1. Introduction
Immersive virtual reality (VR) represents one of the most remarkable technological advancements of the 21st century. A digital interface that promises full immersion into an alternate or simulated environment, VR’s potential has been tapped across an array of disciplines. Entertainment industries have been early adopters, providing audiences with experiences that were once relegated to the realms of imagination [
1,
2,
3]. Concurrently, the education sector has witnessed a paradigm shift, with VR-enhanced pedagogical tools fostering enriched learning experiences [
4,
5,
6].
Furthermore, the domain of professional training has embraced VR to craft realistic scenarios for a myriad of professionals, from manual labourers mastering their craft to surgeons simulating complex procedures [
7,
8,
9]. The medical area is reaping the benefits too. Beyond conventional treatments and therapies, VR is emerging as a powerful adjunctive tool. Pain management, once reliant solely on pharmacological interventions, is now exploring the pain-distracting potential of VR [
10]. Rehabilitation, be it physical or neurological, is experiencing a renaissance with VR-infused therapies [
11,
12]. Neuropsychology, in particular, has found a robust partner in VR, aiding in cognitive assessments [
13,
14], training [
15], and targeted rehabilitation efforts [
16,
17,
18].
Such broad applications further extend their arms to vulnerable populations. The elderly, often considered tech-averse, find solace and cognitive rejuvenation in VR experiences tailored to their needs [
19,
20]. Individuals with Mild Cognitive Impairment (MCI) and/or a type of dementia [
21,
22], or developmental challenges such as Attention-Deficit Hyperactivity Disorder (ADHD) [
23,
24,
25]) and Autism Spectrum Disorder (ASD) [
17,
26,
27] are not mere spectators. VR interventions, designed with sensitivity and precision, are being administered to provide these populations with therapeutic as well as recreational relief. Considering the importance of the aforementioned applications of VR and the fragility of some of the targeted populations, the effective implementation of VR becomes imperative.
While the promise of VR in transforming various domains is indisputable, VR also harbours an inherent limitation—cybersickness, a condition affecting a segment of its users [
28]. Manifesting as a triad of nausea, disorientation, and oculomotor disturbances, cybersickness remains a significant concern. While there is a temptation to draw parallels between cybersickness and simulator sickness, the two display distinct characteristics [
29]. Notably, cybersickness presents with heightened general discomfort, particularly intensified by nausea and disorientation [
29]. Adding another layer to this complex tapestry, cybersickness also stands apart from motion sickness. The former arises primarily from visual cues in VR, whereas the latter emerges from actual physical movement [
30].
Delving into the underlying causes of cybersickness, a comprehensive theoretical understanding is still needed. The sensory conflict theory has gained traction, shedding light on the root of the issue [
28,
30,
31]. According to the neural mismatch theory, an extension of the sensory conflict theory, the symptoms of cybersickness primarily arise from discrepancies between expected and sensed motion, with a significant involvement of the central nervous system (CNS) in the generation of cybersickness [
28,
31]. This theory suggests that cybersickness is not merely a result of sensorial conflict between the visual and vestibular systems but also involves the CNS’s interpretation of these conflicting cues. Our sense of balance and spatial orientation relies on the integration of visual, vestibular, and proprioceptive feedback. In VR environments, these systems often receive conflicting cues, leading to a neural mismatch that causes sensory dissonance and contributes to the onset of cybersickness. For VR, this conflict can be attributed to vection—an illusionary perception of motion [
32]. This illusion, particularly when accompanied by movements like linear and angular accelerations, has been pinpointed as a major instigator of cybersickness in VR [
33,
34]. As VR continues its ascendancy in the tech world, the quest to understand and alleviate cybersickness remains a pressing concern.
Cybersickness in VR appears to be a result of both hardware and software issues, and poses a significant challenge. Hardware problems like latency and refresh rate discrepancies, alongside software issues such as inconsistencies in visual–vestibular integration, contribute to a disorienting VR experience [
35]. The industry is addressing these through advancements in head-mounted displays (HMDs), enhanced display resolutions, and software that aligns more closely with human physiology [
35,
36]. Strategies to mitigate cybersickness symptoms include acclimatization, the use of medications or natural remedies, and innovations in Human–Computer Interactions, although these methods have limitations such as high costs, time consumption, or an impact on immersion [
37,
38,
39,
40,
41].
