Racial and Gender Disparities in Clinical Trial Representation for Age-Related Macular Degeneration Treatments: A Scoping Review
Abstract
1. Introduction
1.1. History of Age-Related Macular Degeneration
1.2. Epidemiology of Age-Related Macular Degeneration
1.3. History of Anti-VEGF Therapy and Its Role in AMD
1.4. Study Rationale
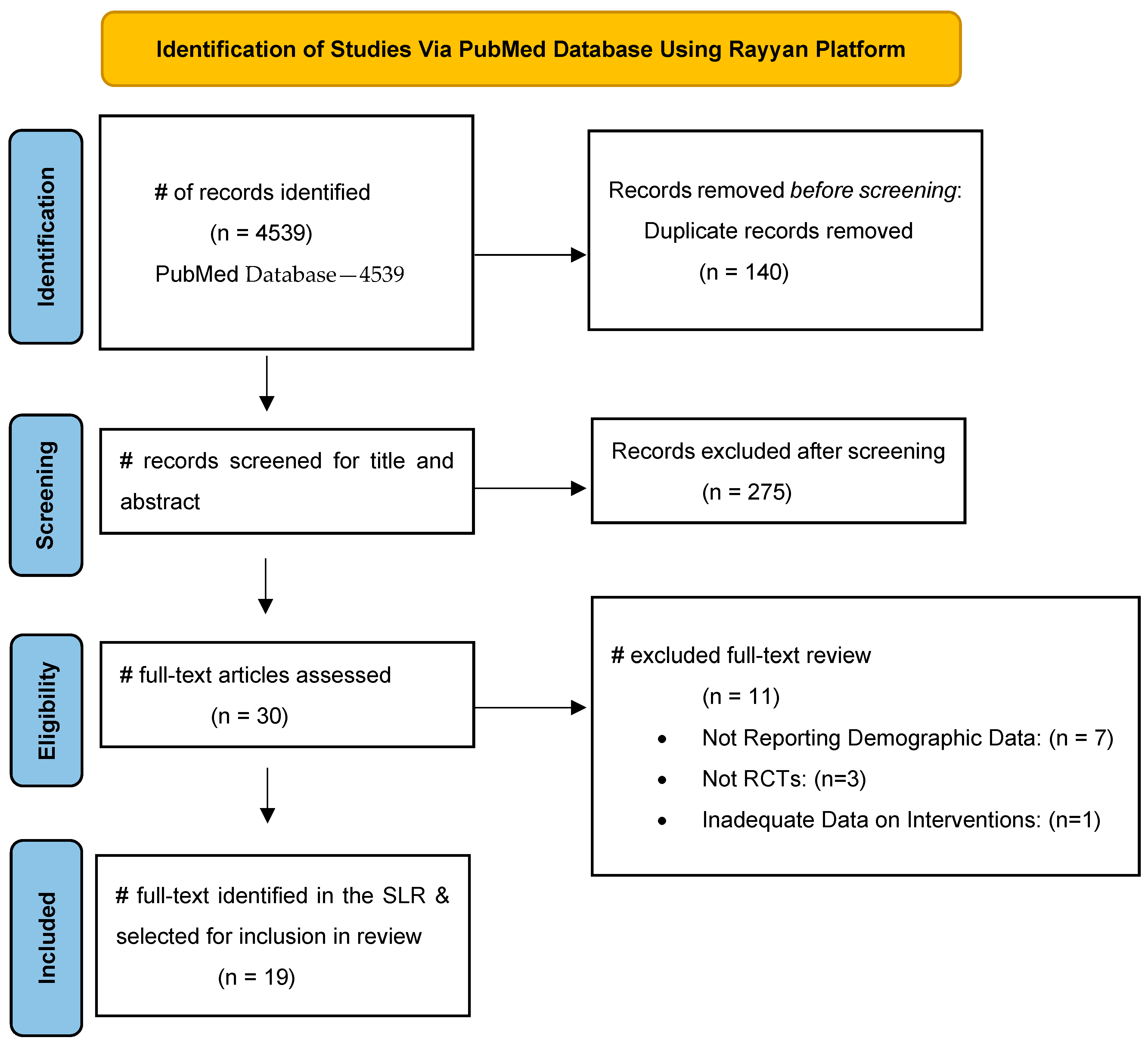
2. Methods
3. Results
4. Discussion
Outcome Statements: Limitations and Assessment of Bias
5. Recommendations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, 106. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- de Jong, P.T.V.M. Elusive drusen and changing terminology of AMD. R. Coll. Ophthalmol. 2018, 32, 904–914. [Google Scholar] [CrossRef]
- de Jong, P.T.V.M. A historical analysis of the quest for the origins of aging macula disorder, the tissues involved, and its terminology. Ophthalmol. Eye Dis. 2016, 8 (Suppl. S1), 5–14. [Google Scholar] [CrossRef]
- Bird, A.C. Age-related macular disease: Aetiology and clinical management. Community Eye Health 1999, 12, 8–9. [Google Scholar] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R., Jr. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Rezkallah, A.; Decullier, E.; Aulagner, G.; Huot, L.; Mathis, T.; GEFAL Study Group. Early predictive factors of visual loss at 1 year in neovascular age-related macular degeneration under anti-vascular endothelial growth factor. Ophthalmol. Retin. 2022, 6, 109–115. [Google Scholar] [CrossRef]
- Tomany, S.C.; Wang, J.J.; Van Leeuwen, R.; Klein, R.; Mitchell, P.; Vingerling, J.R.; Klein, B.E.; Smith, W.; De Jong, P.T. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology 2004, 111, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Tomany, S.C.; Cruickshanks, K.J.; Klein, R.; Klein, B.E.K.; Knudtson, M.D. Sunlight and the 10-year incidence of age-related maculopathy: The beaver dam eye study. Arch. Ophthalmol. 2004, 122, 750–757. [Google Scholar] [CrossRef]
- Whitescarver, T.D.; Hobbs, S.D.; Wade, C.I.; Winegar, J.W.; Colyer, M.H.; Reddy, A.K.; Drayna, P.M.; Justin, G.A. A history of anti-VEGF inhibitors in the ophthalmic literature: A bibliographic review. J. Vitreoretin. Dis. 2020, 5, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.E.; Klein, B.E.K.; Wong, T.Y.; Rotter, J.I.; Li, X.; Shrager, S.; Burke, G.L.; Klein, R.; Cotch, M.F. Incidence of age-related macular degeneration in a multi-ethnic united states population: The multi-ethnic study of atherosclerosis. Ophthalmology 2016, 123, 1297–1308. [Google Scholar] [CrossRef]
- Berkowitz, S.T.; Groth, S.L.; Gangaputra, S.; Patel, S. Racial/ethnic disparities in ophthalmology clinical trials resulting in US food and drug administration drug approvals from 2000 to 2020. JAMA Ophthalmol. 2021, 139, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zheng, Y.; Zhou, M.; Zhang, Y.; Shen, F.; Cao, J.; Xu, L.M. Value of bevacizumab in treatment of colorectal cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 5072–5080. [Google Scholar] [CrossRef]
