The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower
Abstract
1. Introduction
2. Materials and Methods
2.1. Cannabis sativa L. Inflorescence
2.2. Analytical Chemistry Testing
2.3. Volunteers
2.4. Sample Kit Preparation
2.5. Experiential Evaluation
2.6. Measures
2.7. Data Analyses
3. Results
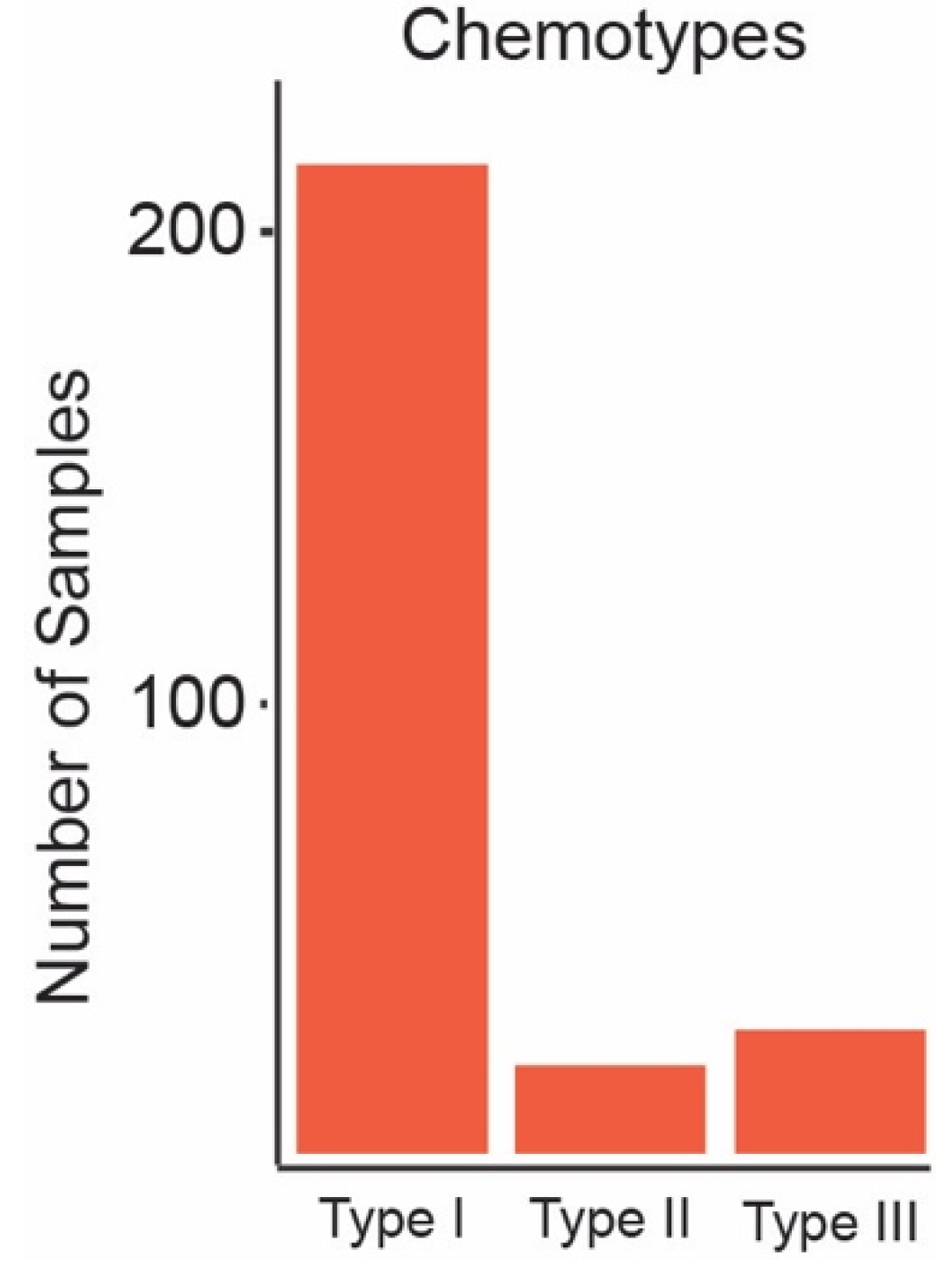
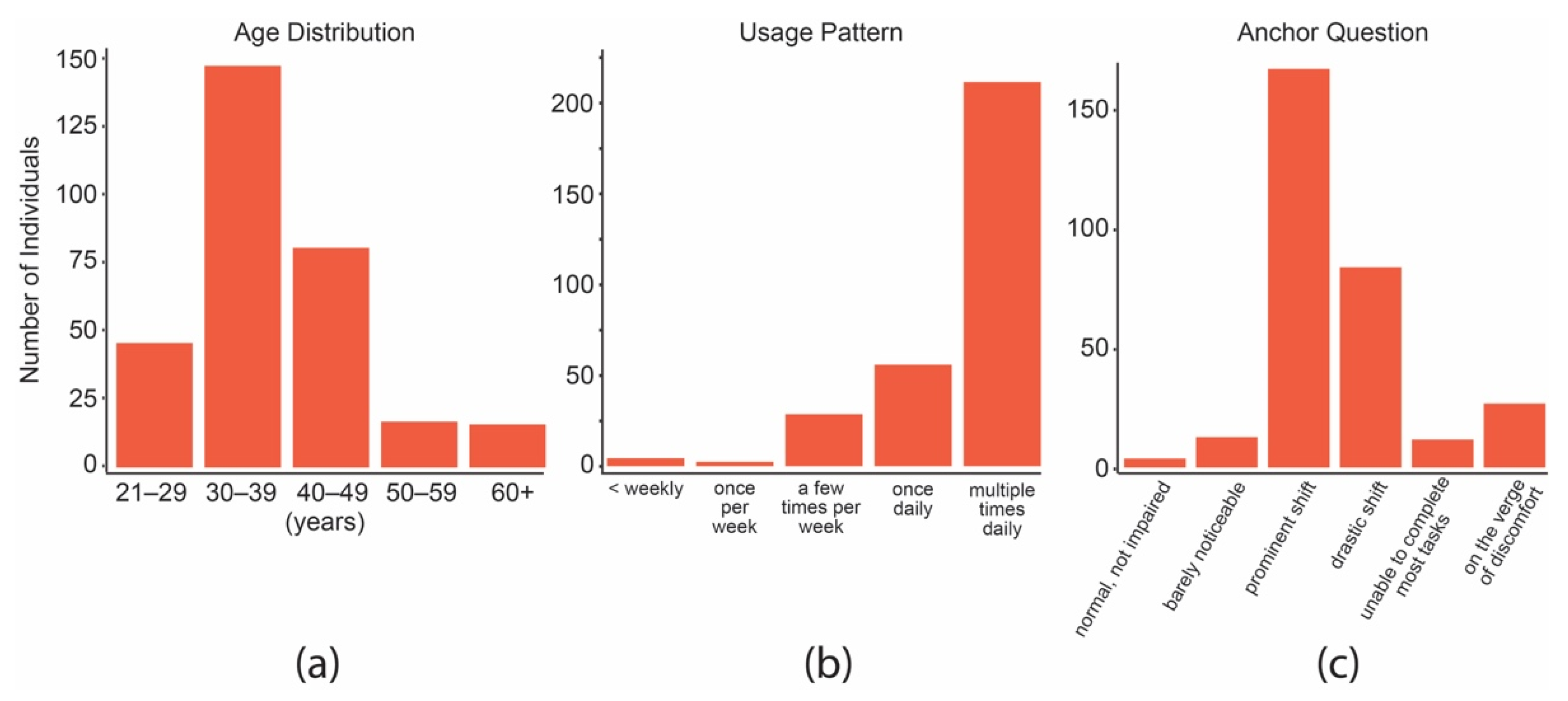
3.1. Volunteer, Consumption, and Inflorescence Characteristics
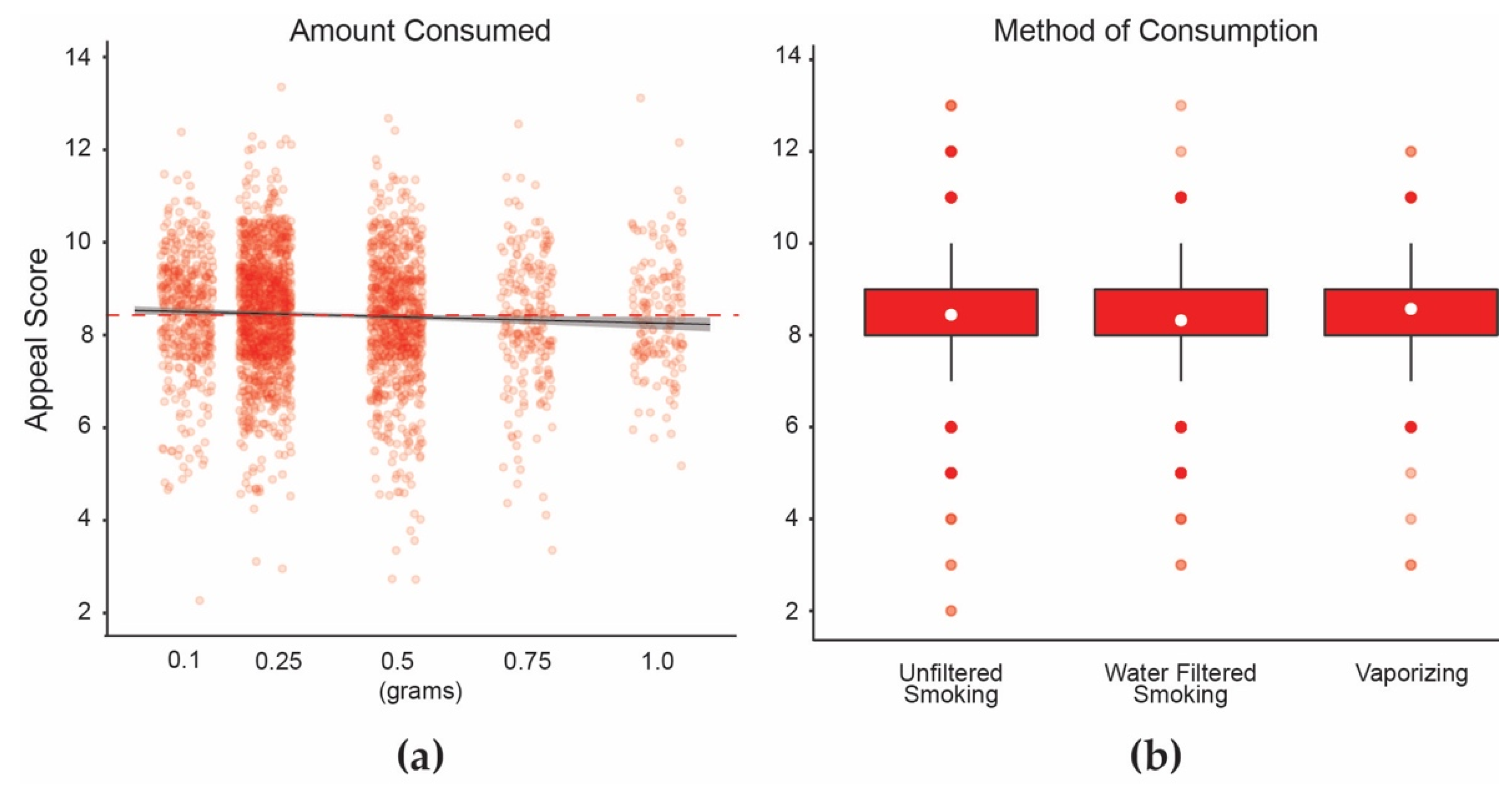
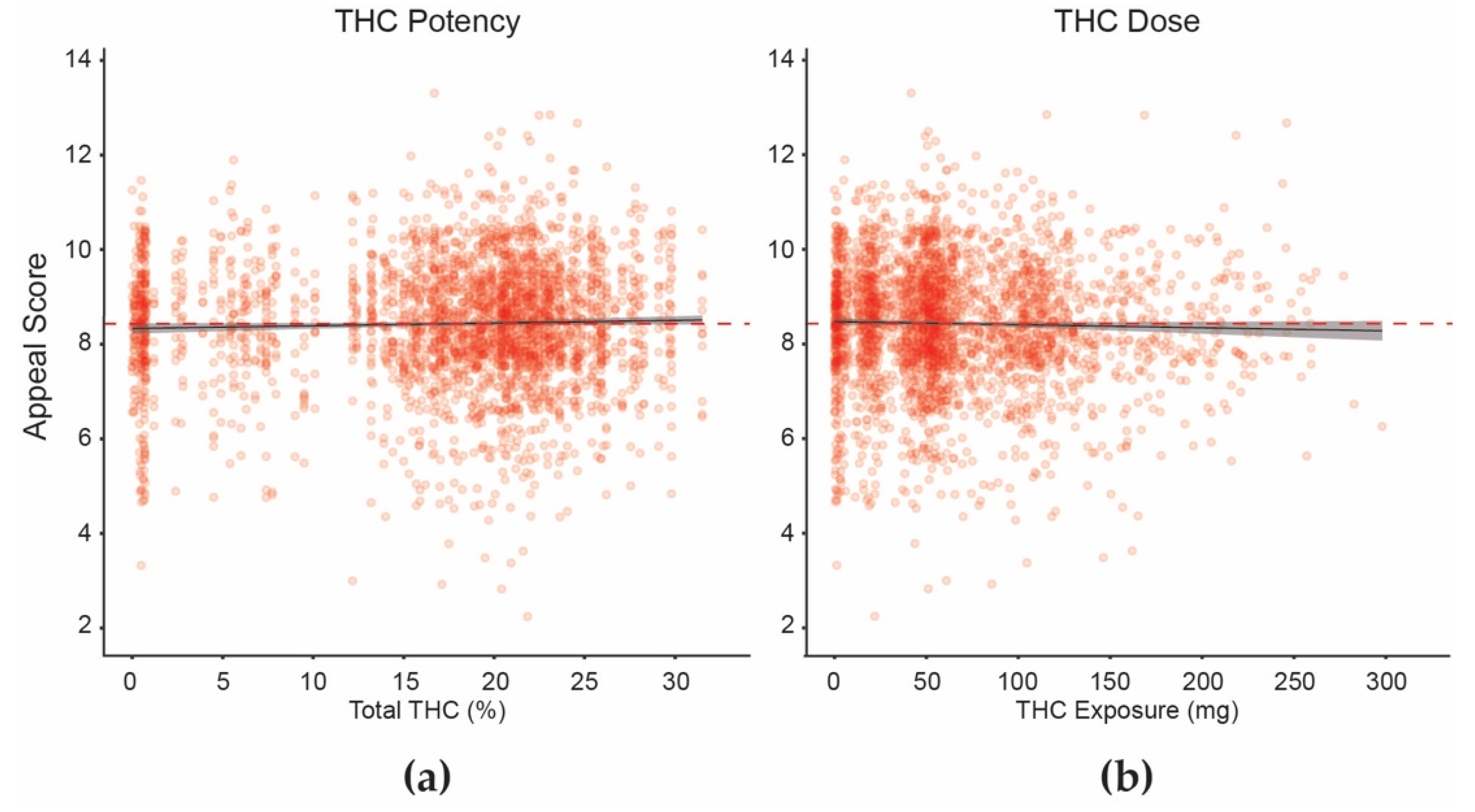
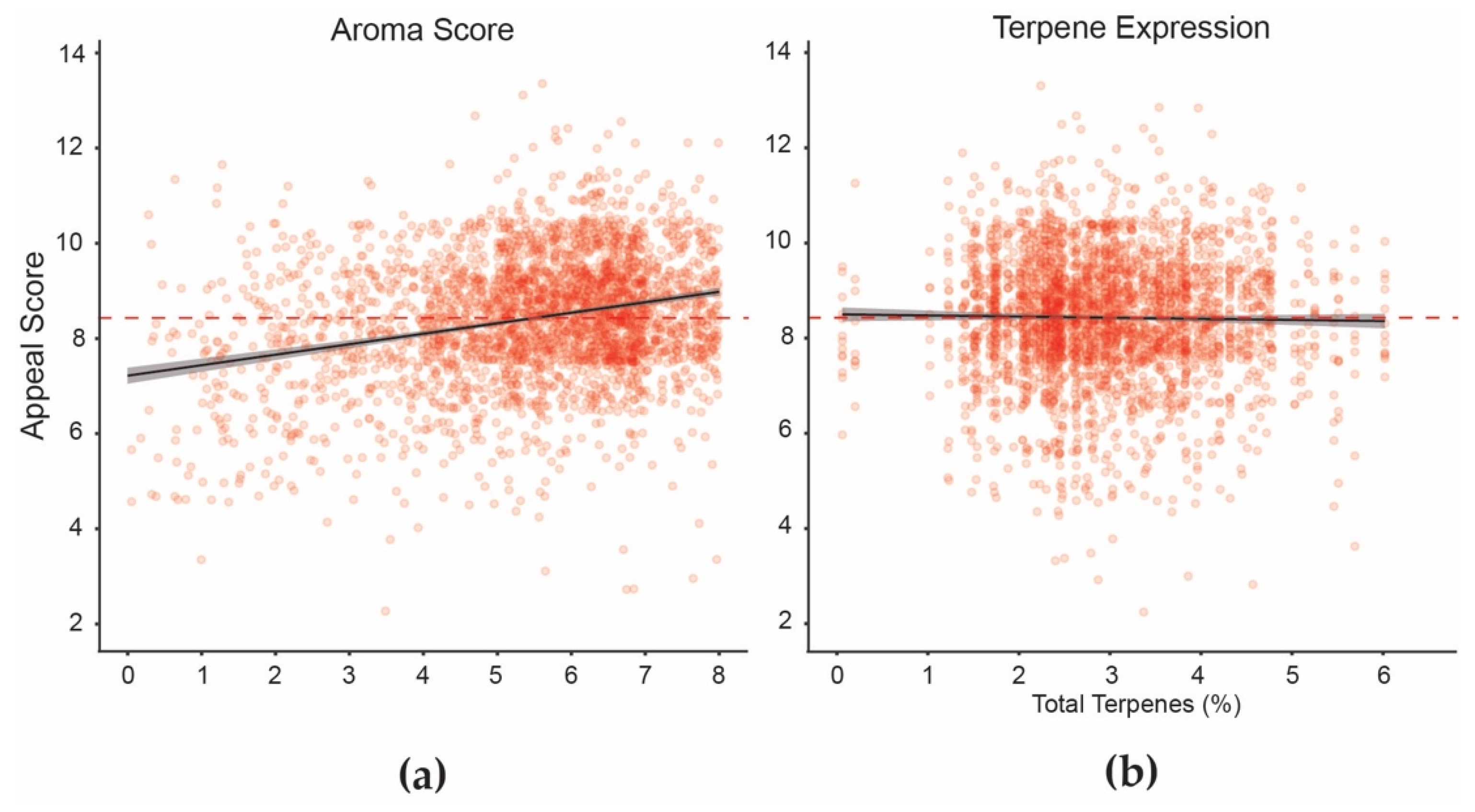
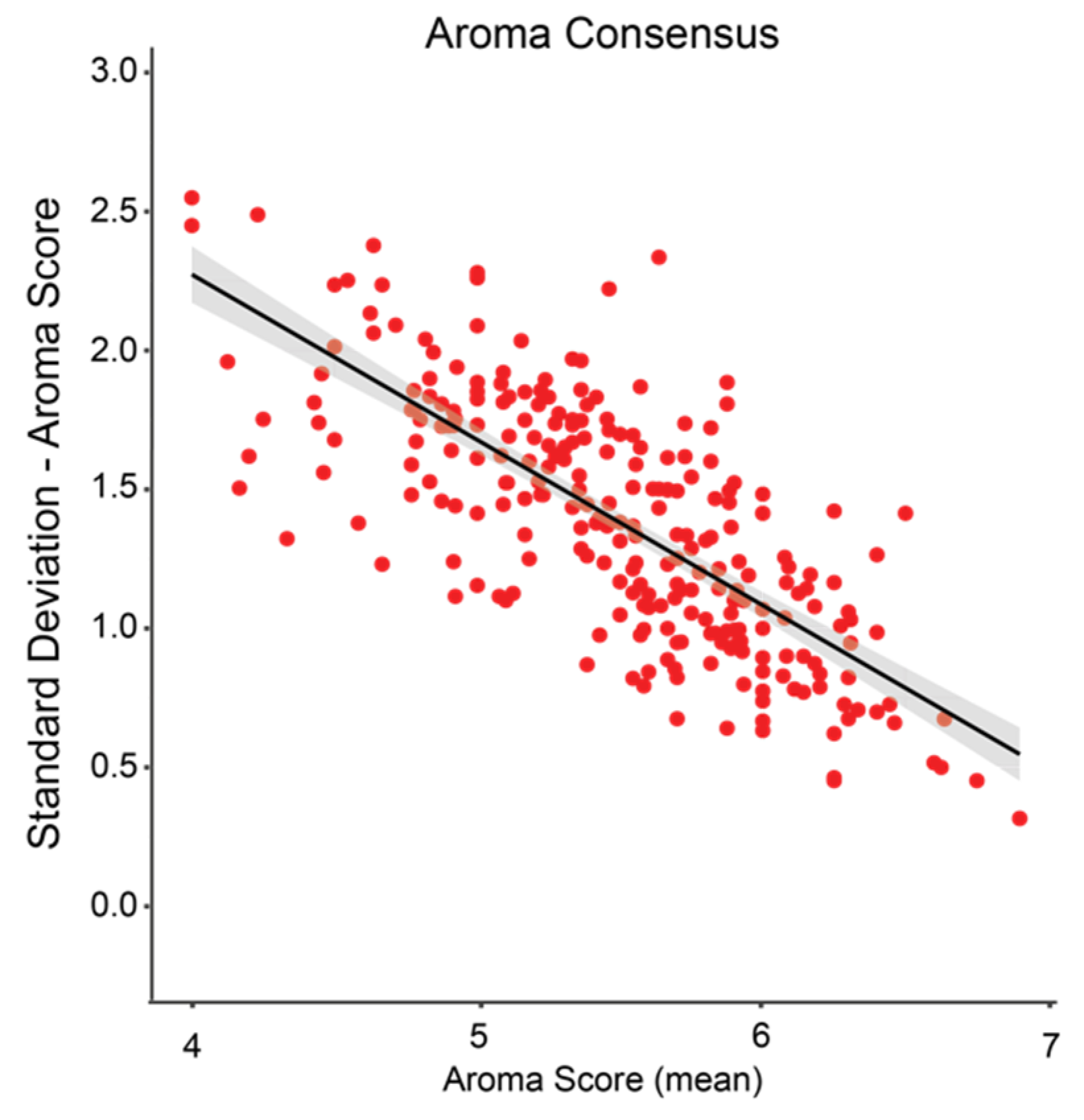
3.2. Data Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drug Enforcement Administration. DEA Continues to Prioritize Efforts to Expand Access to Marijuana for Research in the United States. Available online: https://www.dea.gov/stories/2021/2021-05/2021-05-14/dea-continues-prioritize-efforts-expand-access-marijuana-research (accessed on 25 July 2021).
- Schwabe, A.L.; Hansen, C.J.; Hyslop, R.M.; McGlaughlin, M.E. Comparative Genetic Structure of Cannabis sativa Including Federally Produced, Wild Collected, and Cultivated Samples. Front. Plant Sci. 2021, 12, 675770. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Bidwell, L.C.; Gaudino, R.; Torres, A.; Du, G.; Ruthenburg, T.C.; deCesare, K.; Land, D.P.; Hutchison, K.E.; Kane, N.C. Compromised External Validity: Federally Produced Cannabis Does Not Reflect Legal Markets. Sci. Rep. 2017, 7, 46528. [Google Scholar] [CrossRef] [PubMed]
