Computed Tomography Imaging Features of Pulmonary Sequestration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Data Collection
2.2. CT Scanning and Image Interpretation
2.3. Statistical Analysis
3. Results
3.1. Sex Distribution and Age at Diagnosis
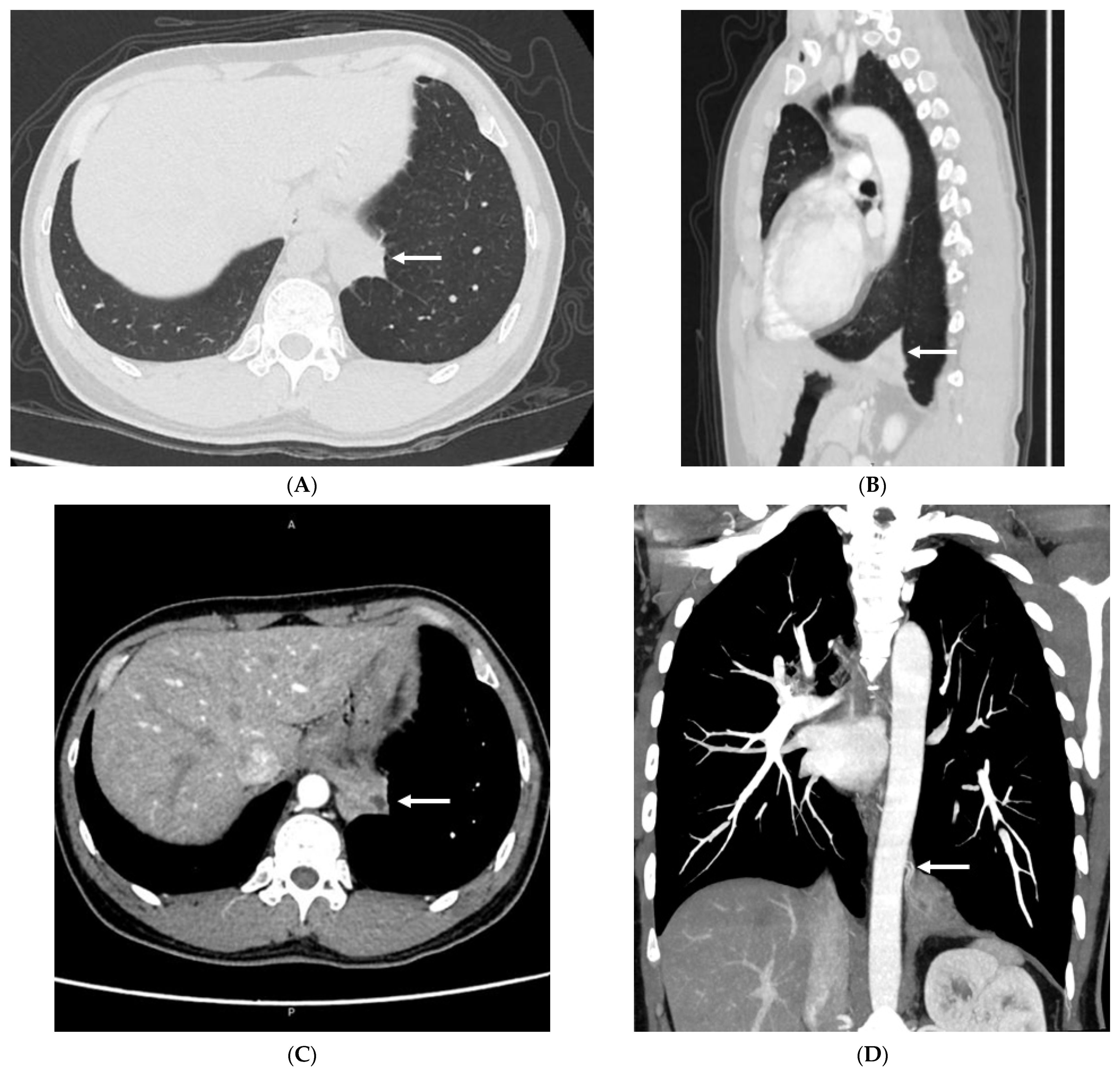
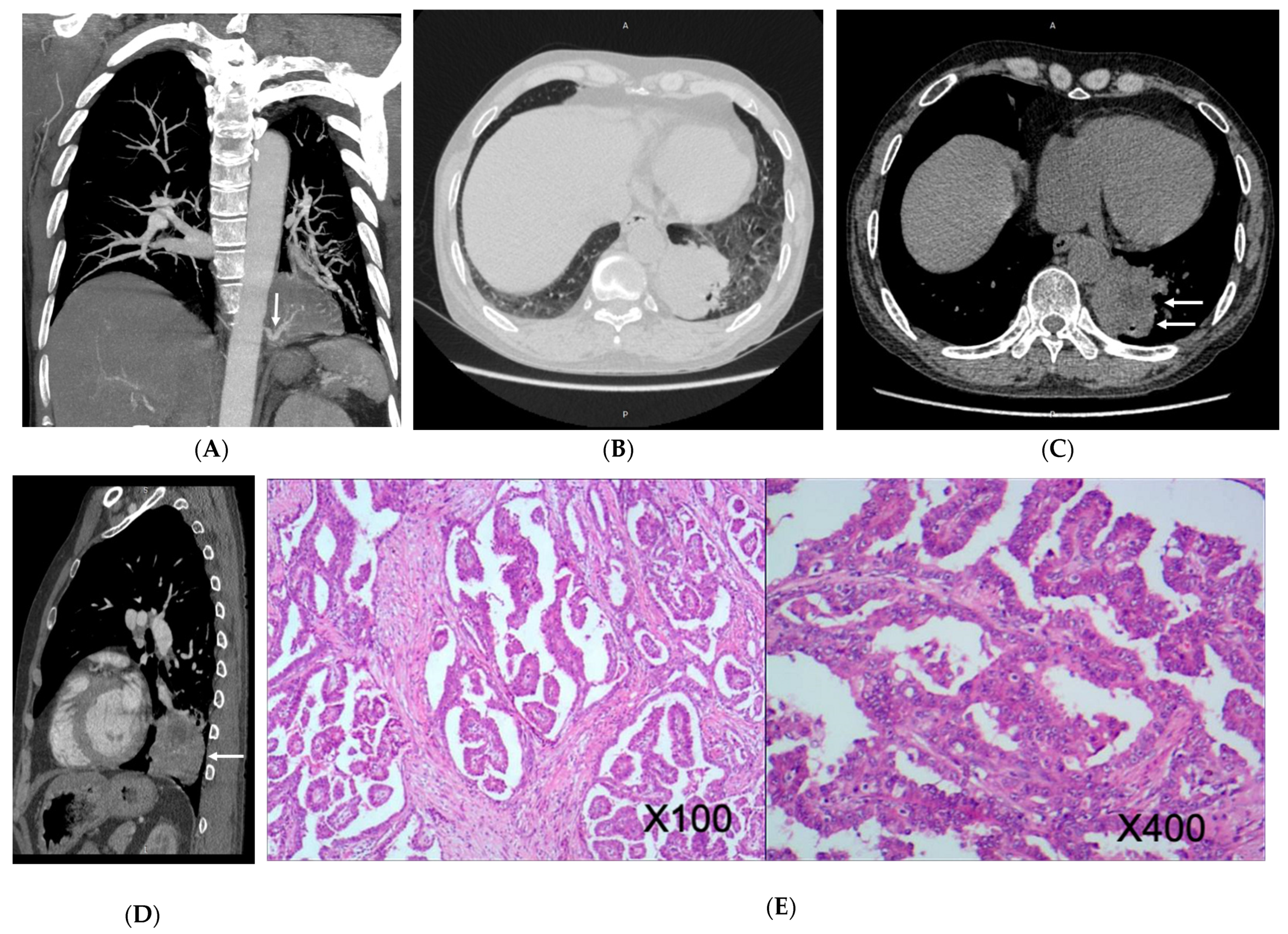
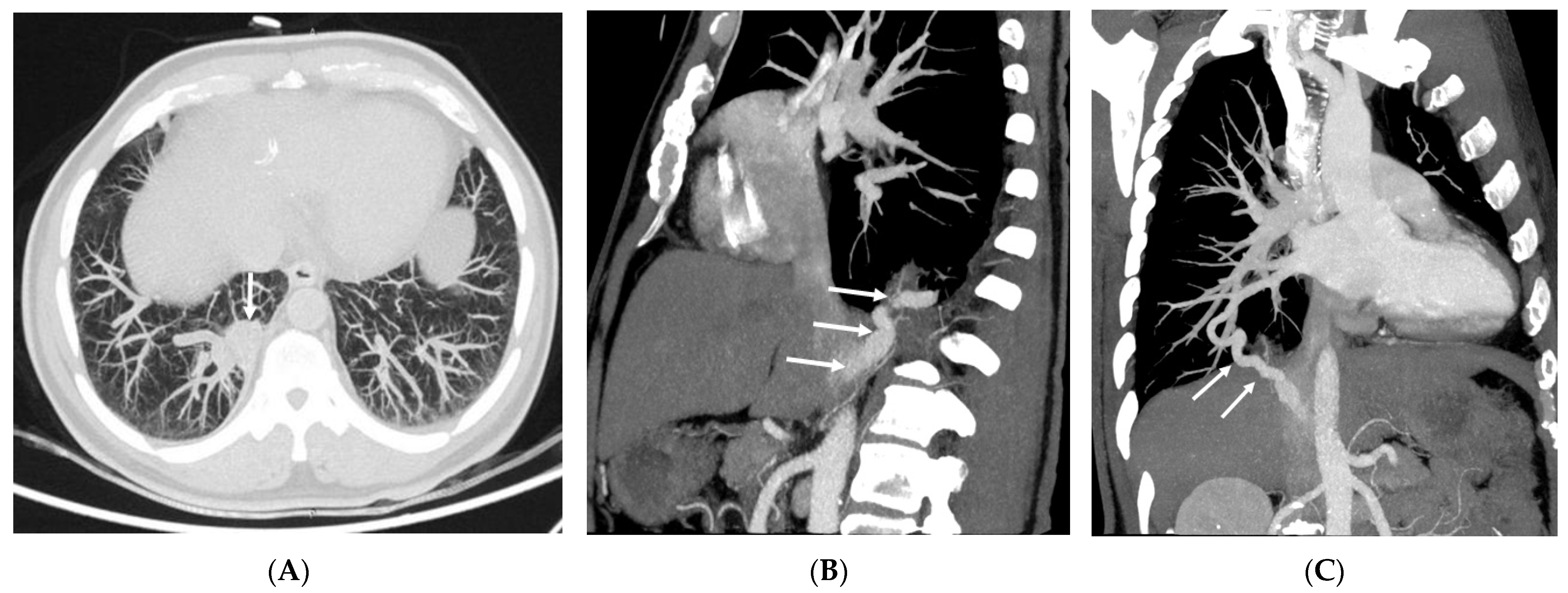
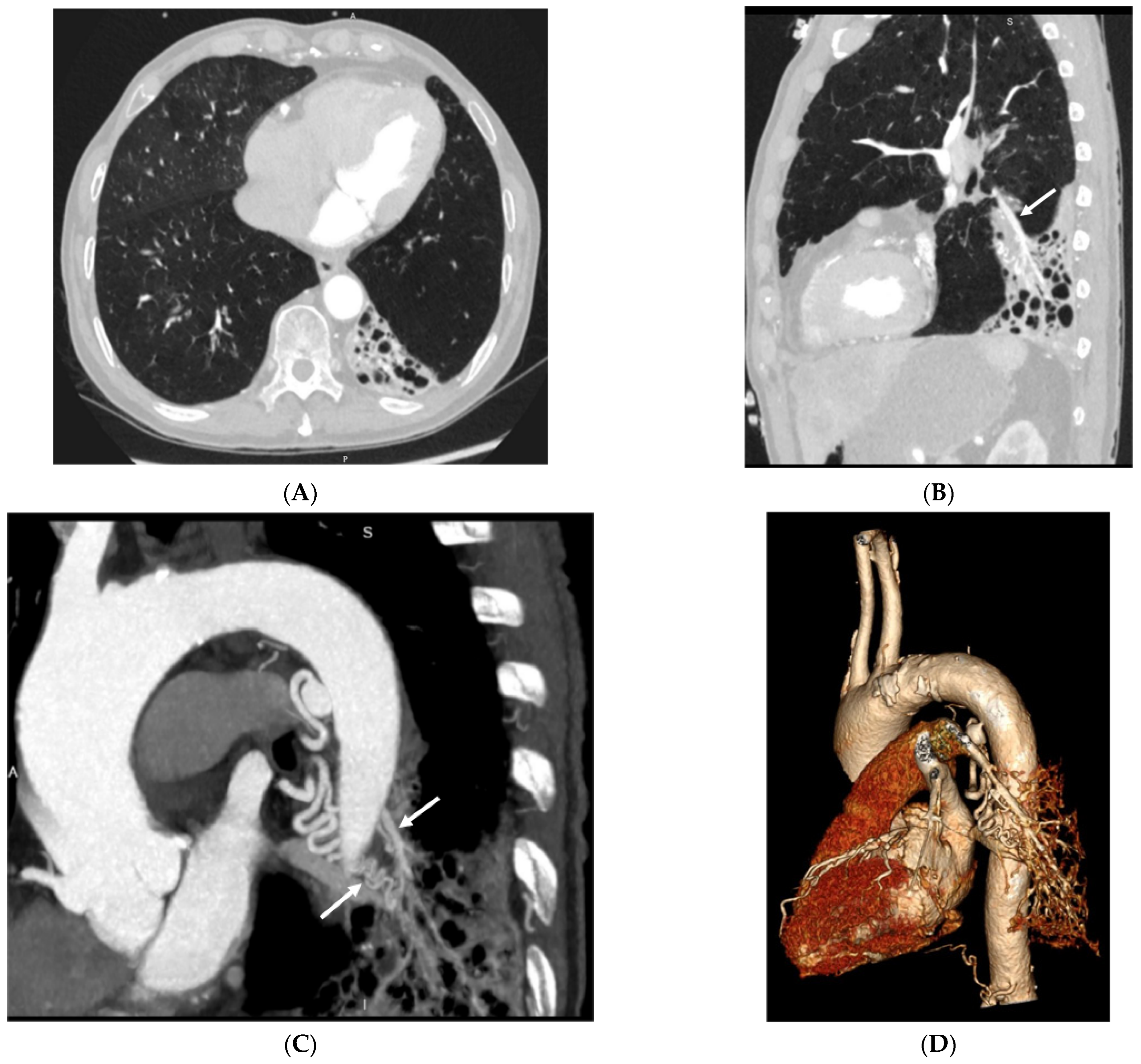
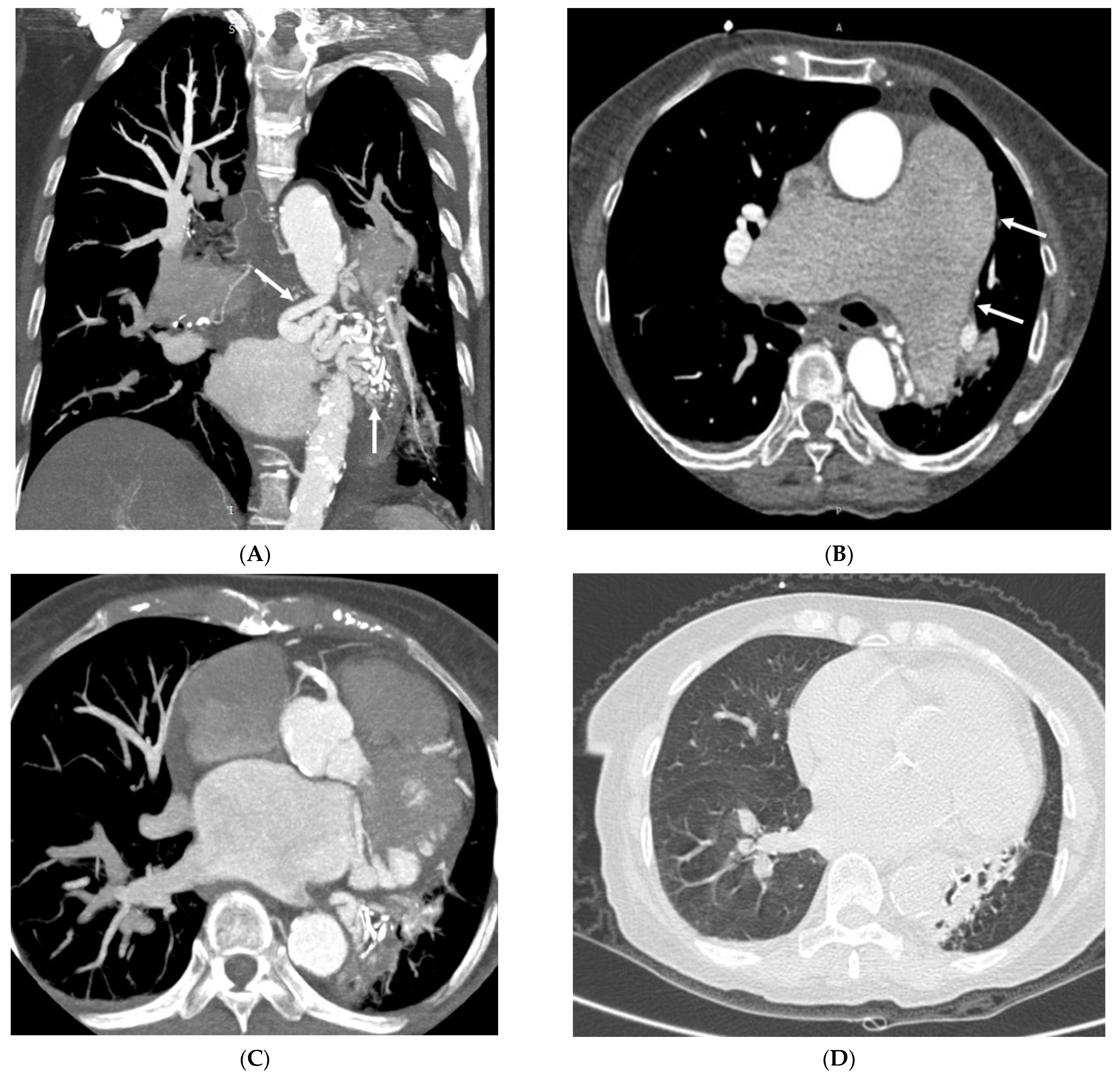
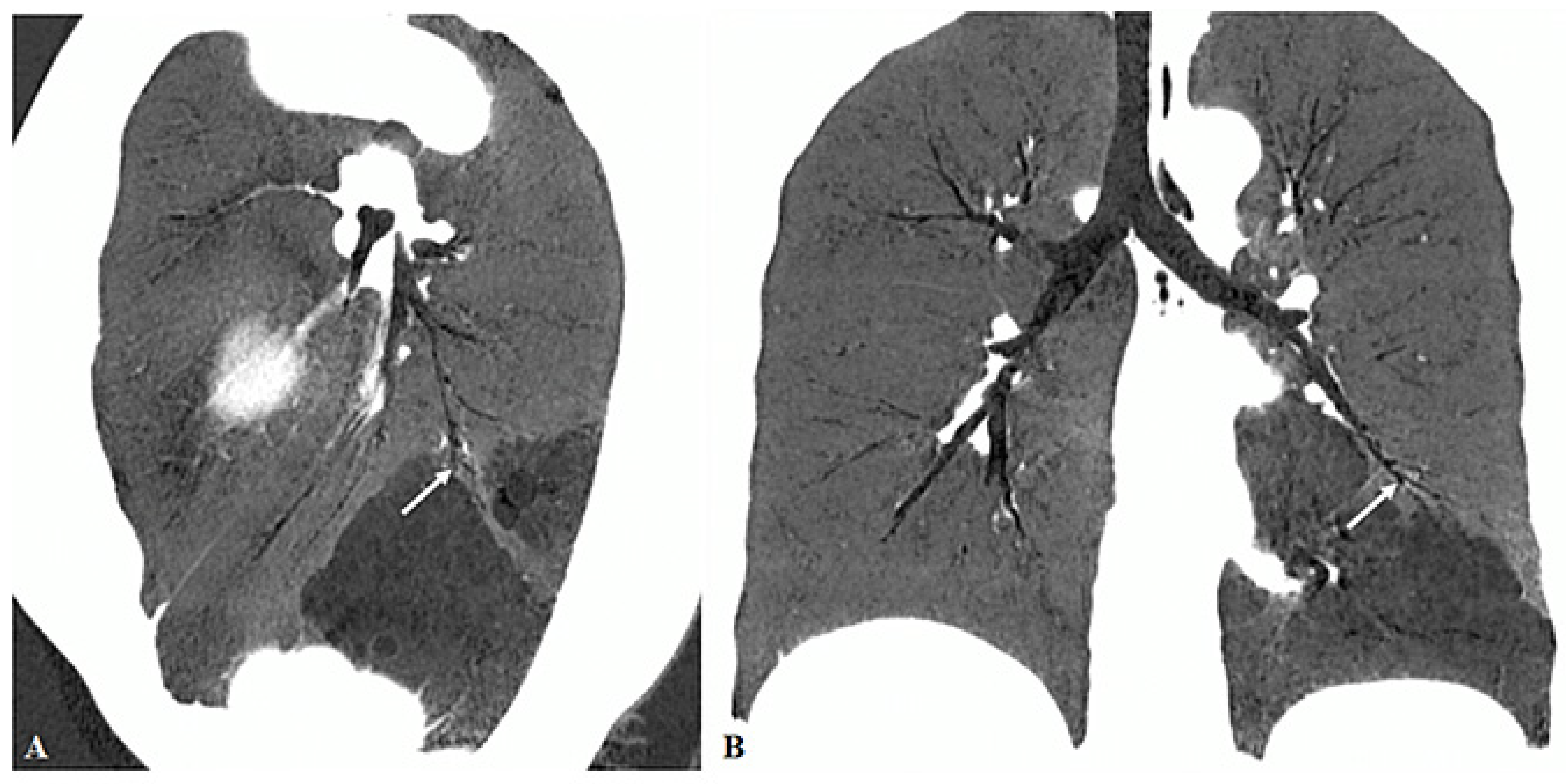
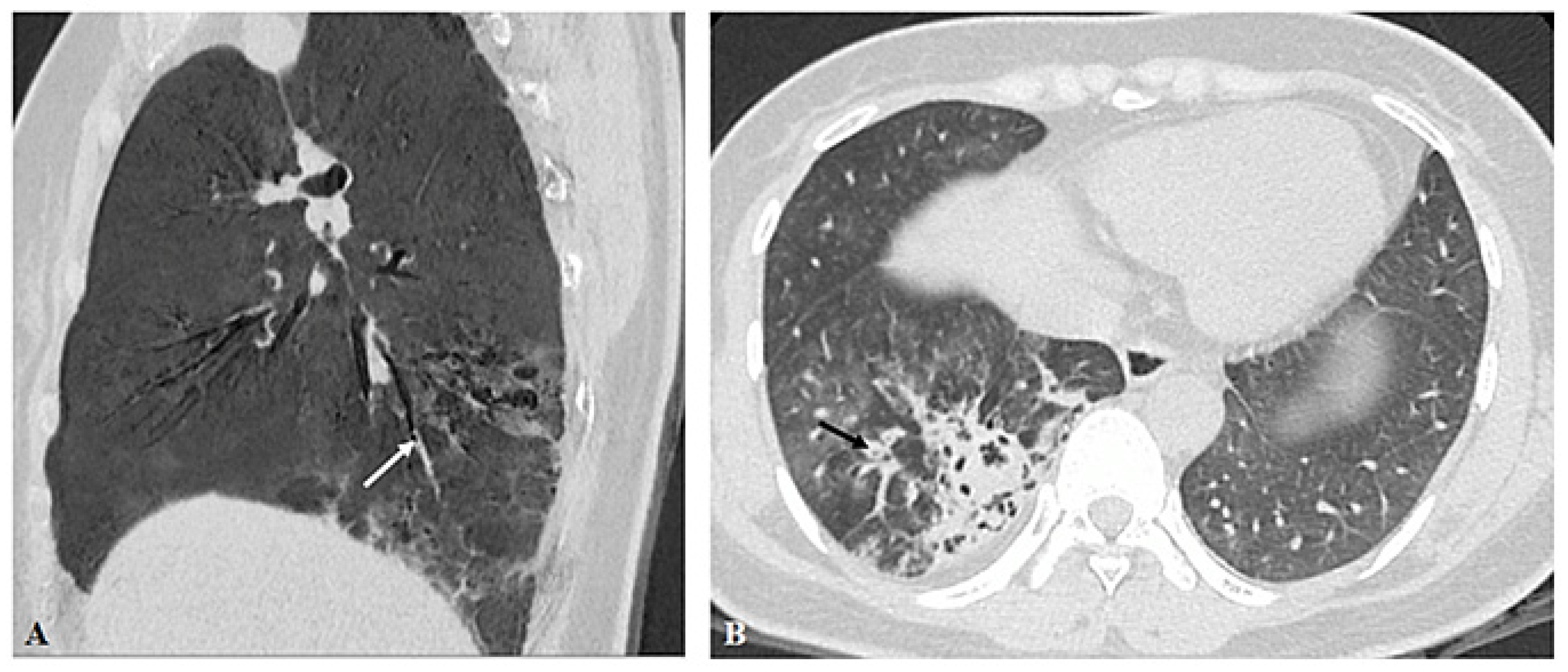
3.2. Imaging Appearances of CTPA
4. Discussion
5. Future Directions of Using CTPA in PS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabelloni, M.; Faggioni, L.; Accogli, S.; Aringheri, G.; Neri, E. Pulmonary sequestration: What the radiologist should know. Clin. Imaging 2021, 73, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, C.C.; Kollias, V.D.; Panayotopoulos, P.P. Pulmonary sequestration. Experience with eight consecutive cases. Scand. Cardiovasc. J. 1997, 31, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Yangui, F.; Charfi, M.; Abouda, M.; Charfi, M.R. Pulmonary sequestration in a healthy teenage girl. Tunis. Med. 2019, 97, 604–605. [Google Scholar]