The measurement of cybersickness has predominantly relied on questionnaires like the Simulator Sickness Questionnaire (SSQ) [
42] and the Virtual Reality Sickness Questionnaire (VRSQ) [
43], which have been criticized for their limited effectiveness [
44,
45,
46,
47]. The Cybersickness in Virtual Reality Questionnaire (CSQ-VR) is recognized as a more effective tool, especially with its integration of eye-tracking technology [
45]. Physiological metrics like electroencephalography (EEG), electrocardiogram (ECG), and galvanic skin response (GSR) have also been used to predict cybersickness, but their widespread use is limited by costs and ergonomic issues [
48,
49,
50]. Individual differences also influence cybersickness experiences, with mixed findings regarding the role of gender and gaming experience [
51,
52,
53,
54]. Furthermore, cybersickness can significantly impair cognitive and motor functions, which are crucial in VR’s application in education, research, and training [
35,
52,
55]. Studies have shown varying impacts of cybersickness on these functions, indicating the need for further research in this area [
51,
56,
57,
58,
59]. Overall, this research highlights the ongoing need for innovation and thorough assessment in mitigating and measuring cybersickness in VR environments.
The current study aims to address several key gaps in the literature regarding the impact of cybersickness on virtual reality (VR) environments, particularly focusing on its effects on cognitive functions and individual differences. The outlined hypotheses contribute to the literature in the following ways:
Pupil Size as a Predictor of Cybersickness Intensity (H1): This hypothesis explores the relationship between physiological responses, specifically pupil size, and the intensity of cybersickness. If confirmed, this would add to the understanding of how physiological markers can be used as reliable predictors of cybersickness, offering a more objective and potentially real-time method for assessing cybersickness severity.
The Link between Motion Sickness Susceptibility and Cybersickness (H2): By investigating the connection between a person’s susceptibility to motion sickness and their experience of cybersickness, this study could provide insights into individual differences in VR experiences. This would contribute to a more personalized approach toVR design and usage, especially for populations that are more prone to motion sickness.
The Impact of Computer and Video Game Experience (H3): This hypothesis examines the role of prior experience with computers and video games in predicting cybersickness. Clarifying this relationship could have implications for understanding how a familiarity with digital interfaces and interactive environments can influence one’s adaptation to VR, potentially guiding training protocols and user onboarding processes in VR applications.
The Effect of Cybersickness on Cognitive and Motor Skills (H4): Investigating the impact of cybersickness on key cognitive functions like verbal and visuospatial working memory, and psychomotor skills, directly addresses the discrepancies noted in previous studies about the extent of cybersickness’s impact. This could significantly enhance our understanding of the cognitive costs of VR usage, informing the design and application of VR in education, training, and rehabilitation.
The Comparison of Cybersickness Intensity During and After VR Exposure (H5): By comparing the intensity of cybersickness during and after VR exposure, this study aims to elucidate the temporal dynamics of cybersickness. This could lead to a better understanding of how cybersickness evolves over time, which is crucial for designing VR sessions and interventions to mitigate its effects.
In this paper, we delve into various aspects of cybersickness and its impact on users during virtual reality (VR) experiences.
Section 2 presents a comprehensive literature review, examining existing research on cybersickness, including its causes, mitigation strategies, and the role of various hardware and software factors in VR.
Section 3, ‘Materials and Methods’, details the VR hardware and software used, the development process of the virtual environment, and the methodologies for assessing cognitive and motor skills, as well as cybersickness in VR. In
Section 4, ‘Results’, we present the findings from our study, including descriptive statistics, regression analysis results, and comparisons of cybersickness intensity during different stages of VR exposure.
Section 5, ‘Discussion’, integrates our findings with the broader body of research, discussing the implications of our results and how they align with or contrast the existing literature. Finally,
Section 6 concludes the paper, summarizing the key insights and contributions of our study to the understanding of cybersickness in VR environments.
4. Results
Descriptive statistics of the data are presented in
Table 1. Demographically, participants were relatively young adults with a wide range of educational backgrounds. Their technology engagement was evident from the experience they had with computing, smartphones, and gaming. This provides an interesting lens through which to understand the effects of VR, given their wide range of familiarity with digital tools. Regarding motion sickness, participants displayed varied susceptibility. Their scores from childhood to adulthood in motion sickness susceptibility showed a notable shift, suggesting that reactions to motion may evolve with age. When diving into the cybersickness, as assessed by the CSQ-VR, it seemed to intensify post VR exposure across all its subcategories: nausea, vestibular, and oculomotor symptoms.
4.1. Linear Regression Analyses: Prediction of Cybersickness Intensity
Linear regression analyses were conducted to detect and compare the significant predictors of overall, and per symptom category, cybersickness intensity.
Table 2 elucidates how various individual factors predict overall cybersickness. In support of H1, the pupil size while responding to the CSQ-VR questionnaire and during the VR ride demonstrated significant cybersickness prediction. In agreement with H2, the most significant predictor is the motion sickness susceptibility score as an adult (MSB-Adult), with an impressive 39% variance (R
2 = 0.39) accounted for. Finally, in line with H3, smartphone experience and gaming experience were notable for their significance in predicting cybersickness.
Table 3 provides insights specific to nausea symptoms. Once again, in line with H1, pupil size, both during CSQ-VR reading and the VR ride, was a significant predictor of nausea symptomatology. Notably, in agreement with H2, the MSB-Adult stands out as the most potent predictor. The child motion sickness score (MSA-Child) and the total motion sickness susceptibility (MSSQ Total) were also significant, hinting that one’s propensity to motion sickness earlier in life could have repercussions in a virtual environment. Finally, in support of H3, experience with using smartphones and playing videogames were significant predictors of the intensity of nausea symptoms.
