D1-Like and D2-Like Dopamine Receptors in the Rat Prefrontal Cortex: Impacts of Genetic Generalized Epilepsies and Social Behavioral Deficits
Abstract
1. Introduction
2. Materials and Methods
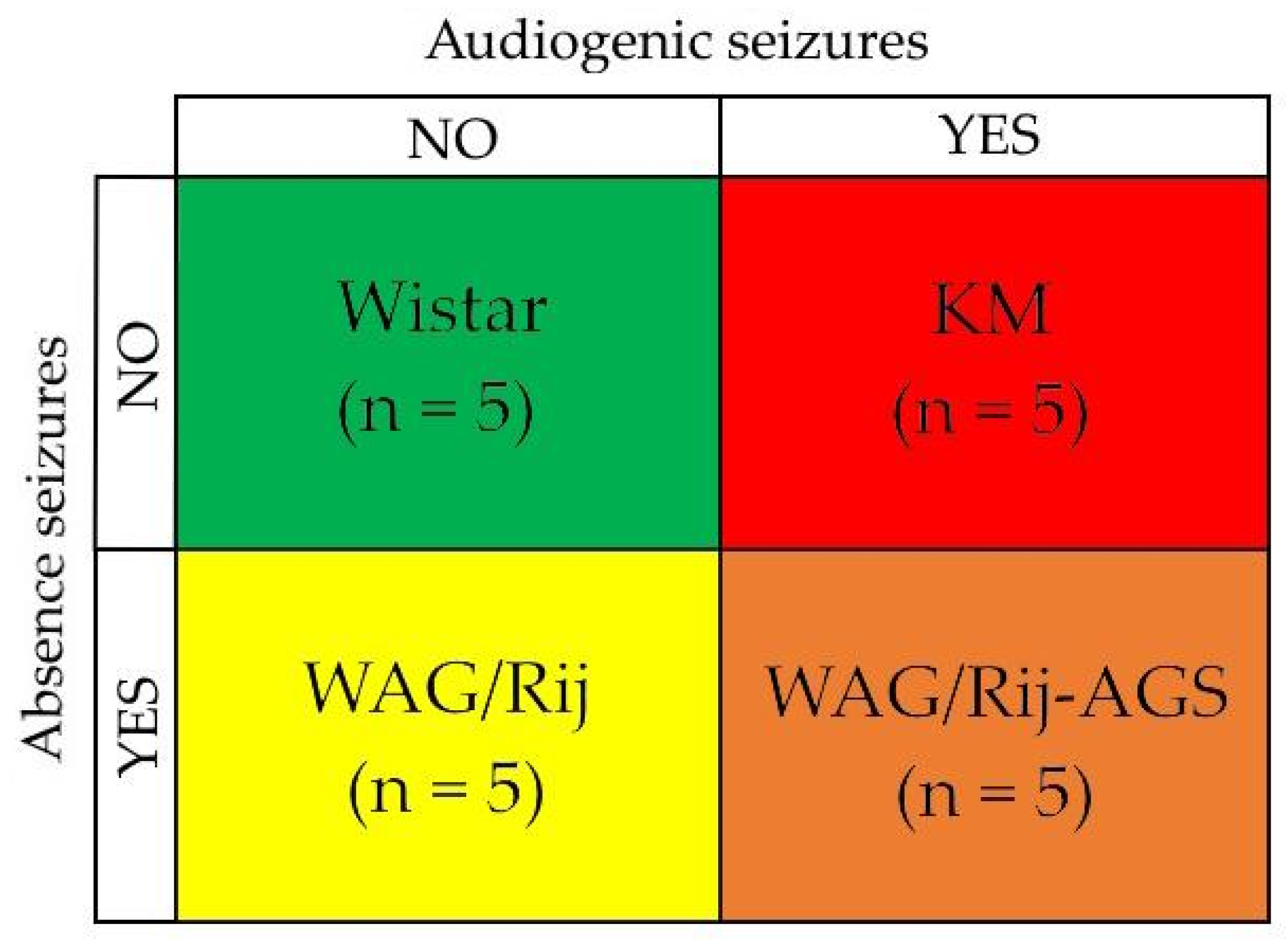
2.1. Animals
2.2. The Audiogenic Seizure Susceptibility Test
2.3. Decapitation and Brain Dissection
2.4. Autoradiography
- For D2DR—0.4 nM [3H]spiperone (tritium-labeled non-selective DR antagonist for the D2 and D3 DR subtypes [8]; specific activity 109.0 Ci/mmol, Amersham) for 60 min at room temperature.
- For D1DR—0.2 nM [3H]SCH 23390 and 10−7 M cis-flupenthixol (non-specific D1/D2-antagonist according to the PDSP Ki Database [57]).
- For D2DR—0.4 nM [3H]spiperone, 10−5 M haloperidol (the inverse D2DR agonist according to the PDSP Ki Database [57]) and 10−5 M ketanserin (the high-affinity non-selective antagonist of 5-HT2 receptors according to the PDSP Ki Database [57], used to block the [3H]spiperone binding to 5-HT2 serotoninergic receptors [58]).
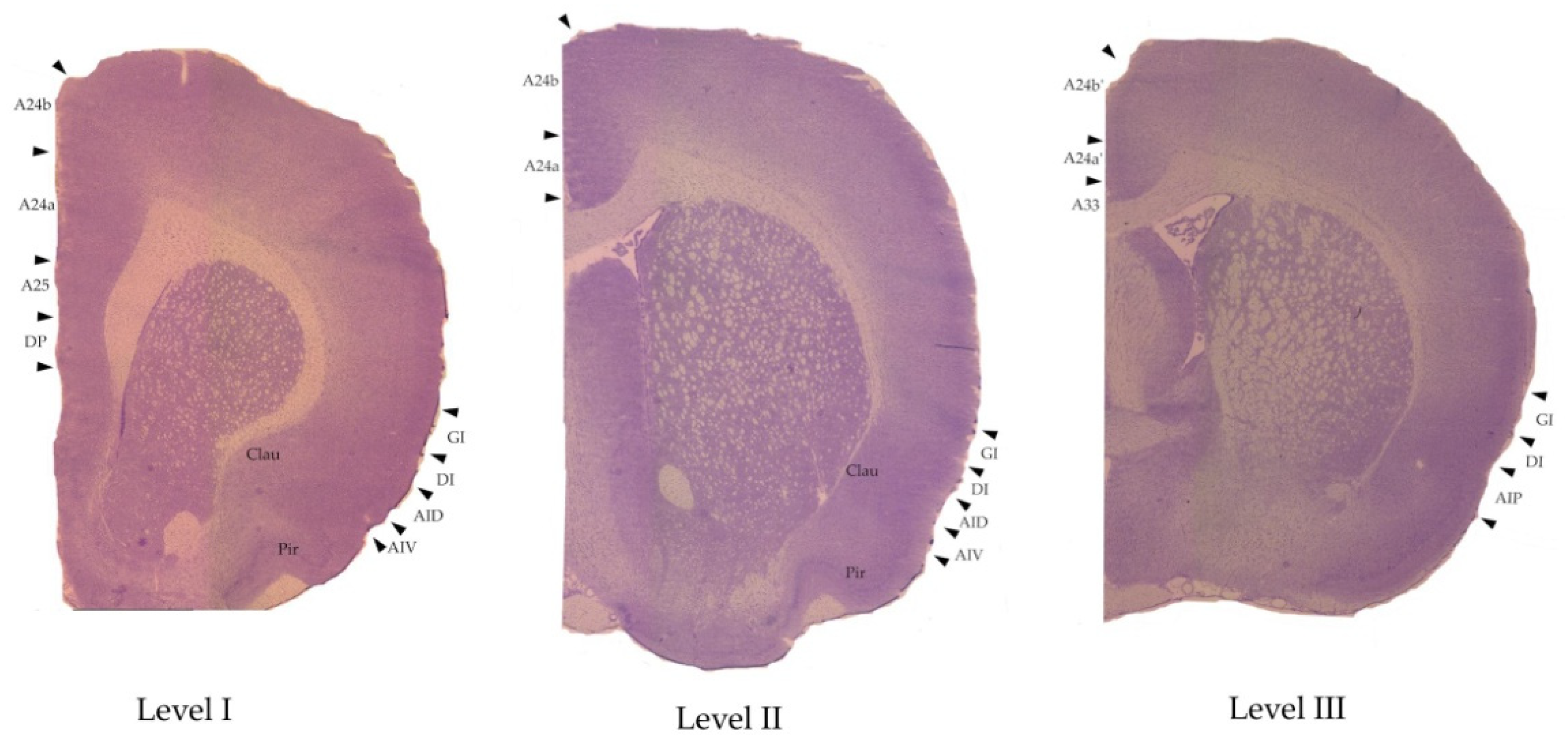
2.5. Measurements
| 6th Edition, 2007, [63] (The “Old” Nomenclature) | 7th Edition, 2014, [52] (The “New” Nomenclature) |
|---|---|
| Medial Prefrontal Cortex | Anterior Cingulate Cortex (ACC) |
| Infralimbic cortex IL | A25 |
| Prelimbic cortex PL | A24a (level I in our study) |
| Primary cingulate cortex Cing1 | A24b |
| Secondary cingulate cortex Cing2 | A24a (level II in our study) |
| Homologue of area 33 A33 | |
| Midcingulate cortex (MCC) | |
| Primary midcingulate cortex mCing1 | A24b’ |
| Secondary midcingulate cortex mCing2 | A24a’ |
| Lateral prefrontal cortex | Insular cortex |
| Granular insular cortex GI | Granular insular cortex GI |
| Dysgranular insular cortex DI | Dysgranular insular cortex DI |
| Dorsal agranular insular cortex AID | Dorsal agranular insular cortex AID |
| Ventral agranular insular cortex AIV | Ventral agranular insular cortex AIV |
| Posterior agranular insular cortex AIP | Posterior agranular insular cortex AIP |
| Adjacent structures | Adjacent structures |
| Dorsal peduncular cortex DP | Dorsal peduncular cortex DP |
| Claustrum Clau | Claustrum Clau |
| Piriform cortex Pir | Piriform cortex Pir |
2.6. The Statistical Analysis
3. Results
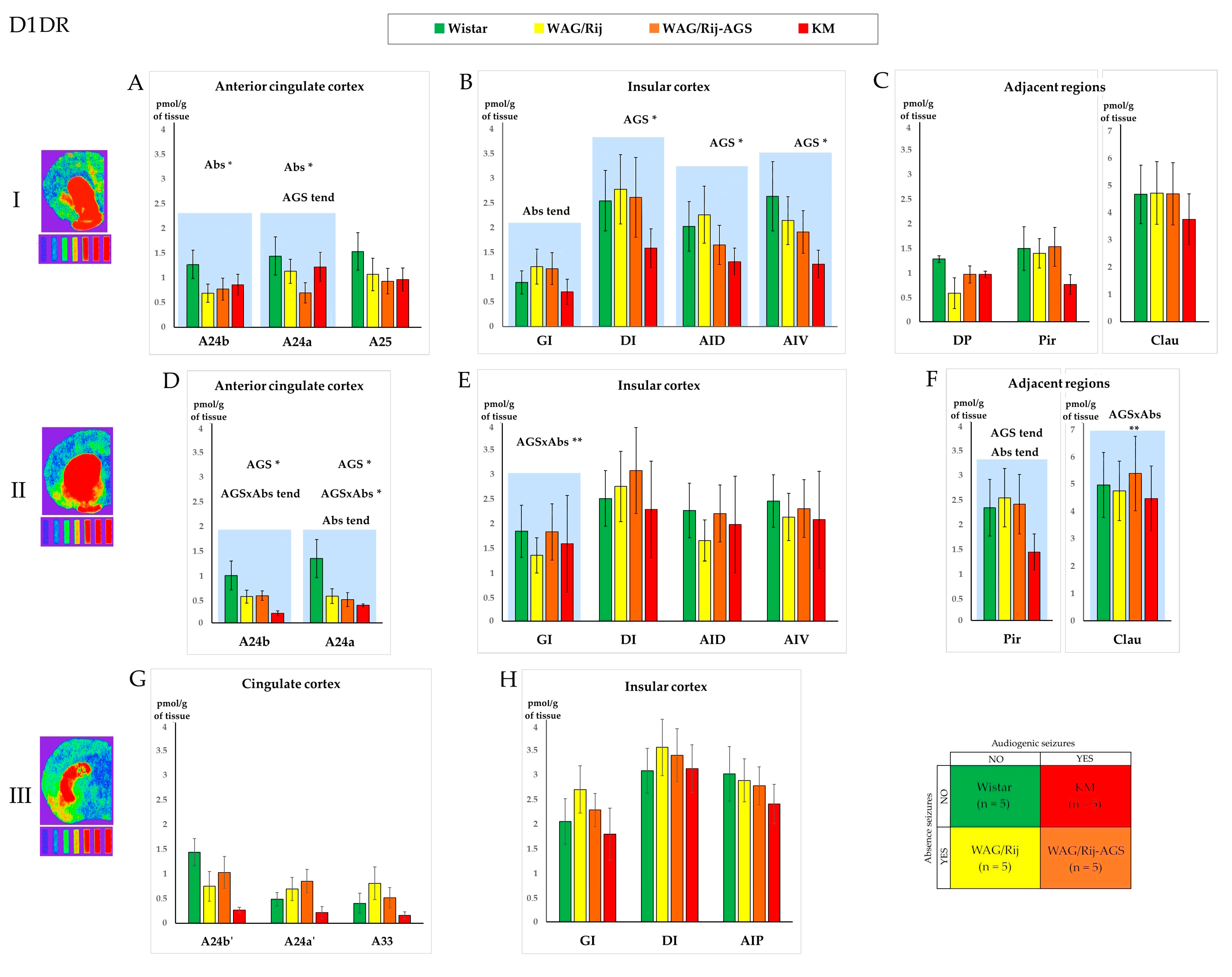
3.1. The D1DR Binding Density
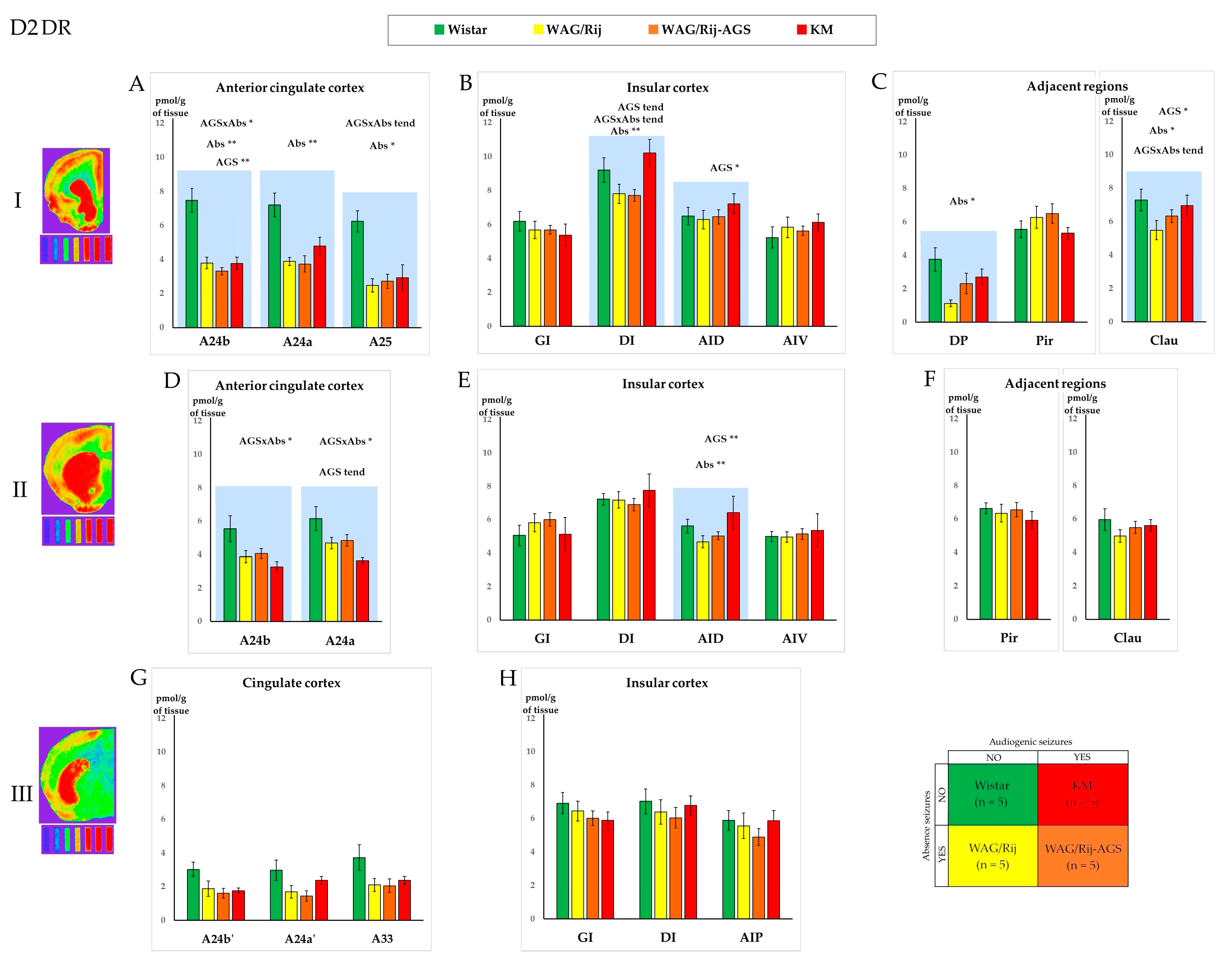
3.2. The D2DR Binding Density
