 8, e82298. [Google Scholar] [CrossRef]
- Reichert, P.; Prosise, W.; Fischmann, T.O.; Scapin, G.; Narasimhan, C.; Spinale, A.; Polniak, R.; Yang, X.; Walsh, E.; Patel, D.; et al. Pembrolizumab Microgravity Crystallization Experimentation. npj Microgravity 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.J.; Grynpas, M.D.; Rosenberg, G.D. Maturation of Bone and Dentin Matrices in Rats Flown on the Soviet Biosatellite Cosmos 1887. FASEB J. 1990, 4, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Slatopolsky, E. Vitamin D Analogs: Perspectives for Treatment. Miner. Electrolyte Metab. 1999, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wastney, M.E.; Morukov, B.V.; Larina, I.M.; Nyquist, L.E.; Abrams, S.A.; Taran, E.N.; Shih, C.-Y.; Nillen, J.L.; Davis-Street, J.E.; et al. Calcium Metabolism before, during, and after a 3-Mo Spaceflight: Kinetic and Biochemical Changes. Am. J. Physiol. Integr. Comp. Physiol. 1999, 277, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Wehland, M.; Schulz, H.; Heer, M.; Infanger, M.; Grimm, D. Microgravity-Related Changes in Bone Density and Treatment Options: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8650. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-Bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in Bone—Role of the Lacuno-Canalicular Network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef]
- Genthial, R.; Gerbaix, M.; Farlay, D.; Vico, L.; Beaurepaire, E.; Débarre, D.; Gourrier, A. Third Harmonic Generation Imaging and Analysis of the Effect of Low Gravity on the Lacuno-Canalicular Network of Mouse Bone. PLoS ONE 2019, 14, e0209079. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, N.; Oganov, V.; Zolotova, N. Ultrastructural Changes in Osteocytes in Microgravity Conditions. Adv. Space Res. 2002, 30, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Shoji-Matsunaga, A.; Ono, T.; Hayashi, M.; Takayanagi, H.; Moriyama, K.; Nakashima, T. Osteocyte Regulation of Orthodontic Force-Mediated Tooth Movement via Rankl Expression. Sci. Rep. 2017, 7, 8753. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Kittaka, M.; Doan, A.A.P.; Urata, R.; Prideaux, M.; Rojas, R.E.; Harding, C.V.; Boom, W.H.; Bonewald, L.F.; Greenfield, E.M.; et al. Osteocytes Directly Regulate Osteolysis via Myd88 Signaling in Bacterial Bone Infection. Nat. Commun. 2022, 13, 6648. [Google Scholar] [CrossRef] [PubMed]
- De Vries, T.J.; Huesa, C. The Osteocyte as a Novel Key Player in Understanding Periodontitis through Its Expression of Rankl and Sclerostin: A Review. Curr. Osteoporos. Rep. 2019, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Cosman, F.; Stad, R.K.; Ferrari, S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Adv. Ther. 2021, 39, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Rengel, A.; Tran, V.; Toh, L.S. Denosumab as a Pharmacological Countermeasure against Osteopenia in Long Duration Spaceflight. Aerosp. Med. Hum. Perform. 2023, 94, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Andersen, J.D. Treatment of Osteoporosis: Unmet Needs and Emerging Solutions. J. Bone Metab. 2018, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.V.; LeBoff, M.S. Osteoanabolic Agents for Osteoporosis. J. Endocr. Soc. 2018, 2, 922–932. [Google Scholar] [CrossRef]