In
Table 4, the focus shifts to vestibular symptoms. While some predictors overlap with those for nausea, in disagreement with H1, the pupil size did not significantly predict vestibular symptoms. However, the MSB-Adult was found to be a robust predictor of nausea, further confirming H2. Finally, it is intriguing to see that smartphone and gaming experience are significant predictors of vestibular symptomatology, which agrees with H3 and implies a potential link between technological experience and experiencing vestibular symptoms in a VR.
Table 5 centres on oculomotor symptoms. Unlike previous findings and discrepantly to H1–H3, no single predictor emerges as significant. The table largely communicates that more conventional metrics (age, education, tech experience) do not have significant associations with oculomotor symptoms in VR. This highlights the complexities of predicting these specific symptoms.
Table 6 aggregates the best models for predicting various aspects of cybersickness. This table reemphasizes the overarching role of the MSB-Adult in determining cybersickness, nausea, and vestibular symptoms. This connotes that the susceptibility to experiencing motion sickness as an adult is robustly associated with experiencing visually induced cybersickness symptoms in VR. Interestingly, no predictors were identified for oculomotor symptoms, indicating a potential gap in our understanding or the need for more refined measures.
In essence, these results underscore the multifaceted nature of cybersickness and its determinants. While some predictors like the MSB-Adult consistently emerge as influential, others show symptom-specific associations. Furthermore, they accentuate the importance of not only considering the user’s history and demographics but also real-time metrics like pupil size in understanding their VR experience.
4.2. Mixed Model Regression Analyses: Effects on Cognitive and Motor Performance
Mixed linear regression model analyses were carried out to determine and evaluate the significant predictors of performance on cognitive and psychomotor skills tasks.
Table 7 and
Table 8 delve into the predictors for verbal short-term and working memory, respectively. In disagreement with H4, the critical observation here is that none of the predictors were significant predictors of verbal short-term and working memory. This might suggest that verbal memory is less susceptible to variations in these predictors, or that other unmeasured factors may have a more substantial impact.
Table 9 elucidates the predictors of visuospatial short-term memory. In contrast with H4, none of the cybersickness measurements was found to be a significant predictor of performance. However, it reveals some compelling findings. Sex, computing experience, and gaming experience were shown as significant predictors of visuospatial short-term memory. Gaming experience, in particular, accounts for a substantial 19% of the variance.
Table 10 examines predictors for visuospatial working memory. Supporting H4, the vestibular symptoms’ intensity was found to be a significant predictor of visuospatial working memory, although other cybersickness metrics did not substantially predict it. Furthermore, sex and gaming experience stand out as significant predictors, indicating that gaming might shape how individuals process and manipulate visual–spatial information.
Table 11, on attentional time, displays that the CSQ-VR—specifically the oculomotor component—has a notable relationship with attentional time. The positive β coefficient suggests a direct correlation, meaning that as cybersickness symptoms increase, attentional time might also increase.
Table 12 targets motor time. Here, gaming experience and the CSQ-VR–Nausea are prominent predictors. This implies that one’s gaming experience might influence motor response times, and individuals experiencing nausea-related cybersickness symptoms might exhibit changes in their motor time.
In
Table 13, the focus is on reaction time (i.e., psychomotor skills). In full support of H4, every cybersickness metric was a significant predictor of psychomotor skills, postulating that overall cybersickness, nausea, vestibular, and oculomotor symptoms play a role in determining reaction times. Interestingly, gaming experience also emerges as a significant negative predictor, likely hinting that frequent gamers might have quicker reaction times.
Lastly,
Table 14 consolidates the most impactful predictors for various cognitive and motor skills. A recurring theme here is the influence of gaming experience on cognitive and motor skills, emphasizing its potential cognitive benefits or the development of specific skills associated with gaming. Interestingly, overall cybersickness and its vestibular and oculomotor components were also included in the best models, postulating the negative effects of cybersickness on cognitive functioning and psychomotor skills.
In summary, these results underscore the potential cognitive and motor influences of digital experiences like gaming. They also hint at the intertwined nature of cybersickness symptoms and cognitive/motor skills, suggesting that our experience in virtual environments can have multifaceted impacts on our cognitive functioning.
4.3. Comparison of Cybersickness during and after Exposure to Virtual Reality
Paired-sample
t-test analyses were performed to examine whether there was a difference in terms of cybersickness symptomatology during VR immersion and after exposure to VR (i.e., immediately after the removal of VR equipment).