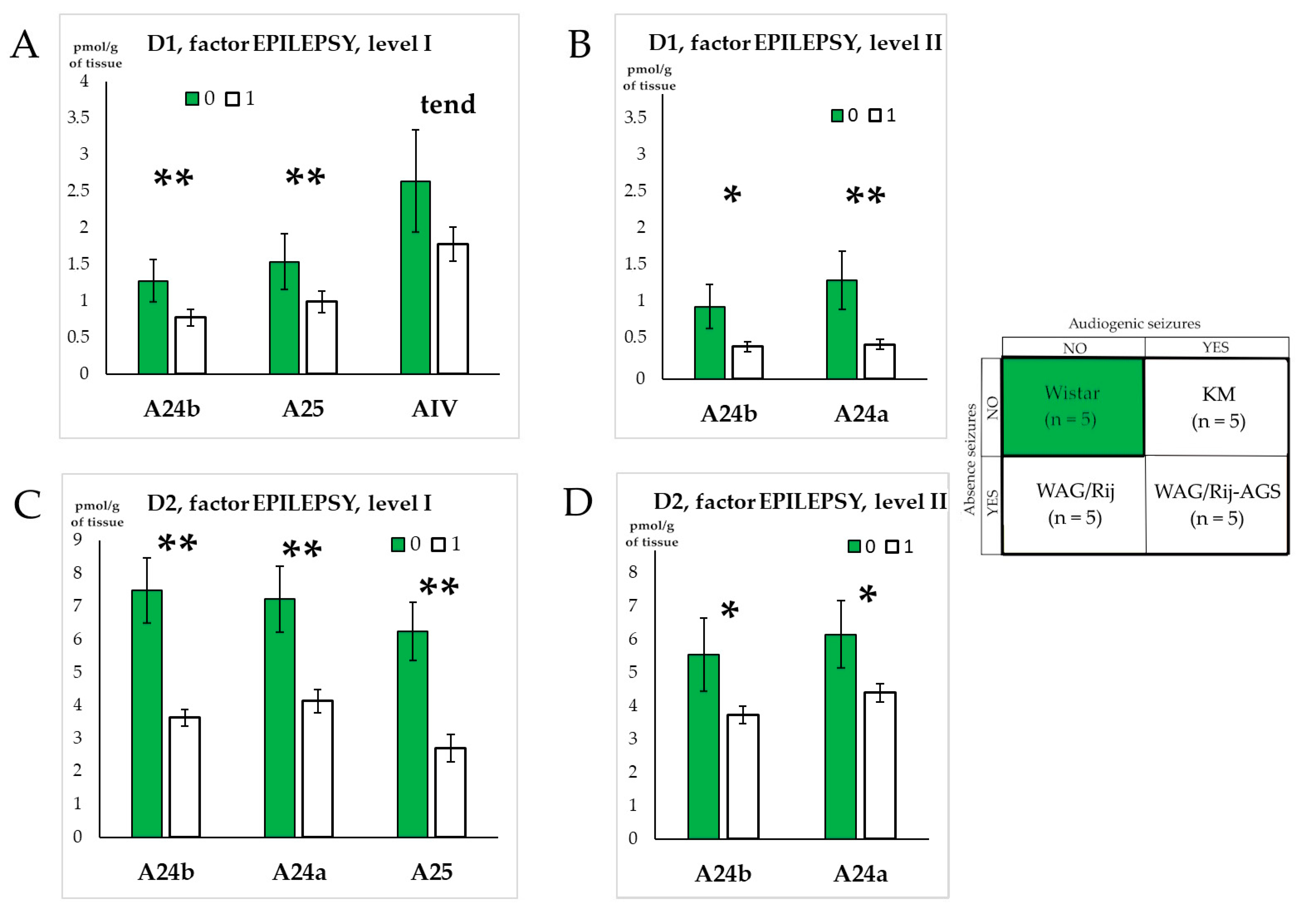
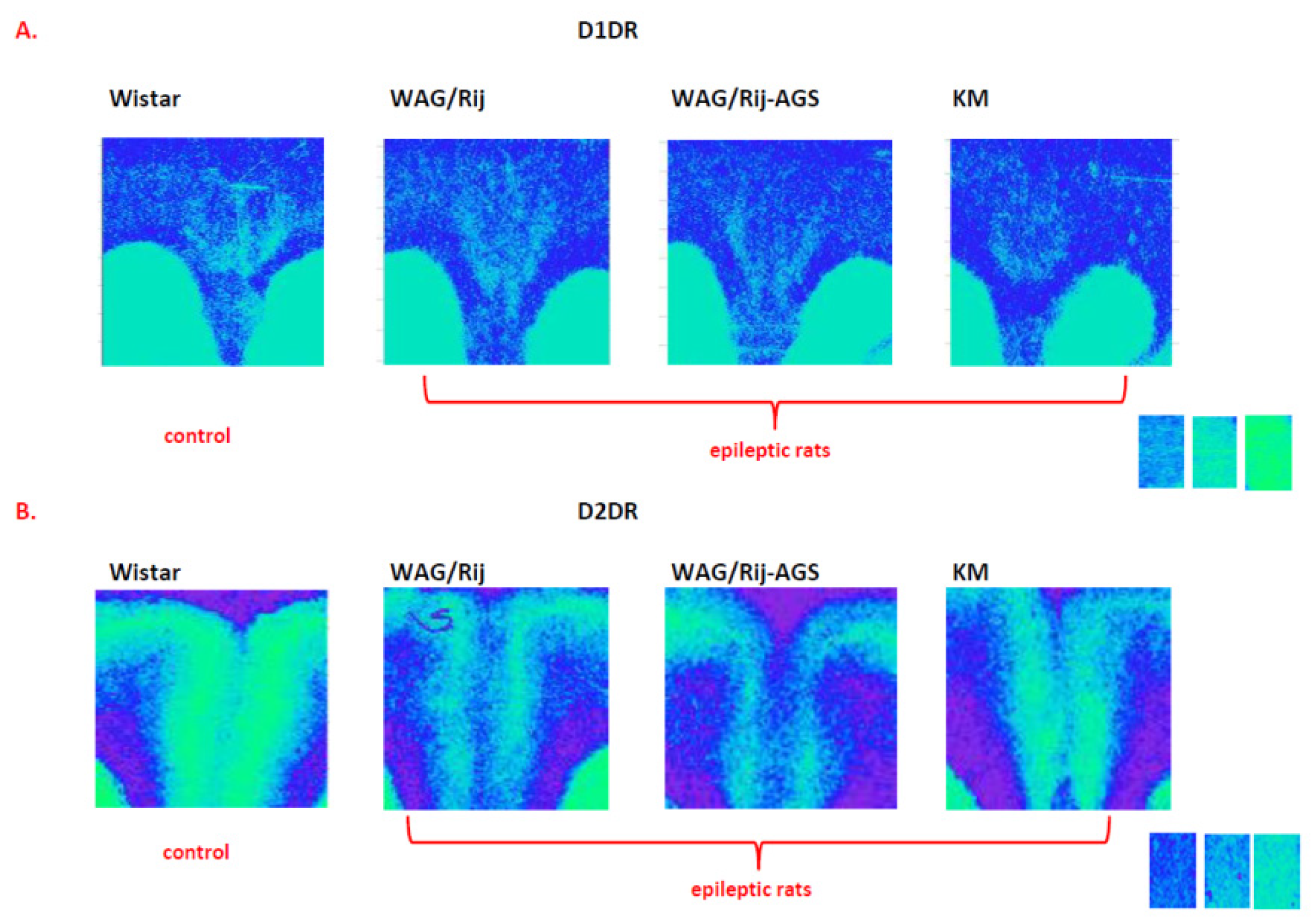
3.3. The General Effect of Epilepsies
3.4. Effect of Social Phenotype
3.5. Correlations between Local Binding Densities to D1DR and D2DR within the Prefrontal Regions
4. Discussion
4.1. Effects of Specific Epilepsy Types
4.2. Effects of Generalized Epilepsies
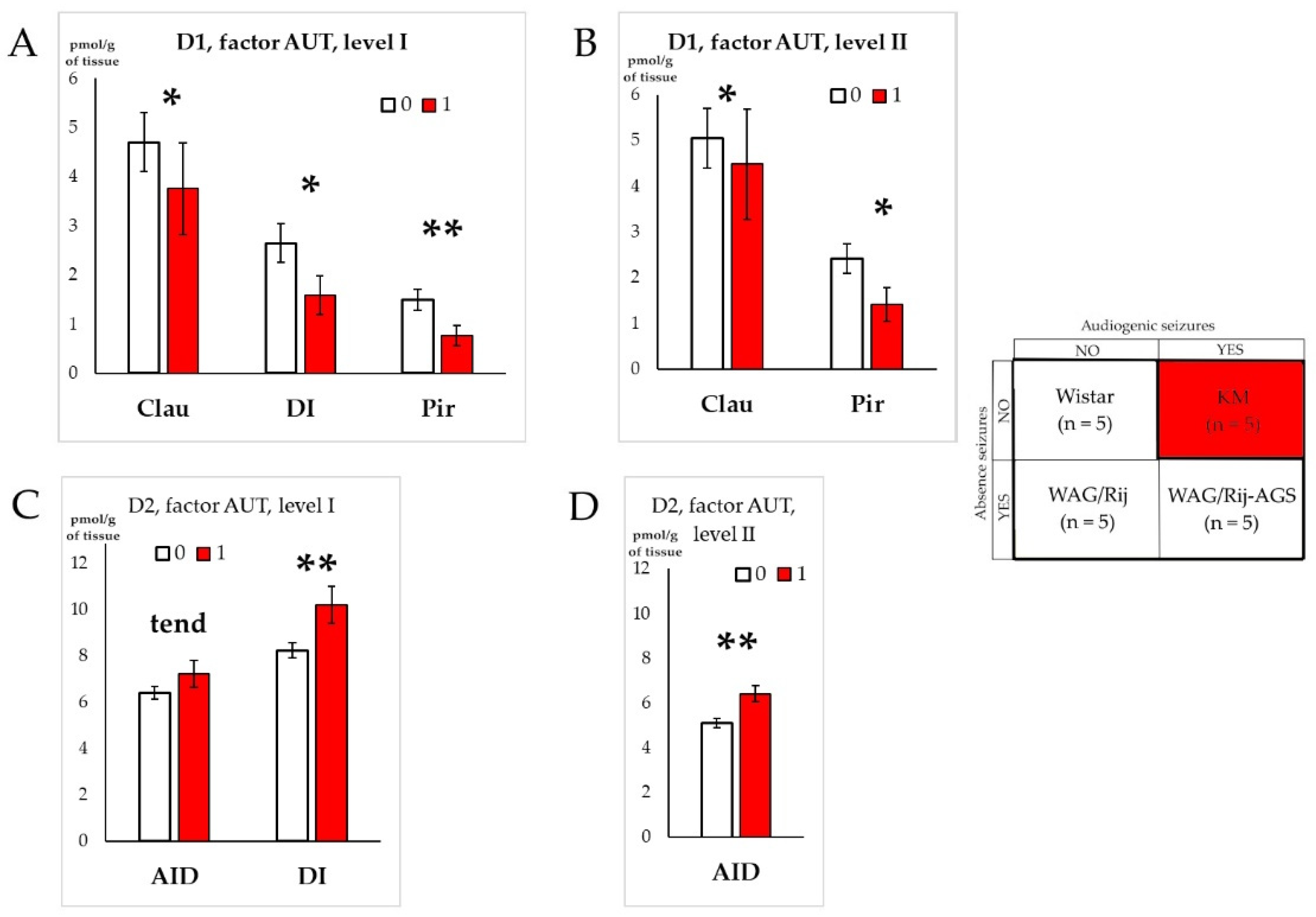
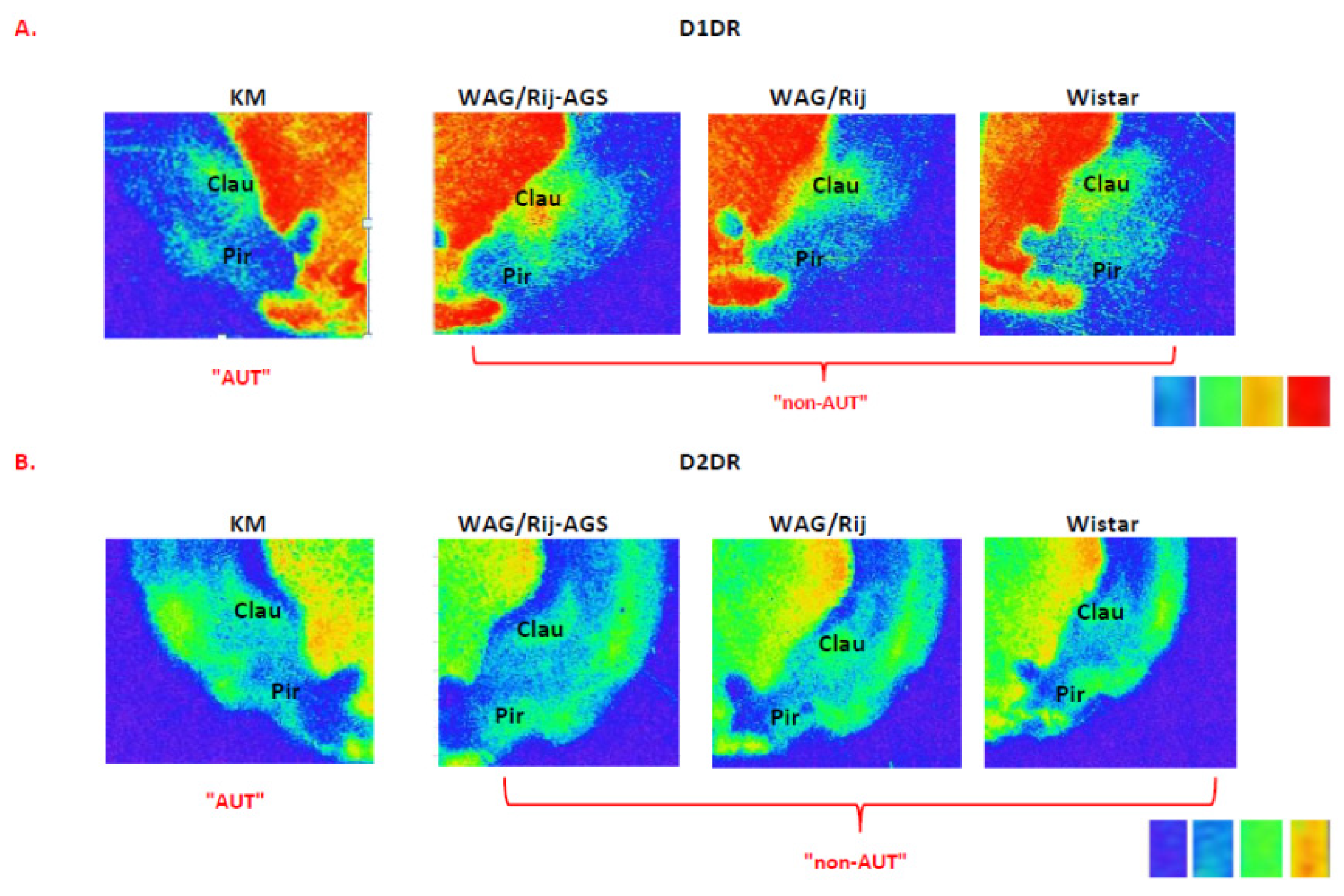
4.3. Effects of the Social Phenotype
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbib, M.A. (Ed.) The Handbook of Brain Theory and Neural Networks, 2nd ed.; MIT Press: Cambridge, MA, USA, 2003; ISBN 978-0-262-01197-6. [Google Scholar]
- Liu, C.; Goel, P.; Kaeser, P.S. Spatial and Temporal Scales of Dopamine Transmission. Nat. Rev. Neurosci. 2021, 22, 345–358. [Google Scholar] [CrossRef]
- Speranza, L.; Di Porzio, U.; Viggiano, D.; De Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W. Dopamine and Cognition. Curr. Opin. Neurol. 2003, 16, S1–S2. [Google Scholar] [CrossRef]
- Tsetsenis, T.; Broussard, J.I.; Dani, J.A. Dopaminergic Regulation of Hippocampal Plasticity, Learning, and Memory. Front. Behav. Neurosci. 2023, 16, 1092420. [Google Scholar] [CrossRef] [PubMed]
- Pezze, M.; Feldon, J. Mesolimbic Dopaminergic Pathways in Fear Conditioning. Prog. Neurobiol. 2004, 74, 301–320. [Google Scholar] [CrossRef]
- Ayano, G. Dopamine: Receptors, Functions, Synthesis, Pathways, Locations and Mental Disorders: Review of Literatures. J. Ment. Disord. Treat. 2016, 2, 2. [Google Scholar] [CrossRef]
- Cumming, P. Imaging Dopamine, 1st ed.; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0-521-79002-4. [Google Scholar]
- Tiberi, M. (Ed.) Dopamine Receptor Technologies; Neuromethods; Humana Press: New York, NY, USA, 2015; ISBN 978-1-4939-2195-9. [Google Scholar]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; White, F.J. Review: D1 Dopamine Receptor—The Search for a Function: A Critical Evaluation of the D1/D2 Dopamine Receptor Classification and Its Functional Implications. Synapse 1987, 1, 347–388. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.C.; McArthur, S.G. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Cramb, K.M.L.; Beccano-Kelly, D.; Cragg, S.J.; Wade-Martins, R. Impaired Dopamine Release in Parkinson’s Disease. Brain 2023, 146, 3117–3132. [Google Scholar] [CrossRef]