- Dickson, S.R.; James, K.E. Medicare Part B Spending on Macular Degeneration Treatments Associated with Manufacturer Payments to Ophthalmologists. JAMA Health Forum. 2023, 4, e232951. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Moshfeghi, A.A.; Puliafito, C.A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging 2005, 36, 331–335. [Google Scholar] [CrossRef]
- Holz, F.G.; Dugel, P.U.; Weissgerber, G.; Hamilton, R.; Silva, R.; Bandello, F.; Larsen, M.; Weichselberger, A.; Wenzel, A.; Schmidt, A.; et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration: A randomized controlled study. Ophthalmology 2016, 123, 1080–1089. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Rapuano, S.B.; Boyer, D. A cost-benefit analysis of VEGF-inhibitor therapy for neovascular age-related macular degeneration in the united states. Am. J. Ophthalmol. 2021, 223, 405–429. [Google Scholar] [CrossRef]
- Lushchyk, T.; Amarakoon, S.; Martinez-Ciriano, J.P.; van den Born, L.I.; Baarsma, G.S.; Missotten, T. Bevacizumab in age-related macular degeneration: A randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmol. 2013, 91, 456. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.M.E.; Dijkman, G.; Hooymans, J.M.; Verbraak, F.D.; Hoyng, C.B.; Dijkgraaf, M.G.; Peto, T.; Vingerling, J.R.; Schlingemann, R.O. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. the BRAMD study. PLoS ONE 2016, 11, e0153052. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Wells, J.A.; Glassman, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; Browning, D.J.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Holz, F.G. Preclinical aspects of anti-VEGF agents for the treatment of wet AMD: Ranibizumab and bevacizumab. Eye 2011, 25, 661–672. [Google Scholar] [CrossRef]
- Heier, J.S.; Wykoff, C.C.; Waheed, N.K.; Kitchens, J.W.; Patel, S.S.; Vitti, R.; Perlee, L.; Chu, K.W.; Leal, S.; Asmus, F.; et al. Intravitreal combined aflibercept + anti-platelet-derived growth factor receptor β for neovascular age-related macular degeneration: Results of the phase 2 CAPELLA trial. Ophthalmology 2020, 127, 211–220. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Grégoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Féart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean diet and incidence of advanced age-related macular degeneration: The EYE-RISK consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase IIb, multicenter, randomized controlled trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef]