- Bullock, W.A.; Pavalko, F.M.; Robling, A.G. Osteocytes and Mechanical Loading: The Wnt Connection. Orthod. Craniofacial Res. 2019, 22, 175–179. [Google Scholar] [CrossRef]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/Beta-Catenin Signaling Is Required for Normal Bone Homeostasis. Mol. Cell Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Vaughan, T.J.; McNamara, L.M. The Role of Integrin Alpha(V)Beta(3) in Osteocyte Mechanotransduction. J. Mech. Behav. Biomed. Mater. 2015, 42, 67–75. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Z.; Yang, D.; He, T.; Xu, Z.; Zhang, P.; Chen, D.; Yi, W.; Xiao, G. Osteocyte Beta3 Integrin Promotes Bone Mass Accrual and Force-Induced Bone Formation in Mice. J. Orthop. Translat 2023, 40, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A.; Almeida, E.A.; Hill, E.L.; Aguirre, J.I.; Rivera, M.F.; Nachbandi, I.; Wronski, T.J.; van der Meulen, M.C.; Globus, R.K. Role for Beta1 Integrins in Cortical Osteocytes during Acute Musculoskeletal Disuse. Matrix Biol. 2008, 27, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, T.; Yang, D.; Wang, Y.; Li, Z.; Yan, Q.; Zhang, P.; Chen, Z.; Lin, S.; Gao, H.; et al. Osteocyte Beta1 Integrin Loss Causes Low Bone Mass and Impairs Bone Mechanotransduction in Mice. J. Orthop. Translat. 2022, 34, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Litzenberger, J.B.; Kim, J.B.; Tummala, P.; Jacobs, C.R. Beta1 Integrins Mediate Mechanosensitive Signaling Pathways in Osteocytes. Calcif. Tissue Int. 2010, 86, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte Apoptosis Is Induced by Weightlessness in Mice and Precedes Osteoclast Recruitment and Bone Loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Mathov, I.; Aguirre, J.I.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Mechanical Stimulation Prevents Osteocyte Apoptosis: Requirement of Integrins, Src Kinases, and Erks. Am. J. Physiol. Physiol. 2005, 289, C633–C643. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Holliday, L.S.; Patel, S.S.; Rody, W.J., Jr. Rankl and Rank in Extracellular Vesicles: Surprising New Players in Bone Remodeling. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 11. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Peng, Y.; Wu, Y.; Ding, Y.; Jiang, Y.; Shen, Z.; Fu, Q. Osteoblast-Derived Microvesicles: A Novel Mechanism for Communication between Osteoblasts and Osteoclasts. Bone 2015, 79, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Loftus, A.; Muraca, M.; Maurizi, A.; Rucci, N.; Teti, A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J. Bone Miner. Res. 2017, 33, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Muraca, M.; Teti, A.; Rucci, N. Circulating Extracellular Vesicles Express Receptor Activator of Nuclear Factor Kappab Ligand and Other Molecules Informative of the Bone Metabolic Status of Mouse Models of Experimentally Induced Osteoporosis. Calcif. Tissue Int. 2023, 112, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of Bone Resorption and Formation by Rankl Reverse Signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J., Jr.; McHugh, K.P.; Holliday, L.S. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, F.; Russo, A.; Wan, Y. Proteomic Analysis of Extracellular Vesicles Derived from Mda-Mb-231 Cells in Microgravity. Protein J. 2021, 40, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Sahana, J.; Neviani, P.; Corydon, T.J.; Schulz, H.; Wehland, M.; Infanger, M.; Grimm, D. Prolonged Exposure to Simulated Microgravity Changes Release of Small Extracellular Vesicle in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 16095. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosome Release in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 2132. [Google Scholar] [CrossRef] [PubMed]