- Menon, V.; Palaniyappan, L.; Supekar, K. Integrative Brain Network and Salience Models of Psychopathology and Cognitive Dysfunction in Schizophrenia. Biol. Psychiatry 2023, 94, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Jayanti, S.; Dalla Verde, C.; Tiribelli, C.; Gazzin, S. Inflammation, Dopaminergic Brain and Bilirubin. Int. J. Mol. Sci. 2023, 24, 11478. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, F. The Interplay of Dopamine Metabolism Abnormalities and Mitochondrial Defects in the Pathogenesis of Schizophrenia. Transl. Psychiatry 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.S. The Role of Dopamine in Epilepsy. Synapse 1996, 22, 159–194. [Google Scholar] [CrossRef]
- Bozzi, Y.; Borrelli, E. The Role of Dopamine Signaling in Epileptogenesis. Front. Cell. Neurosci. 2013, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, C.; Sadat-Hossieny, Z.; Kanner, A.M.; Meador, K.J. Cognitive Disorders in Epilepsy II: Clinical Targets, Indications and Selection of Test Instruments. Seizure 2020, 83, 223–231. [Google Scholar] [CrossRef]
- Keele, N.B. The Role of Serotonin in Impulsive and Aggressive Behaviors Associated with Epilepsy-like Neuronal Hyperexcitability in the Amygdala. Epilepsy Behav. 2005, 7, 325–335. [Google Scholar] [CrossRef]
- Noeker, M.; Haverkamp-Krois, A.; Haverkamp, F. Development of Mental Health Dysfunction in Childhood Epilepsy. Brain Dev. 2005, 27, 5–16. [Google Scholar] [CrossRef]
- Fuster, J.M. The Prefrontal Cortex, 5th ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-408060-7. [Google Scholar]
- Preuss, T.M.; Wise, S.P. Evolution of Prefrontal Cortex. Neuropsychopharmacology 2022, 47, 3–19. [Google Scholar] [CrossRef]
- McGregor, M.S.; LaLumiere, R.T. Still a “Hidden Island”? The Rodent Insular Cortex in Drug Seeking, Reward, and Risk. Neurosci. Biobehav. Rev. 2023, 153, 105334. [Google Scholar] [CrossRef]
- Inoyama, K.; Devinsky, O. Cingulate Seizures and Recent Treatment Strategies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 166, pp. 341–353. ISBN 978-0-444-64196-0. [Google Scholar]