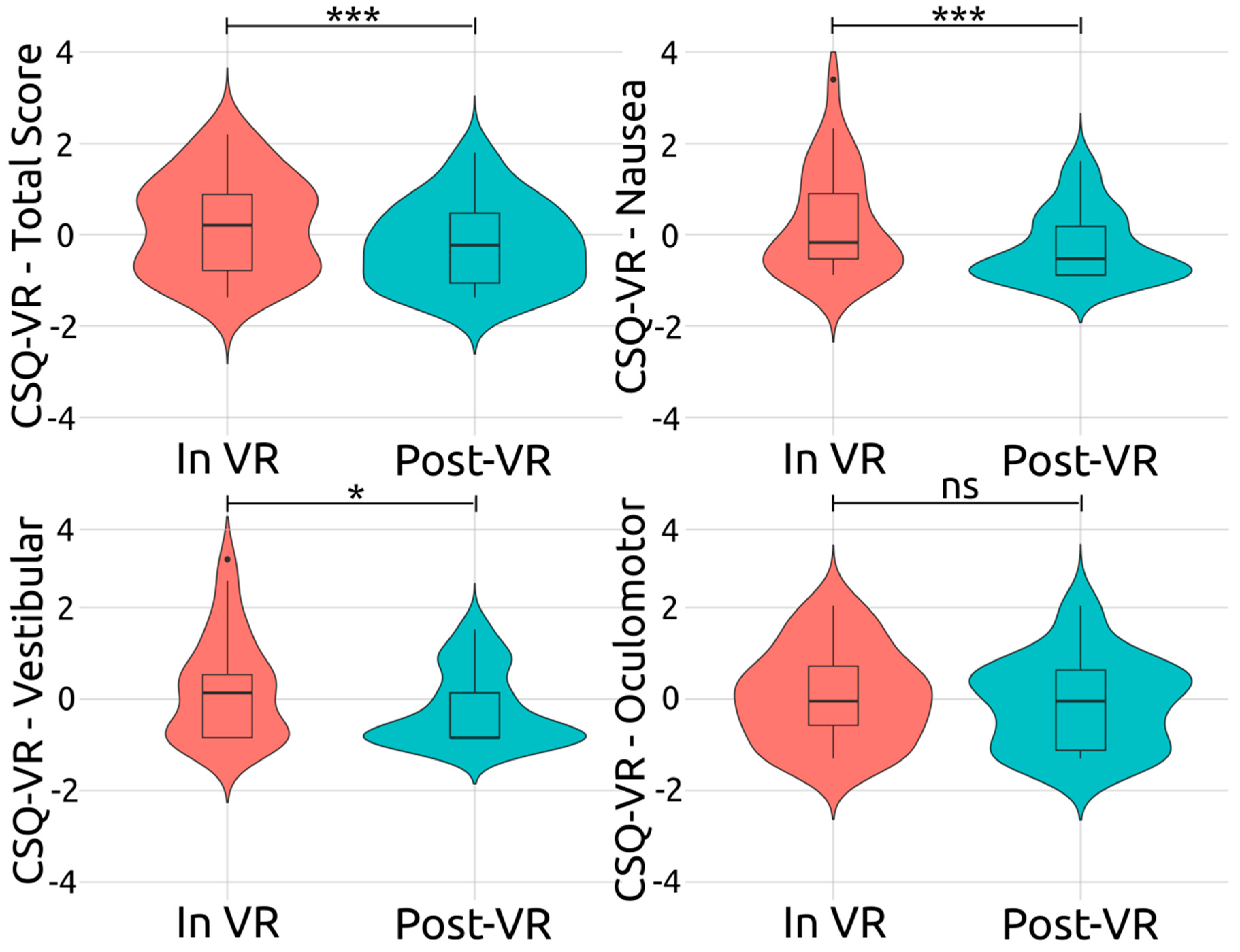
Figure 4 illustrates the z-scores, representing the intensity of the overall and various cybersickness symptoms experienced by participants both during their immersion in VR and after their exposure to VR (i.e., after the removal of the VR headset). In line with H5, the overall cybersickness intensity was found to have a significant and large decrease after the removal of the VR headset (i.e., re-adaptation to the physical world),
t(29) = 3.59,
p < 0.001,
Hedges g = 0.64. Furthermore, other cybersickness symptoms showed significant decreases after removing the VR headset and transitioning from the virtual to the physical environment, further supporting H5.
For vestibular symptoms, after exposure to VR (Post-VR), a noticeable shift towards negative z-scores was identified, indicating that the experience of vestibular symptoms was less intense. This difference between the two stages was confirmed to be statistically significant and large, t(29) = 2.74, p = 0.010, Hedges g = 0.49. Regarding nausea, post-VR, a slightly broader spread of z-scores, predominantly on the negative side, was detected. This decrease in the nausea symptoms’ intensity was deemed significant and large, t(29) = 3.12, p < 0.001, Hedges g = 0.56. Finally, for oculomotor symptoms, after exposure to VR, the distribution pattern of the z-scores remained largely unchanged, t(29) = 1.22, p = 0.230, Hedges g = 0.22, indicating that the removal of the headset and re-adaptation to the physical world had a non-significant effect on the oculomotor symptoms’ intensity. To summarize, of the three cybersickness symptom categories examined, the vestibular and nausea symptoms exhibited a statistically significant decrease after headset removal (i.e., post VR exposure). It was determined, however, that the oculomotor symptoms’ intensity remained relatively stable after exposure to VR.