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S.V. A Systematic Review and Meta-Analysis of Bone Loss in Space Travelers. npj Microgravity 2020, 6, 13. [Google Scholar] [CrossRef]
- Oganov, V.S.; Bakulin, A.V.; Novikov, V.E.; Murashko, L.M.; Kabitskaia, O.E.; Morgun, V.V.; Voronin, L.I.; Schneider, V.; Shakelford, L.; LeBlanc, A. [Reactions of the Human Bone System in Space Flight: Phenomenology]. Aviakosm Ekolog Med. 2006, 39, 3–9. [Google Scholar]
- Mack, P.B.; Vogt, F.B. Roentgenographic Bone Density Changes in Astronauts during Representative Apollo Space Flight. Am. J. Roentgenol. 1971, 113, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Whittle, M.; Whittle, J.V.A.M.; Fisher, A.J.; Fleishman, M.J.; Hancock, D.; Kaude, J.V.; Smith, T.R.; Berkowitz, D.; Frost, A.; et al. Proceedings: Bone Mineral Content Changes in the Skylab Astronauts. Am. J. Roentgenol. 1976, 126, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J. Bone Mineral Measurement: Skylab Experiment M-078. Acta Astronaut. 1975, 2, 129–139. [Google Scholar] [CrossRef]
- Dietlein, L.F.; Johnston, R.S. U.S. Manned Space Flight: The First Twenty Years, a Biomedical Status Report. Acta Astronaut. 1981, 8, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Shigematsu, T.; Fukunaga, T.; Kawakami, K.; Mukai, C.; Sekiguchi, C. Medical Baseline Data Collection on Bone and Muscle Change with Space Flight. Bone 1998, 22, 79S–82S. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The Mechanostat: A Proposed Pathogenic Mechanism of Osteoporoses and the Bone Mass Effects of Mechanical and Nonmechanical Agents. Bone Miner. 1987, 2, 73–85. [Google Scholar]
- Frost, H.M. Bone’s Mechanostat: A 2003 Update. Anat. Rec. Part A Discov. Mol. Cell Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Frost, H.M. Bone Mass and the Mechanostat: A Proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heer, M.; Kamps, N.; Biener, C.; Korr, C.; Boerger, A.; Zittermann, A.; Stehle, P.; Drummer, C. Calcium Metabolism in Microgravity. Eur. J. Med. Res. 1999, 4, 357–360. [Google Scholar] [PubMed]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity Stress: Bone and Connective Tissue. Compr. Physiol. 2016, 6, 645–686. [Google Scholar]
- Nguyen, H.P.; Tran, P.H.; Kim, K.-S.; Yang, S.-G. The Effects of Real and Simulated Microgravity on Cellular Mitochondrial Function. npj Microgravity 2021, 7, 44. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R. Nutritional Biochemistry of Spaceflight. Adv. Clin. Chem. 2008, 46, 87–130. [Google Scholar] [PubMed]
- Dickerson, B.L.; Sowinski, R.; Kreider, R.B.; Wu, G. Impacts of Microgravity on Amino Acid Metabolism during Spaceflight. Exp. Biol. Med. 2023, 248, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K. Il-6 and the Dysregulation of Immune, Bone, Muscle, and Metabolic Homeostasis during Spaceflight. npj Microgravity 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How Does Spaceflight Affect the Acquired Immune System? npj Microgravity 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kuang, Y.; Zuo, Z. The Emerging Role of Macrophages in Immune System Dysfunction under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2021, 22, 2333. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Medvedev, A.E. Countermeasures for Ameliorating in-Flight Immune Dysfunction. J. Leukoc. Biol. 1993, 54, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tahimic, C.G.T.; Globus, R.K. Redox Signaling and Its Impact on Skeletal and Vascular Responses to Spaceflight. Int. J. Mol. Sci. 2017, 18, 2153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Dong, D.; Shang, P. Effects of Iron Overload and Oxidative Damage on the Musculoskeletal System in the Space Environment: Data from Spaceflights and Ground-Based Simulation Models. Int. J. Mol. Sci. 2018, 19, 2608. [Google Scholar] [CrossRef]