- Li, M.; Ma, X.; Mai, C.; Fan, Z.; Wang, Y.; Ren, Y. Knowledge Atlas of Insular Epilepsy: A Bibliometric Analysis. Neuropsychiatr. Dis. Treat. 2022, 18, 2891–2903. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; van Luijtelaar, E.L.J.M. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Zobeiri, M. Progress and Outlooks in a Genetic Absence Epilepsy Model (WAG/Rij). Curr. Med. Chem. 2014, 21, 704–721. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; van Oijen, G. Establishing Drug Effects on Electrocorticographic Activity in a Genetic Absence Epilepsy Model: Advances and Pitfalls. Front. Pharmacol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Kuznetsova, G.D. Audiogenic seizures in rats of different genetic strains. Zh. Vyssh. Nerv. Deiat. Im. I. P. Pavlova 1998, 48, 143–152. [Google Scholar]
- Midzyanovskaya, I.S.; Kuznetsova, G.D.; Vinogradova, L.V.; Shatskova, A.B.; Coenen, A.M.L.; van Luijtelaar, G. Mixed Forms of Epilepsy in a Subpopulation of WAG/Rij Rats. Epilepsy Behav. 2004, 5, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Poletaeva, I.I.; Surina, N.M.; Kostina, Z.A.; Perepelkina, O.V.; Fedotova, I.B. The Krushinsky-Molodkina Rat Strain: The Study of Audiogenic Epilepsy for 65 Years. Epilepsy Behav. 2017, 71, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, I.B.; Surina, N.M.; Nikolaev, G.M.; Revishchin, A.V.; Poletaeva, I.I. Rodent Brain Pathology, Audiogenic Epilepsy. Biomedicines 2021, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, D.G.; Davletshin, A.A.; Litvinova, S.A.; Fedotova, I.B.; Surina, N.M.; Poletaeva, I.I. Rodent Models of Audiogenic Epilepsy: Genetic Aspects, Advantages, Current Problems and Perspectives. Biomedicines 2022, 10, 2934. [Google Scholar] [CrossRef] [PubMed]
- Midzyanovskaya, I.S.; Shatskova, A.B.; Sarkisova, K.Y.; van Luijtelaar, G.; Tuomisto, L.; Kuznetsova, G.D. Convulsive and Nonconvulsive Epilepsy in Rats: Effects on Behavioral Response to Novelty Stress. Epilepsy Behav. 2005, 6, 543–551. [Google Scholar] [CrossRef]
- Midzyanovskaya, I.S.; Kuznetsova, G.D.; van Luijtelaar, E.L.J.M.; Van Rijn, C.M.; Tuomisto, L.; MacDonald, E. The Brain 5HTergic Response to an Acute Sound Stress in Rats with Generalized (Absence and Audiogenic) Epilepsy. Brain Res. Bull. 2006, 69, 631–638. [Google Scholar] [CrossRef]
- Midzyanovskaya, I.S.; Birioukova, L.M.; Shatskova, A.B.; van Luijtelaar, G.; Tuomisto, L.M. H1 Histamine Receptor Densities Are Increased in Brain Regions of Rats with Genetically Generalized Epilepsies. Epilepsy Res. 2016, 127, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Midzyanovskaya, I.S.; Petrenko, T.E.; Birioukova, L.M.; Tuomisto, L.M. Reduced H3 Histamine Receptor Binding Densities in the Upper Layers of Motor Cortex in Rats Prone to Audiogenic Convulsive Seizures. Epilepsy Res. 2021, 170, 106543. [Google Scholar] [CrossRef]