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.M.; Largent-Milnes, T.M. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 98–126. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.; Arkell, T.R.; Irwin, C.; McGregor, I.S. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: A systematic and meta-analytic review. Neurosci. Biobehav. Rev. 2021, 126, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Jarbe, T.U.; LeMay, B.J.; Halikhedkar, A.; Wood, J.; Vadivel, S.K.; Zvonok, A.; Makriyannis, A. Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats. Psychopharmacology 2014, 231, 489–500. [Google Scholar] [CrossRef]
- Li, X.; Vigil, J.M.; Stith, S.S.; Brockelman, F.; Keeling, K.; Hall, B. The effectiveness of self-directed medical cannabis treatment for pain. Complement. Ther. Med. 2019, 46, 123–130. [Google Scholar] [CrossRef]
- Greis, A.; Larsen, E.; Liu, C.; Renslo, B.; Radakrishnan, A.; Wilson-Poe, A.R. Perceived Efficacy, Reduced Prescription Drug Use, and Minimal Side Effects of Cannabis in Patients with Chronic Orthopedic Pain. Cannabis Cannabinoid Res. 2021; Epub ahead of printing. [Google Scholar] [CrossRef]
- Arkell, T.R.; Lintzeris, N.; Kevin, R.C.; Ramaekers, J.G.; Vandrey, R.; Irwin, C.; Haber, P.S.; McGregor, I.S. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 2019, 236, 2713–2724. [Google Scholar] [CrossRef]
- DeVuono, M.V.; Parker, L.A. Cannabinoid Hyperemesis Syndrome: A Review of Potential Mechanisms. Cannabis Cannabinoid Res. 2020, 5, 132–144. [Google Scholar] [CrossRef]
- Rossi, G.; Beck, M. A Little Dab Will Do: A Case of Cannabis-Induced Psychosis. Cureus 2020, 12, e10311. [Google Scholar] [CrossRef]
- Arterberry, B.J.; Treloar Padovano, H.; Foster, K.T.; Zucker, R.A.; Hicks, B.M. Higher average potency across the United States is associated with progression to first cannabis use disorder symptom. Drug Alcohol Depend. 2019, 195, 186–192. [Google Scholar] [CrossRef]
- Russo, E.B. Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues. Front. Pharmacol. 2016, 7, 309. [Google Scholar] [CrossRef]
- Russo, E.B.; Spooner, C.; May, L.; Leslie, R.; Whiteley, V.L. Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation. Cannabis Cannabinoid Res. 2022, 7, 336–344. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef]
- Freeman, T.P.; Winstock, A.R. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol. Med. 2015, 45, 3181–3189. [Google Scholar] [CrossRef]
- Cuttler, C.; LaFrance, E.M.; Stueber, A. Acute effects of high-potency cannabis flower and cannabis concentrates on everyday life memory and decision making. Sci. Rep. 2021, 11, 13784. [Google Scholar] [CrossRef]