- Berna, P.; Lebied, E.I.D.; Assouad, J.; Foucault, C.; Danel, C.; Riquet, M. Pulmonary sequestration and aspergillosis. Eur. J. Cardiothroac. Surg. 2005, 27, 28–31. [Google Scholar] [CrossRef]
- Salvati, F. Cardiovascular, broncho-parenchima and neoplastic anomalies related to pulmonary sequestration: A critical review. Recenti. Prog. Med. 2018, 109, 388–392. [Google Scholar]
- Franko, J.; Bell, K.; Pezzi, C.M. Intraabdominal pulmonary sequestration. Curr. Surg. 2006, 63, 35–38. [Google Scholar] [CrossRef]
- Ulys, A.; Samalavicius, N.; Cicenas, S.; Petraitis, T.; Trakymas, M.; Sheinin, D.; Gatijatullin, L. Extralobar pulmonary sequestration. Int. Med. Case. Rep. J. 2011, 4, 21–23. [Google Scholar]
- Hirai, S.; Hamanaka, Y.; Mitsui, N.; Uegami, S.; Matsuura, Y. Surgical treatment of infected intralobar pulmonary sequestration: A collective review of patients older than 50 years reported in the literature. Ann. Thorac. Cardiovasc. Surg. 2007, 13, 331–334. [Google Scholar]
- Adzic-Vukicevic, T.N.; Radovanovic, D.V.; Acimovic, B.D.; Popovic, M.P. Pulmonary sequestration mimicring lung cancer: A case report. Vojnosanit. Pregl. 2016, 73, 1060–1063. [Google Scholar] [CrossRef]
