Difluprednate and Loratadine in the Treatment of Pachychoroid Disease Spectrum
Abstract
1. Introduction
2. Materials and Methods
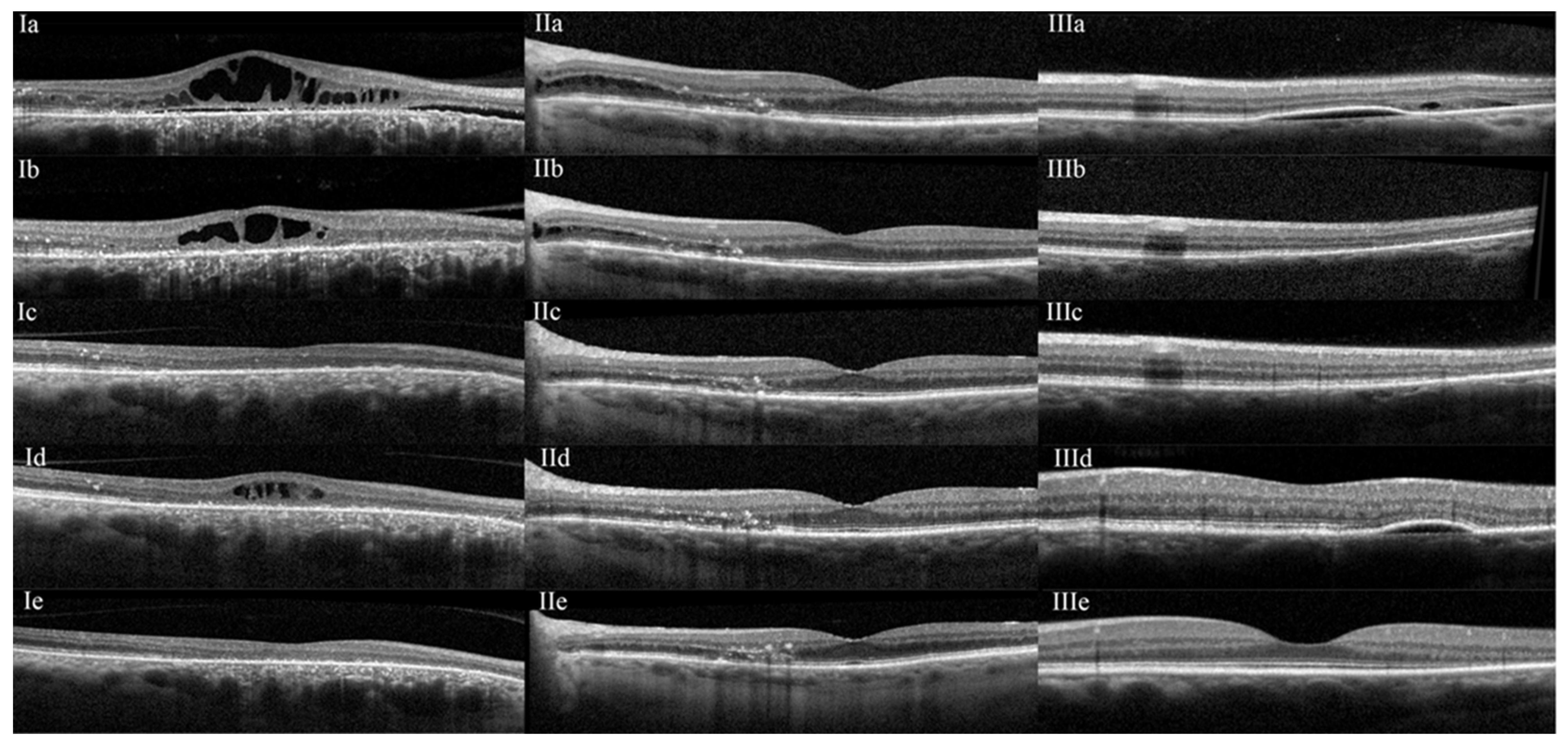
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haimovici, R.; Koh, S.; Gagnon, D.R.; Lehrfeld, T.; Wellik, S. Risk factors for central serous chorioretinopathy: A case-control study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef]
- van den Tillaart, F.M.; Temmerman, I.M.; Hartgers, F.; Yzer, S. Prednisolone Eye Drops as a Potential Treatment in Nonneovascular Pachychoroid-Related Diseases. Retina 2024, 44, 1371–1378. [Google Scholar] [CrossRef]
- Carvalho-Recchia, C.A.; Yannuzzi, L.A.; Negrão, S.; Spaide, R.F.; Freund, K.B.; Rodriguez-Coleman, H.; Lenharo, M.; Iida, T. Corticosteroids and central serous chorioretinopathy. Ophthalmology 2002, 109, 1834–1837. [Google Scholar] [CrossRef]
- Kim, L.A.; Maguire, M.G.; Weng, C.Y.; Smith, J.R.; Jain, N.; Flaxel, C.J.; Patel, S.; Kim, S.J.; Yeh, S. Therapies for Central Serous Chorioretinopathy: A Report by the American Academy of Ophthalmology. Ophthalmology 2025, 132, 343–353. [Google Scholar] [CrossRef]
- Vilstrup, G. Studies on the vascular capacity and tissue fluid content of the choroid and their variations under treatment with histamine. Acta Ophthalmol. 1952, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Larsen, G. An autoradiographic study on the uptake of S35-labeled sodium sulfate; in the eyes of normal, scorbutic, and hormonal treated guinea pigs. Am. J. Ophthalmol. 1959, 47, 519–529; discussion 529–530. [Google Scholar] [CrossRef]
- Ashton, N.; Cunha-Vaz, J.G. Effect of Histamine on the Permeability of the Ocular Vessels. Arch. Ophthalmol. 1965, 73, 211–223. [Google Scholar] [CrossRef]
- Smith, R.S.; Trokel, S. Effects of mast-cell degranulation on the choroid. Arch. Ophthalmol. 1966, 75, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, W.A. Characterization of the choroidal mast cell. Trans. Am. Ophthalmol. Soc. 1987, 85, 557–599. [Google Scholar] [PubMed]
- Schlaegel, T.F., Jr. Histamine and uveal infiltration. Am. J. Ophthalmol. 1949, 32, 1331–1336. [Google Scholar] [CrossRef]
- Bousquet, E.; Zhao, M.; Thillaye-Goldenberg, B.; Lorena, V.; Castaneda, B.; Naud, M.C.; Bergin, C.; Besson-Lescure, B.; Behar-Cohen, F.; de Kozak, Y. Choroidal mast cells in retinal pathology: A potential target for intervention. Am. J. Pathol. 2015, 185, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Akin, C. Mast cell activation syndromes. J. Allergy Clin. Immunol. 2017, 140, 349–355. [Google Scholar] [CrossRef]