- Midzyanovskaya, I.S.; Birioukova, L.M.; Storvik, M.; van Luijtelaar, G.; Tuomisto, L.M. The Prefrontal Cortex Shows Widespread Decrease in H3 Histamine Receptor Binding Densities in Rats with Genetic Generalized Epilepsies. Epilepsy Res. 2022, 182, 106921. [Google Scholar] [CrossRef] [PubMed]
- Tsyba, E.T.; Midzyanovskaya, I.S.; Birioukova, L.M.; Tuomisto, L.M.; van Luijtelaar, G.; Abbasova, K.R. Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies. Diagnostics 2023, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Rebik, A.A.; Riga, V.D.; Smirnov, K.S.; Sysoeva, O.V.; Midzyanovskaya, I.S. Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy. J. Pers. Med. 2022, 12, 2062. [Google Scholar] [CrossRef]
- Rebik, A.; Broshevitskaya, N.; Kuzhuget, S.; Aleksandrov, P.; Abbasova, K.; Zaichenko, M.; Midzyanovskaya, I. Audiogenic Seizures and Social Deficits: No Aggravation Found in Krushinsky–Molodkina Rats. Biomedicines 2023, 11, 2566. [Google Scholar] [CrossRef]
- Besag, F. Epilepsy in Patients with Autism: Links, Risks and Treatment Challenges. Neuropsychiatr. Dis. Treat. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Brooks-Kayal, A. Epilepsy and Autism Spectrum Disorders: Are There Common Developmental Mechanisms? Brain Dev. 2010, 32, 731–738. [Google Scholar] [CrossRef]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological Bases of Autism—Epilepsy Comorbidity: A Focus on Excitation/Inhibition Imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering Animal Models Used to Study Autism Spectrum Disorder: Current State and Optimizing Future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef]
- Sierra-Arregui, T.; Llorente, J.; Giménez Minguez, P.; Tønnesen, J.; Peñagarikano, O. Neurobiological Mechanisms of Autism Spectrum Disorder and Epilepsy, Insights from Animal Models. Neuroscience 2020, 445, 69–82. [Google Scholar] [CrossRef]
- Haratizadeh, S.; Parvan, M.; Mohammadi, S.; Shabani, M.; Nozari, M. An Overview of Modeling and Behavioral Assessment of Autism in the Rodent. Int. J. Dev. Neurosci. 2021, 81, 221–228. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Text with EEA Relevance. Off. J. Eur. Union 2010, 276, 33–79.
