Aerobic Exercise, Training Dose, and Cardiorespiratory Fitness: Effects and Relationships with Resting Plasma Neurotrophic Factors in Alzheimer’s Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Sample
2.3. Experimental Protocol
- AEx
- Stretching
2.4. Outcome Variables
- Blood Draws/Biomarkers
- Exercise Dose
- CRF
2.5. Statistical Analyses
3. Results
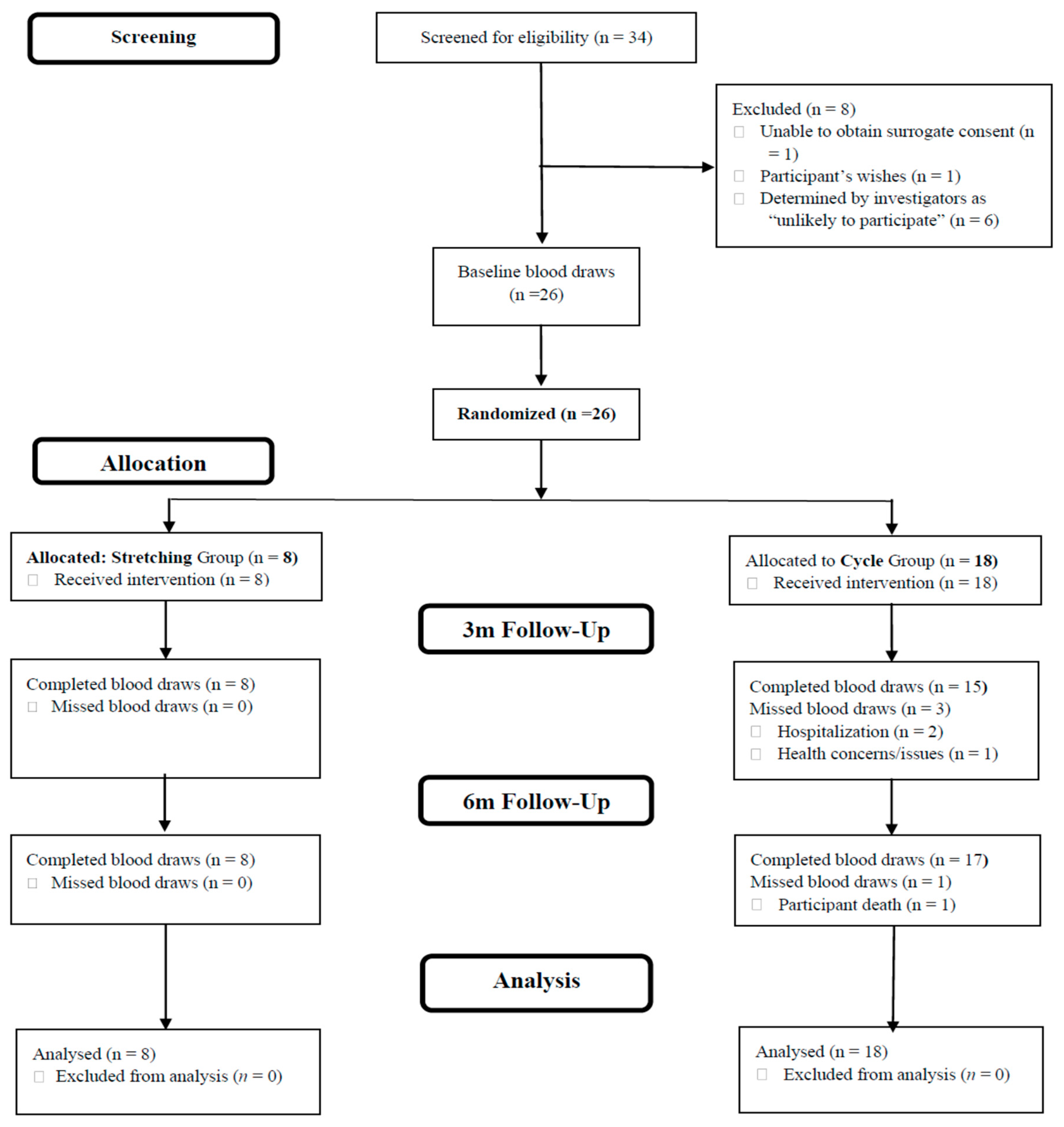
3.1. Sample and Intervention Descriptors
3.2. Plasma Neurotrophic Biomarker Changes
3.3. Exercise Dose and Biomarker Change
3.4. Change in CRF and Biomarker Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. 2022. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 29 June 2022).
