Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System
Abstract
1. Introduction
2. Inflammation in the Central Nervous System
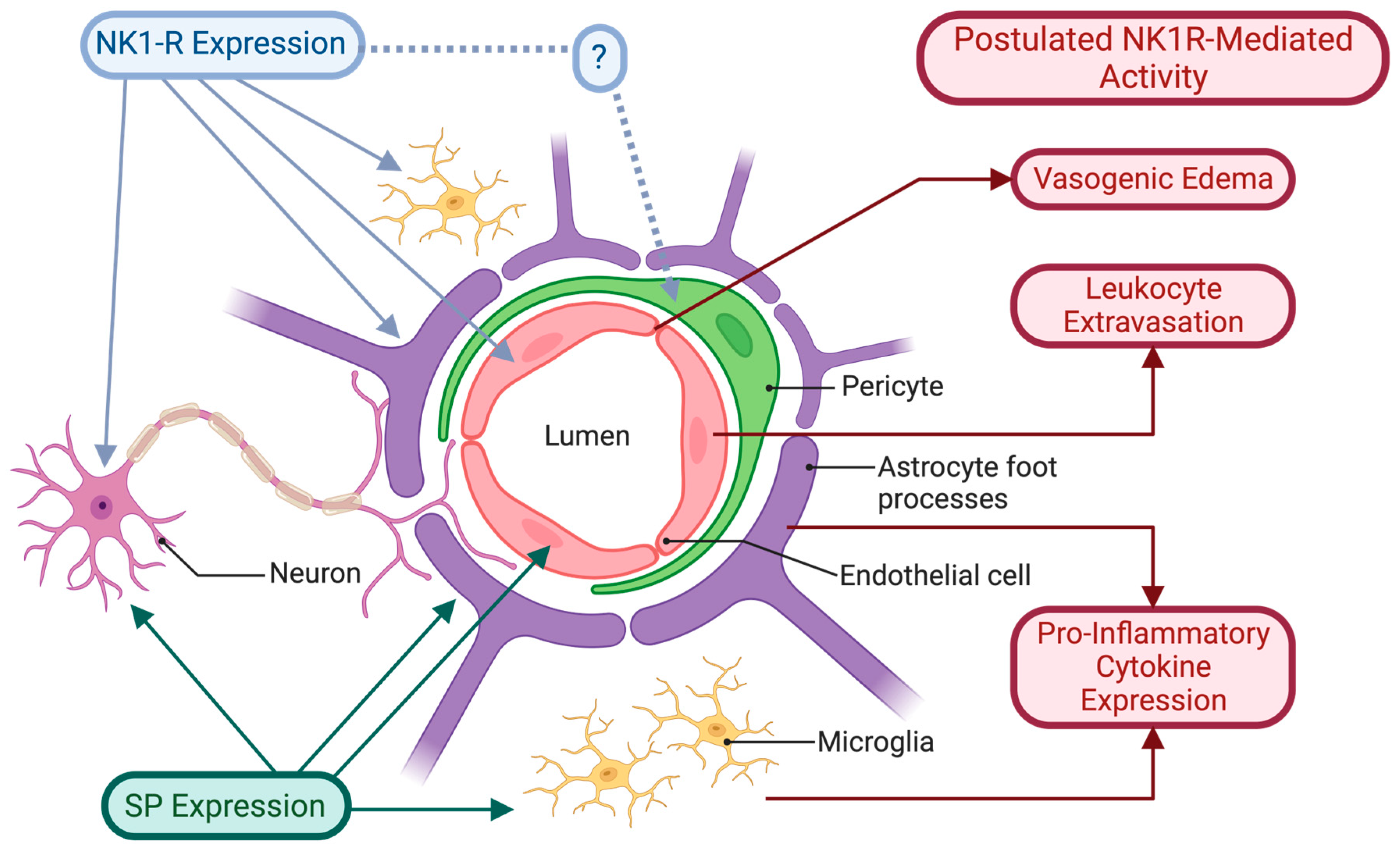
3. The Blood–Brain Barrier
4. Perivascular Innervation and Neurogenic Inflammation
Role of the NK1R in Neurogenic Inflammation
5. Distribution of SP and the NK1R in the CNS
6. Non-Neuronal NK1 Receptor Activity in the CNS
| CNS Pathology | Model | Drug | Mechanism of Action | Reference |
|---|---|---|---|---|
| Ischaemic Stroke | Rat—internal carotid A | SR140333 | Selective NK1 antagonist | Yu et al. [118] |
| Rat—transient middle cerebral (tMC) | NAT | ? NK1 antagonist? * | Turner et al. [116] | |
| Rat—tMC + tPA | NAT | ? NK1 antagonist? * | Turner et al. [119] | |
| Sheep—tMC | EU-C-001 | Selective NK1 antagonist | Sorby-Adams et al. [115] | |
| Rat—bilateral common carotid | Aprepitant | Selective NK1 antagonist | Kaur et al. [120] | |
| Traumatic Brain Injury | Rat—diffuse axonal injury | Capsaicin | TRPV1 agonist | Nimmo et al. [54] |
| Rat—diffuse axonal injury | NAT | ? NK1 antagonist? * | Donkin et al. [113] | |
| Rat—diffuse axonal injury | EU-C-001 | Selective NK1 antagonist | Hoffmann et al. [114] | |
| Rat—Blast injury and CTE | EU-C-001 | Selective NK1 antagonist | Corrigan et al. [121] | |
| Mouse—Cortical impact | L-733,060 | Selective NK1 antagonist | Li et al. [122] | |
| Sheep—Diffuse axonal injury | NAT | ? NK1 antagonist? * | Vink et al. [123,124] | |
| Hematoma | Mouse—intracerebral hemorrhage | Aprepitant | Selective NK1 antagonist | Jin et al. [125] |
| Spinal Cord Injury | Rat—contusion/compression SCI | EU-C-001 | Selective NK1 antagonist | Zheng et al. [126] |
| Rat—Autonomic dysreflexia SCI | GR205171 | Selective NK1 antagonist | Rupniak et al. [127] | |
| Bacterial Infection | Mouse—N. meningitidis and B. burgdorferi | L 703,606 | Selective NK1 antagonist | Chauhan et al. [102] |
| Mouse—S. pneumoniae | L 703,606 | Selective NK1 antagonist | Chauhan et al. [128] | |
| Primary mouse microglia—B. burgdorferi | L 703,606 | Selective NK1 antagonist | Rasley et al. [129] | |
| Viral Infection | Human mononuclear phagocytes—HIV | CP-96,345 | Selective NK1 antagonist | Lai et al. [130] |
| Microglia/macrophages—HIV | Aprepitant | Selective NK1 antagonist | Wang et al. [131] | |
| Peripheral blood monocytes—HIV | Aprepitant | Selective NK1 antagonist | Manak et al. [132] | |
| Mouse—WNV neuroinvasive disease | Ro 67,5930 | Selective NK1 antagonist | Ronca et al. [133] | |
| Parasitic Infection | Mouse—Trypanosoma brucei brucei | RP 67,580 | Selective NK1 antagonist | Kennedy et al. [134] |
7. NK1R and SP Activity in Models of Acute CNS Inflammation
7.1. Stroke
7.2. Traumatic Brain Injury
7.3. Intracerebral Hemorrhage
7.4. Spinal Cord Injury
7.5. Bacterial Infections
7.6. Viral Infections
7.7. Parasitic Infections
8. The Dual Role of the NK1R in Inflammation
9. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, K.; Mutlak, H.; Smith, W.R.; Shohami, E.; Stahel, P.F. Pharmacology of traumatic brain injury: Where is the “golden bullet”? Mol. Med. 2008, 14, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocritical Care 2020, 32, 647–666. [Google Scholar] [CrossRef] [PubMed]
- ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin. Trials 2006, 1, e33. [Google Scholar]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Vink, R.; Nimmo, A.J. Multifunctional drugs for head injury. Neurotherapeutics 2009, 6, 28–42. [Google Scholar] [CrossRef]
- Bouras, M.; Asehnoune, K.; Roquilly, A. Immune modulation after traumatic brain injury. Front. Med. 2022, 9, 995044. [Google Scholar] [CrossRef]
- Corrigan, F.; Vink, R.; Turner, R.J. Inflammation in acute CNS injury: A focus on the role of substance P. Br. J. Pharmacol. 2016, 173, 703–715. [Google Scholar] [CrossRef]
