The Activity of Substance P (SP) on the Corneal Epithelium
Abstract
1. Introduction
2. Discovery of Substance P
3. Molecular Biology of Substance P
4. Vasoactive Effects of Substance P
5. Secretory Effects of Substance P
6. Mitogenic Effects of Substance P
7. Muscle Contraction with Substance P Stimulation
8. Substance P as a Neurotransmitter
9. Ocular Distribution of Substance P
10. Ocular Smooth Muscle Contraction
11. Substance P and Corneal Pain
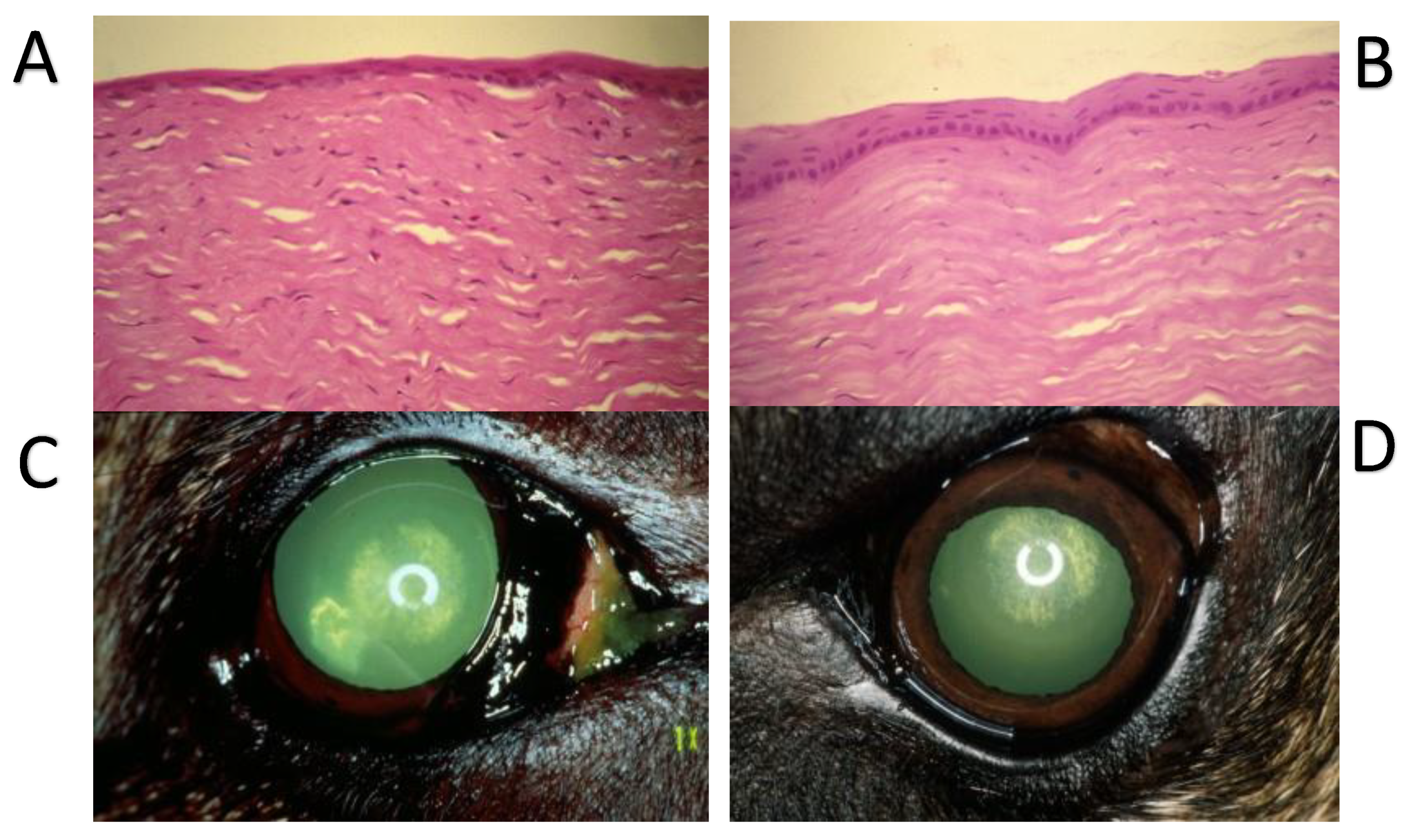
12. Substance P and Corneal Epithelium Healing
13. Therapeutic Applications of SP in Cornea Epithelium
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.B.; Naderi, A.; Cho, W.; Ortiz, G.; Musayeva, A.; Dohlman, T.H.; Chen, Y.; Ferrari, G.; Dana, R. Modulating the tachykinin: Role of substance P and neurokinin receptor expression in ocular surface disorders. Ocul. Surf. 2022, 25, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Patacchini, R.; Rovero, P.; Giachetti, A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993, 13, 23–93. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef]
- Chang, M.M.; Leeman, S.E. Isolation of a Sialogogic Peptide from Bovine Hypothalamic Tissue and Its Characterization as Substance P. J. Biol. Chem. 1970, 245, 4784–4790. [Google Scholar] [CrossRef]
- Stone, R.A.; Kuwayama, Y.; Laties, A.M. Regulatory peptides in the eye. Experientia 1987, 43, 791–800. [Google Scholar] [CrossRef]
- Euler, U.S.V.; Gaddum, J.H. An unidentified depressor substance in certain tissue extracts. J. Physiol. 1931, 72, 74–87. [Google Scholar] [CrossRef]
- Nawa, H.; Kotani, H.; Nakanishi, S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature 1984, 312, 729–734. [Google Scholar] [CrossRef]
- Krause, J.E.; Blount, P.; Sachais, B.S. Molecular Biology of Receptors. In The Tachykinin Receptors; Humana Press: Totowa, NJ, USA, 1994; pp. 165–218. [Google Scholar]
- Kotani, H.; Hoshimaru, M.; Nawa, H.; Nakanishi, S. Structure and gene organization of bovine neuromedin K precursor. Proc. Natl. Acad. Sci. USA 1986, 83, 7074–7078. [Google Scholar] [CrossRef]
- Carter, M.S.; Krause, J.E. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J. Neurosci. 1990, 10, 2203–2214. [Google Scholar] [CrossRef]
- Harmar, A.J.; Hyde, V.; Chapman, K. Identification and cDNA sequence of δ-preprotachykinin, a fourth splicing variant of the rat substance P precursor. FEBS Lett. 1990, 275, 22–24. [Google Scholar] [CrossRef]
- Krause, J.E.; Chirgwin, J.M.; Carter, M.S.; Xu, Z.S.; Hershey, A.D. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc. Natl. Acad. Sci. USA 1987, 84, 881–885. [Google Scholar] [CrossRef]
- Krause, J.E.; Cremins, J.D.; Carter, M.S.; Brown, E.R.; MacDonald, M.R. Solution hybridization-nuclease protection assays for sensitive detection of differentially spliced substance P- and neurokinin A-encoding messenger ribonucleic acids. In Methods Enzymology; Elsevier: Amsterdam, The Netherlands, 1989; pp. 634–652. [Google Scholar]