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Montagne, A.; Sagare, A.P.; Nation, D.A.; Schneider, L.S.; Chui, H.C.; Harrington, M.G.; Pa, J.; Law, M.; Wang, D.J.J.; et al. Vascular dysfunction-The Disregarded Partner of Alzheimer’s Disease. Alzheimers Dement. 2019, 15, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic Relationship between Alzheimer’s Disease, Cerebrovascular Disease, and Cardiovascular Risk: A Review and Synthesis. Alzheimers Dement. 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lyu, D.; Li, Y.; Li, W.; Wang, Q.; Qin, Q.; Wang, X.; Gong, M.; Jiao, H.; et al. Cerebral Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 71, 101450. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.A.; Khabour, O.F.; Maikano, A.; Alawneh, K. Vascular Function and Brain-Derived Neurotrophic Factor: The Functional Capacity Factor. Vasc. Med. 2015, 20, 518–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Long, X.X.; Fang, Q.C.; Jia, W.P.; Li, H.T. The role of FGF21 in the Pathogenesis of Cardiovascular Disease. Chin. Med. J. 2021, 134, 2931–2943. [Google Scholar] [CrossRef]
- Fu, J.; Li, F.; Tang, Y.; Cai, L.; Zeng, C.; Yang, Y.; Yang, J. The Emerging Role of Irisin in Cardiovascular Diseases. J. Am. Heart Assoc. 2021, 10, e022453. [Google Scholar] [CrossRef]
- Higashi, Y.; Gautam, S.; Delafontaine, P.; Sukhanov, S. IGF-1 and Cardiovascular Disease. Growth Horm. IGF Res. 2019, 45, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.Y.; Delafontaine, P. Aging, Atherosclerosis, and IGF-1. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Pius-Sadowska, E.; Machaliński, B. BDNF—A Key Player in Cardiovascular System. J. Mol. Cell. Cardiol. 2017, 110, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Qin, X.Y.; Cao, C.; Cawley, N.X.; Liu, T.T.; Yuan, J.; Loh, Y.P.; Cheng, Y. Decreased Peripheral Brain-Derived Neurotrophic Factor Levels in Alzheimer’s Disease: A Meta-Analysis Study (N = 7277). Mol. Psychiatry 2017, 22, 312–320. [Google Scholar] [CrossRef]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating Brain-Derived Neurotrophic Factor Concentrations and the Risk of Cardiovascular Disease in the Community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, B.; Wang, X. Lower Irisin Levels in Coronary Artery Disease: A Meta-Analysis. Minerva Endocrinol. 2020, 45, 61–69. [Google Scholar] [CrossRef]
- Panza, G.A.; Taylor, B.A.; MacDonald, H.V.; Johnson, B.T.; Zaleski, A.L.; Livingston, J.; Thompson, P.D.; Pescatello, L.S. Can Exercise Improve Cognitive Symptoms of Alzheimer’s Disease? J. Am. Geriatr. Soc. 2018, 66, 487–495. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise Interventions for Cognitive Function in Adults Older than 50: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbaek, G.; Kivipelto, M.; Moreira, J.B.N.; et al. Are the Neuroprotective Effects of Exercise Training Systemically Mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo-Garcia, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise Benefits on Alzheimer’s Disease: State-of-the-Science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef] [PubMed]
- Jandova, T.; Buendía-Romero, A.; Polanska, H.; Hola, V.; Rihova, M.; Vetrovsky, T.; Courel-Ibáñez, J.; Steffl, M. Long-Term Effect of Exercise on Irisin Blood Levels-Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1438. [Google Scholar] [CrossRef]