5. Discussion
Considering that VR is implemented in educational, professional, research, and clinical settings, the present study aimed to examine cybersickness symptoms during immersion in VR. The study explored the role of several factors pertinent to individual differences, such as motion sickness susceptibility, experience with playing videogames, experience using computers, sex, and age, in the experiencing of cybersickness symptomatology and its intensity. For predicting cybersickness overall, and the symptoms’ intensities, eye-tracking metrics, such as pupil dilation, were also considered. Furthermore, the study aimed to determine the effects of cybersickness symptoms on cognitive functions and motor skills. Finally, given that users experience a readjustment to physical space while removing the VR HMD and transitioning from the virtual to the physical environment, the current study also examined the differences in cybersickness intensity during and after immersion. A comprehensive discussion is offered by integrating findings from the current study with the insights from the reviewed literature.
5.1. Pupil Dilation as a Biomarker of Cybersickness
Pupil dilation has been previously seen as a biomarker of an affective state, where a bigger size indicates a positive (e.g., joy) and a smaller size indicates a negative affective state (e.g., fear) [
106]. Our results demonstrated a comparable pattern in cybersickness, where more intense cybersickness induced a smaller diameter (i.e., more negative affective state), and less intense cybersickness a bigger diameter of the pupils (i.e., more positive affective state). In VR, pupil size has been previously incorporated into a deep fusion model to predict cybersickness [
79]. However, this previous study did not assess pupil size’s association, predictive capacity, or role within this model, making it challenging to determine whether pupil size acts as a biomarker for cybersickness. However, in our previous studies, evidence was offered that pupil dilation while reading the questions of the CSQ-VR substantially predicts cybersickness intensity [
45,
51]. In line with all the studies mentioned above, pupil dilation was found to be a significant predictor of cybersickness in this study. However, the pupil dilation was measured during reading of the CSQ-VR questions, as well as during the ride. The pupil size during the ride was deemed a significant predictor of overall cybersickness and nausea symptoms, while the pupil size while responding to the CSQ-VR significantly predicted only overall cybersickness. Also, for overall cybersickness intensity, pupil dilation during the ride was a substantially better predictor than pupil dilation during the CSQ-VR. This suggests that pupil size is a more reliable biomarker during the triggering and experience of cybersickness than post exposure to stimuli eliciting symptomatology.
5.2. Modulators of Cybersickness: Sex, Smartphone Experience, and Videogame Experience
None of the demographics appeared to be a significant predictor of cybersickness intensity. Notably, age was not a significant predictor of cybersickness. Given the younger demographic of the participants, it is worth investigating these effects in a broader age range. Also, experience using computers failed to predict cybersickness. Finally, in disagreement with previous studies [
54,
107], sex (i.e., male, female) did not predict cybersickness’s intensity or symptoms, postulating that cybersickness is not more frequent or intense in either sex. Interestingly, Stanney et al. [
54] proposed that the variations stemmed from the VR headset’s InterPupillary Distance (IPD). In their subsequent experiment, no disparities between the two sexes were observed when participants deemed the IPD agreeable. In our research, the HTC Vive Pro Eye was employed, known for its universal comfort. Each participant underwent an eye-tracking calibration to fine-tune the IPD, ensuring its appropriateness and comfort. Also, in our previous study [
51], the differences between sexes in terms of cybersickness were eliminated when we controlled for gaming experience (i.e., male and female users with the same experience playing videogames). In this study, a balanced sample was attempted, with both female and male participants with a comparable level of (e.g., high/low) gaming experience. Therefore, the calibration of the IPD via eye-tracking, as well as the balance in terms of gaming experience among participants, may explain the non-significant effect of sex on experiencing cybersickness. In combination, the findings of this study and our previous study [
51], along with the findings of Stanney et al., [
54] suggest that the sex/gender of the participant does not modulate the intensity of their cybersickness symptomatology.
On the contrary, experience with playing videogames and experience using apps on a smartphone, for improving everyday functionality, significantly predicted the intensity of the perceived cybersickness symptoms. Notably, our study is the first to show that experience with smartphones may be a predictor of cybersickness. Specifically, more experience with smartphones was associated with a substantially lower intensity of cybersickness symptomatology. Interestingly, the usage of smartphones for performing various tasks (e.g., surfing on the internet, sending emails, writing documents, editing photos and videos, etc.), which traditionally were performed on a computer, is currently significantly higher compared to the usage of computers/laptops for performing the same tasks [
108]. Note that visually induced cybersickness can be elicited by exposure to any screen, including smartphones [
109,
110,
111]. Also, exposure to and experience with tech mediums with screens is associated with cultivating tolerance towards experiencing cybersickness symptomatology due to the user adapting to their spatial requirements and motion [
32]. Thus, the finding of this study regarding smartphone experience for developing a resilience to cybersickness is aligned with the relevant literature.