- Böhni, S.C.; Bittner, M.; Howell, J.P.; Bachmann, L.M.; Faes, L.; Schmid, M.K. Comparison of eylea® with lucentis® as first-line therapy in patients with treatment-naïve neovascular age-related macular degeneration in real-life clinical practice: Retrospective case-series analysis. BMC Ophthalmol. 2015, 15, 109. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Meer, E.A.; Oh, D.H.; Brodie, F.L. Time and distance cost of longer acting anti-VEGF therapies for macular degeneration: Contributions to drug cost comparisons. Clin. Ophthalmol. 2022, 16, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Eldem, B.M.; Muftuoglu, G.; Topbaş, S.; Çakir, M.; Kadayifcilar, S.; Özmert, E.; Bahçecioğlu, H.; Sahin, F.; Sevgi, S.; SALUTE Study Group. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmol. 2015, 93, 458. [Google Scholar] [CrossRef]
- Lally, D.R.; Hill, L.; Amador-Patarroyo, M.J. Subretinal fluid resolution and visual acuity in patients with neovascular age-related macular degeneration: A HARBOR post hoc analysis. Ophthalmol. Retin. 2022, 6, 1054–1060. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: The STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020, 138, 964–972. [Google Scholar] [CrossRef]
- Ross, E.L.; Hutton, D.W.; Stein, J.D.; Bressler, N.M.; Jampol, L.M.; Glassman, A.R.; Diabetic Retinopathy Clinical Research Network. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: Analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016, 134, 888–896. [Google Scholar] [CrossRef]
- Jackson, T.L.; Slakter, J.; Buyse, M.; Wang, K.; Dugel, P.U.; Wykoff, C.C.; Boyer, D.S.; Gerometta, M.; Baldwin, M.E.; Price, C.F. A randomized controlled trial of OPT-302, a VEGF-C/D inhibitor for neovascular age-related macular degeneration. Ophthalmology 2023, 130, 588–597. [Google Scholar] [CrossRef]
- Kertes, P.J.; Galic, I.J.; Greve, M.; Williams, G.; Baker, J.; Lahaie, M.; Sheidow, T. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: A randomized clinical trial. JAMA Ophthalmol. 2020, 138, 244–250. [Google Scholar] [CrossRef]
- Poor, S.H.; Weissgerber, G.; Adams, C.M.; Bhatt, H.; Browning, D.J.; Chastain, J.; Ciulla, T.A.; Ferriere, M.; Gedif, K.; Glazer, L.C.; et al. A randomized, double-masked, multicenter trial of topical acrizanib (LHA510), a tyrosine kinase VEGF-receptor inhibitor, in treatment-experienced subjects with neovascular age-related macular degeneration. Am. J. Ophthalmol. 2022, 239, 180–189. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- CATT Research Group; Martin, D.F.; Maguire, M.G.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mehta, A.; Steel, D.H.; Muldrew, A.; Peto, T.; Reeves, B.C.; Evans, R.; Chakravarthy, U.; IVAN Study Investigators. Associations and Outcomes of Patients with Submacular Hemorrhage Secondary to Age-related Macular Degeneration in the IVAN Trial. Am. J. Ophthalmol. 2022, 236, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Galdas, P.M.; Cheater, F.; Marshall, P. Men and health help-seeking behaviour: Literature review. J. Adv. Nurs. 2005, 49, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.N.; Sivaprasad, S.; Cheung, C.M.G. Gender and ethnic diversity in randomised clinical trials in age-related macular degeneration and diabetic macular oedema. Eye 2025, 39, 1249–1253. [Google Scholar] [CrossRef]