- Tashtoush, B.; Memarpour, R.; Gonzalez, J.; Gleason, J.B.; Hadeh, A. Pulmonary sequestration: A 29 patient case series and review. J. Clin. Diagn. Res. 2015, 9, AC05-8. [Google Scholar] [CrossRef]
- Cong, C.V.; Ly, T.T.; Minh, N.M. Intralobar pulmonary sequestration supplied by vessel from the inferior vena cava: Literature overview and case report. Radiol. Case Rep. 2022, 17, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Shafig, M.; Ali, A.; Dawar, U.; Setty, N. Rare cause of haemoptysis: Bronchopulmonary sequestration. BMJ Case Rep. 2021, 14, e239140. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xiao, Y. Pulmonary sequestration in adult patients: A retrospective study. Eur. J. Cardiothorac. Surg. 2015, 48, 279–282. [Google Scholar] [CrossRef]
- Long, Q.; Zha, Y.; Yang, Z. Evaluation of pulmonary sequestration with multidetector computed tomography angiography in a select cohort of patients: A retrospective study. Clinics 2016, 71, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, Q.; Yu, J.; Zhang, X. Distribution, diagnosis, and treatment of pulmonary sequestration: Report of 208 cases. J. Pediatr. Surg. 2019, 54, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, F. Pulmonary sequestration: A retrospective analysis of 2625 cases in China. Eur. J. Cardiothorac. Surg. 2011, 40, e39–e42. [Google Scholar] [CrossRef]