In the same direction, the current study showed that more experience with playing videogames predicts a higher resilience to cybersickness. This finding corroborates with the findings of our previous studies [
45,
51] and a large-scale study by Weech et al., [
84], as well as other studies on cybersickness [
112] and simulator sickness [
113]. Therefore, a more significant gaming background seems to act as a protective factor against cybersickness, whereas a limited one might heighten vulnerability. Notably, similar to our previous studies on cybersickness [
45,
51], in this study, experience with playing videogames considered both proficiency and frequency, which appears to be a refined measure of gaming experience. However, videogames are clustered under several diverse genres (e.g., action, first-person shooting, role-playing, etc.), and each one may modulate the physiological and biochemical state [
114], as well as cognitive functioning and brain structure [
115], in a significantly different way. Thus, further research is required for examining the effects of gaming on cybersickness, through considering experience with playing games of each genre as distinct metrics.
5.3. Cybersickness and Susceptibility to Motion Sickness
Susceptibility to motion sickness during adulthood (i.e., the MSB-Adult score of the MSSQ) emerged as the best predictor of cybersickness intensity. Except for oculomotor symptoms, the MSB-Adult was a significant predictor of overall cybersickness, nausea symptoms, and vestibular symptoms. Notably, the single-predictor models with the MSB-Adult (i.e., with only the MSB-Adult as a predictor) were found to be the best regression models for predicting the respective scores of the CSQ-VR. Considering that motion sickness and cybersickness induced by vection share common characteristics, such as motion cues acting as elicitors of sickness, it comes with no surprise that the findings of previous studies postulated that visually induced cybersickness and motion sickness demonstrate similar patterns of susceptibility among individuals [
85,
86]. The findings of this study agree with the findings of the aforementioned studies, since the MSB-Adult was the most prominent predictor of overall cybersickness, as well as nausea and vestibular symptoms.
However, in our previous study, the MSSQ scores failed to predict cybersickness’s intensity and symptoms [
51]. Nonetheless, in this previous study, the MSSQ was also used to exclude participants who demonstrated a high susceptibility to motion sickness. This may explain why the MSSQ scores were not identified as significant predictors in our previous study. Finally, taken together, the MSSQ scores, especially the MSB-Adult, may be used to identify individuals who are prone to experience a vection-induced cybersickness. However, the Visual Induced MSSQ (VIMSSQ) has been recently developed and validated, which is specific to exposure to screens (e.g., computers, tablets, and VR HMDs) [
111,
116]. Also, it should be noted that vection is only one of the reasons for experiencing cybersickness in VR [
31,
35,
36,
45]. Thus, further research is required to examine whether MSSQ and/or VIMSSQ scores may predict cybersickness induced by other factors such as low latency or latency fluctuations, non-ergonomic navigation, and low-quality graphics.
5.4. Cybersickness’s Effects on Verbal Short-Term Memory and Working Memory
The study of Dahlman et al. [
55] posited a direct negative effect of motion sickness on verbal working memory. Also, significant negative effects of cybersickness on verbal working memory were observed in our previous study [
51]. Discrepantly to these previous studies, cybersickness was seen to significantly affect neither verbal short-term memory nor verbal working memory. Regarding short-term and working memory, there is consensus that they are two different cognitive constructs stemming from the activation of diverse brain regions, where the former requires substantially fewer cognitive resources than the latter [
117,
118]. Hence, short-term memory may not appear to decrease due to low difficulty. Furthermore, the study of Dahlman et al. [
55] was on motion sickness. While motion sickness and cybersickness share some similarities, they are substantially different in terms of symptoms’ frequency and intensity [
29,
30]. Thus, this difference between cybersickness and motion sickness may explain the disagreement between the findings of this study and the findings of Dahlman et al.’ study.
Furthermore, in our previous study, only working memory was examined [
51]. Also, the order of the tasks was not counterbalanced, as the verbal working memory task was always the first to be performed after exposure to linear and angular accelerations. Finally, the size effect of the performance decrease during the verbal working memory task was small [
51]. These limitations of our previous study may thus explain the discrepancy between the findings of the two studies. Nonetheless, given that cybersickness effects are transient and of relatively short duration [
44], if performing a task immediately after exposure to stimuli inducing cybersickness does indeed have an impact, then this poses a severe methodological consideration that should be further explored in future studies. However, in this study, in line with the design of the original tests (see [
95,
97]), short-term memory tasks always preceded working memory tasks. Thus, if the order had a significant impact, the cybersickness effects should have also been observed during short-term memory tasks.