- Krause, J.E.; Bu, J.Y.; Takeda, Y.; Blount, P.; Raddatz, R.; Sachais, B.S.; Chou, K.B.; Takeda, J.; McCarson, K.; DiMaggio, D. Structure, expression and second messenger-mediated regulation of the human and rat substance P receptors and their genes. Regul. Pept. 1993, 46, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Ostalska-Nowicka, D.; Kondraciuk, B.; Miskowiak, B. The significance of substance P in physiological and malignant haematopoiesis. J. Clin. Pathol. 2007, 60, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Bény, J.L. Effect of substance P on the membrane potential of coronary arterial endothelial cellsin situ. Agents Actions 1990, 31, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.; Ralevic, V.; Hopwood, A.M.; Fehér, E.; Lincoln, J.; Kirkpatrick, K.A.; Burnstock, G. Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia 1989, 45, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Milner, P.; Hudlická, O.; Kristek, F.; Burnstock, G. Substance P is released from the endothelium of normal and capsaicin-treated rat hind-limb vasculature, in vivo, by increased flow. Circ. Res. 1990, 66, 1178–1183. [Google Scholar] [CrossRef]
- Kuo, L.; Chilian, W.M.; Davis, M.J. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H1706–H1715. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.G.; Misra, S.; Grider, J.R.; Makhlouf, G.M. Functional difference between SP and NKA: Relaxation of gastric muscle by SP is mediated by VIP and NO. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 264, G678–G685. [Google Scholar] [CrossRef]
- Sandberg, B.E.B.; Iversen, L.L. Substance P. J. Med. Chem. 1982, 25, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Öhlén, A.; Thureson-Klein, Å.; Lindbom, L.; Persson, M.G.; Hedqvist, P. Substance P Activates Leukocytes and Platelets in Rabbit Microvessels. J. Vasc. Res. 1989, 26, 84–94. [Google Scholar] [CrossRef]
- White, D.M.; Helme, R.D. Release of substance P from peripheral nerve terminals following electrical stimulation of the sciatic nerve. Brain Res. 1985, 336, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lembeck, F.; Donnerer, J.; Tsuchiya, M.; Nagahisa, A. The non-peptide tachykinin antagonist, CP-96,345, is a potent inhibitor of neurogenic inflammation. Br. J. Pharmacol. 1992, 105, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Mathison, R.; Davison, J.S. Altered vascular permeability responses to substance P in diabetic rats: Interactions with a nitric oxide synthesis inhibitor. Eur. J. Pharmacol. 1993, 240, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Snyder, G.D.; De Palatis, L.; Ho, R.H.; Xu, R.K.; Pan, G.; McCann, S.M. Role of substance P in suppressing growth hormone release in the rat. Proc. Natl. Acad. Sci. USA 1989, 86, 7290–7294. [Google Scholar] [CrossRef]
- Chihara, K.; Arimura, A.; Coy, D.H.; Schally, A.V. Studies on the Interaction of Endorphins, Substance P, and Endogenous Somatostatin in Growth Hormone and Prolactin Release in Rats. Endocrinology 1978, 102, 281–290. [Google Scholar] [CrossRef]
- Eckstein, N.; Wehrenberg, W.B.; Louis, K.; Carmel, P.W.; Zimmermann, E.A.; Frantz, A.G.; Ferin, M. Effects of Substance P on Anterior Pituitary Secretion in the Female Rhesus Monkey. Neuroendocrinology 1980, 31, 338–342. [Google Scholar] [CrossRef]
- Hasséssian, H.; Couture, R.; de Champlain, J. Sympathoadrenal Mechanisms Underlying Cardiovascular Responses to Intrathecal Substance P in Conscious Rats. J. Cardiovasc. Pharmacol. 1990, 15, 736–744. [Google Scholar] [CrossRef]
- Yashpal, K.; Gauthier, S.G.; Henry, J.L. Substance P given intrathecally at the spinal T9 level increases adrenal output of adrenaline and noradrenaline in the rat. Neuroscience 1985, 15, 529–536. [Google Scholar] [CrossRef]