- Morimoto, K.; Fahnestock, M.; Racine, R.J. Kindling and Status Epilepticus Models of Epilepsy: Rewiring the Brain. Prog. Neurobiol. 2004, 73, 1–60. [Google Scholar] [CrossRef]
- Vinogradova, L.V. Audiogenic Kindling and Secondary Subcortico-Cortical Epileptogenesis: Behavioral Correlates and Electrographic Features. Epilepsy Behav. 2017, 71, 142–153. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. Paxino’s and Watson’s The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier/AP, Academic Press is an imprint of Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2014; ISBN 978-0-12-391949-6. [Google Scholar]
- Paxinos, G.; Kassem, M.S.; Kirkcaldie, M.; Carrive, P. Chemoarchitectonic Atlas of the Rat Brain, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; ISBN 0-12-818959-2. [Google Scholar]
- Blunt, S.B.; Jenner, P.; Marsden, C.D. Autoradiographic Study of Striatal D1 and D2 Dopamine Receptors in 6-OHDA-Lesioned Rats Receiving Foetal Ventral Mesencephalic Grafts and Chronic Treatment WithL-DOPA and Carbidopa. Brain Res. 1992, 582, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Birioukova, L.M.; Midzyanovskaya, I.S.; Lensu, S.; Tuomisto, L.; van Luijtelaar, G. Distribution of D1-like and D2-like Dopamine Receptors in the Brain of Genetic Epileptic WAG/Rij Rats. Epilepsy Res. 2005, 63, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.A. SCH 23390: The First Selective Dopamine D1-Like Receptor Antagonist. CNS Drug Rev. 2006, 7, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Lopez, E.; Patel, S.; Kroeze, W.K. The Multiplicity of Serotonin Receptors: Uselessly Diverse Molecules or an Embarrassment of Riches? Neuroscientist 2000, 6, 252–262. [Google Scholar] [CrossRef]
- Janowsky, A.; Neve, K.A.; Kinzie, J.M.; Taylor, B.; de Paulis, T.; Belknap, J.K. Extrastriatal Dopamine D2 Receptors: Distribution, Pharmacological Characterization and Region-Specific Regulation by Clozapine. J. Pharmacol. Exp. Ther. 1992, 261, 1282–1290. [Google Scholar] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The Role of the Medial Prefrontal Cortex in Cognition, Ageing and Dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021, 44, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Kolk, S.M.; Rakic, P. Development of Prefrontal Cortex. Neuropsychopharmacology 2022, 47, 41–57. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands; Heidelberg, Germany, 2007; ISBN 978-0-12-374121-9. [Google Scholar]
- Zilles, K. The Cortex of the Rat; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-642-70575-5. [Google Scholar]
- Vogt, B.A.; Paxinos, G. Cytoarchitecture of Mouse and Rat Cingulate Cortex with Human Homologies. Brain Struct. Funct. 2014, 219, 185–192. [Google Scholar] [CrossRef]
- Swanson, L.W. Brain Maps 4.0—Structure of the Rat Brain: An Open Access Atlas with Global Nervous System Nomenclature Ontology and Flatmaps. J. Comp. Neurol. 2018, 526, 935–943. [Google Scholar] [CrossRef]
- Vogt, B.A. Cingulate Cortex and Pain Architecture. In The Rat Nervous System; Paxinos, G., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2015; pp. 575–599. ISBN 978-0-12-374245-2. [Google Scholar]
- Laubach, M.; Amarante, L.M.; Swanson, K.; White, S.R. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018, 5, ENEURO.0315-18.2018. [Google Scholar] [CrossRef]
- Weible, A.P. Remembering to Attend: The Anterior Cingulate Cortex and Remote Memory. Behav. Brain Res. 2013, 245, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of Anterior Cingulate Cortex to Behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The Cingulate Cortex and Limbic Systems for Emotion, Action, and Memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. The Insular Cortex. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 195, pp. 123–163. ISBN 978-0-444-53860-4. [Google Scholar]
- Gehrlach, D.A.; Weiand, C.; Gaitanos, T.N.; Cho, E.; Klein, A.S.; Hennrich, A.A.; Conzelmann, K.-K.; Gogolla, N. A Whole-Brain Connectivity Map of Mouse Insular Cortex. eLife 2020, 9, e55585. [Google Scholar] [CrossRef]
- Lamm, C.; Singer, T. The Role of Anterior Insular Cortex in Social Emotions. Brain Struct. Funct. 2010, 214, 579–591. [Google Scholar] [CrossRef]
- Shura, R.D.; Hurley, R.A.; Taber, K.H. Insular Cortex: Structural and Functional Neuroanatomy. J. Neuropsychiatry Clin. Neurosci. 2014, 26, iv-282. [Google Scholar] [CrossRef]
- Frank, S.; Kullmann, S.; Veit, R. Food Related Processes in the Insular Cortex. Front. Hum. Neurosci. 2013, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Droutman, V.; Read, S.J.; Bechara, A. Revisiting the Role of the Insula in Addiction. Trends Cogn. Sci. 2015, 19, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Chemical Neurotransmission. In The Prefrontal Cortex; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–131. ISBN 978-0-12-407815-4. [Google Scholar]
- Islam, K.U.S.; Meli, N.; Blaess, S. The Development of the Mesoprefrontal Dopaminergic System in Health and Disease. Front. Neural Circuits 2021, 15, 746582. [Google Scholar] [CrossRef]
- Labrakakis, C. The Role of the Insular Cortex in Pain. Int. J. Mol. Sci. 2023, 24, 5736. [Google Scholar] [CrossRef] [PubMed]
- De Caro, C.; Di Cesare Mannelli, L.; Branca, J.J.V.; Micheli, L.; Citraro, R.; Russo, E.; De Sarro, G.; Ghelardini, C.; Calignano, A.; Russo, R. Pain Modulation in WAG/Rij Epileptic Rats (A Genetic Model of Absence Epilepsy): Effects of Biological and Pharmacological Histone Deacetylase Inhibitors. Front. Pharmacol. 2020, 11, 549191. [Google Scholar] [CrossRef]
- Meeren, H.; van Luijtelaar, G.; Lopes Da Silva, F.; Coenen, A. Evolving Concepts on the Pathophysiology of Absence Seizures: The Cortical Focus Theory. Arch. Neurol. 2005, 62, 371. [Google Scholar] [CrossRef]
- Buzsáki, G.; Smith, A.; Berger, S.; Fisher, L.J.; Gage, F.H.; Aston-Jones, G.; Bloom, F.E. Petit Mal Epilepsy and Parkinsonian Tremor: Hypothesis of a Common Pacemaker. Neuroscience 1990, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bernedo Paredes, V.E.; Buchholz, H.-G.; Gartenschläger, M.; Breimhorst, M.; Schreckenberger, M.; Werhahn, K.J. Reduced D2/D3 Receptor Binding of Extrastriatal and Striatal Regions in Temporal Lobe Epilepsy. PLoS ONE 2015, 10, e0141098. [Google Scholar] [CrossRef]