- Martinez, A.N.; Philipp, M.T. Substance P and Antagonists of the Neurokinin-1 Receptor in Neuroinflammation Associated with Infectious and Neurodegenerative Diseases of the Central Nervous System. J. Neurol. Neuromed. 2016, 1, 29–36. [Google Scholar]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell. Neurosci. 2016, 10, 296. [Google Scholar] [CrossRef]
- DeWire, S.M.; Ahn, S.; Lefkowitz, R.J.; Shenoy, S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007, 69, 483–510. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.D.; Lieu, T.; Halls, M.L.; Veldhuis, N.A.; Imlach, W.L.; Mai, Q.N.; Poole, D.P.; Quach, T.; Aurelio, L.; Conner, J.; et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 2017, 9, eaal3447. [Google Scholar] [CrossRef]
- Lai, J.P.; Lai, S.; Tuluc, F.; Tansky, M.F.; Kilpatrick, L.E.; Leeman, S.E.; Douglas, S.D. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 12605–12610. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.M.; Anderson, S.A.; Yu, H.; Huang, R.R.; Strader, C.D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992, 41, 24–30. [Google Scholar] [PubMed]
- Gillespie, E.; Leeman, S.E.; Watts, L.A.; Coukos, J.A.; O’Brien, M.J.; Cerda, S.R.; Farraye, F.A.; Stucchi, A.F.; Becker, J.M. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 17420–17425. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S232–S240. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Sharma, K.; Agyeah, G.; Kruger, R.; Grunewald, A.; Fitzgerald, J.C. Neurodegeneration and Neuroinflammation in Parkinson’s Disease: A Self-Sustained Loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, S.M.; Ong, L.K.; Collins-Praino, L.E.; Turner, R.J. Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke? Int. J. Mol. Sci. 2021, 22, 13101. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.P.; Simoes, C.J.V.; Brito, R.M.M. The Therapeutic Prospects of Targeting IL-1R1 for the Modulation of Neuroinflammation in Central Nervous System Disorders. Int. J. Mol. Sci. 2022, 23, 1731. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef]
- Millan Solano, M.V.; Salinas Lara, C.; Sanchez-Garibay, C.; Soto-Rojas, L.O.; Escobedo-Avila, I.; Tena-Suck, M.L.; Ortiz-Butron, R.; Choreno-Parra, J.A.; Romero-Lopez, J.P.; Melendez Camargo, M.E. Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 11902. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Koizumi, T.; Kerkhofs, D.; Mizuno, T.; Steinbusch, H.W.M.; Foulquier, S. Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia. Front. Neurosci. 2019, 13, 1291. [Google Scholar] [CrossRef]
- Balança, B.; Desmurs, L.; Grelier, J.; Perret-Liaudet, A.; Lukaszewicz, A.-C. DAMPs and RAGE Pathophysiology at the Acute Phase of Brain Injury: An Overview. Int. J. Mol. Sci. 2021, 22, 2439. [Google Scholar] [CrossRef]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bacha, R.S.; Baez, E.; Garcia-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Dos Santos, J.P.; Leffa, D.T.; Bobermin, L.D.; Roppa, P.H.A.; da Silva Torres, I.L.; Goncalves, C.A.; Souza, D.O.; Quincozes-Santos, A. Systemic Inflammation as a Driver of Brain Injury: The Astrocyte as an Emerging Player. Mol. Neurobiol. 2018, 55, 2685–2695. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link Between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef] [PubMed]