- Oh, S.S.; Galanter, J.; Thakur, N.; Pino-Yanes, M.; Barcelo, N.E.; White, M.J.; de Bruin, D.M.; Greenblatt, R.M.; Bibbins-Domingo, K.; Wu, A.H.; et al. Diversity in clinical and biomedical research: A promise yet to be fulfilled. PLoS Med. 2015, 12, e1001918. [Google Scholar] [CrossRef]
- Schaneman, J.; Kagey, A.; Soltesz, S.; Stone, J. The role of comprehensive eye exams in the early detection of diabetes and other chronic diseases in an employed population. Popul. Health Manag. 2010, 13, 195–199. [Google Scholar] [CrossRef]
- Mohamed, R.; Gadhvi, K.; Mensah, E. What Effect Does Ethnicity Have on the Response to Ranibizumab Therapy in Neovascular Age-Related Macular Degeneration? Ophthalmologica 2018, 240, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Guymer, R.H.; Chong, E.W. Modifiable risk factors for age-related macular degeneration. Med. J. Aust. 2006, 184, 455–458. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Dangas, K.; Ortega, R.; Vogel, B.; Batchelor, W.B.; Douglas, P.S.; Mehran, R. Preserving and Promoting Clinical Trial Representativeness: A Review of Existing Strategies and the Path Forward. JAMA Cardiol. 2025. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.G.; Greene, L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e218348. [Google Scholar] [CrossRef]
- Greenlee, T.E.; Malhotra, N.A.; Iyer, A.I.; Conti, T.F.; Chen, A.X.; Singh, R.P. Association of Socioeconomic Health Care Disparities with Use of Anti-Vascular Endothelial Growth Factor and Visual Acuity Outcomes in Patients with Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Guttman Krader, C. Identifying Gaps in Anti-VEGF Treatment Among Minorities with Diabetic Macular Edema. Modern Retina. 1 December 2020. Available online: https://www.modernretina.com/view/identifying-gaps-in-anti-vegf-treatment-among-minorities-with-dme (accessed on 7 May 2025).
- Feldman, R.M.; Cioffi, G.A.; Liebmann, J.M.; Weinreb, R.N. Current knowledge and attitudes concerning cost-effectiveness in glau-coma pharmacotherapy: A glaucoma specialists focus group study. Clin. Ophthalmol. 2020, 14, 729–739. [Google Scholar] [CrossRef]
- Lee, P.P.; Walt, J.G.; Doyle, J.J.; Kotak, S.V.; Evans, S.J.; Budenz, D.L.; Chen, P.P.; Coleman, A.L.; Feldman, R.M.; Jampel, H.D.; et al. A Multicenter, Retrospective Pilot Study of Resource Use and Costs Associated with Severity of Disease in Glaucoma. Arch. Ophthalmol. 2006, 124, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Low, S.; Kumari, N.; Wang, J.; Ang, K.; Yeo, D.; Yip, C.C.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Direct medical cost associated with diabetic retinopathy severity in type 2 diabetes in sin-gapore. PLoS ONE 2017, 12, e0180949. [Google Scholar] [CrossRef]
- ROBIS (Risk of Bias in Systematic Reviews). Available online: https://www.bristol.ac.uk/population-health-sciences/projects/robis/ (accessed on 7 May 2025).