- Kirsch, K.A.; Baartz, F.-J.; Gunga, H.-C.; Wicke, H.J.; Röcker, L.; Bünsch, B. Fluid Shifts into and out of Superficial Tissues under Microgravity and Terrestrial Conditions. Clin. Investig. 1993, 71, 687–689. [Google Scholar] [CrossRef]
- Wei, F.; Flowerdew, K.; Kinzel, M.; Perotti, L.E.; Asiatico, J.; Omer, M.; Hovell, C.; Reumers, V.; Coathup, M.J. Changes in Interstitial Fluid Flow, Mass Transport and the Bone Cell Response in Microgravity and Normogravity. Bone Res. 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Shaik, T.; Kaur, I.P.; Gupta, V.M.; Shaik, A.; Anamika, F.M.; Garg, N.; Jain, R. Cardiovascular Challenges Beyond Earth: Investigating the Impact of Space Travel on Astronauts’ Cardiovascular Health. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.B. The Bearable Lightness of Being: Bones, Muscles, and Spaceflight. Anat. Rec. 1998, 253, 24–27. [Google Scholar] [CrossRef]
- Willey, J.S.; Lloyd, S.A.; Nelson, G.A.; Bateman, T.A. Space Radiation and Bone Loss. Gravit. Space Biol. Bull. 2011, 25, 14–21. [Google Scholar] [PubMed]
- Willey, J.S.; Lloyd, S.A.; Nelson, G.A.; Bateman, T.A. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin. Rev. Bone Miner. Metab. 2011, 9, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.-T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites during the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Gabel, L.; Liphardt, A.-M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Incomplete Recovery of Bone Strength and Trabecular Microarchitecture at the Distal Tibia 1 Year after Return from Long Duration Spaceflight. Sci. Rep. 2022, 12, 9446. [Google Scholar] [CrossRef]
- Zeineddine, Y.; Friedman, M.A.; Buettmann, E.G.; Abraham, L.B.; Hoppock, G.A.; Donahue, H.J. Genetic Diversity Modulates the Physical and Transcriptomic Response of Skeletal Muscle to Simulated Microgravity in Male Mice. npj Microgravity 2023, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Buettmann, E.G.; Zeineddine, Y.; Abraham, L.B.; Hoppock, G.A.; Meas, S.J.; Zhang, Y.; Farber, C.R.; Donahue, H.J. Genetic Variation Influences the Skeletal Response to Hindlimb Unloading in the Eight Founder Strains of the Diversity Outbred Mouse Population. J. Orthop. Res. 2023, 42, 134–140. [Google Scholar] [CrossRef]
- Gabel, L.; Liphardt, A.M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Pre-Flight Exercise and Bone Metabolism Predict Unloading-Induced Bone Loss Due to Spaceflight. Br. J. Sports Med. 2021, 56, 196–203. [Google Scholar] [CrossRef]
- Zhao, C.; Oztekin, A.; Cheng, X. Gravity-Induced Swirl of Nanoparticles in Microfluidics. J. Nanoparticle Res. 2013, 15, 1611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morier, P.; Vollet, C.; Michel, P.E.; Reymond, F.; Rossier, J.S. Gravity-Induced Convective Flow in Microfluidic Systems: Electrochemical Characterization and Application to Enzyme-Linked Immunosorbent Assay Tests. Electrophoresis 2004, 25, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, D.; Bennett, J.P.; Wong, M.C.; Quon, B.K.; Liu, Y.E.; Kelly, N.N.; Kelly, T.; Schoeller, D.A.; Heymsfield, S.B.; Shepherd, J.A. Accuracy and Precision of Multiple Body Composition Methods and Associations with Muscle Strength in Athletes of Varying Hydration: The Da Kine Study. Clin. Nutr. 2023, 43, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.J. Potential Renovascular Hypertension, Space Missions, and the Role of Magnesium. Int. J. Nephrol. Renovasc Dis. 2009, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Atomi, Y. Gravitational Effects on Human Physiology. Subcell. Biochem. 2015, 72, 627–659. [Google Scholar] [PubMed]