- Sabato, V.; Beyens, M.; Toscano, A.; Van Gasse, A.; Ebo, D.G. Mast Cell-Targeting Therapies in Mast Cell Activation Syndromes. Curr. Allergy Asthma Rep. 2024, 24, 63–71. [Google Scholar] [CrossRef]
- Chang, Y.S.; Weng, S.F.; Chang, C.; Wang, J.J.; Wang, J.Y.; Jan, R.L. Associations Between Topical Ophthalmic Corticosteroids and Central Serous Chorioretinopathy: A Taiwanese Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4083–4089. [Google Scholar] [CrossRef]
- Chang, Y.S.; Weng, S.F.; Wang, J.J.; Jan, R.L. Temporal Association between Topical Ophthalmic Corticosteroid and the Risk of Central Serous Chorioretinopathy. Int. J. Environ. Res. Public Health 2020, 17, 9455. [Google Scholar] [CrossRef]
- Wong, A.; Zhu, D.; Li, A.S.; Lee, J.G.; Ferrone, P.J. Topical Dexamethasone as an Adjuvant to Mineralocorticoid Receptor Antagonists for the Treatment of Recalcitrant Central Serous Chorioretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 659–665. [Google Scholar] [CrossRef]
- Bae, S.H.; Heo, J.; Kim, C.; Kim, T.W.; Shin, J.Y.; Lee, J.Y.; Song, S.J.; Park, T.K.; Moon, S.W.; Chung, H. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: One-year results of a randomized trial. Ophthalmology 2014, 121, 558–565. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.M.; Keunen, J.E.E.; Peters, P.J.H.; Dijkman, G.; Souied, E.H.; MacLaren, R.E. Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Nicolo, M.; Eandi, C.M.; Alovisi, C.; Grignolo, F.M.; Traverso, C.E.; Musetti, D.; Cardillo Piccolino, F. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2014, 157, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Adriono, G.A.; Triyoga, I.F.; Kadharusman, M.M.; Victor, A.A.; Djatikusumo, A.; Yudantha, A.R.; Hutapea, M.M. Efficacy and Safety of Ophthalmic Steroids in the Management of Polypoidal Choroidal Vasculopathy: A Systematic Review. Clin. Ophthalmol. 2025, 19, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Kamppeter, B.A. Intravitreal triamcinolone acetonide and central serous chorioretinopathy. Br. J. Ophthalmol. 2005, 89, 386–387. [Google Scholar] [CrossRef]
- Goldsmith, P.; McGarity, B.; Walls, A.F.; Church, M.K.; Millward-Sadler, G.H.; Robertson, D.A. Corticosteroid treatment reduces mast cell numbers in inflammatory bowel disease. Dig. Dis. Sci. 1990, 35, 1409–1413. [Google Scholar] [CrossRef]
- Belvisi, M.G. Regulation of inflammatory cell function by corticosteroids. Proc. Am. Thorac. Soc. 2004, 1, 207–214. [Google Scholar] [CrossRef]