- Cridland, R.A.; Henry, J.L. An adrenal-mediated, naloxone-reversible increase in reaction time in the tail-flick test following intrathecal administration of substance p at the lower thoracic spinal level in the rat. Neuroscience 1988, 26, 243–251. [Google Scholar] [CrossRef]
- Dreux, C.; Imhoff, V.; Mauduit, P.; Rossignol, B. Substance P Effect on the Secretory Process in Rat Parotid Gland. In Substance P and Neurokinins; Springer: New York, NY, USA, 1987; pp. 195–196. [Google Scholar]
- Gardner, J.D.; Jackson, M.J. Regulation of amylase release from dispersed pancreatic acinar cells. J. Physiol. 1977, 270, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Vigna, S.R.; Mantyh, C.R.; Soll, A.H.; Maggio, J.E.; Mantyh, P.W. Substance P receptors on canine chief cells: Localization, characterization, and function. J. Neurosci. 1989, 9, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, K.; Fujiwara, M. Effects of substance-P(SP) on parasympathetically stimulated gastric acid secretion and mucosal blood flow(MBF) in rats. Jpn. J. Pharmacol. 1984, 36, 250. [Google Scholar] [CrossRef]
- Chiba, K.; Kontani, K.; Tadenuma, H.; Katada, T.; Hoshi, M. Induction of starfish oocyte maturation by the beta gamma subunit of starfish G protein and possible existence of the subsequent effector in cytoplasm. Mol. Biol. Cell 1993, 4, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, A.; Kaneko, T.; Kajinuma, H.; Kosaka, K. Effects of Substance P and Neurotensin Infused Intrapancreatically on Glucagon and Insulin Secretion. Endocrinology 1978, 102, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Lowman, M.A.; Robinson, C.; Holgate, S.T.; Benyon, C. Interaction of Neuropeptides with Human Mast Cells. Int. Arch. Allergy Immunol. 1989, 88, 70–78. [Google Scholar] [CrossRef]
- Iwamoto, I.; Nakagawa, N.; Yamazaki, H.; Kimura, A.; Tomioka, H.; Yoshida, S. Mechanism for substance P-induced activation of human neutrophils and eosinophils. Regul. Pept. 1993, 46, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Payan, D.G.; Brewster, D.R.; Missirian-Bastian, A.; Goetzl, E.J. Substance P recognition by a subset of human T lymphocytes. J. Clin. Investig. 1984, 74, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.O. Improved final height in girls with Turner’s syndrome treated with growth hormone and oxandrolone. J. Clin. Endocrinol. Metab. 1996, 81, 635–640. [Google Scholar] [CrossRef]
- Nilsson, J.; von Euler, A.M.; Dalsgaard, C.-J. Stimulation of connective tissue cell growth by substance P and substance K. Nature 1985, 315, 61–63. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Dolara, P.; Maggi, C.A. NK1-receptors mediate the proliferative response of human fibroblasts to tachykinins. Br. J. Pharm. 1990, 100, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of Neuropeptides on Production of Inflammatory Cytokines by Human Monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Carson, D.A.; Vaughan, J.H. Substance P Activation of Rheumatoid Synoviocytes: Neural Pathway in Pathogenesis of Arthritis. Science 1987, 235, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, M.; Payan, D.G. The mitogenic effects of vasoactive neuropeptides on cultured smooth muscle cell lines. Life Sci. 1987, 40, 853–861. [Google Scholar] [CrossRef]