- Ellwardt, E.; Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp. Neurol. 2014, 262 Pt A, 8–17. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12, 688254. [Google Scholar] [CrossRef] [PubMed]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Uprety, A.; Kang, Y.; Kim, S.Y. Blood-brain barrier dysfunction as a potential therapeutic target for neurodegenerative disorders. Arch. Pharmacal Res. 2021, 44, 487–498. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Pacheco-Herrero, M.; Martinez-Gomez, P.A.; Campa-Cordoba, B.B.; Apatiga-Perez, R.; Villegas-Rojas, M.M.; Harrington, C.R.; de la Cruz, F.; Garces-Ramirez, L.; Luna-Munoz, J. The Neurovascular Unit Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2022. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, P.; Cioni, C.; Santonini, R.; Paccagnini, E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J. Neuroimmunol. 2002, 131, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Hickey, W.F. Leukocyte traffic in the central nervous system: The participants and their roles. Semin. Immunol. 1999, 11, 125–137. [Google Scholar] [CrossRef]
- Jansson, D.; Rustenhoven, J.; Feng, S.; Hurley, D.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.; Dragunow, M. A role for human brain pericytes in neuroinflammation. J. Neuroinflamm. 2014, 11, 104. [Google Scholar] [CrossRef]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnar, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Valente, J.; Brain, S.D. A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin. Immunopathol. 2018, 40, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int. J. Mol. Sci. 2017, 18, 1788. [Google Scholar] [CrossRef]
- Hu, D.E.; Easton, A.S.; Fraser, P.A. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br. J. Pharmacol. 2005, 146, 576–584. [Google Scholar] [CrossRef]
- Kargbo, R.B. TRPV1 Modulators for the Treatment of Pain and Inflammation. ACS Med. Chem. Lett. 2019, 10, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.; Keen, P. Synthesis, and central and peripheral axonal transport of substance P in a dorsal root ganglion-nerve preparation in vitro. Brain Res. 1982, 231, 379–385. [Google Scholar] [CrossRef]
- Aalkjær, C.; Nilsson, H.; De Mey, J.G.R. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol. Rev. 2021, 101, 495–544. [Google Scholar] [CrossRef]
- Garret, C.; Carruette, A.; Fardin, V.; Moussaoui, S.; Peyronel, J.F.; Blanchard, J.C.; Laduron, P.M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc. Natl. Acad. Sci. USA 1991, 88, 10208–10212. [Google Scholar] [CrossRef]
- Andrews, P.V.; Helme, R.D.; Thomas, K.L. NK-1 receptor mediation of neurogenic plasma extravasation in rat skin. Br. J. Pharmacol. 1989, 97, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Saria, A.; Brodin, E.; Rosell, S.; Folkers, K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc. Natl. Acad. Sci. USA 1983, 80, 1120–1124. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Bekkemeyer, H.; Oehme, P.; Foreman, J.C. Accidental injection of substance P. J. R. Soc. Med. 1983, 76, 801–802. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W.; van Nieuw Amerongen, G.P. Endothelial hyperpermeability in vascular leakage. Vascul. Pharmacol. 2002, 39, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Hirata, A.; Thurston, G.; Fujiwara, T.; Neal, C.R.; Michel, C.C.; McDonald, D.M. Endothelial gaps: Time course of formation and closure in inflamed venules of rats. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 272, L155–L170. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.J.; Baluk, P.; Lefevre, P.M.; Vigna, S.R.; McDonald, D.M. Substance P (NK1) receptor immunoreactivity on endothelial cells of the rat tracheal mucosa. Am. J. Physiol. 1996, 270, L404–L414. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Leroux, A.; Paiva Dos Santos, B.; Leng, J.; Oliveira, H.; Amedee, J. Sensory neurons from dorsal root ganglia regulate endothelial cell function in extracellular matrix remodelling. Cell Commun. Signal. 2020, 18, 162. [Google Scholar] [CrossRef]