- Zarnegar, A.; Ong, J.; Matsyaraja, T.; Arora, S.; Chhablani, J. Pathomechanisms in central serous chorioretinopathy: A recent update. Int. J. Retin. Vitr. 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Miyake, M.; Momozawa, Y.; Arakawa, S.; Maruyama-Inoue, M.; Endo, M.; Iwasaki, Y.; Ishigaki, K.; Matoba, N.; Okada, Y. Genome-Wide Association Study of Age-Related Macular Degeneration Reveals 2 New Loci Implying Shared Genetic Components with Central Serous Chorioretinopathy. Ophthalmology 2023, 130, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Kondo, N.; Yanagisawa, S.; Bessho, H.; Honda, S.; Negi, A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology 2014, 121, 1067–1072. [Google Scholar] [CrossRef]
- Hosoda, Y.; Yoshikawa, M.; Miyake, M.; Tabara, Y.; Ahn, J.; Woo, S.J.; Honda, S.; Sakurada, Y.; Shiragami, C.; Nakanishi, H. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc. Natl. Acad. Sci. USA 2018, 115, 6261–6266. [Google Scholar] [CrossRef]
- de Jong, E.K.; Breukink, M.B.; Schellevis, R.L.; Bakker, B.; Mohr, J.K.; Fauser, S.; Keunen, J.E.; Hoyng, C.B.; den Hollander, A.I.; Boon, C.J. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology 2015, 122, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ramo, J.T.; Abner, E.; van Dijk, E.H.C.; Wang, X.; Brinks, J.; Nikopensius, T.; Nõukas, M.; Marjonen, H.; Silander, K.; Jukarainen, S. Overlap of Genetic Loci for Central Serous Chorioretinopathy With Age-Related Macular Degeneration. JAMA Ophthalmol. 2023, 141, 449–457. [Google Scholar] [CrossRef]
- Zon, L.I.; Gurish, M.F.; Stevens, R.L.; Mather, C.; Reynolds, D.S.; Austen, K.F.; Orkin, S.H. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J. Biol. Chem. 1991, 266, 22948–22953. [Google Scholar] [CrossRef]
- Plotkin, J.D.; Elias, M.G.; Fereydouni, M.; Daniels-Wells, T.R.; Dellinger, A.L.; Penichet, M.L.; Kepley, C.L. Human Mast Cells From Adipose Tissue Target and Induce Apoptosis of Breast Cancer Cells. Front. Immunol. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Casado-Bedmar, M.; Heil, S.D.S.; Myrelid, P.; Söderholm, J.D.; Keita, Å.V. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterol. Motil. 2019, 31, e13503. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, J.; Zhu, Y.; Zhang, Y.; Chen, L.; Hu, C. Mast cell promotes obesity by activating microglia in hypothalamus. Front. Endocrinol. 2025, 16, 1544213. [Google Scholar] [CrossRef]
- Bhutto, I.A.; McLeod, D.S.; Jing, T.; Sunness, J.S.; Seddon, J.M.; Lutty, G.A. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 720–726. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 27 Eyes, 19 Patients) | ||
|---|---|---|
| Mean age ± STD | 64.3 ± 13.1 | |
| Gender | M (%) | 24 (88.9) |
| F (%) | 3 (11.1) | |
| Mean BMI ± STD | 26.7 ± 4.7 | |
| Exogenous steroid (%) | 8 (29.6) | |