- Matthews, M.R.; Connaughton, M.; Cuello, A.C. Ultrastructure and distribution of substance P-immunoreactive sensory collaterals in the guinea pig prevertebral sympathetic ganglia. J. Comp. Neurol. 1987, 258, 28–51. [Google Scholar] [CrossRef]
- Maggi, C.A.; Giuliani, S.; Santicioli, P.; Abelli, L.; Regoli, D.; Meli, A. Further studies on the mechanisms of the tachykinin-induced activation of micturition reflex in rats: Evidence for the involvement of the capsaicin-sensitive bladder mechanoreceptors. Eur. J. Pharmacol. 1987, 136, 189–205. [Google Scholar] [CrossRef]
- Maggi, C.A. The role of peptides in the regulation of the micturition reflex: An update. Gen. Pharmacol. Vasc. Syst. 1991, 22, 1–24. [Google Scholar] [CrossRef]
- Maggi, C.A.; Theodorsson, E.; Santicioli, P.; Giuliani, S. Tachykinins and calcitonin gene-related peptide as co-transmitters in local motor responses produced by sensory nerve activation in the guinea-pig isolated renal pelvis. Neuroscience 1992, 46, 549–559. [Google Scholar] [CrossRef]
- Shinkai, M.; Takayanagi, I. Characterization of Tachykinin Receptors in Urinary Bladder from Guinea Pig. Jpn. J. Pharmacol. 1990, 54, 241–243. [Google Scholar] [CrossRef]
- D’Orléans-Juste, P.; Dion, S.; Drapeau, G.; Regoli, D. Different receptors are involved in the endothelium-mediated relaxation and the smooth muscle contraction of the rabbit pulmonary artery in response to substance and related neurokinins. Eur. J. Pharmacol. 1986, 125, 37–44. [Google Scholar] [CrossRef]
- Mastrangelo, D.; Mathison, R.; Huggel, H.J.; Dion, S.; D’Orléans-Juste, P.; Rhaleb, N.E.; Drapeau, G.; Rovero, P.; Regoli, D. The rat isolated portal vein: A preparation sensitive to neurokinins, particularly neurokinin B. Eur. J. Pharmacol. 1987, 134, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Barthó, L.; Holzer, P.; Lembeck, F.; Szolcsányi, J. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to release of substance P. J. Physiol. 1982, 332, 157–167. [Google Scholar] [CrossRef]
- Barthó, L.; Holzer, P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience 1985, 16, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.E.; Collins, S.M.; Fox, J.A.E.T.; Huizinga, J.D. Pharmacology of neuroendocrine peptides. In Comprehensive Physiology; Wiley: New York, NY, USA, 1989; pp. 759–816. [Google Scholar]
- Chahl, L.A. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to substance P release. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1982, 319, 212–215. [Google Scholar] [CrossRef] [PubMed]
- BjÖRkroth, U.; Rosell, S.; Xu, J.-C.; Folkers, K. Pharmacological characterization of four related substance P antagonist. Acta Physiol. Scand. 1982, 116, 167–173. [Google Scholar] [CrossRef]
- Amin, A.H.; Crawford, T.B.B.; Gaddum, J.H. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J. Physiol. 1954, 126, 596–618. [Google Scholar] [CrossRef]
- Kopera, H.; Lazarini, W. Zur Frage der zentralen Übertragung afferenter Impulse. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1953, 219, 197–213. [Google Scholar] [CrossRef]
- Lembeck, F. Zur Frage der zentralen Übertragung afferenter Impulse. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1953, 219, 214–222. [Google Scholar] [CrossRef]