- Oyer, R.A.; Hurley, P.; Boehmer, L.; Steeby Bruinooge, S.; Levit, K.; Barrett, N.; Benson, A.B.; Bernick, L.A.; Byatt, L.; Charlot, M.; et al. Increasing Racial and Ethnic Diversity in Cancer Clinical Trials: An American Society of Clinical Oncology and Association of Community Cancer Centers Joint Research Statement. J. Clin. Oncol. 2022, 40, 2163–2171. [Google Scholar] [CrossRef]
- Cruz, J.G.; Patel, D.; Allison, K. Diabetic Retinopathy and Vision Loss: A Public Health Concern. J. Biomed. Res. Environ. Sci. 2024, 5, 691–698. [Google Scholar] [CrossRef]

| Risk Factor | Description |
|---|---|
| Age | Age is the most significant risk factor for the development of ARMD. The incidence of ARMD increases with age, particularly after the age of 50. Diagnosis is most common in individuals over the age of 65 specifically |
| Genetic Factors | Family history of exudative ARMD significantly increases the risk. Specific genetic variations have also been linked to higher susceptibility (CFH, ARMS2) |
| Female Gender | Women are more likely to develop ARMD than men, likely in part due to longer life expectancy and hormonal factors (increased estrogen) |
| Smoking | Smoking is one of the proven modifiable risk factors in the development of ARMD. There is a two- to three-fold increased risk of ARMD among smokers |
| Race and Ethnicity | Caucasians are at a higher risk compared to other racial groups. Hispanics and African Americans have a lower risk of a development. |
| Hypertension (High Blood Pressure) | Uncontrolled sustained hypertension can result in microvascular retinal complications, increasing the risk of ARMD secondary to pre-existing retinal damage. |
| Obesity | Obesity results in microvascular and macrovascular complications systemically, as well as in the retina. Associated presence of metabolic syndrome and high cholesterol also slightly increases the risk |
| Dietary Factors | Low intake of antioxidants, including, vitamins C, E, zinc, and omega-3 fatty acids increases the risk. A poor diet lacking green leafy vegetables and high in saturated fats has been linked with a higher prevalence |
| Prolonged Sunlight Exposure | Ultraviolet light from the sun (UV-A and UV-B) increases the risk of ARMD, due to the cumulative retina damage over time. |
| Light-Colored Eyes | Similar to prolonged sunlight exposure, light-colored eyes (blue, green, etc.) have an increased risk due to a decreased amount of UV protection |