- Nessler, S.; Stadelmann, C.; Bittner, A.; Schlegel, K.; Gronen, F.; Brueck, W.; Hemmer, B.; Sommer, N. Suppression of autoimmune encephalomyelitis by a neurokinin-1 receptor antagonist—A putative role for substance P in CNS inflammation. J. Neuroimmunol. 2006, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.C.; Jordan, C.C.; Oehme, P.; Renner, H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J. Physiol. 1983, 335, 449–465. [Google Scholar] [CrossRef]
- Forsythe, P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019, 42, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Hokfelt, T.; Kellerth, J.O.; Nilsson, G.; Pernow, B. Substance p: Localization in the central nervous system and in some primary sensory neurons. Science 1975, 190, 889–890. [Google Scholar] [CrossRef]
- Mantyh, P.W.; Johnson, D.J.; Boehmer, C.G.; Catton, M.D.; Vinters, H.V.; Maggio, J.E.; Too, H.P.; Vigna, S.R. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc. Natl. Acad. Sci. USA 1989, 86, 5193–5197. [Google Scholar] [CrossRef]
- Mai, J.K.; Stephens, P.H.; Hopf, A.; Cuello, A.C. Substance P in the human brain. Neuroscience 1986, 17, 709–739. [Google Scholar] [CrossRef]
- Mantyh, P.W. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry 2002, 63 (Suppl. S11), 6–10. [Google Scholar]
- Dudas, B.; Merchenthaler, I. Substance P-Immunoreactive Fiber Varicosities Appear to Innervate Galaninergic Perikarya in the Human Hypothalamus. Brain Connect. 2021, 11, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brown, J.L.; Jasmin, L.; Maggio, J.E.; Vigna, S.R.; Mantyh, P.W.; Basbaum, A.I. Synaptic relationship between substance P and the substance P receptor: Light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc. Natl. Acad. Sci. USA 1994, 91, 1009–1013. [Google Scholar] [CrossRef]
- Iftikhar, K.; Siddiq, A.; Baig, S.G.; Zehra, S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides 2020, 79, 101993. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Kramer, M.S. NK1 receptor antagonists for depression: Why a validated concept was abandoned. J. Affect. Disord. 2017, 223, 121–125. [Google Scholar] [CrossRef]
- Frick, A.; Ahs, F.; Linnman, C.; Jonasson, M.; Appel, L.; Lubberink, M.; Långström, B.; Fredrikson, M.; Furmark, T. Increased neurokinin-1 receptor availability in the amygdala in social anxiety disorder: A positron emission tomography study with [11C]GR205171. Transl. Psychiatry 2015, 5, e597. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Tattersall, F.D.; Rycroft, W.; Hill, R.G.; Hargreaves, R.J. Enantioselective inhibition of apomorphine-induced emesis in the ferret by the neurokinin1 receptor antagonist CP-99,994. Neuropharmacology 1994, 33, 259–260. [Google Scholar] [CrossRef]
- Aapro, M.; Carides, A.; Rapoport, B.L.; Schmoll, H.J.; Zhang, L.; Warr, D. Aprepitant and fosaprepitant: A 10-year review of efficacy and safety. Oncologist 2015, 20, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Takano, Y.; Kamiya, H.O. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J. Pharmacol. Sci. 2003, 91, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, A.R.; Johnson, M.B.; Chauhan, V.S.; Moerdyk-Schauwecker, M.J.; Young, A.D.; Cooley, I.D.; Martinez, A.N.; Ramesh, G.; Philipp, M.T.; Marriott, I. Human microglia and astrocytes constitutively express the neurokinin-1 receptor and functionally respond to substance P. J. Neuroinflamm. 2017, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Spitsin, S.; Pappa, V.; Douglas, S.D. Truncation of neurokinin-1 receptor-Negative regulation of substance P signaling. J. Leukoc. Biol. 2018, 103, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Tuluc, F.; Lai, J.P.; Kilpatrick, L.E.; Evans, D.L.; Douglas, S.D. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009, 30, 271–276. [Google Scholar] [CrossRef]