- Ruiz-González, D.; Hernández-Martínez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of Physical Exercise on Plasma Brain-Derived Neurotrophic Factor in Neurodegenerative Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.M.; Coelho, F.G.M.; Vital-Silva, T.M.; Rueda, A.V.; Pereira, J.R.; Deslandes, A.C.; Camarini, R.; Santos Galduróz, R.F. Aerobic Training and Circulating Neurotrophins in Alzheimer’s Disease Patients: A Controlled Trial. Exp. Aging Res. 2022, 49, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Enette, L.; Vogel, T.; Merle, S.; Valard-Guiguet, A.G.; Ozier-Lafontaine, N.; Neviere, R.; Leuly-Joncart, C.; Fanon, J.L.; Lang, P.O. Effect of 9 weeks Continuous vs. Interval Aerobic Training on Plasma BDNF Levels, Aerobic Fitness, Cognitive Capacity and Quality of Life among Seniors with Mild to Moderate Alzheimer’s Disease: A Randomized Controlled Trial. Eur. Rev. Aging Phys. Act. 2020, 17, 2. [Google Scholar] [CrossRef]
- Church, T.S.; Earnest, C.P.; Skinner, J.S.; Blair, S.N. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness among Sedentary, Overweight or Obese Postmenopausal Women with Elevated Blood Pressure: A Randomized Controlled Trial. JAMA 2007, 297, 2081–2091. [Google Scholar] [CrossRef]
- Li, D.; Thomas, R.; Tsai, M.Y.; Li, L.; Vock, D.M.; Greimel, S.; Yu, F. Vascular Biomarkers to Predict Response to Exercise in Alzheimer’s Disease: The study protocol. BMJ Open 2016, 6, e011054. [Google Scholar] [CrossRef]
- Yu, F.; Vock, D.M.; Zhang, L.; Salisbury, D.; Nelson, N.W.; Chow, L.S.; Smith, G.; Barclay, T.R.; Dysken, M.; Wyman, J.F. Cognitive Effects of Aerobic Exercise in Alzheimer’s Disease: A Pilot Randomized Controlled Trial. J. Alzheimers Dis. 2021, 80, 233–244. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Effects of Physical Exercise on Alzheimer’s Disease Biomarkers: A Systematic Review of Intervention Studies. J. Alzheimers Dis. 2018, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Reijs, B.L.R.; Vos, S.J.B.; Soininen, H.; Lötjonen, J.; Koikkalainen, J.; Pikkarainen, M.; Hall, A.; Vanninen, R.; Liu, Y.; Herukka, S.K.; et al. Association Between Later Life Lifestyle Factors and Alzheimer’s Disease Biomarkers in Non-Demented Individuals: A Longitudinal Descriptive Cohort Study. J. Alzheimers Dis. 2017, 60, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Andersson, E.; Janelidze, S.; Ossenkoppele, R.; Insel, P.; Strandberg, O.; Zetterberg, H.; Rosen, H.J.; Rabinovici, G.; Chai, X.; et al. Aβ deposition is Associated with Increases in Soluble and Phosphorylated Tau that Precede a Positive Tau PET in Alzheimer’s Disease. Sci. Adv. 2020, 6, eaaz2387. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G. (Eds.) American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Piccinni, A.; Marazziti, D.; Del Debbio, A.; Bianchi, C.; Roncaglia, I.; Mannari, C.; Origlia, N.; Catena Dell’Osso, M.; Massimetti, G.; Domenici, L.; et al. Diurnal Variation of Plasma Brain-Derived Neurotrophic Factor (BDNF) in Humans: An Analysis of Sex Differences. Chronobiol. Int. 2008, 25, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Beck-Nielsen, H.; Højlund, K. Plasma FGF21 Displays a Circadian Rhythm during a 72-h Fast in Healthy Female Volunteers. Clin. Endocrinol. 2011, 75, 514–519. [Google Scholar] [CrossRef]