5.5. Cybersickness’s Effects on Visuospatial Short-Term Memory and Working Memory
Cybersickness was found to have a significant negative effect on visuospatial working memory. This finding aligns with the findings of the study of Mittelstaedt et al. [
56], where performance on the visuospatial working memory task was substantially decreased. The two studies hence postulate that cybersickness does indeed have a negative impact on visuospatial working memory. However, again, there was no effect on visuospatial short-term memory. Considering that the administration of the CBT tasks, the forward (short-term memory) and backward (working memory) recall tasks, should be in this order (i.e., forward recall and then backward recall) [
97,
98], the absence of an effect on short-term memory or a significant effect on working memory further supports that order of the tasks does not have an impact on observing the effects of cybersickness on cognitive performance. Furthermore, similar to verbal short-term and working memory, it is widely agreed that visuospatial short-term memory and working memory are distinct cognitive processes facilitated by different brain structures, with the former requiring fewer cognitive resources than the latter [
118,
119]. This difference between the two explains why visuospatial short-term memory was left intact by cybersickness while visuospatial working memory was substantially decreased.
Moreover, in this study, vestibular symptomatology, which implies a transient dysfunction of the vestibular system, was found to have a significant negative impact on visuospatial working memory. The functioning of visuospatial working memory pertains to the processing of visuospatial information [
118,
119]. The vestibular system has been suggested to have an important implication in visuospatial cognitive functioning [
120]. Thus, the decrease in visuospatial working memory by predominantly vestibular symptomatology that was observed in this study further supports the importance of the vestibular system to visuospatial information processing. Nevertheless, the best model for predicting visuospatial working memory also included gaming experience, which revealed a positive effect on working memory. Notably, gaming experience was also included in the best model of visuospatial short-term memory. These findings are in line with the relevant literature, which suggests that gamers have enhanced short-term and working memory abilities [
121]. In the investigation of cybersickness, these findings indicate that gaming experience should always be considered (e.g., as a covariate or an additional factor) when examining the effects of cybersickness on cognition.
5.6. Cybersickness’s Effects on Psychomotor Skills: Reaction, Attention, and Motor Speed
Psychomotor skills, such as attentional speed, motor speed, and overall reaction time were found to be substantially negatively affected by cybersickness’s symptomatology and intensity. In this study, the VR version of the DLRT was implemented, which, in contrast with the traditional version that produces a single score (i.e., reaction time), produces three metrics corresponding to attentional speed, motor speed, and overall reaction time. Since the previous studies on cybersickness (e.g., [
34,
56,
57]) used the traditional version, and assessed participants after immersion, the current study may further decipher the effects of cybersickness on psychomotor skills. The observed significant deceleration of overall reaction speed is in line with our [
45,
51] and other previous studies [
34,
56,
57]. Additionally, the current and previous findings thus offer robust evidence that cybersickness substantially compromised psychomotor skills. However, experience with playing videogames was also included in the best model, where a significant positive effect on reaction time (i.e., acceleration of reaction speed) was detected. This finding aligns with the previous literature pertaining to the effects of gaming experience on psychomotor speed [
122,
123,
124,
125]. In the context of cybersickness, this outcome indicates that the gaming experience has to be considered (e.g., as a covariate) to effectively examine the effects of cybersickness on psychomotor skills.
While the effects on overall reaction speed are well established by the current and previous findings, the cybersickness’s effects on the components of psychomotor skills (i.e., attentional and motor speed) still need to be investigated in depth. In this study, the attentional speed was found to be significantly decelerated by the oculomotor symptoms’ intensity. This outcome is in agreement with previous studies that revealed a significant negative effect on attentional processing speed [
56,
58]. However, these previous studies attributed the deceleration of attentional speed to overall cybersickness. In the current study, while both overall cybersickness intensity and the oculomotor symptoms’ intensity were deemed significant predictors of attentional speed, only the oculomotor symptomatology was incorporated in the respective best model. This outcome is in line with the established understanding that the oculomotor system is essential for facilitating visual attention functioning, especially for orienting attention [
126,
127]. Hence, our findings connote that a transient dysfunction of the oculomotor system (e.g., eye fatigue or strain) substantially compromises attentional speed.
However, the deceleration of the motor speed was found to be predominantly attributed to nausea symptomatology. This finding is aligned with the current understanding of the negative effects of nausea on motor coordination and skills due to a modulation of the activation of sensorimotor brain regions [
128,
129]. Nevertheless, gaming experience was also included in the best predictive model of motor speed, where a significant acceleration of motor speed was observed due to more gaming experience. This aligns with the relevant literature that postulates that gaming experience promotes an enhanced motor speed, especially for fine motor functions [
130,
131]. Thus, this finding suggests that gaming experience should be considered in the examination of cybersickness effects on motor speed. In summary, gaming experience appears central to enhancing psychomotor speed, while cybersickness substantially decelerates overall reaction speed. Regarding the components of psychomotor skills, the intensity of oculomotor and nausea symptoms significantly decelerates attentional and motor speed, respectively.