- Pernow, B. Substance P. Pharmacol. Rev. 1983, 35, 85–141. [Google Scholar]
- Otsuka, M.; Konishi, S.; Takahashi, T. A Further Study of the Motoneuron-Depolarizing Peptide Extracted from Dorsal Roots of Bovine Spinal Nerves. Proc. Jpn. Acad. 1972, 48, 747–752. [Google Scholar] [CrossRef]
- Takahashi, T.; Konishi, S.; Powell, D.; Leeman, S.E.; Otsuka, M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Res. 1974, 73, 59–69. [Google Scholar] [CrossRef]
- De Biasi, S.; Rustioni, A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc. Natl. Acad. Sci. USA 1988, 85, 7820–7824. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-da-silva, A.; Tagari, P.; Cuello, A.C. Morphological characterization of substance P-like immunoreactive glomeruli in the superficial dorsal horn of the rat spinal cord and trigeminal subnucleus caudalis: A quantitative study. J. Comp. Neurol. 1989, 281, 497–515. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.W.; Lawson, S.N. Cell type and conduction velocity of rat primary sensory neurons with substance p-like immunoreactivity. Neuroscience 1989, 28, 745–753. [Google Scholar] [CrossRef]
- Gamse, R.; Molnar, A.; Lembeck, F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979, 25, 629–636. [Google Scholar] [CrossRef]
- Theriault, E.; Otsuka, M.; Jessell, T. Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res. 1979, 170, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Jessell, T.M.; Gamse, R.; Mudge, A.W.; Leeman, S.E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature 1980, 286, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Otsuka, M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature 1974, 252, 734–735. [Google Scholar] [CrossRef]
- Otsuka, M.; Konishi, S. Substance P and Excitatory Transmitter of Primary Sensory Neurons. Cold Spring Harb. Symp. Quant. Biol. 1976, 40, 135–143. [Google Scholar] [CrossRef]
- Elbadri, A.A.; Shaw, C.; Johnston, C.F.; Archer, D.B.; Buchanan, K.D. The distribution of neuropeptides in the ocular tissues of several mammals: A comparative study. Comp. Biochem. Physiol. C Comp. Pharmacol. 1991, 100, 625–627. [Google Scholar] [CrossRef]
- Brecha, N.; Sharma, S.C.; Karten, H.J. Localization of substance P-like immunoreactivity in the adult and developing goldfish retina. Neuroscience 1981, 6, 2737–2746. [Google Scholar] [CrossRef]
- Brecha, N.; Johnson, D.; Bolz, J.; Sharma, S.; Parnavelas, J.G.; Lieberman, A.R. Substance P-immunoreactive retinal ganglion cells and their central axon terminals in the rabbit. Nature 1987, 327, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Kuwayama, Y.; Shiosaka, S.; Ishimoto, I.; Shimizu, Y.; Takagi, H.; Inagaki, S.; Sakanaka, M.; Semba, E.; Takatsuki, K.; et al. Demonstration of a substance P-like immunoreactivity in retinal cells of the rat. Neurosci. Lett. 1981, 23, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N. Substance P in the bovine retina: Localization, identification, release, uptake and receptor analysis. J. Physiol. 1984, 349, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, R.A.; Miller, R.F. The physiology of substance P in the rabbit retina. J. Neurosci. 1990, 10, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Otori, Y.; Tominaga, K.; Fukuhara, C.; Yang, J.; Yamazaki, S.; Cagampang, F.R.A.; Okamura, H.; Inouye, S.-I.T. Substance P-like immunoreactivity in the suprachiasmatic nucleus of the rat. Brain Res. 1993, 619, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, I.L.; Morns, J.L. Co-existence of neuropeptides in sympathetic, cranial autonomic and sensory neurons innervating the iris of the guinea-pig. J. Auton. Nerv. Syst. 1987, 21, 67–82. [Google Scholar] [CrossRef]