- Rocha, L.; Alonso-Vanegas, M.; Villeda-Hernández, J.; Mújica, M.; Cisneros-Franco, J.M.; López-Gómez, M.; Zavala-Tecuapetla, C.; Frías-Soria, C.L.; Segovia-Vila, J.; Borsodi, A. Dopamine Abnormalities in the Neocortex of Patients with Temporal Lobe Epilepsy. Neurobiol. Dis. 2012, 45, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yakushev, I.Y.; Dupont, E.; Buchholz, H.; Tillmanns, J.; Debus, F.; Cumming, P.; Heimann, A.; Fellgiebel, A.; Luhmann, H.J.; Landvogt, C.; et al. In Vivo Imaging of Dopamine Receptors in a Model of Temporal Lobe Epilepsy. Epilepsia 2010, 51, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Firstova, J.J.; Abaimov, D.A.; Surina, N.M.; Poletaeva, I.I.; Fedotova, I.B.; Kovalev, G.I. Binding of Specific Ligand by D2- and NMDA-Receptors of Striatum Cells in Two Rat Strains Predisposed and Resistant to Audiogenic Seizures. Bull. Exp. Biol. Med. 2012, 154, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Gonzalez, D.; Floran, B.; Escartin, E.; Rocha, L. Changes on D2-like Receptor Induced Gi Protein Activation and Hippocampal Dopamine Release in Kindled Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 246–251. [Google Scholar] [CrossRef]
- De Bruin, N.M.W.J.; van Luijtelaar, E.L.J.M.; Cools, A.R.; Ellenbroek, B.A. Dopamine Characteristics in Rat Genotypes with Distinct Susceptibility to Epileptic Activity: Apomorphine-Induced Stereotyped Gnawing and Novelty/Amphetamine-Induced Locomotor Stimulation. Behav. Pharmacol. 2001, 12, 517–525. [Google Scholar] [CrossRef]
- Kuznetsova, G.D.; Petrova, E.V.; Coenen, A.M.L.; van Luijtelaar, E.L.J.M. Generalized Absence Epilepsy and Catalepsy in Rats. Physiol. Behav. 1996, 60, 1165–1169. [Google Scholar] [CrossRef]
- Kalimullina, L.B.; Musina, A.M.; Kuznetsova, G.D. Experimental approaches to investigation into the role of the genotype by the locus TAG 1A of dopamine D2-receptors in epileptogenesis. Ross. Fiziol. Zh. Im. I M Sechenova 2012, 98, 177–185. [Google Scholar]
- Midzyanovskaya, I.S.; Shatskova, A.B.; MacDonald, E.; van Luijtelaar, G.; Tuomisto, L. Brain Aminergic Deficiency in Absence Epileptic Rats: Dependency on Seizure Severity and Their Functional Coupling at Rest. J. Behav. Brain Sci. 2020, 10, 29–45. [Google Scholar] [CrossRef][Green Version]
- Bentivoglio, M.; Morelli, M. Chapter I The Organization and Circuits of Mesencephalic Dopaminergic Neurons and the Distribution of Dopamine Receptors in the Brain. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 21, pp. 1–107. ISBN 978-0-444-51778-4. [Google Scholar]
- Ottman, R.; Lipton, R.B.; Ettinger, A.B.; Cramer, J.A.; Reed, M.L.; Morrison, A.; Wan, G.J. Comorbidities of Epilepsy: Results from the Epilepsy Comorbidities and Health (EPIC) Survey. Epilepsia 2011, 52, 308–315. [Google Scholar] [CrossRef]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij Strain: A Genetic Animal Model of Absence Epilepsy with Comorbidity of Depressiony. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.Y.; Fedotova, I.B.; Surina, N.M.; Nikolaev, G.M.; Perepelkina, O.V.; Kostina, Z.A.; Poletaeva, I.I. Genetic Background Contributes to the Co-Morbidity of Anxiety and Depression with Audiogenic Seizure Propensity and Responses to Fluoxetine Treatment. Epilepsy Behav. 2017, 68, 95–102. [Google Scholar] [CrossRef]
- Sarkisova, K.Y.; Kulikov, M.A.; Kudrin, V.S.; Narkevich, V.B.; Midzianovskaia, I.S.; Biriukova, L.M.; Folomkina, A.A.; Basian, A.S. Neurochemical mechanisms of depression-like behavior in WAG/Rij rats. Zh. Vyssh. Nerv. Deiat. Im. P. Pavlova 2013, 63, 303–315. [Google Scholar] [CrossRef]
- Lörincz, M.; Oláh, M.; Baracskay, P.; Szilágyi, N.; Juhász, G. Propagation of Spike and Wave Activity to the Medial Prefrontal Cortex and Dorsal Raphe Nucleus of WAG/Rij Rats. Physiol. Behav. 2007, 90, 318–324. [Google Scholar] [CrossRef]
- Bianchin, M.M.; Londero, R.G.; Lima, J.E.; Bigal, M.E. Migraine and Epilepsy: A Focus on Overlapping Clinical, Pathophysiological, Molecular, and Therapeutic Aspects. Curr. Pain Headache Rep. 2010, 14, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, Z.; Gambeta, E.; Xu, S.C.; Thomas, C.; Godfrey, N.; Chen, L.; M’Dahoma, S.; Borgland, S.L.; Zamponi, G.W. Dopamine Inputs from the Ventral Tegmental Area into the Medial Prefrontal Cortex Modulate Neuropathic Pain-Associated Behaviors in Mice. Cell Rep. 2020, 31, 107812. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.; Artigas, F. Laminar and Cellular Distribution of Monoamine Receptors in Rat Medial Prefrontal Cortex. Front. Neuroanat. 2017, 11, 87. [Google Scholar] [CrossRef]
- Flik, G.; Folgering, J.H.A.; Cremers, T.I.H.F.; Westerink, B.H.C.; Dremencov, E. Interaction Between Brain Histamine and Serotonin, Norepinephrine, and Dopamine Systems: In Vivo Microdialysis and Electrophysiology Study. J. Mol. Neurosci. 2015, 56, 320–328. [Google Scholar] [CrossRef]
- Surina, N.M.; Fedotova, I.B.; Nikolaev, G.M.; Grechenko, V.V.; Gankovskaya, L.V.; Ogurtsova, A.D.; Poletaeva, I.I. Neuroinflammation in Pathogenesis of Audiogenic Epilepsy: Altered Proinflammatory Cytokine Levels in the Rats of Krushinsky–Molodkina Seizure-Prone Strain. Biochem. Mosc. 2023, 88, 481–490. [Google Scholar] [CrossRef]
- Aygun, H.; Akin, A.T.; Kızılaslan, N.; Sumbul, O.; Karabulut, D. Probiotic Supplementation Alleviates Absence Seizures and Anxiety- and Depression-like Behavior in WAG/Rij Rat by Increasing Neurotrophic Factors and Decreasing Proinflammatory Cytokines. Epilepsy Behav. 2022, 128, 108588. [Google Scholar] [CrossRef]
- Russo, E.; Andreozzi, F.; Iuliano, R.; Dattilo, V.; Procopio, T.; Fiume, G.; Mimmi, S.; Perrotti, N.; Citraro, R.; Sesti, G.; et al. Early Molecular and Behavioral Response to Lipopolysaccharide in the WAG/Rij Rat Model of Absence Epilepsy and Depressive-like Behavior, Involves Interplay between AMPK, AKT/mTOR Pathways and Neuroinflammatory Cytokine Release. Brain. Behav. Immun. 2014, 42, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Nesci, V.; Tallarico, M.; Amodio, N.; Gallo Cantafio, E.M.; De Sarro, G.; Constanti, A.; Russo, E.; Citraro, R. IL-6 Receptor Blockade by Tocilizumab Has Anti-Absence and Anti-Epileptogenic Effects in the WAG/Rij Rat Model of Absence Epilepsy. Neurotherapeutics 2020, 17, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Besag, F.; Ettinger, A.B.; Mula, M.; Gobbi, G.; Comai, S.; Aldenkamp, A.P.; Steinhoff, B.J. Epilepsy, Antiepileptic Drugs, and Aggression: An Evidence-Based Review. Pharmacol. Rev. 2016, 68, 563–602. [Google Scholar] [CrossRef] [PubMed]
- Elnazer, H.Y.; Agrawal, N. Aggressive Behavior. In Neuropsychiatric Symptoms of Epilepsy; Mula, M., Ed.; Neuropsychiatric Symptoms of Neurological Disease; Springer International Publishing: Cham, Switzerland, 2016; pp. 99–116. ISBN 978-3-319-22158-8. [Google Scholar]
- Zhu, Y.; Feng, J.; Ji, J.; Hou, H.; Chen, L.; Wu, S.; Liu, Q.; Yao, Q.; Du, P.; Zhang, K.; et al. Alteration of Monoamine Receptor Activity and Glucose Metabolism in Pediatric Patients with Anticonvulsant-Induced Cognitive Impairment. J. Nucl. Med. 2017, 58, 1490–1497. [Google Scholar] [CrossRef]
- Kodama, T.; Watanabe, M. Interaction of Dopamine and Glutamate Release in the Primate Prefrontal Cortex in Relation to Working Memory and Reward. In The Prefrontal Cortex as an Executive, Emotional, and Social Brain; Watanabe, M., Ed.; Springer: Tokyo, Japan, 2017; pp. 77–102. ISBN 978-4-431-56506-2. [Google Scholar]