- Wang, D.; Wheeler, W.B. A hybrid lesion of intralobar sequestration with mixed features of CPAM type I and type II umasked following SARS-CoV-2 infection: Case report and literature review. Int. J. Surg. Case Rep. 2022, 96, 107336. [Google Scholar] [CrossRef]
- Kunisaki, S.M. Narrative review of congenital lung lesions. Transl. Pediatr. 2021, 10, 1418–1431. [Google Scholar] [CrossRef]
- Fukui, T.; Hakiri, S.; Yokoi, K. Extralobar pulmonary sequestration in the middle mediastinum. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 481–483. [Google Scholar] [CrossRef]
- Yue, S.W.; Guo, H.; Zhang, Y.G.; Gao, J.B.; Ma, X.X.; Ding, P.X. The clinical value of computer tomographic angiography for the diagnosis and therapeutic planning of patients with pulmonary sequestration. Eur. J. Cardiothorac. Surg. 2013, 43, 946–951. [Google Scholar] [CrossRef]
- Naumeri, F.; Sajjad, M.N. Hybrid lesion: Extralobar sequestration with cystic adenomatoid malformation-misdiagnosed as pulmonary tuberculosis. J. Coll. Physicians Surg. Pak. 2018, 28, S204–S206. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.V.; Kumar, A.; Ocazionez, D.; Estrada-Y-Martin, R.M.; Restrepo, C.S. Developmental lung anomalies in adults: A pictorial review. Respir. Med. 2019, 155, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Riedlinger, W.F.; Vargas, S.O.; Jennings, R.W.; Estroff, J.A.; Barnewolt, C.E.; Lillehei, C.W.; Wilson, J.M.; Colin, A.A.; Reid, L.M.; Kozakewich, H.P. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr. Dev. Pathol. 2006, 9, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, F.; Cerillo, A.G.; Chiappino, D. The levoatriocardinal vein. Pediatr. Cardiol. 2005, 26, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.P.; Mahani, M.G.; Lu, J.C.; Dorfman, A.L. Levoatriocardinal vein and mimics: Spectrum of imaging findings. AJR Am. J. Roentgenol. 2015, 205, W162–W171. [Google Scholar] [CrossRef]
- Saremi, F.; Ho, S.Y. Extracardiac pulmonary-systematic connection via persistent levoatriocardinal vein in adults. Ann. Vasc. Surg. 2016, 34, 269.e1–269.e7. [Google Scholar] [CrossRef] [PubMed]
- Shet, N.; Maldjian, P. Levoatriocardinal vein: An unusual cause of right-left shunting. J. Clin. Imaging Sci. 2014, 4, 68. [Google Scholar]