- Lai, J.P.; Cnaan, A.; Zhao, H.; Douglas, S.D. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J. Neurosci. Methods 2008, 168, 127–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stumm, R.; Culmsee, C.; Schafer, M.K.; Krieglstein, J.; Weihe, E. Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium. J. Neurosci. 2001, 21, 798–811. [Google Scholar] [CrossRef]
- Hamel, E. Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 2006, 100, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Markowitz, S.; Saito, K.; Moskowitz, M.A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 1987, 7, 4129–4136. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Saria, A.; Lundberg, J.M.; Skofitsch, G.; Lembeck, F. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983, 324, 212–218. [Google Scholar] [CrossRef]
- Cyrino, L.A.; Cardoso, R.C.; Hackl, L.P.; Nicolau, M. Effect of quercetin on plasma extravasation in rat CNS and dura mater by ACE and NEP inhibition. Phytother. Res. 2002, 16, 545–549. [Google Scholar] [CrossRef]
- Annunziata, P.; Cioni, C.; Toneatto, S.; Paccagnini, E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS 1998, 12, 2377–2385. [Google Scholar] [CrossRef]
- Cioni, C.; Renzi, D.; Calabro, A.; Annunziata, P. Enhanced secretion of substance P by cytokine-stimulated rat brain endothelium cultures. J. Neuroimmunol. 1998, 84, 76–85. [Google Scholar] [CrossRef]
- Marriott, D.R.; Wilkin, G.P. Substance P receptors on O-2A progenitor cells and type-2 astrocytes in vitro. J. Neurochem. 1993, 61, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.S.; Sterka, D.G., Jr.; Gray, D.L.; Bost, K.L.; Marriott, I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J. Immunol. 2008, 180, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Lieb, K.; Fiebich, B.L.; Berger, M.; Bauer, J.; Schulze-Osthoff, K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J. Immunol. 1997, 159, 4952–4958. [Google Scholar] [CrossRef]
- Rasley, A.; Bost, K.L.; Olson, J.K.; Miller, S.D.; Marriott, I. Expression of functional NK-1 receptors in murine microglia. Glia 2002, 37, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.M.; Segui, M.; Blazy, C.; Emonds-Alt, X.; Le Fur, G.; Brelire, J.C.; Casellas, P. Effect of substance P on cytokine production by human astrocytic cells and blood mononuclear cells: Characterization of novel tachykinin receptor antagonists. FEBS Lett. 1996, 399, 321–325. [Google Scholar] [CrossRef]
- Vo, T.T.; Im, G.H.; Han, K.; Suh, M.; Drew, P.J.; Kim, S.G. Parvalbumin interneuron activity drives fast inhibition-induced vasoconstriction followed by slow substance P-mediated vasodilation. Proc. Natl. Acad. Sci. USA 2023, 120, e2220777120. [Google Scholar] [CrossRef]
- Vruwink, M.; Schmidt, H.H.; Weinberg, R.J.; Burette, A. Substance P and nitric oxide signaling in cerebral cortex: Anatomical evidence for reciprocal signaling between two classes of interneurons. J. Comp. Neurol. 2001, 441, 288–301. [Google Scholar] [CrossRef]
- Guo, C.J.; Douglas, S.D.; Gao, Z.; Wolf, B.A.; Grinspan, J.; Lai, J.P.; Riedel, E.; Ho, W.Z. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia 2004, 48, 259–266. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Blum, A.; Metwali, A.; Elliott, D.; Arsenescu, R. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J. Immunol. 2003, 170, 5003–5007. [Google Scholar] [CrossRef]
- MacLeod, A.M.; Merchant, K.J.; Brookfield, F.; Kelleher, F.; Stevenson, G.; Owens, A.P.; Swain, C.J.; Casiceri, M.A.; Sadowski, S.; Ber, E.; et al. Identification of L-tryptophan derivatives with potent and selective antagonist activity at the NK1 receptor. J. Med. Chem. 1994, 37, 1269–1274. [Google Scholar] [CrossRef]