- Petrilli, K.; Ofori, S.; Hines, L.; Taylor, G.; Adams, S.; Freeman, T.P. Association of cannabis potency with mental ill health and addiction: A systematic review. Lancet Psychiatry 2022, 9, 736–750. [Google Scholar] [CrossRef]
- Block, W. Drug Prohibition: A Legal and Economic Analysis. J. Bus. Ethics 1993, 12, 689–700. [Google Scholar] [CrossRef]
- Cole, J.C.; Goudie, A.J.; Field, M.; Loverseed, A.C.; Charlton, S.; Sumnall, H.R. The effects of perceived quality on the behavioural economics of alcohol, amphetamine, cannabis, cocaine, and ecstasy purchases. Drug Alcohol Depend. 2008, 94, 183–190. [Google Scholar] [CrossRef]
- Vincent, P.C.; Collins, R.L.; Liu, L.; Yu, J.; De Leo, J.A.; Earleywine, M. The effects of perceived quality on behavioral economic demand for marijuana: A web-based experiment. Drug Alcohol Depend. 2017, 170, 174–180. [Google Scholar] [CrossRef]
- Donnan, J.; Shogan, O.; Bishop, L.; Swab, M.; Najafizada, M. Characteristics that influence purchase choice for cannabis products: A systematic review. J. Cannabis Res. 2022, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cao, Y.; Shang, C.; Pacula, R.L. The impacts of potency, warning messages, and price on preferences for Cannabis flower products. Int. J. Drug Policy 2019, 74, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Guo, H.; Cao, Y.; An, R.; Shi, Y. Perceived Importance of Factors in Cannabis Purchase Decisions: A Best-worst Scaling Experiment. Int. J. Drug Policy 2021, 91, 102793. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.; Caulkins, J.P.; Kilmer, B.; Davenport, S.; Midgette, G. Variation in cannabis potency and prices in a newly legal market: Evidence from 30 million cannabis sales in Washington state. Addiction 2017, 112, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- Sainz-Cort, A.; Jimenez-Garrido, D.; Munoz-Marron, E.; Viejo-Sobera, R.; Heeroma, J.; Bouso, J.C. Opposite Roles for Cannabidiol and delta-9-Tetrahydrocannabinol in Psychotomimetic Effects of Cannabis Extracts: A Naturalistic Controlled Study. J. Clin. Psychopharmacol. 2021, 41, 561–570. [Google Scholar] [CrossRef]
- Gibson, L.P.; Karoly, H.C.; Ellingson, J.M.; Klawitter, J.; Sempio, C.; Squeri, J.E.; Bryan, A.D.; Bidwell, L.C.; Hutchison, K.E. Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addict. Biol. 2022, 27, e13092. [Google Scholar] [CrossRef]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2013, 27, 19–27. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. [Google Scholar] [CrossRef]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids From Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ9-THC at Human CB1 and CB2 Receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef]
- Bueno, J.; Leuer, E.; Kearney, M., Jr.; Green, E.H.; Greenbaum, E.A. The preservation and augmentation of volatile terpenes in cannabis inflorescence. J. Cannabis Res. 2020, 2, 27. [Google Scholar] [CrossRef]
- Oswald, I.W.H.; Ojeda, M.A.; Pobanz, R.J.; Koby, K.A.; Buchanan, A.J.; Del Rosso, J.; Guzman, M.A.; Martin, T.J. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6, 31667–31676. [Google Scholar] [CrossRef]
- Kwasnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczynski, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Rozanski, H. Volatile Composition and Sensory Properties as Quality Attributes of Fresh and Dried Hemp Flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Oklahoma Medical Marijuana Authority. Testing Information Chart. Available online: https://oklahoma.gov/content/dam/ok/en/omma/docs/Testing%20Processes.pdf (accessed on 25 July 2022).
- Jikomes, N.; Zoorob, M. The Cannabinoid Content of Legal Cannabis in Washington State Varies Systematically Across Testing Facilities and Popular Consumer Products. Sci. Rep. 2018, 8, 4519. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef] [PubMed]
- United States Census. QuickFacts Portland City, Oregon. Available online: https://www.census.gov/quickfacts/portlandcityoregon (accessed on 25 July 2022).