| OSA (%) | 5 (18.5) | |
| Stress (%) | 4 (21.1) | |
| Allergies (%) | 9 (33.3) | |
| Prior unsuccessful anti-VEGF (%) | 6 (22.2) | |
| Prior unsuccessful PDT (%) | 9 (33.3) | |
| Diabetes (%) | 4 (14.8) | |
| Mean chronicity ± STD (Months) | 36.5 ± 44.5 | |
| Mean follow-up ± STD (Months) | 20.2 ± 10.8 | |
| Mean baseline VA ± STD (logMAR) | 0.3 ± 0.3 | |
| Mean final VA ± STD (logMAR) | 0.3 ± 0.4 | |
| Case Eye | Prior Treatments | Location of Fluid | Follow-Up (Months) | Time to Improve (Months) | Time to Resolve (Months) | Recurrence (Reason) |
|---|---|---|---|---|---|---|
| 1 OS | PDT | Temporal parafoveal SRF | 47.4 | 1.2 | 1.2 | No |
| 2 OS | Anti-VEGF | Subfoveal SRF | 13.5 | 1.2 | 2.3 | No |
| 3 OS | PDT, Anti-VEGF | Subfoveal SRF, CME | 20.5 | 0.6 | 5.4 | Yes (Non-compliance) |
| 4 OD | None | Subfoveal SRF | 26.8 | 1.2 | - | No |
| 5 OD | Anti-VEGF | Temporal subfoveal SRF | 23.3 | 0.9 | 0.9 | Yes (LTFU) |
| 5 OS | Anti-VEGF | Peripapillary SRF | 23.3 | 0.9 | 0.9 | Yes (LTFU) |
| 6 OD | None | Subfoveal SRF, CME | 33.9 | 0.5 | 3 | No |
| 7 OD | None | Central and peripapillary SRF, CME | 20.5 | 1.2 | 3.5 | Yes (Tapering) |
| 7 OS | None | Subfoveal SRF | 18.2 | 1.2 | 1.2 | Yes (Tapering) |
| 8 OS | None | Diffuse SRF (emanating from nerve) | 8.4 | 1.4 | 4 | No |
| 9 OD | PDT | Diffuse SRF | 19.6 | 1.2 | - | No |
| 9 OS | PDT | Parafoveal SRF | 18.4 | 6.5 | - | No |
| 10 OD | PDT, AntiVEGF | Central SRF, peripapillary CME | 34.6 | 1 | 3.8 | No |
| 10 OS | PDT, AntiVEGF | Peripapillary CME | 34.6 | 1 | 3.8 | Yes (Tapering) |
| 11 OD | None | Central SRF + CME | 29.8 | 13.3 | 3 | Yes (Tapering) |
| 12 OD | None | Central SRF, CME | 7 | 1.4 | 1.4 | No |
| 12 OS | None | Mild central SRF | 7 | 1.4 | 3.7 | No |
| 13 OS | PDT | Subfoveal SRF | 3 | 0.7 | - | No |
| 14 OD | None | Subfoveal SRF | 4.2 | 0.7 | 4.2 | No |
| 15 OS | PDT | Central SRF | 16.6 | 0.6 | 0.6 | No |
| 16 OS | None | Central SRF | 51 | 0.7 | 1.9 | No |
| 17 OD | None | Peripapillary CME | 16.3 | 5.8 | 5.8 | Yes (Holiday) |
| 17 OS | None | Peripapillary CME | 16.3 | 2.3 | 2.3 | No |
| 18 OD | None | Peripapillary CME | 21.4 | 0.9 | 3.6 | Yes (Holiday) |
| 18 OS | None | Peripapillary CME | 21.4 | 0.9 | 3.6 | Yes (Holiday) |
| 19 OD | None | Peripapillary CME | 31.3 | 0.6 | 6.5 | Yes (Tapering) |
| 19 OS | None | Peripapillary CME | 31.3 | 1.1 | 1.1 | Yes (Tapering) |
| Case Eye | Existing Glaucoma | Base IOP (mmHg) | Maximum IOP (mmHg) | Final IOP (mmHg) | Number of Drops | Drops | Surgical Management |
|---|---|---|---|---|---|---|---|
| 1 OS | No | 14 | 19 | 11 | 0 | No | |