- Yu, H.; Xia, F.; Lam, K.S.; Wang, Y.; Bao, Y.; Zhang, J.; Gu, Y.; Zhou, P.; Lu, J.; Jia, W.; et al. Circadian Rhythm of Circulating Fibroblast Growth Factor 21 is Related to Diurnal Changes in Fatty Acids in Humans. Clin. Chem. 2011, 57, 691–700. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in Healthy, Young Individuals: Day-Night Rhythm, Effects of Food Intake and Exercise, and Associations with Gender, Physical Activity, Diet, and Body Composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Begliuomini, S.; Lenzi, E.; Ninni, F.; Casarosa, E.; Merlini, S.; Pluchino, N.; Valentino, V.; Luisi, S.; Luisi, M.; Genazzani, A.R. Plasma Brain-Derived Neurotrophic Factor Daily Variations in Men: Correlation with Cortisol Circadian Rhythm. J. Endocrinol. 2008, 197, 429–435. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic Growth Factor Responses to Lower Body Resistance Training in Older Adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor: A Systematic Review of Experimental Studies in Human Subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A Transient Elevated Irisin Blood Concentration in Response to Prolonged, Moderate Aerobic Exercise in Young Men and Women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.L.; O’Connor, D.P.; Herring, M.P.; Sailors, M.H.; Jackson, A.S.; Dishman, R.K.; Bray, M.S. Exercise Dose, Exercise Adherence, and Associated Health Outcomes in the TIGER Study. Med. Sci. Sports Exerc. 2014, 46, 69–75. [Google Scholar] [CrossRef]
- Salisbury, D.; Yu, F. Establishing Reference Cardiorespiratory Fitness Parameters in Alzheimer’s Disease. Sports Med. Int. Open 2020, 4, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Edelmayer, R.M.; Boxer, A.L.; Carrillo, M.C.; Mielke, M.M.; Rabinovici, G.D.; Salloway, S.; Sperling, R.; Zetterberg, H.; Teunissen, C.E. The Alzheimer’s Association Appropriate use Recommendations for Blood Biomarkers in Alzheimer’s Disease. Alzheimers Dement. 2022, 18, 2669–2686. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic Exercise Training and Circulating Irisin in Adults: A Meta-Analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, F.; Sadeghi, E.; Afshari-Safavi, A. Comparative Impact of Various Exercises on Circulating Irisin in Healthy Subjects: A Systematic Review and Network Meta-Analysis. Oxid. Med. Cell. Longev. 2022, 2022, 8235809. [Google Scholar] [CrossRef]
- Mohammad Rahimi, G.R.; Hejazi, K.; Hofmeister, M. The effect of Exercise Interventions on Irisin Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. EXCLI J. 2022, 21, 524–539. [Google Scholar]
- Pimenta, N.M.; Santa-Clara, H.; Sardinha, L.B.; Fernhall, B. Body Fat Responses to a 1-Year Combined Exercise Training Program in Male Coronary Artery Disease Patients. Obesity 2013, 21, 723–730. [Google Scholar] [CrossRef]