| Cardiovascular Disease | Presence of diagnosed cardiovascular diseases including, heart disease, atherosclerosis, hyper cholesterol, etc., can impact retinal blood flow, contributing to ARMD development |
| Prolonged Use of Specific Medications | Vasodilators: Prolonged use of vasodilating agents (ex: nitrates, beta blockers, calcium channel blockers) can impact retinal circulation long term Beta Blockers: Similar to vasodilators as above, decreased pressure and circulating volume can result in decreased retinal circulation, increasing risk of ARMD Calcium Channel Blockers: Both dihydropyridine and non-dihydropyridine subtypes may increase vasculature leak, contributing to the pathogenesis of ARMD Thiazide Diuretics: Long term use of thiazide diuretics can impact electrolyte balance directly, resulting in aberrant retinal circulation |
| Low Serum Levels of Lutein and Zeaxanthin | Carotenoids found in green leafy vegetables can help to protect the retina from oxidative damage long-term. Low serum levels of carotenoids for an extended period of time can result in increased risk of ARMD development |
| Cataract Surgery | Cataract surgery can change the mechanism of retinal exposure to UV exposure from sunlight. Specifically, patients who underwent cataract surgery at a young age have a slightly increased risk of ARMD development |
| Chronic Systemic and/or Ocular Inflammation | Prolonged systemic and/or retinal inflammation have both been linked to a slightly increased risk over ARMD development. The low-grade background inflammation is suspected to contribute to eventual retinal damage |
| Properties | Ranibizumab (Lucentis) | Bevacizumab (Avastin) | Aflibercept (Eylea) | Faricimab (Vabysmo) | RTH258 (Brolucizumab) | Acrizanib |
|---|---|---|---|---|---|---|
| Year of FDA Approval | 2006 | N/A | 2011 | 2022 | 2019 | N/A |
| Class | Antibody Fragment | Monoclonal Antibody | Fusion Protein | Antibody Fragment | Antibody Fragment | Protein Tyrosine Kinase |
| MW (KDa) | 48 | 149 | 115 | 149 | 26 | 0.4454 |
| Net Charge | Negative | Negative | Slightly Positive | Negative | Negative | Negative |
| Binding Target | VEGF-A | VEGF-A | VEGF-A, VEGF-B, PlGF | VEGF-A, Ang-2 | VEGF-A | VEGFR-1, EGFR |
| Therapy | Mean Spending per Unit, USD | Mean Units per Claim | Mean Spending per Claim, USD | Mean No. of Claims per Beneficiary per Year | Mean Annual Spending per Beneficiary, USD | Mean Annual Add-On Payment per Beneficiary, USD |
|---|---|---|---|---|---|---|