- Kanner, A.M. Management of Psychiatric and Neurological Comorbidities in Epilepsy. Nat. Rev. Neurol. 2016, 12, 106–116. [Google Scholar] [CrossRef]
- Epps, S.A.; Weinshenker, D. Rhythm and Blues: Animal Models of Epilepsy and Depression Comorbidity. Biochem. Pharmacol. 2013, 85, 135–146. [Google Scholar] [CrossRef]
- Mazarati, A.; Sankar, R. Common Mechanisms Underlying Epileptogenesis and the Comorbidities of Epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022798. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The Hidden Island of Addiction: The Insula. Trends Neurosci. 2009, 32, 56–67. [Google Scholar] [CrossRef]
- Krushinsky, L.V.; Molodkina, L.N.; Fless, D.A.; Dobrokhotova, L.P.; Steshenko, A.P.; Semiokhina, A.F.; Zorina, Z.A.; Romanova, L.G. The Functional State of the Brain during Sonic Stimulation. In Physiological Effects of Noise; Welch, B.L., Welch, A.S., Eds.; Springer: Boston, MA, USA, 1970; pp. 159–183. ISBN 978-1-4684-8809-8. [Google Scholar]
- Fadyukova, O.E.; Storozhevykh, T.P.; Pinelis, V.G.; Koshelev, V.B. Ischemic and Hemorrhagic Disturbances in Cerebral Circulation Alter Contractile Responses of the Rat Middle Cerebral Artery. Brain Res. 2004, 995, 145–149. [Google Scholar] [CrossRef]
- Ruggiero, D.A.; Mraovitch, S.; Granata, A.R.; Anwar, M.; Reis, D.J. A Role of Insular Cortex in Cardiovascular Function. J. Comp. Neurol. 1987, 257, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.V.; Saper, C.B.; Hurley, K.M.; Cechetto, D.F. Organization of Visceral and Limbic Connections in the Insular Cortex of the Rat. J. Comp. Neurol. 1991, 311, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Livneh, Y.; Andermann, M.L. Cellular Activity in Insular Cortex across Seconds to Hours: Sensations and Predictions of Bodily States. Neuron 2021, 109, 3576–3593. [Google Scholar] [CrossRef] [PubMed]
- DuBois, D.; Ameis, S.H.; Lai, M.; Casanova, M.F.; Desarkar, P. Interoception in Autism Spectrum Disorder: A Review. Int. J. Dev. Neurosci. 2016, 52, 104–111. [Google Scholar] [CrossRef]
- Muskens, J.B.; Velders, F.P.; Staal, W.G. Medical Comorbidities in Children and Adolescents with Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorders: A Systematic Review. Eur. Child Adolesc. Psychiatry 2017, 26, 1093–1103. [Google Scholar] [CrossRef]
- Caria, A.; De Falco, S. Anterior Insular Cortex Regulation in Autism Spectrum Disorders. Front. Behav. Neurosci. 2015, 9, 38. [Google Scholar] [CrossRef]
| A. non-epileptic rats | |||||||||||||||||||
| D1 DR | D2 DR | ||||||||||||||||||
| A24b | A24a | A25 | DP | GI | DI | AID | AIV | Clau | A24b | A24a | A25 | DP | GI | DI | AID | AIV | Clau | ||
| D1DR Cing/A24b | 0.84 | 0.94 | 0.95 | 0.91 | 0.86 | 0.96 | 0.80 | 0.64 | |||||||||||
| D1DR PL/A24b | 0.84 | 0.87 | 0.88 | 0.86 | 0.83 | 0.86 | 0.87 | 0.68 | 0.83 | ||||||||||
| D1DR IL/A25 | 0.94 | 0.87 | 0.94 | 0.94 | 0.85 | 0.92 | 0.88 | 0.71 | 0.66 | ||||||||||
| D1DR DP | 0.95 | 0.88 | 0.94 | 0.90 | 0.84 | 0.96 | 0.90 | 0.72 | |||||||||||
| D1DR GI | 0.84 | 0.73 | |||||||||||||||||
| D1DR DI | 0.91 | 0.86 | 0.94 | 0.90 | 0.98 | 0.96 | 0.84 | 0.73 | 0.73 | ||||||||||
| D1DR AID | 0.86 | 0.83 | 0.85 | 0.84 | 0.98 | 0.94 | 0.80 | 0.67 | 0.67 | ||||||||||
| D1DR AIV | 0.96 | 0.86 | 0.92 | 0.96 | 0.96 | 0.94 | 0.84 | 0.69 | 0.69 | ||||||||||
| D1DR Clau | 0.80 | 0.87 | 0.88 | 0.90 | 0.84 | 0.80 | 0.84 | 0.71 | 0.63 | ||||||||||
| D2DR Cing/A24b | 0.68 | 0.68 | 0.68 | 0.75 | |||||||||||||||
| D2DR PL/A24b | 0.64 | 0.83 | 0.71 | 0.72 | 0.84 | 0.73 | 0.67 | 0.69 | 0.71 | 0.68 | |||||||||
| D2DR IL/A25 | 0.66 | 0.73 | 0.73 | 0.67 | 0.69 | 0.63 | 0.75 | 0.83 | |||||||||||
| D2DR DP | 0.87 | 0.90 | 0.72 | 0.70 | 0.65 | ||||||||||||||
| D2DR GI | 0.87 | 0.95 | 0.82 | 0.81 | 0.76 | ||||||||||||||
| D2DR DI | 0.90 | 0.95 | 0.76 | 0.82 | 0.77 | ||||||||||||||
| D2DR AID | 0.72 | 0.82 | 0.76 | 0.92 | 0.90 | ||||||||||||||
| D2DR AIV | 0.70 | 0.81 | 0.82 | 0.92 | 0.96 | ||||||||||||||
| D2DR Clau | 0.65 | 0.76 | 0.77 | 0.90 | 0.96 | ||||||||||||||
| B. epileptic rats | |||||||||||||||||||
| D1 DR | D2 DR | ||||||||||||||||||
| A24b | A24a | A25 | DP | GI | DI | AID | AIV | Clau | A24b | A24a | A25 | DP | GI | DI | AID | AIV | Clau | ||
| D1DR Cing/A24b | 0.84 | 0.87 | 0.82 | 0.56 | 0.72 | 0.69 | 0.89 | 0.40 | 0.42 | ||||||||||
| D1DR PL/A24b | 0.84 | 0.83 | 0.74 | 0.72 | 0.75 | 0.78 | 0.83 | ||||||||||||
| D1DR IL/A25 | 0.87 | 0.83 | 0.81 | 0.57 | 0.74 | 0.74 | 0.73 | 0.82 | 0.40 | 0.41 | 0.36 | ||||||||
| D1DR DP | 0.82 | 0.74 | 0.81 | 0.47 | 0.68 | 0.69 | 0.71 | 0.78 | 0.40 | ||||||||||
| D1DR GI | 0.56 | 0.72 | 0.57 | 0.47 | 0.75 | 0.75 | 0.74 | 0.74 | |||||||||||
| D1DR DI | 0.75 | 0.75 | 0.74 | 0.68 | 0.75 | 0.96 | 0.95 | 0.93 | 0.46 | ||||||||||
| D1DR AID | 0.72 | 0.78 | 0.74 | 0.69 | 0.75 | 0.96 | 0.97 | 0.91 | 0.46 | ||||||||||
| D1DR AIV | 0.69 | 0.73 | 0.73 | 0.71 | 0.74 | 0.95 | 0.97 | 0.92 | 0.41 | ||||||||||
| D1DR Clau | 0.84 | 0.83 | 0.82 | 0.78 | 0.74 | 0.93 | 0.91 | 0.92 | 0.42 | ||||||||||
| D2DR Cing/A24b | 0.48 | 0.47 | 0.51 | 0.72 | 0.47 | ||||||||||||||
| D2DR PL/A24b | 0.40 | 0.50 | 0.49 | 0.46 | 0.48 | 0.39 | |||||||||||||
| D2DR IL/A25 | 0.50 | 0.64 | |||||||||||||||||
| D2DR DP | 0.49 | 0.64 | |||||||||||||||||
| D2DR GI | 0.40 | 0.41 | 0.46 | 0.46 | 0.41 | 0.42 | 0.48 | 0.55 | 0.52 | 0.63 | 0.61 | ||||||||
| D2DR DI | 0.47 | 0.46 | 0.55 | 0.75 | 0.79 | 0.93 | |||||||||||||
| D2DR AID | 0.51 | 0.48 | 0.52 | 0.75 | 0.72 | 0.71 | |||||||||||||
| D2DR AIV | 0.72 | 0.63 | 0.79 | 0.72 | 0.78 | ||||||||||||||
| D2DR Clau | 0.42 | 0.36 | 0.40 | 0.47 | 0.39 | 0.61 | 0.93 | 0.71 | 0.78 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birioukova, L.M.; van Luijtelaar, G.; Midzyanovskaya, I.S. D1-Like and D2-Like Dopamine Receptors in the Rat Prefrontal Cortex: Impacts of Genetic Generalized Epilepsies and Social Behavioral Deficits. Receptors 2024, 3, 36-57. https://doi.org/10.3390/receptors3010004
Birioukova LM, van Luijtelaar G, Midzyanovskaya IS. D1-Like and D2-Like Dopamine Receptors in the Rat Prefrontal Cortex: Impacts of Genetic Generalized Epilepsies and Social Behavioral Deficits. Receptors. 2024; 3(1):36-57. https://doi.org/10.3390/receptors3010004
Chicago/Turabian StyleBirioukova, Lidia M., Gilles van Luijtelaar, and Inna S. Midzyanovskaya. 2024. "D1-Like and D2-Like Dopamine Receptors in the Rat Prefrontal Cortex: Impacts of Genetic Generalized Epilepsies and Social Behavioral Deficits" Receptors 3, no. 1: 36-57. https://doi.org/10.3390/receptors3010004
APA StyleBirioukova, L. M., van Luijtelaar, G., & Midzyanovskaya, I. S. (2024). D1-Like and D2-Like Dopamine Receptors in the Rat Prefrontal Cortex: Impacts of Genetic Generalized Epilepsies and Social Behavioral Deficits. Receptors, 3(1), 36-57. https://doi.org/10.3390/receptors3010004