- Canan, A.; Aziz, M.U.; Abbara, S. A rate pulmonary-systemic connection: Levoatriocardinal vein. Radiol. Cardiothorac. Imaging 2020, 2, e190228. [Google Scholar] [CrossRef]
- Karangelis, D.; Avramidis, D.; Mousiama, T.; Karamitsos, T.D.; Mitropoulos, F.; Tzifa, A. Levoatriocardinal vein: A rarely recognized cause of recurrent cardiac and cerebral thromboembolic events. Can. J. Cardiol. 2020, 36, 589.e9–589.e11. [Google Scholar] [CrossRef]
- Hertzog, P.; Roujeau, J.; Marcou, J. Epidermoid cancer developed on a sequestration. J. Fr. Med. Chir. Thorac. 1963, 17, 33–38. [Google Scholar]
- Koons, E.; VanMeter, P.; Rajendran, K.; Yu, L.; McCollough, C.; Leng, S. Improved quantification of coronary artery luminal stenosis in the presence of heavy calcifications using photon-counting detector CT. Proc. SPIE Int. Soc. Opt. Eng. 2022, 12031, 120311A. [Google Scholar] [CrossRef] [PubMed]
- Zsarnoczay, E.; Fink, N.; Schoepf, U.J.; O’Doherty, J.; Allmendinger, T.; Hagenauer, J.; Wolf, E.V.; Griffith, J.P., 3rd; Maurovich-Horvat, P.; Varga-Szemes, A.; et al. Ultra-high resolution photon-counting coronary CT angiography improves coronary stenosis quantification over a wide range of heart rates-A dynamic phantom study. Eur. J. Radiol. 2023, 161, 110746. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT angiography with photon-counting CT: First-in-human results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Ran, A.; Taron, J.; Weiss, J.; Stein, T.; Faby, S.; von zur Mueblen, C.; Ruile, P.; et al. Accuracy of ultrahigh-resolution photon-counting CT for detecting coronary artery disease in a high-risk population. Radiology 2023, 307, e223305. [Google Scholar] [CrossRef] [PubMed]
- Soschynski, M.; Hagen, F.; Baumann, S.; Hagar, M.T.; Weiss, J.; Krauss, T.; Schlett, C.L.; von zur Muhlen, C.; Nikolaou, K.; Greulich, S.; et al. High temporal resolution dual-source photon-counting CT for coronary artery disease: Initial multicenter clinical experience. J. Clin. Med. 2022, 11, 6003. [Google Scholar] [CrossRef]
- Gaillandre, Y.; Duhamel, A.; Flohr, T.; Falvre, J.-B.; Khung, S.; Hutt, A.; Felloni, P.; Remy, J.; Remy-Jardin, M. Ultra-high resolution CT imaging of interstitial lung disease: Impact of photon-counting CT in 112 patients. Eur. Radiol. 2023, 33, 5528–5539. [Google Scholar] [CrossRef]
| Imaging Appearances | Subtype | Number of Cases |
|---|---|---|
| Subtype of disease | ILS | 54 (98.18%) |
| ELS | 1 (1.82%) | |
| Location of disease | LLL | 42 (76.36%) |
| RLL | 12 (21.82%) | |