- Miller, A.; Costa, M.; Furness, J.B.; Chubb, I.W. Substance P immunoreactive sensory nerves supply the rat iris and cornea. Neurosci. Lett. 1981, 23, 243–249. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ishimoto, I.; Shiosaka, S.; Kuwayama, Y.; Fukuda, M.; Inagaki, S.; Sakanaka, M.; Tohyama, M. A direct contact of substance P-containing nerve fibers with pupillary sphincter muscle of the rat: An immunohistochemical analysis. Neurosci. Lett. 1982, 33, 25–28. [Google Scholar] [CrossRef]
- Stone, R.A.; Laties, A.M.; Brecha, N.C. Substance P-like immunoreactive nerves in the anterior segment of the rabbit, cat and monkey eye. Neuroscience 1982, 7, 2459–2468. [Google Scholar] [CrossRef]
- Tervo, K.; Tervo, T.; Eränkö, L.; Vannas, A.; Cuello, A.C.; Eränkö, O. Substance P-immunoreactive nerves in the human cornea and iris. Investig. Ophthalmol. Vis. Sci. 1982, 23, 671–674. [Google Scholar]
- Tornqvist, K.; Mandahl, A.; Leander, S.; Lorén, I.; Håkanson, R.; Sundler, F. Substance P-immunoreactive nerve fibres in the anterior segment of the rabbit eye. Cell Tissue Res. 1982, 222, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sasaoka, A.; Ishimoto, I.; Kuwayama, Y.; Sakiyama, T.; Manabe, R.; Shiosaka, S.; Inagaki, S.; Tohyama, M. Overall distribution of substance P nerves in the rat cornea and their three-dimensional profiles. Investig. Ophthalmol. Vis. Sci. 1984, 25, 351–356. [Google Scholar]
- Unger, W.G.; Butler, J.M.; Cole, D.F.; Bloom, S.R.; McGregor, G.P. Substance P, vasoactive intestinal polypeptide (VIP) and somatostatin levels in ocular tissue of normal and sensorily denervated rabbit eyes. Exp. Eye Res. 1981, 32, 797–801. [Google Scholar] [CrossRef]
- Keen, P.; Tullo, A.B.; Blyth, W.A.; Hill, T.J. Substance P in the mouse cornea: Effects of chemical and surgical denervation. Neurosci. Lett. 1982, 29, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Powell, D.; Unger, W.G. Substance P levels in normal and sensorily denervated rabbit eyes. Exp. Eye Res. 1980, 30, 311–313. [Google Scholar] [CrossRef]
- Murphy, C.J.; Marfurt, C.F.; McDermott, A.; Bentley, E.; Abrams, G.A.; Reid, T.W.; Campbell, S. Spontaneous Chronic Corneal Epithelial Defects (SCCED) in Dogs: Clinical Features, Innervation, and Effect of Topical SP, with or without IGF-1. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2252–2261. [Google Scholar]
- Igić, R. Substance P Inactivation by Aqueous Humor. Exp. Eye Res. 1993, 57, 415–417. [Google Scholar] [CrossRef]
- Jackman, H.L.; Tan, F.L.; Tamei, H.; Beurling-Harbury, C.; Li, X.Y.; Skidgel, R.A.; Erdös, E.G. A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal “protective protein”. J. Biol. Chem. 1990, 265, 11265–11272. [Google Scholar] [CrossRef]
- Gamse, R.; Leeman, S.E.; Holzer, P.; Lembeck, F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1981, 317, 140–148. [Google Scholar] [CrossRef]