- Matalinska, J.; Lipinski, P.F.J. Correcting a widespread error: Neuroprotectant N-acetyl-L-tryptophan does not bind to the neurokinin-1 receptor. Mol. Cell. Neurosci. 2022, 120, 103728. [Google Scholar] [CrossRef]
- Satarker, S.; Maity, S.; Mudgal, J.; Nampoothiri, M. In silico screening of neurokinin receptor antagonists as a therapeutic strategy for neuroinflammation in Alzheimer’s disease. Mol. Divers. 2022, 26, 443–466. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Nimmo, A.; Sleight, A.; Vankan, P.; Vink, R. Method of Treatment and/or Prevention of Brain, Spinal or Nerve Injury. U.S. Patent US20030083345A1, 1 May 2003. [Google Scholar]
- Sorby-Adams, A.J.; Leonard, A.V.; Hoving, J.W.; Yassi, N.; Vink, R.; Wells, A.J.; Turner, R.J. NK1-r Antagonist Treatment Comparable to Decompressive Craniectomy in Reducing Intracranial Pressure Following Stroke. Front. Neurosci. 2019, 13, 681. [Google Scholar] [CrossRef]
- Turner, R.J.; Helps, S.C.; Thornton, E.; Vink, R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res. 2011, 1393, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.J.; Vink, R. Recent patents in CNS drug discovery: The management of inflammation in the central nervous system. Recent Pat. CNS Drug Discov. 2009, 4, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cheng, G.; Huang, X.; Li, K.; Cao, X. Neurokinin-1 receptor antagonist SR140333: A novel type of drug to treat cerebral ischemia. Neuroreport 1997, 8, 2117–2119. [Google Scholar] [CrossRef]
- Turner, R.J.; Vink, R. Combined tissue plasminogen activator and an NK1 tachykinin receptor antagonist: An effective treatment for reperfusion injury following acute ischemic stroke in rats. Neuroscience 2012, 220, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sharma, S.; Sandhu, M.; Sharma, S. Neurokinin-1 receptor inhibition reverses ischaemic brain injury and dementia in bilateral common carotid artery occluded rats: Possible mechanisms. Inflammopharmacology 2016, 24, 133–143. [Google Scholar] [CrossRef]
- Corrigan, F.; Cernak, I.; McAteer, K.; Hellewell, S.C.; Rosenfeld, J.V.; Turner, R.J.; Vink, R. NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury. Sci. Rep. 2021, 11, 8861. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X.; Yang, Y.; Zhang, Y.; He, F.; Xu, X.; Zhang, Z.; Tao, L.; Luo, C. Tachykinin NK1 receptor antagonist L-733,060 and substance P deletion exert neuroprotection through inhibiting oxidative stress and cell death after traumatic brain injury in mice. Int. J. Biochem. Cell Biol. 2019, 107, 154–165. [Google Scholar] [CrossRef]
- Vink, R.; Gabrielian, L.; Thornton, E. The Role of Substance P in Secondary Pathophysiology after Traumatic Brain Injury. Front. Neurol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Gabrielian, L.; Helps, S.C.; Thornton, E.; Turner, R.J.; Leonard, A.V.; Vink, R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir. Suppl. 2013, 118, 201–204. [Google Scholar]
- Jin, P.; Deng, S.; Sherchan, P.; Cui, Y.; Huang, L.; Li, G.; Lian, L.; Xie, S.; Lenahan, C.; Travis, Z.D.; et al. Neurokinin Receptor 1 (NK1R) Antagonist Aprepitant Enhances Hematoma Clearance by Regulating Microglial Polarization via PKC/p38MAPK/NFκB Pathway After Experimental Intracerebral Hemorrhage in Mice. Neurotherapeutics 2021, 18, 1922–1938. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Harms, A.K.; Tail, M.; Zhang, H.; Nimmo, A.; Skutella, T.; Kiening, K.; Unterberg, A.; Zweckberger, K.; Younsi, A. Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model. Front. Mol. Neurosci. 2023, 16, 1128545. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Fernandes, S.; Hou, S.; Thor, K.B.; Marson, L. Effect of GR205171 on autonomic dysreflexia induced by colorectal distension in spinal cord injured rats. Spinal Cord 2023, 61, 499–504. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Kluttz, J.M.; Bost, K.L.; Marriott, I. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. J. Immunol. 2011, 186, 7255–7263. [Google Scholar] [CrossRef]