- Sesti, G.; Andreozzi, F.; Fiorentino, T.V.; Mannino, G.C.; Sciacqua, A.; Marini, M.A.; Perticone, F. High Circulating Irisin Levels are Associated with Insulin Resistance and Vascular Atherosclerosis in a Cohort of Nondiabetic Adult Subjects. Acta Diabetol. 2014, 51, 705–713. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, H.; Slettaløkken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Rønnestad, B.R.; Ellefsen, S. Irisin in Blood Increases Transiently after Single Sessions of Intense Endurance Exercise and Heavy Strength training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery Responses of Testosterone, Growth Hormone, and IGF-1 after Resistance Exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The Effect of Acute Exercise on Blood Concentrations of Brain-Derived Neurotrophic Factor in Healthy Adults: A Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Shobeiri, P.; Karimi, A.; Momtazmanesh, S.; Teixeira, A.L.; Teunissen, C.E.; van Wegen, E.E.H.; Hirsch, M.A.; Yekaninejad, M.S.; Rezaei, N. Exercise-Induced Increase in Blood-Based Brain-Derived Neurotrophic Factor (BDNF) in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis of Exercise Intervention Trials. PLoS ONE 2022, 17, e0264557. [Google Scholar] [CrossRef]
- Allard, J.S.; Ntekim, O.; Johnson, S.P.; Ngwa, J.S.; Bond, V.; Pinder, D.; Gillum, R.F.; Fungwe, T.V.; Kwagyan, J.; Obisesan, T.O. APOEε4 Impacts Up-Regulation of Brain-Derived Neurotrophic Factor after a Six-Month Stretch and Aerobic Exercise Intervention in Mild Cognitively Impaired Elderly African Americans: A Pilot Study. Exp. Gerontol. 2017, 87 Pt A, 129–136. [Google Scholar] [CrossRef]
- Hu, H.Y.; Zhang, Y.R.; Aerqin, Q.; Ou, Y.N.; Wang, Z.T.; Cheng, W.; Feng, J.F.; Tan, L.; Yu, J.T. Association Between Multimorbidity Status and Incident Dementia: A Prospective Cohort Study of 245,483 Participants. Transl. Psychiatry 2022, 12, 505. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Soontornpun, A.; Wongpakaran, T.; Wongpakaran, N.; Tanprawate, S.; Nadsasarn, A.; Pinyopornpanish, M. Impact of Behavioral and Psychological Symptoms of Alzheimer’s Disease on Caregiver Outcomes. Sci. Rep. 2022, 12, 14138. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and Cellular Mechanisms Underlying the Pathogenesis of Alzheimer’s Disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE Isoforms Differentially Regulates Cleavage and Secretion of BDNF. Mol. Brain 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Traylor, M.K.; Bauman, A.J.; Saiyasit, N.; Frizell, C.A.; Hill, B.D.; Nelson, A.R.; Keller, J.L. An Examination of the Relationship among Plasma Brain Derived Neurotropic Factor, Peripheral Vascular Function, and Body Composition with Cognition in Midlife African Americans/Black Individuals. Front. Aging Neurosci. 2022, 14, 980561. [Google Scholar] [CrossRef]
- Saiyasit, N.; Butlig, E.R.; Chaney, S.D.; Traylor, M.K.; Hawley, N.A.; Randall, R.B.; Bobinger, H.V.; Frizell, C.A.; Trimm, F.; Crook, E.D.; et al. Neurovascular Dysfunction in Diverse Communities With Health Disparities-Contributions to Dementia and Alzheimer’s Disease. Front. Neurosci. 2022, 16, 915405. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.L.; Hausdorff, J.M. The Role of Higher-Level Cognitive Function in Gait: Executive Dysfunction Contributes to Fall Risk in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2007, 24, 125–137. [Google Scholar] [CrossRef] [PubMed]
| All (n = 26) | Cycling (n = 18) | Stretch (n = 8) | T Value or χ2 | p | |
|---|---|---|---|---|---|