- Mochizuki-Oda, N.; Nakajima, Y.; Nakanishi, S.; Ito, S. Substance P-induced elevation of intracellular calcium in transfected Chinese hamster ovary cells: Role of inositol trisphosphate. Regul. Pept. 1993, 46, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki-Oda, N.; Nakajima, Y.; Nakanishi, S.; Ito, S. Characterization of the substance P receptor-mediated calcium influx in cDNA transfected Chinese hamster ovary cells. A possible role of inositol 1,4,5-trisphosphate in calcium influx. J. Biol. Chem. 1994, 269, 9651–9658. [Google Scholar] [CrossRef]
- Tachado, S.D.; Akhtar, R.A.; Yousufzai, S.Y.K.; Abdel-Latif, A.A. Species differences in the effects of substance P on inositol trisphosphate accumulation and cyclic AMP formation, and on contraction in isolated iris sphincter of the mammalian eye: Differences in receptor density. Exp. Eye Res. 1991, 53, 729–739. [Google Scholar] [CrossRef]
- Holmdahl, G.; Håkanson, R.; Leander, S.; Rosell, S.; Folkers, K.; Sundler, F. A Substance P Antagonist, [D-Pro2, D-Trp 7,9]SP, Inhibits Inflammatory Responses in the Rabbit Eye. Science 1981, 214, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Oksala, O.; Stjernschantz, J.; von Dickhoff, K. Characterization of the Mechanism of Acute Ocular Irritation to YAG Laser Capsulotomy in Rabbits: Effects of Substance P Antagonists, Met-Enkephalin, Tetracaine and Tetrodotoxin. Ophthalmic Res. 1989, 21, 360–368. [Google Scholar] [CrossRef]
- Mandahl, A.; Bill, A. Effects of the substance P antagonist (D-Arg1, D-Pro2, D-Trp7, 9, Leu11)-SP on the miotic response to substance P, antidromic trigeminal nerve stimulation, capsaicin, prostaglandin E1, compound 48/80 and histamine. Acta Physiol. Scand. 1984, 120, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kunitomo, M.; Imaizumi, N.; Sameshima, E.; Fujiwara, M. Pharmacological analysis of receptors involved in tachykininergic contraction induced by electrical transmural stimulation in the rabbit iris sphincter muscle. Regul. Pept. 1993, 46, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.G.; Tighe, J. The response of the isolated iris sphincter muscle of various mammalian species to substance P. Exp. Eye Res. 1984, 39, 677–684. [Google Scholar] [CrossRef]
- Anderson, J.A.; Malfroy, B.; Richard, N.R.; Kullerstrand, L.; Lucas, C.; Binder, P.S. Substance P contracts the human iris sphincter: Possible modulation by endogenous enkephalinase. Regul. Pept. 1990, 29, 49–58. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Håkanson, R. The rabbit iris sphincter contains NK1 and NK3 but not NK2 receptors: A study with selective agonists and antagonists. Regul. Pept. 1993, 44, 269–275. [Google Scholar] [CrossRef]
- Hall, J.M.; Mitchell, D.; Morton, I.K.M. Tachykinin receptors mediating responses to sensory nerve stimulation and exogenous tachykinins and analogues in the rabbit isolated iris sphincter. Br. J. Pharmacol. 1993, 109, 1008–1013. [Google Scholar] [CrossRef]
- Gitter, B.D.; Waters, D.C.; Bruns, R.F.; Mason, N.R.; Nixon, J.A.; Howbert, J.J. Species differences in affinities of non-peptide antagonists for substance p receptors. Eur. J. Pharmacol. 1991, 197, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, I.; Nakanishi, S.; Fujiwara, M. Comparison of the Responses to the Sensory Neuropeptides, Substance P, Neurokinin A, Neurokinin B and Calcitonin Gene-Related Peptide and to Trigeminal Nerve Stimulation in the Iris Sphincter Muscle of the Rabbit. Jpn. J. Pharmacol. 1987, 44, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.E.; Almegård, B. CGRP(8–37) and CGRP(32–37) contract the iris sphincter in the rabbit eye: Antagonism by spantide and GR82334. Regul. Pept. 1993, 49, 73–80. [Google Scholar] [CrossRef]