5.7. Cybersickness Symptoms and Their Intensity during and after Immersion
The intensities of overall cybersickness, nausea symptoms, and vestibular symptoms were substantially decreased after immersion (i.e., immediately after removing the headset). However, oculomotor symptoms did not decrease, which suggests that they may have a more lasting impact. This appears to be similar to simulator sickness, where oculomotor symptoms like eye strain persist long after the exposure to the simulator [
132]. In contrast, vestibular and nausea symptoms are the most frequent and predominant in cybersickness [
29,
71,
133]. Thus, these findings draw a stark contrast between the experience of cybersickness during immersion in a virtual environment and after immersion. This agrees with the suggestion that the human body and mind strive to readjust to the physical environment immediately after the removal of the VR headset [
59].
Based on our results, after the exposure to stimuli inducing cybersickness, and during this transitory period of readapting to the physical space and body, the cybersickness’s intensity and symptoms substantially fade away. This also agrees with the current predominant understanding that cybersickness symptoms and effects are transient [
44]. However, there is no consensus concerning how long cybersickness symptoms may persist, with some previous reviews suggesting that they may last for up to 12 h after exposure to VR [
31,
134]. However, our results suggest that the period of experiencing a substantial alleviation of cybersickness intensity commences during immersion (i.e., after exposure to stimuli inducing cybersickness, such as linear and angular accelerations) and that users attained a significant decrease after immersion, during their readaptation to the physical body and environment. Hence, this natural process counteracts or alleviates some of the dissonances experienced in the virtual environment during immersion. However, the cybersickness was measured after the ride (i.e., not during the exposure to the stimuli inducing vection and cybersickness), and not during the ride, which allows room for further research.
Nevertheless, our findings posit fundamental ramifications for the methodology used in VR research on cybersickness. To the best of our knowledge, except for the current and our previous studies [
45,
51], the studies on cybersickness (e.g., [
28,
39,
40,
41,
43,
68]) or cybersickness’s effects on human physiology or cognitive functioning or psychomotor skills (e.g., [
34,
50,
56,
57,
58,
72,
73,
133]) measure cybersickness’s intensity, symptomatology, and/or its effects, after immersion. Our findings indicate that this approach may only capture a part of the overall picture, or even worse, a substantially distorted picture.
If cybersickness symptoms subside or change in intensity immediately after VR exposure, then solely post-immersion evaluations could lead to unreliable conclusions. For instance, evaluations performed post immersion might underestimate the true intensity of the symptoms experienced during VR exposure, as well as the effects of cybersickness on cognition and motor skills. From a research design standpoint, these insights underscore the importance of adopting a more temporally accurate approach. Hence, in studies attempting to attain a holistic view of cybersickness, researchers should endeavour to capture data at multiple points—before, during, and after VR exposure. On the other hand, studies attempting to examine cybersickness aftereffects or the persistence of symptomatology may perform their examination after immersion. Lastly, it is crucial for studies striving to assess cybersickness’s intensity and symptomatology, as well as its effects on physiology (e.g., autonomic responses such as heart rate and temperature), cognition, and motor skills, to perform their examination during the VR immersion.
5.8. Limitations and Future Studies
This study also has some limitations that should be considered. While allowing for the conduction of the required statistical analyses, the sample size was relatively small. Also, the sample consisted predominantly of young adults aged 20–45 years old. A future study should incorporate a larger and/or more age-diverse sample (e.g., considering adolescents and/or older adults). Moreover, this study utilized the MSSQ and did not consider the VIMSSQ, which is specific to the vection elicited by screen-based mediums. Using both in a future study may assist in deciphering whether generic motion sickness susceptibility or a susceptibility that is specific to vection is an indicator for experiencing cybersickness’s symptomatology and intensity. Also, overall gaming experience was used for investigating gaming experience’s effects on cybersickness, cognitive functioning, and psychomotor skills. Since each genre of videogames offers diverse content that may stimulate distinct physiological and cognitive aspects, future studies should attempt to examine the effects of gaming experience by genre.
Moreover, this study explored the cybersickness intensity and symptoms in VR that were induced by vection. While vection is indeed one of the main reasons for cybersickness, several factors (e.g., low latency or latency fluctuations, non-ergonomic navigation, and low-quality graphics) may induce cybersickness. Future research should explore the intensity and symptomatology of cybersickness induced by each factor. The current study included several cognitive and psychomotor tasks. Given that a task’s characteristics may modulate cybersickness [
135], future studies should either consider a single task or examine each task’s effects on cybersickness. Finally, considering that significant differences were found during and post immersion, future attempts should consider multiple assessment points of cybersickness to effectively scrutinize the cybersickness’s symptoms and intensity before, during (i.e., with several assessments), and after immersion.