| Age | 77.6 (6.9) | 76.8 (7.6) | 79.3 (5.5) | −0.81 | 0.423 |
| Sex (Female) | 9 (34.6) | 7 (38.9) | 2 (25.0) | 0.472 | 0.492 |
| Race/Ethnicity | 2.72 | 0.256 | |||
| non-Hispanic White | 24 (92.3) | 17 (94.4) | 7 (87.5) | ||
| Hispanic White | 1 (3.8) | 1 (5.5) | - | ||
| Black American | 1 (3.8) | - | 1 (12.5) | ||
| Education (years) | 15.4 (2.9) | 15.9 (3.2) | 14.5 (2.5) | 1.05 | 0.361 |
| APOE genotype | 2.88 | 0.224 | |||
| E2/E3 | 3.8% | 5.6% | 0% | ||
| E3/E4 | 42.3% | 33.3% | 62.5% | ||
| E2/E4 | 26.9% | 33.3% | 12.5% | ||
| E4/E4 | 26.9% | 27.8% | 25.0% | ||
| BMI | 27.7 (4.4) | 26.8 (4.1) | 29.7 (4.7) | −1.58 | 0.126 |
| MMSE | 21.6 (3.3) | 21.3 (3.7) | 22.3 (2.3) | −0.96 | 0.348 |
| CVD | 6 (23.1) | 4 (22.2) | 2 (25.0) | FET | 0.651 |
| Beta blocker | 1 (3.8) | - | 1 (12.5) | FET | 0.308 |
| AD medications | 17 (65.4) | 11 (61.1) | 6 (75.0) | FET | 0.413 |
| VO2Peak (mL/kg/min) | 18.3 (4.6) | 18.4 (5.0) | 18.0 (3.8) | 0.20 | 0.842 |
| 3 Months vs. Baseline | 6 Months vs. Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Group | Baseline | 3 Months M (SD) | 6 Months M (SD) | Mean Difference a (95% CI) | d b | p | Mean Difference a (95% CI) | d b | p |
| BDNF-free (pg/mL) | Cycling | 999.50 (722.30) | 1481.00 (1273.37) | 1201.44 (958.92) | −230.13 (−1443.59, 983.34) | −0.18 | 0.696 | 18.82 (−854.88, 892.52) | 0.02 | 0.965 |
| Stretching | 1408.38 (845.63) | 1824.86 (1095.39) | 1671.75 (1603.10) | |||||||
| BDNF-total (pg/mL) | Cycling | 1166.67 (840.37) | 1531.87 (1520.52) | 1240.75 (730.17) | 97.666 (−1197.24, 1392.57) | 0.07 | 0.876 | −142.71 (−1051.38, 765.96) | −0.14 | 0.747 |
| Stretching | 1560.25 (957.02) | 1629.43 (863.76) | 1709.25 (1672.96) | |||||||
| Irisin (ng/mL) | Cycling | 5.00 (2.69) | 4.39 (0.52) | 4.35 (0.53) | −0.17 (−0.57, 0.23) | −0.40 | 0.395 | −0.186 (−0.56, 0.19) | −0.44 | 0.320 |
| Stretching | 4.27 (0.49) | 4.52 (0.46) | 4.44 (0.49) | |||||||
| FGF-21 (pg/mL) | Cycling | 299.50 (213.60) | 261.53 (164.04) | 258.12 (128.44) | −60.52 (−218.49, 97.46) | −0.37 | 0.582 | 14.56 (−109.498, 138.612) | 0.11 | 0.990 |
| Stretching | 343.88 (266.26) | 328.14 (278.15) | 264.75 (153.87) | |||||||
| IGF-1 (pg/mL) | Cycling | 98.18 (31.68) | 95.91 (29.34) | 100.96 (36.98) | −4.05 (−19.17, 11.06) | −0.25 | 0.190 | −0.08 (−12.74, 12.59) | −0.01 | 0.993 |
| Stretching | 100.35 (38.83) | 103.11 (37.51) | 102.05 (39.51) |
| Cycling Group 3 Months vs. Baseline | Cycling Group 6 Months vs. Baseline | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | n | Mean Difference (95% CI) | d | p | n | Mean Difference (95% CI) | d | p |
| BDNF-free (pg/mL) | 15 | −385.40 (−1155.49, 384.69) | −0.29 | 0.301 | 16 | −250.00 (−670.22, 170.22) | −0.33 | 0.224 |
| BDNF-total (pg/mL) | 15 | −285.93 (−1170.96, 599.09) | −0.19 | 0.500 | 16 | −148.69 (−556.59, 259.21) | −0.20 | 0.449 |
| Irisin (ng/mL) | 15 | −0.01 (−0.25, 0.22) | −0.03 | 0.899 | 16 | 0.05 (−0.17, 0.27) | 0.12 | 0.635 |
| FGF-21 (pg/mL) | 15 | 3.27 (−87.88, 94.41) | 0.02 | 0.940 | 16 | −5.75 (−90.30, 78.80) | −0.04 | 0.887 |
| IGF-1 (pg/mL) | 15 | 0.49 (−9.23, 10.22) | 0.03 | 0.915 | 17 | −1.60 (−8.11, 4.90) | −0.13 | 0.609 |
| Stretching Group 3 Months vs. Baseline | Stretching Group 6 Months vs. Baseline | |||||||