- Hettrick, H.; Aviles, F. Microgravity and Lymphatics: Why Space Programs Need Lymphedema Physiology Specialists. Lymphat. Res. Biol. 2023, 21, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Belgrado, J.P.; Bonetti, G.; Maloizelle-Delaunay, J.; Stoichkova, V.; Tartaglia, G.M.; Chiurazzi, P.; Cecchin, S.; Bertelli, M. Lymphatic Circulation in Astronauts: Basic Knowledge, Challenges and Perspectives. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 119–126. [Google Scholar] [PubMed]
- Ly, V.; Velichala, S.R.; Hargens, A.R. Cardiovascular, Lymphatic, and Ocular Health in Space. Life 2022, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; Gibson, C.R.; Mader, T.H. The Odyssey of the Ocular and Cerebrospinal Fluids during a Mission to Mars: The Ocular Glymphatic System under Pressure. Eye 2021, 36, 686–691. [Google Scholar] [CrossRef]
- Miglietta, S.; Cristiano, L.; Espinola, M.S.B.; Masiello, M.G.; Micara, G.; Battaglione, E.; Linari, A.; Palmerini, M.G.; Familiari, G.; Aragona, C.; et al. Effects of Simulated Microgravity In Vitro on Human Metaphase Ii Oocytes: An Electron Microscopy-Based Study. Cells 2023, 12, 1346. [Google Scholar] [CrossRef]
- Mao, X.; Chen, Z.; Luo, Q.; Zhang, B.; Song, G. Simulated Microgravity Inhibits the Migration of Mesenchymal Stem Cells by Remodeling Actin Cytoskeleton and Increasing Cell Stiffness. Cytotechnology 2016, 68, 2235–2243. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. The Role of Signaling Pathways in Osteoblast Gravity Perception. J. Gravit. Physiol. 2002, 9, P257–P260. [Google Scholar]
- Smith, J.K. Osteoclasts and Microgravity. Life 2020, 10, 207. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Zhivodernikov, I.; Ratushnyy, A.; Buravkova, L. Simulated Microgravity Remodels Extracellular Matrix of Osteocommitted Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2021, 22, 5428. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Gilbertson, V.; Guignandon, A.; Faure, C.; Neutelings, T.; Rattner, A.; Mineur, P.; Linossier, M.-T.; Laroche, N.; Lambert, C.; et al. Osteoblast Fibronectin Mrna, Protein Synthesis, and Matrix Are Unchanged after Exposure to Microgravity. FASEB J. 1999, 13, S121–S127. [Google Scholar] [CrossRef]
- Chatziravdeli, V.; Katsaras, G.N.; Lambrou, G.I. Gene Expression in Osteoblasts and Osteoclasts under Microgravity Conditions: A Systematic Review. Curr. Genom. 2019, 20, 184–198. [Google Scholar] [CrossRef]
- Zhivodernikov, I.V.; Ratushnyy, A.Y.; Matveeva, D.K.; Buravkova, L.B. Extracellular Matrix Proteins and Transcription of Matrix-Associated Genes in Mesenchymal Stromal Cells during Modeling of the Effects of Microgravity. Bull. Exp. Biol. Med. 2020, 170, 230–232. [Google Scholar] [CrossRef]
- Genchi, G.G.; Sinibaldi, E.; Ceseracciu, L.; Labardi, M.; Marino, A.; Marras, S.; De Simoni, G.; Mattoli, V.; Ciofani, G. Ultrasound-Activated Piezoelectric P(Vdf-Trfe)/Boron Nitride Nanotube Composite Films Promote Differentiation of Human Saos-2 Osteoblast-like Cells. Nanomedicine 2018, 14, 2421–2432. [Google Scholar] [CrossRef]