- Rasley, A.; Marriott, I.; Halberstadt, C.R.; Bost, K.L.; Anguita, J. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J. Immunol. 2004, 172, 5707–5713. [Google Scholar] [CrossRef]
- Lai, J.P.; Ho, W.Z.; Zhan, G.X.; Yi, Y.; Collman, R.G.; Douglas, S.D. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 3970–3975. [Google Scholar] [CrossRef]
- Wang, X.; Douglas, S.D.; Song, L.; Wang, Y.J.; Ho, W.Z. Neurokinin-1 receptor antagonist (aprepitant) suppresses HIV-1 infection of microglia/macrophages. J. Neuroimmune Pharmacol. 2008, 3, 257–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manak, M.M.; Moshkoff, D.A.; Nguyen, L.T.; Meshki, J.; Tebas, P.; Tuluc, F.; Douglas, S.D. Anti-HIV-1 activity of the neurokinin-1 receptor antagonist aprepitant and synergistic interactions with other antiretrovirals. AIDS 2010, 24, 2789–2796. [Google Scholar] [CrossRef]
- Ronca, S.E.; Gunter, S.M.; Kairis, R.B.; Lino, A.; Romero, J.; Pautler, R.G.; Nimmo, A.; Murray, K.O. A Potential Role for Substance P in West Nile Virus Neuropathogenesis. Viruses 2022, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.; Rodgers, J.; Jennings, F.W.; Murray, M.; Leeman, S.E.; Burke, J.M. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc. Natl. Acad. Sci. USA 1997, 94, 4167–4170. [Google Scholar] [CrossRef]
- Vinet-Oliphant, H.; Alvarez, X.; Buza, E.; Borda, J.T.; Mohan, M.; Aye, P.P.; Tuluc, F.; Douglas, S.D.; Lackner, A.A. Neurokinin-1 receptor (NK1-R) expression in the brains of SIV-infected rhesus macaques: Implications for substance P in NK1-R immune cell trafficking into the CNS. Am. J. Pathol. 2010, 177, 1286–1297. [Google Scholar] [CrossRef]
- Bruno, G.; Tega, F.; Bruno, A.; Graf, U.; Corelli, F.; Molfetta, R.; Barucco, M. The role of substance P in cerebral ischemia. Int. J. Immunopathol. Pharmacol. 2003, 16, 67–72. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bös, M.; Stadler, H.; Schnider, P.; Hunkeler, W.; Godel, T.; Galley, G.; Ballard, T.M.; Higgins, G.A.; Poli, S.M.; et al. Design and synthesis of a novel, achiral class of highly potent and selective, orally active neurokinin-1 receptor antagonists. Bioorg Med. Chem. Lett. 2006, 16, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.; Koggel, M.; Blaauw, J.; van der Horn, H.J.; Jacobs, B.; van der Naalt, J. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review. Neurosci. Biobehav. Rev. 2022, 132, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Dennis, J.A. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst. Rev. 2019, 12, Cd003983. [Google Scholar] [CrossRef]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflamm. 2016, 13, 264. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Leonard, A.V.; Manavis, J.; Blumbergs, P.C.; Vink, R. Changes in substance P and NK1 receptor immunohistochemistry following human spinal cord injury. Spinal Cord 2014, 52, 17–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leonard, A.V.; Thornton, E.; Vink, R. NK1 receptor blockade is ineffective in improving outcome following a balloon compression model of spinal cord injury. PLoS ONE 2014, 9, e98364. [Google Scholar] [CrossRef] [PubMed]
- Bystritsky, R.J.; Chow, F.C. Infectious Meningitis and Encephalitis. Neurol. Clin. 2022, 40, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Cnaan, A.; Lynch, K.G.; Benton, T.; Zhao, H.; Gettes, D.R.; Evans, D.L. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res. Hum. Retroviruses 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Douglas, S.D.; Ho, W.Z.; Gettes, D.R.; Cnaan, A.; Zhao, H.; Leserman, J.; Petitto, J.M.; Golden, R.N.; Evans, D.L. Elevated substance P levels in HIV-infected men. AIDS 2001, 15, 2043–2045. [Google Scholar] [CrossRef]