| 2 OS | No | 14 | 19 | 19 | 0 | No | |
| 3 OS | OHTN | 21 | 44 | 14 | 2 | Dorzolamide Timolol | No |
| 4 OD | OHTN | 14 | 38 | 16 | 3 | Dorzolamide Timolol Bimatoprost | No |
| 5 OD | No | 15 | 46 | 10 | 4 | Dorzolamide Timolol Brimonidine Rhopressa | CEIOL + OMNI |
| 5 OS | No | 17 | 47 | 11 | 3 | Dorzolamide Timolol Rhopressa | No |
| 6 OD | No | 8 | 20 | 13 | 0 | No | |
| 7 OD | No | 21 | 37 | 16 | 4 | Dorzolamide Timolol Brimonidine Latanoprost | CEIOL + iStent |
| 7 OS | No | 19 | 28 | 16 | 4 | Dorzolamide Timolol Brimonidine Latanoprost | CEIOL + iStent |
| 8 OS | POAG | 19 | 26 | 19 | 1 | Dorzolamide | No |
| 9 OD | No | 14 | 18 | 16 | 2 | Dorzolamide Timolol | No |
| 9 OS | No | 14 | 21 | 15 | 2 | Dorzolamide Timolol | No |
| 10 OD | No | 14 | 27 | 10 | 0 | No | |
| 10 OS | No | 22 | 30 | 8 | 0 | No | |
| 11 OD | POAG | 13 | 18 | 7 | 2 | Dorzolamide Timolol | No |
| 12 OD | No | 12 | 26 | 18 | 0 | No | |
| 12 OS | No | 12 | 23 | 18 | 0 | No | |
| 13 OS | No | 17 | 28 | 26 | 2 | Dorzolamide Timolol | No |
| 14 OD | No | 17 | 23 | 23 | 0 | No | |
| 15 OS | No | 19 | 38 | 11 | 3 | Dorzolamide Timolol Latanoprost | No |
| 16 OS | No | 15 | 22 | 14 | 0 | No | |
| 17 OD | No | 16 | 33 | 12 | 3 | Brimonidine Latanoprost | OMNI (HSV) |
| 17 OS | No | 17 | 49 | 22 | 3 | Brimonidine Latanoprost | OMNI |
| 18 OD | No | 18 | 34 | 18 | 4 | Dorzolamide Timolol Brimonidine Latanoprost | SLT |
| 18 OS | No | 19 | 38 | 23 | 4 | Dorzolamide Timolol Brimonidine Latanoprost | SLT |
| 19 OD | PXS | 17 | 35 | 11 | 3 | Dorzolamide Timolol Rhopressa | No |
| 19 OS | PXS | 20 | 32 | 13 | 3 | Dorzolamide Timolol Rhopressa | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Vieta-Ferrer, E.R.; Au, A.; Ahn, J.; Gorin, M.B. Difluprednate and Loratadine in the Treatment of Pachychoroid Disease Spectrum. J. Clin. Transl. Ophthalmol. 2026, 4, 2. https://doi.org/10.3390/jcto4010002
Vieta-Ferrer ER, Au A, Ahn J, Gorin MB. Difluprednate and Loratadine in the Treatment of Pachychoroid Disease Spectrum. Journal of Clinical & Translational Ophthalmology. 2026; 4(1):2. https://doi.org/10.3390/jcto4010002
Chicago/Turabian StyleVieta-Ferrer, Emile R., Adrian Au, Jeeyun Ahn, and Michael B. Gorin. 2026. "Difluprednate and Loratadine in the Treatment of Pachychoroid Disease Spectrum" Journal of Clinical & Translational Ophthalmology 4, no. 1: 2. https://doi.org/10.3390/jcto4010002
APA StyleVieta-Ferrer, E. R., Au, A., Ahn, J., & Gorin, M. B. (2026). Difluprednate and Loratadine in the Treatment of Pachychoroid Disease Spectrum. Journal of Clinical & Translational Ophthalmology, 4(1), 2. https://doi.org/10.3390/jcto4010002