| Bevacizumab (Avastin) | 76.32 | 14 | 1068.48 | 3.9 | 4167.07 | 171.80 |
| Aflibercept (Eylea) | 923.56 | 2 | 1847.12 | 5.0 | 9235.60 | 380.76 |
| Ranibizumab (Lucentis) | 333.55 | 5 | 1667.75 | 5.1 | 8505.52 | 350.66 |
| First Author, Year Published | Mean Age (Years) | Male (%) | Female (%) | Intravitreal Drug Used | Final Data Stratified by Ethnicity or Race |
|---|---|---|---|---|---|
| Rosenfeld, 2006 | 77 | 35.2 | 64.8 | Ranibizumab | No |
| Heier, 2012 | 76 | 42.9 | 57.1 | Ranibizumab/Aflibercept | No |
| Heier, 2022 | >50 | 37.3 | 62.7 | Faricimab | No |
| Lushchyk, 2013 | >65 | 40 | 60 | Bevacizumab | No |
| Holz, 2016 | 76 | 46.1 | 53.9 | RTH258 | No |
| Schauwvlieghe, 2016 | 78 | 45 | 55 | Bevacizumab/Ranibizumab | No |
| Khanani, 2020 | 79 | 42 | 58 | Ranibizumab/Faricimab | No |
| Eldem, 2015 | 70 | 52 | 48 | Ranibizumab | No |
| Kertes, 2020 | 79 | 39.7 | 60.3 | Ranibizumab | No |
| Lally, 2022 | 77 | 40.6 | 59.4 | Ranibizumab | No |
| Jaffee, 2017 | 78 | 38 | 62 | Ranibizumab | No |
| Poor, 2022 | 77 | 54.4 | 45.6 | Topical Acrizanib | No |
| Jackson, 2023 | 76 | 39.7 | 60.3 | Ranibizumab | No |
| Khanani, 2022 | 76 | 45.2 | 54.8 | Brolucizumab/Aflibercept | No |
| Nguyen, 2012 | 75 | 34.5 | 65.5 | Ranibizumab | No |
| Mohamed, 2018 | 86 | 33 | 67 | Ranibizumab | Yes |
| Heier, 2020 | 79 | 40 | 60 | Ranibizumab/Aflibercept | No |
| Gillies, 2020 | 77 | 49 | 51 | Ranibizumab/Aflibercept | No |
| Mehta, 2022 | 79 | 39 | 61 | Bevacizumab/Ranibizumab | No |
| First Author, Year Published | Study Design | Trial Duration (Months) | Number of Patients Analyzed | White (%) | Black or African American (%) | Hispanic or Latino (%) | Asian (%) | American Indian or Alaska Native (%) | Multiple (%) | Not Reported or Other (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rosenfeld, 2006 | RCT | 24 | 716 | 96.6 | 0 | 0 | 0 | 0 | 0 | 3.4 |
| Heier, 2012 | RCT | 12 | 2419 | 84.7 | 0.3 | 11.2 | 0 | 0 | 0 | 3.8 |
| Heier, 2022 | RCT | 28 | 133 | 93.62 | 1.4 | 0 | 3.2 | 0.2 | 0.4 | 0.4 |
| Lushchyk, 2013 | RCT | 12 | 120 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Holz, 2016 | RCT | 6 | 194 | 98.6 | 0 | 0 | 0 | 1.4 | 0 | 0 |
| Schauwvlieghe, 2016 | RCT | 12 | 327 | 98 | 0 | 0 | 0 | 0 | 2 | 0 |
| Khanani, 2020 | RCT | 12 | 76 | 97.6 | 1.4 | 0 | 1.1 | 0 | 0 | 0 |
| Eldem, 2015 | RCT | 12 | 77 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kertes, 2020 | RCT | 24 | 466 | 94.3 | 0 | 0 | 0 | 0 | 5.7 | 0 |
| Lally, 2022 | RCT | 18 | 349 | 97.4 | 0.2 | 3.6 | 2.1 | 0.2 | 0.3 | 0 |