- Cooper, Z.D.; Comer, S.D.; Haney, M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 2013, 38, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Cortes-Briones, J.A.; Ranganathan, M.; Thurnauer, H.; Creatura, G.; Surti, T.; Planeta, B.; Neumeister, A.; Pittman, B.; Normandin, M.; et al. Rapid Changes in CB1 Receptor Availability in Cannabis Dependent Males after Abstinence from Cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Messner, M.; Pollatos, O. Improvement of Interoceptive Processes after an 8-Week Body Scan Intervention. Front. Hum. Neurosci. 2017, 11, 452. [Google Scholar] [CrossRef]
- Cuttler, C.; Spradlin, A. Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU). PLoS ONE 2017, 12, e0178194. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Højsgaard, S.; Halekoh, U.; Yan, J. The R Package geepack for Generalized Estimating Equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar]
- Small, E.; Beckstead, H.D. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 1973, 36, 144–165. [Google Scholar]
- de Meijer, E.P.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.; Ranalli, P.; Mandolino, G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Bidwell, L.C.; Ellingson, J.M.; Karoly, H.C.; YorkWilliams, S.L.; Hitchcock, L.N.; Tracy, B.L.; Klawitter, J.; Sempio, C.; Bryan, A.D.; Hutchison, K.E. Association of Naturalistic Administration of Cannabis Flower and Concentrates With Intoxication and Impairment. JAMA Psychiatry 2020, 77, 787–796. [Google Scholar] [CrossRef]
- Ramesh, D.; Haney, M.; Cooper, Z.D. Marijuana’s dose-dependent effects in daily marijuana smokers. Exp. Clin. Psychopharmacol. 2013, 21, 287–293. [Google Scholar] [CrossRef]
- Cash, M.C.; Cunnane, K.; Fan, C.; Romero-Sandoval, E.A. Mapping cannabis potency in medical and recreational programs in the United States. PLoS ONE 2020, 15, e0230167. [Google Scholar] [CrossRef]
- FiveThirtyEight. America’s Pot Labs Have a THC Problem. Available online: https://fivethirtyeight.com/features/americas-pot-labs-have-a-thc-problem/ (accessed on 25 July 2022).
- Statista. Marijuana Retail Price Per Gram in the United States in 2020, by THC Levels. Available online: https://www.statista.com/statistics/1251356/cannabis-retail-price-by-potency-us/ (accessed on 25 July 2022).
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef]
- Zeng, L.; Lytvyn, L.; Wang, X.; Kithulegoda, N.; Agterberg, S.; Shergill, Y.; Esfahani, M.A.; Heen, A.F.; Agoritsas, T.; Guyatt, G.H.; et al. Values and preferences towards medical cannabis among people living with chronic pain: A mixed-methods systematic review. BMJ Open 2021, 11, e050831. [Google Scholar] [CrossRef]
- Miller, R.E.; Brown, T.L.; Lee, S.; Tibrewal, I.; Gaffney, G.G.; Milavetz, G.; Hartman, R.L.; Gorelick, D.A.; Compton, R.; Huestis, M.A. Impact of cannabis and low alcohol concentration on divided attention tasks during driving. Traffic Inj. Prev. 2020, 21, S123–S129. [Google Scholar] [CrossRef]
- Fogel, J.S.; Kelly, T.H.; Westgate, P.M.; Lile, J.A. Sex differences in the subjective effects of oral Δ9-THC in cannabis users. Pharmacol. Biochem. Behav. 2017, 152, 44–51. [Google Scholar] [CrossRef]
- Sholler, D.J.; Strickland, J.C.; Spindle, T.R.; Weerts, E.M.; Vandrey, R. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict. Biol. 2021, 26, e12968. [Google Scholar] [CrossRef]
- Gibson, L.P.; Gust, C.J.; Ellingson, J.M.; YorkWilliams, S.L.; Sempio, C.; Klawitter, J.; Bryan, A.D.; Hutchison, K.E.; Cinnamon Bidwell, L. Investigating sex differences in acute intoxication and verbal memory errors after ad libitum cannabis concentrate use. Drug Alcohol Depend. 2021, 223, 108718. [Google Scholar] [CrossRef]
- Sharpe, L.; Sinclair, J.; Kramer, A.; de Manincor, M.; Sarris, J. Cannabis, a cause for anxiety? A critical appraisal of the anxiogenic and anxiolytic properties. J. Transl. Med. 2020, 18, 374. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Rubio-Casillas, A. Biphasic effects of THC in memory and cognition. Eur. J. Clin. Investig. 2018, 48, e12920. [Google Scholar] [CrossRef]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimaraes, F.S.; Crippa, J.A. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz. J. Psychiatry 2019, 41, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimaraes, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021, 11, 8232. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; LaFrance, E.M.; Craft, R.M. A Large-Scale Naturalistic Examination of the Acute Effects of Cannabis on Pain. Cannabis Cannabinoid Res. 2022, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Russell, A.; Brown, T.; Friedman, K.; Wrobel, J.; Schwarz, J.; Dooley, G.; Ryall, K.A.; Steinhart, B.; Amioka, E.; Milavetz, G.; et al. Simulated driving performance among daily and occasional cannabis users. Accid. Anal. Prev. 2021, 160, 106326. [Google Scholar] [CrossRef]
- Sexton, M.; Shelton, K.; Haley, P.; West, M. Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Med. 2018, 84, 234–241. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Geerts, P.; Membrebe, B.N.; Keulemans, J.; NicolaÏ, B.M.; Hertog, M.L.A.T.M. Journeys through aroma space: A novel approach towards the selection of aroma-enriched strawberry cultivars in breeding programmes. Plant Breed. 2013, 132, 217–223. [Google Scholar] [CrossRef]