- Ahn, A.C.; Grodzinsky, A.J. Relevance of Collagen Piezoelectricity to Wolff’s Law: A Critical Review. Med. Eng. Phys. 2009, 31, 733–741. [Google Scholar] [CrossRef]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The Mechanosensitive Piezo1 Channel Is Required for Bone Formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical Sensing Protein Piezo1 Regulates Bone Homeostasis via Osteoblast-Osteoclast Crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Moussa, M.; Goldsmith, M.; Komarova, S. Craniofacial Bones and Teeth in Spacefarers: Systematic Review and Meta-Analysis. JDR Clin. Transl. Res. 2022, 8, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.S.; Pishak, S.A.; Mastro, A.M. The Effect of a 10-Day Space Flight on the Function, Phenotype, and Adhesion Molecule Expression of Splenocytes and Lymph Node Lymphocytes. Exp. Cell Res. 1995, 219, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; Michaletti, A.; Scimeca, M.; Marini, M.; Tarantino, U.; Zolla, L.; Coletta, M. Simulated Microgravity Induces a Cellular Regression of the Mature Phenotype in Human Primary Osteoblasts. Cell Death Discov. 2018, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Zayzafoon, M.; Meyers, V.E.; McDonald, J.M. Microgravity: The Immune Response and Bone. Immunol. Rev. 2005, 208, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.J.; Gombocz, E.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Insight in Adhesion Protein Sialylation and Microgravity Dependent Cell Adhesion—An Omics Network Approach. Int. J. Mol. Sci. 2020, 21, 1749. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Z.; Cooper, D.M.L.; Magnus, A.; Harrison, K.; Eames, B.F.; Chibbar, R.; Groot, G.; Huang, J.; Genth, H.; et al. Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Beta-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 5593. [Google Scholar] [CrossRef]
- Ingram, M.; Techy, G.B.; Saroufeem, R.; Yazan, O.; Narayan, K.S.; Goodwin, T.J.; Spaulding, G.F. Three-Dimensional Growth Patterns of Various Human Tumor Cell Lines in Simulated Microgravity of a Nasa Bioreactor. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 459–466. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in Mcf7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef] [PubMed]
- Sahana, J.; Corydon, T.J.; Wehland, M.; Krüger, M.; Kopp, S.; Melnik, D.; Kahlert, S.; Relja, B.; Infanger, M.; Grimm, D. Alterations of Growth and Focal Adhesion Molecules in Human Breast Cancer Cells Exposed to the Random Positioning Machine. Front. Cell Dev. Biol. 2021, 9, 672098. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The Effects of Microgravity on Differentiation and Cell Growth in Stem Cells and Cancer Stem Cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human Osteogenic Differentiation in Space: Proteomic and Epigenetic Clues to Better Understand Osteoporosis. Sci. Rep. 2019, 9, 8343. [Google Scholar] [CrossRef] [PubMed]
- Wahl, J.R.; Vivek, A.; Palomino, S.M.; Almuslim, M.; Cottier, K.E.; Langlais, P.R.; Streicher, J.M.; Vanderah, T.W.; Liktor-Busa, E.; Largent-Milnes, T.M. Extracellular Alterations in Ph and K+ Modify the Murine Brain Endothelial Cell Total and Phospho-Proteome. Pharmaceutics 2022, 14, 1469. [Google Scholar] [CrossRef]
- Pavlakou, P.; Dounousi, E.; Roumeliotis, S.; Eleftheriadis, T.; Liakopoulos, V. Oxidative Stress and the Kidney in the Space Environment. Int. J. Mol. Sci. 2018, 19, 3176. [Google Scholar] [CrossRef]