| Jaffee, 2017 | RCT | 6 | 449 | 97.6 | 0 | 0 | 0 | 0 | 0 | 2.4 |
| Poor, 2022 | RCT | 3 | 90 | 87.8 | 0 | 0 | 8.9 | 0 | 0 | 0 |
| Jackson, 2023 | RCT | 11 | 366 | 98.6 | 0 | 0 | 0 | 0 | 0 | 1.4 |
| Khanani, 2022 | RCT | 24 | 535 | 97.8 | 0.6 | 0 | 0.9 | 0 | 0 | 0.6 |
| Nguyen, 2012 | RCT | 10 | 151 | 75.9 | 0 | 0 | 24.1 | 0 | 0 | 0 |
| Mohamed, 2018 | RCT | 5 | 217 | 84 | 0 | 0 | 0 | 0 | 0 | 16 |
| Heier, 2020 | RCT | 2 | 505 | 93.6 | 0.6 | 0 | 4.7 | 0.2 | 0.4 | 0 |
| Gillies, 2020 | RCT | 3 | 278 | 93.3 | 0.4 | 0 | 5.3 | 0 | 0 | 1 |
| Mehta, 2022 | RCT | 2 | 535 | 99 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sponsorship Source | Number of Studies | Total Patients | Gender Distribution (Number of Patients) | Racial Distribution (# of Patients) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Caucasian | African American | Hispanic | Asian | AI * | M ** | NR *** | |||
| Private | 2 | 836 | 300 | 536 | 812 | 0 | 0 | 0 | 0 | 0 | 24 |
| Public | 4 | 1273 | 517 | 756 | 1223 | 0 | 0 | 0 | 3 | 0 | 47 |
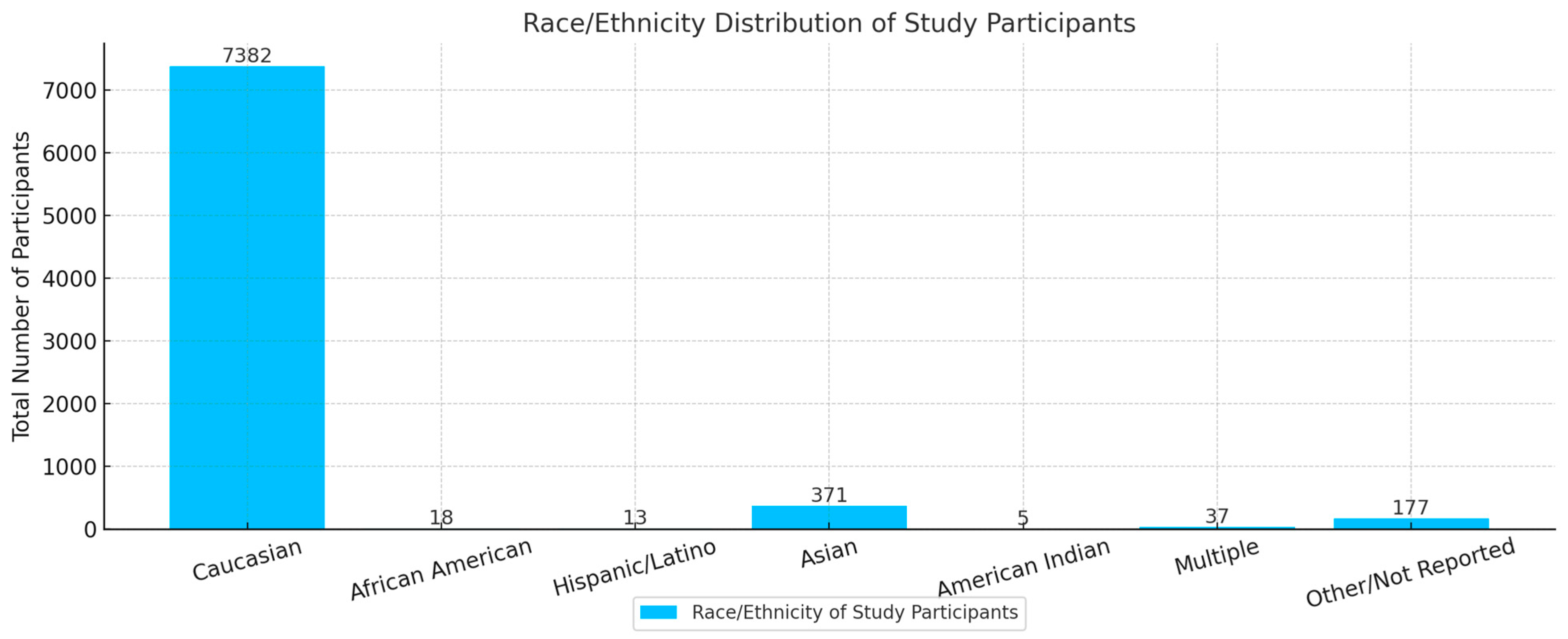
| Pharmaceutical | 13 | 5894 | 2483 | 3411 | 5347 | 18 | 13 | 371 | 2 | 37 | 106 |
| Total | 19 | 8003 | 3300 | 4703 | 7382 | 18 | 13 | 371 | 5 | 37 | 177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiee, A.; Juran, T.; Zabaneh, I.; Patel, D.; Allison, K. Racial and Gender Disparities in Clinical Trial Representation for Age-Related Macular Degeneration Treatments: A Scoping Review. J. Clin. Transl. Ophthalmol. 2025, 3, 16. https://doi.org/10.3390/jcto3030016
Shafiee A, Juran T, Zabaneh I, Patel D, Allison K. Racial and Gender Disparities in Clinical Trial Representation for Age-Related Macular Degeneration Treatments: A Scoping Review. Journal of Clinical & Translational Ophthalmology. 2025; 3(3):16. https://doi.org/10.3390/jcto3030016
Chicago/Turabian StyleShafiee, Amirmohammad, Taylor Juran, Iza Zabaneh, Deepkumar Patel, and Karen Allison. 2025. "Racial and Gender Disparities in Clinical Trial Representation for Age-Related Macular Degeneration Treatments: A Scoping Review" Journal of Clinical & Translational Ophthalmology 3, no. 3: 16. https://doi.org/10.3390/jcto3030016
APA StyleShafiee, A., Juran, T., Zabaneh, I., Patel, D., & Allison, K. (2025). Racial and Gender Disparities in Clinical Trial Representation for Age-Related Macular Degeneration Treatments: A Scoping Review. Journal of Clinical & Translational Ophthalmology, 3(3), 16. https://doi.org/10.3390/jcto3030016