| BLL | 1 (1.82%) | |
| Supplying artery | Aorta A | 1 (1.82%) |
| Aorta D | 47 (85.45%) | |
| CA | 7 (12.73%) | |
| BA | 1 (1.82%) | |
| Draining vessels | PV | 49 (89.09%) |
| UV | 1 (1.82%) | |
| IV | 1 (1.82%) | |
| PA | 11 (20.00%) | |
| NF | 2 (3.64%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Wang, Z.; Qiang, J.; Mao, Q.; Kong, S.; Sun, Z.; Li, Y. Computed Tomography Imaging Features of Pulmonary Sequestration. J. Vasc. Dis. 2023, 2, 367-380. https://doi.org/10.3390/jvd2040028
Yang T, Wang Z, Qiang J, Mao Q, Kong S, Sun Z, Li Y. Computed Tomography Imaging Features of Pulmonary Sequestration. Journal of Vascular Diseases. 2023; 2(4):367-380. https://doi.org/10.3390/jvd2040028
Chicago/Turabian StyleYang, Tingqian, Zhaoyu Wang, Jun Qiang, Qinxiang Mao, Shufeng Kong, Zhonghua Sun, and Yu Li. 2023. "Computed Tomography Imaging Features of Pulmonary Sequestration" Journal of Vascular Diseases 2, no. 4: 367-380. https://doi.org/10.3390/jvd2040028
APA StyleYang, T., Wang, Z., Qiang, J., Mao, Q., Kong, S., Sun, Z., & Li, Y. (2023). Computed Tomography Imaging Features of Pulmonary Sequestration. Journal of Vascular Diseases, 2(4), 367-380. https://doi.org/10.3390/jvd2040028