- Versari, S.; Longinotti, G.; Barenghi, L.; Maier, J.A.M.; Bradamante, S. The Challenging Environment on Board the International Space Station Affects Endothelial Cell Function by Triggering Oxidative Stress through Thioredoxin Interacting Protein Overexpression: The Esa-Sphinx Experiment. FASEB J. 2013, 27, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- De Santo, N.G.; Cirillo, M.; Valenti, G.; Perna, A.; Anastasio, P.; Drummer, C. Renal Function in Space: The Link between Osteoporosis, Hypercalciuria, and Aquaporins. J. Ren. Nutr. 2005, 15, 183–188. [Google Scholar] [CrossRef]
- Doty, S.; Seagrave, R. Human Water, Sodium, and Calcium Regulation during Space Flight and Exercise. Acta Astronaut. 2000, 46, 591–604. [Google Scholar] [CrossRef]
- Seo, H.; Itoh, T.; Murata, Y.; Ohmori, S.; Kambe, F.; Mohri, M.; Sekiguchi, C.; Matsui, N. Changes in Urinary Excretion of Pyridinium Cross-Links during Spacelab-J. Biol. Sci. Space 1997, 11, 321–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The Calcium-Sensing Receptor in Physiology and in Calcitropic and Noncalcitropic Diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Jüppner, H.; Abou-Samra, A.-B.; Freeman, M.; Kong, X.F.; Schipani, E.; Richards, J.; Kolakowski, L.F., Jr.; Hock, J.; Potts, J.T.; Kronenberg, H.M.; et al. A G Protein-Linked Receptor for Parathyroid Hormone and Parathyroid Hormone-Related Peptide. Science 1991, 254, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Thadhani, R.; Slatopolsky, E. Vitamin D Receptor and Analogs. Semin. Nephrol. 2004, 24, 10–16. [Google Scholar] [CrossRef]
- Chappell, M.C. Biochemical Evaluation of the Renin-Angiotensin System: The Good, Bad, and Absolute? Am. J. Physiol. Circ. Physiol. 2016, 310, H137–H152. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-L.; Shen, H.; Liu, A.; Dong, S.-S.; Zhang, L.; Deng, F.-Y.; Zhao, Q.; Deng, H.-W. A Road Map for Understanding Molecular and Genetic Determinants of Osteoporosis. Nat. Rev. Endocrinol. 2019, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Moayyeri, A.; Cheung, C.L.; Tan, K.C.; Morris, J.A.; Cerani, A.; Mohney, R.P.; Richards, J.B.; Hammond, C.; Spector, T.D.; Menni, C. Metabolomic Pathways to Osteoporosis in Middle-Aged Women: A Genome-Metabolome-Wide Mendelian Randomization Study. J. Bone Miner. Res. 2017, 33, 643–650. [Google Scholar] [CrossRef]
- Zhou, S.; Sosina, O.A.; Bovijn, J.; Laurent, L.; Sharma, V.; Akbari, P.; Forgetta, V.; Jiang, L.; Kosmicki, J.A.; Banerjee, N.; et al. Converging Evidence from Exome Sequencing and Common Variants Implicates Target Genes for Osteoporosis. Nat. Genet. 2023, 55, 1277–1287. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An Atlas of Genetic Influences on Osteoporosis in Humans and Mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Singh, S.; Chen, Y.; Hamadeh, I.S.; Langaee, T.; McDonough, C.W.; Holliday, L.S.; Lamba, J.K.; Moreb, J.S.; Katz, J.; et al. Pharmacogenomics of Osteonecrosis of the Jaw. Bone 2019, 124, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Nicole, L.; Guido, A.; Pizzol, D. Spaceflight Osteoporosis: Current State and Future Perspective. Endocr. Regul. 2015, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.L.; Blue, R.S.; Jennings, R.T.; Tarver, W.J.; Gray, G.W. Astronaut Medical Selection during the Shuttle Era: 1981–2011. Aviat. Space, Environ. Med. 2014, 85, 823–827. [Google Scholar] [CrossRef]
- Loriè, E.P.; Baatout, S.; Choukér, A.; Buchheim, J.-I.; Baselet, B.; Russo, C.D.; Wotring, V.; Monici, M.; Morbidelli, L.; Gagliardi, D.; et al. The Future of Personalized Medicine in Space: From Observations to Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 739747. [Google Scholar]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology: A Cellular and Molecular Approach; Elsevier/Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).