- Lal, R.K.; Chanotiya, C.S.; Singh, V.R.; Gupta, P.; Mishra, A.; Srivastava, S.; Dwivedi, A. Patchouli (Pogostemon cablin (Blanco) Benth) essential oil yield stability with the unique aroma of ar-curcumene and genotype selection over the years. Acta Ecol. Sin. 2021; Epub ahead of print. [Google Scholar] [CrossRef]
- Hagenguth, J.; Kanski, L.; Kahle, H.; Naumann, M.; Pawelzik, E.; Becker, H.C.; Horneburg, B. Breeders’ Sensory Test: A new tool for early selection in breeding for tomato (Solanum lycopersicum) flavour. Plant Breed. 2022, 141, 96–107. [Google Scholar] [CrossRef]
- Su, X.; Yin, Y. Aroma characterization of regional Cascade and Chinook hops (Humulus lupulus L.). Food Chem. 2021, 364, 130410. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Dry-Hopping to Modify the Aroma of Alcohol-Free Beer on a Molecular Level-Loss and Transfer of Odor-Active Compounds. J. Agric. Food Chem. 2020, 68, 8602–8612. [Google Scholar] [CrossRef]
- Kishimoto, T.; Wanikawa, A.; Kono, K.; Shibata, K. Comparison of the odor-active compounds in unhopped beer and beers hopped with different hop varieties. J. Agric. Food Chem. 2006, 54, 8855–8861. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Prediction of coffee aroma from single roasted coffee beans by hyperspectral imaging. Food Chem. 2022, 371, 131159. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, X.; Shao, Y.; Cheng, Y.; Yang, Y.; Zhang, M.; Hua, J.; Li, J.; Deng, Y.; Wang, J.; et al. Quality Evaluation of Green and Dark Tea Grade Using Electronic Nose and Multivariate Statistical Analysis. J. Food Sci. 2019, 84, 3411–3417. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A. Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis. PLoS ONE 2015, 10, e0144160. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Nogueira, I.; Faria, R.P.V. Perfume and Flavor Engineering: A Chemical Engineering Perspective. Molecules 2021, 26, 3095. [Google Scholar] [CrossRef]
- Gilbert, A.N.; DiVerdi, J.A. Consumer perceptions of strain differences in Cannabis aroma. PLoS ONE 2018, 13, e0192247. [Google Scholar] [CrossRef]
- Bult, J.H.; Schifferstein, H.N.; Roozen, J.P.; Voragen, A.G.; Kroeze, J.H. The influence of olfactory concept on the probability of detecting sub- and peri-threshold components in a mixture of odorants. Chem. Senses 2001, 26, 459–469. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC-MS, GC-O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Voleti, V.; Zou, D.J.; Hillman, E.M.C.; Firestein, S. Widespread receptor-driven modulation in peripheral olfactory coding. Science 2020, 368, eaaz5390. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.I.; Curtis, J.A.; Vearrier, D. The perception of odor is not a surrogate marker for chemical exposure: A review of factors influencing human odor perception. Clin. Toxicol. 2013, 51, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Hoberg, E. Rapid Methods in Plant Aroma Analysis—Mass Spectrometric Sensor Measurements on Strawberries. Acta Hortic. 2000, 538, 443–446. [Google Scholar] [CrossRef]
- Hawko, C.; Verriele, M.; Hucher, N.; Crunaire, S.; Leger, C.; Locoge, N.; Savary, G. A review of environmental odor quantification and qualification methods: The question of objectivity in sensory analysis. Sci. Total Environ. 2021, 795, 148862. [Google Scholar] [CrossRef]
- Clepce, M.; Neumann, K.; Martus, P.; Nitsch, M.; Wielopolski, J.; Koch, A.; Kornhuber, J.; Reich, K.; Thuerauf, N. The psychophysical assessment of odor valence: Does an anchor stimulus influence the hedonic evaluation of odors? Chem. Senses 2014, 39, 17–25. [Google Scholar] [CrossRef][Green Version]
- Tjeerdema, R.S. The pyrolysis of cannabinoids. Rev. Environ. Contam. Toxicol. 1987, 99, 61–81. [Google Scholar] [CrossRef]
- Czogala, J.; Koszowski, B.; Goniewicz, M.L.; Zielinska-Danch, W.; Sobczak, A. Influence of smoking topography on respirabile suspended particulates concentrations in main and side stream. Przeglad Lek. 2010, 67, 940–943. [Google Scholar]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plumb, J.; Demirel, S.; Sackett, J.L.; Russo, E.B.; Wilson-Poe, A.R. The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower. Psychoactives 2022, 1, 70-86. https://doi.org/10.3390/psychoactives1020008
Plumb J, Demirel S, Sackett JL, Russo EB, Wilson-Poe AR. The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower. Psychoactives. 2022; 1(2):70-86. https://doi.org/10.3390/psychoactives1020008
Chicago/Turabian StylePlumb, Jeremy, Shaban Demirel, Jeremy L. Sackett, Ethan B. Russo, and Adrianne R. Wilson-Poe. 2022. "The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower" Psychoactives 1, no. 2: 70-86. https://doi.org/10.3390/psychoactives1020008
APA StylePlumb, J., Demirel, S., Sackett, J. L., Russo, E. B., & Wilson-Poe, A. R. (2022). The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower. Psychoactives, 1(2), 70-86. https://doi.org/10.3390/psychoactives1020008