| Biomarker | n | Mean difference (95% CI) | d | p | n | Mean difference (95% CI) | d | p |
| BDNF-free (pg/mL) | 7 | −339.00 (−1413.98, 735.98) | −0.32 | 0.470 | 8 | −263.38 (−1201.54, 674.79) | −0.25 | 0.528 |
| BDNF-total (pg/mL) | 7 | 34.14 (−526.14, 594.43) | 0.06 | 0.886 | 8 | −149.00 (−1270.22, 972.22) | −0.12 | 0.763 |
| Irisin (ng/mL) | 7 | −0.20 (−0.63, 0.23) | −0.47 | 0.294 | 8 | −0.17 (−0.52, 0.18) | −0.42 | 0.299 |
| FGF-21 (pg/mL) | 7 | −56.43 (−199.19, 86.33) | −0.39 | 0.371 | 8 | 79.12 (−125.14, 283.39) | 0.35 | 0.390 |
| IGF-1 (pg/mL) | 8 | −2.76 (−17.59, 12.06) | −0.17 | 0.673 | 8 | −1.70 (−15.52, 12.12) | −0.11 | 0.780 |
| 3-Month Dose (Intensity-Minutes) | 6-Month Dose (Intensity-Minutes) | |||||
|---|---|---|---|---|---|---|
| Biomarker | b | r2 | p | b | r2 | p |
| BDNF-free (pg/mL) | −0.726 (−2.419, 0.968) | 0.04 | 0.381 | −0.052 (−0.645, 0.540) | 0.00 | 0.856 |
| BDNF-total (pg/mL) | −0.440 (−2.240, 1.359) | 0.01 | 0.614 | −0.159 (−0.765, 0.448) | 0.01 | 0.592 |
| Irisin (ng/mL) | 0.000 (−0.001, 0.001) | 0.00 | 0.840 | 0.000 (0.000,0.000) | 0.02 | 0.461 |
| FGF-21 (pg/mL) | −0.066 (−0.280, 0.149) | 0.01 | 0.531 | 0.000 (−0.083, 0.083) | 0.00 | 0.999 |
| IGF-1 (pg/mL) | −0.008 (−0.029, 0.012) | 0.01 | 0.396 | 0.003 (−0.006, 0.011) | 0.00 | 0.557 |
| Baseline-3 Months | Baseline-6 Months | All Time Points | ||||
|---|---|---|---|---|---|---|
| Biomarker | r | p | r | p | r | p |
| BDNF-free (pg/mL) | −0.016 (−0.627, 0.554) | 0.942 | −0.032 (−0.484, 0.342) | 0.881 | −0.133 (−0.451, 0.197) | 0.372 |
| BDNF-total (pg/mL) | −0.134 (−0.631, 0.371) | 0.542 | −0.100 (−0.540, 0.297) | 0.634 | −0.223 (−0.427, −0.006) | 0.133 |
| Irisin (ng/mL) | −0.284 (−0.718, 0.308) | 0.189 | 0.019 (−0.349, 0.535) | 0.927 | −0.125 (−0.406, 0.250) | 0.403 |
| FGF-21 (pg/mL) | 0.038 (−0.604, 0.634) | 0.860 | 0.272 (−0.018, 0.572) | 0.179 | 0.135 (−0.161, 0.420) | 0.356 |
| IGF-1 (pg/mL) | 0.241 (−0.262, 0.523) | 0.268 | 0.013 (−0.612, 0.425) | 0.951 | 0.073 (−0.311, 0.280) | 0.624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salisbury, D.L.; Li, D.; Todd, M.; Ng, T.K.S.; Yu, F. Aerobic Exercise, Training Dose, and Cardiorespiratory Fitness: Effects and Relationships with Resting Plasma Neurotrophic Factors in Alzheimer’s Dementia. J. Vasc. Dis. 2023, 2, 351-366. https://doi.org/10.3390/jvd2030027
Salisbury DL, Li D, Todd M, Ng TKS, Yu F. Aerobic Exercise, Training Dose, and Cardiorespiratory Fitness: Effects and Relationships with Resting Plasma Neurotrophic Factors in Alzheimer’s Dementia. Journal of Vascular Diseases. 2023; 2(3):351-366. https://doi.org/10.3390/jvd2030027
Chicago/Turabian StyleSalisbury, Dereck L., Danni Li, Michael Todd, Ted K. S. Ng, and Fang Yu. 2023. "Aerobic Exercise, Training Dose, and Cardiorespiratory Fitness: Effects and Relationships with Resting Plasma Neurotrophic Factors in Alzheimer’s Dementia" Journal of Vascular Diseases 2, no. 3: 351-366. https://doi.org/10.3390/jvd2030027
APA StyleSalisbury, D. L., Li, D., Todd, M., Ng, T. K. S., & Yu, F. (2023). Aerobic Exercise, Training Dose, and Cardiorespiratory Fitness: Effects and Relationships with Resting Plasma Neurotrophic Factors in Alzheimer’s Dementia. Journal of Vascular Diseases, 2(3), 351-366. https://doi.org/10.3390/jvd2030027