- Bost, K.L. Tachykinin-modulated anti-viral responses. Front. Biosci. 2004, 9, 1994–1998. [Google Scholar] [CrossRef]
- Schwartz, L.; Spitsin, S.V.; Meshki, J.; Tuluc, F.; Douglas, S.D.; Wolfe, J.H. Substance P enhances HIV-1 infection in human fetal brain cell cultures expressing full-length neurokinin-1 receptor. J. Neurovirol. 2013, 19, 219–227. [Google Scholar] [CrossRef][Green Version]
- Robinson, P.; White, A.C.; Lewis, D.E.; Thornby, J.; David, E.; Weinstock, J. Sequential expression of the neuropeptides substance P and somatostatin in granulomas associated with murine cysticercosis. Infect. Immun. 2002, 70, 4534–4538. [Google Scholar] [CrossRef]
- Garza, A.; Tweardy, D.J.; Weinstock, J.; Viswanathan, B.; Robinson, P. Substance P signaling contributes to granuloma formation in Taenia crassiceps infection, a murine model of cysticercosis. J. Biomed. Biotechnol. 2010, 2010, 597086. [Google Scholar] [CrossRef]
- Garza, A.; Weinstock, J.; Robinson, P. Absence of the SP/SP receptor circuitry in the substance P-precursor knockout mice or SP receptor, neurokinin (NK)1 knockout mice leads to an inhibited cytokine response in granulomas associated with murine Taenia crassiceps infection. J. Parasitol. 2008, 94, 1253–1258. [Google Scholar] [CrossRef]
- Elsawa, S.F.; Taylor, W.; Petty, C.C.; Marriott, I.; Weinstock, J.V.; Bost, K.L. Reduced CTL response and increased viral burden in substance P receptor-deficient mice infected with murine gamma-herpesvirus 68. J. Immunol. 2003, 170, 2605–2612. [Google Scholar] [CrossRef]
- Blum, A.M.; Metwali, A.; Kim-Miller, M.; Li, J.; Qadir, K.; Elliott, D.E.; Lu, B.; Fabry, Z.; Gerard, N.; Weinstock, J.V. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. J. Immunol. 1999, 162, 6080–6085. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.M.; Elliott, D.E.; Metwali, A.; Li, J.; Qadir, K.; Weinstock, J.V. Substance P regulates somatostatin expression in inflammation. J. Immunol. 1998, 161, 6316–6322. [Google Scholar] [CrossRef]
- Kincy-Cain, T.; Bost, K.L. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J. Immunol. 1996, 157, 255–264. [Google Scholar] [CrossRef]
- Taylor, J.M.; Moore, Z.; Minter, M.R.; Crack, P.J. Type-I interferon pathway in neuroinflammation and neurodegeneration: Focus on Alzheimer’s disease. J. Neural Transm. (Vienna) 2018, 125, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. Biomed Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef]
- Marriott, D.; Wilkin, G.P.; Coote, P.R.; Wood, J.N. Eicosanoid synthesis by spinal cord astrocytes is evoked by substance P; possible implications for nociception and pain. Adv. Prostaglandin Thromboxane Leukot. Res. 1991, 21B, 739–741. [Google Scholar] [PubMed]
- Nakagawa, N.; Sano, H.; Iwamoto, I. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides 1995, 16, 721–725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, R.J.; Nimmo, A.J. Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System. Receptors 2023, 2, 232-250. https://doi.org/10.3390/receptors2040016
Turner RJ, Nimmo AJ. Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System. Receptors. 2023; 2(4):232-250. https://doi.org/10.3390/receptors2040016
Chicago/Turabian StyleTurner, Renée J., and Alan J. Nimmo. 2023. "Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System" Receptors 2, no. 4: 232-250. https://doi.org/10.3390/receptors2040016
APA StyleTurner, R. J., & Nimmo, A. J. (2023). Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System. Receptors, 2(4), 232-250. https://doi.org/10.3390/receptors2040016