- Boscan, P.; Paton, J.F. Integration of cornea and cardiorespiratory afferents in the nucleus of the solitary tract of the rat. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1278–H1287. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Bereiter, D.F.; Hathaway, C.B. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity in central trigeminal neurons and blocks select endocrine and autonomic responses to corneal stimulation in the rat. Pain 1996, 64, 179–189. [Google Scholar] [CrossRef]
- Acosta, M.C.; Tan, M.E.; Belmonte, C.; Gallar, J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2063–2067. [Google Scholar]
- Belmonte, C.; Acosta, M.C.; Schmelz, M.; Gallar, J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Investig. Ophthalmol. Vis. Sci. 1999, 40, 513–519. [Google Scholar]
- Beuerman, R.W.; Tanelian, D.L. Corneal pain evoked by thermal stimulation. Pain 1979, 7, 1–14. [Google Scholar] [CrossRef]
- Kenshalo, D.R. Comparison of thermal sensitivity of the forehead, lip, conjunctiva and cornea. J. Appl. Physiol. 1960, 15, 987–991. [Google Scholar] [CrossRef]
- Guerrero-Moreno, A.; Baudouin, C.; Melik Parsadaniantz, S.; Réaux-Le Goazigo, A. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, C.; Herrero, J.F.; O’Brien, J.A.; Palmer, J.A.; Doyle, C.A.; Smith, A.J.; Laird, J.M.; Belmonte, C.; Cervero, F.; Hunt, S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998, 392, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Motterle, L.; Diebold, Y.; Enríquez de Salamanca, A.; Saez, V.; Garcia-Vazquez, C.; Stern, M.E.; Calonge, M.; Leonardi, A. Altered expression of neurotransmitter receptors and neuromediators in vernal keratoconjunctivitis. Arch. Ophthalmol. 2006, 124, 462–468. [Google Scholar] [CrossRef]
- He, J.; Bazan, H.E. Neuroanatomy and Neurochemistry of Mouse Cornea. Investig. Ophthalmol. Vis. Sci. 2016, 57, 664–674. [Google Scholar] [CrossRef]
- He, J.; Pham, T.L.; Kakazu, A.; Bazan, H.E.P. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment. Diabetes 2017, 66, 2511–2520. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- He, J.; Pham, T.L.; Kakazu, A.H.; Bazan, H.E.P. Remodeling of Substance P Sensory Nerves and Transient Receptor Potential Melastatin 8 (TRPM8) Cold Receptors After Corneal Experimental Surgery. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Magendie, P. De l’influence de la cinquieme paire de nerfs sur la nutrition et les fonctions de l’oeil. J. Physiol. 1824, 4, 176–177. [Google Scholar]
- Bee, J.A.; Kuhl, U.; Edgar, D.; von der Mark, K. Avian corneal nerves: Co-distribution with collagen type IV and acquisition of substance P immunoreactivity. Investig. Ophthalmol. Vis. Sci. 1988, 29, 101–107. [Google Scholar]
- Denis, P.; Fardin, V.; Nordmann, J.P.; Elena, P.P.; Laroche, L.; Saraux, H.; Rostene, W. Localization and characterization of substance P binding sites in rat and rabbit eyes. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1894–1902. [Google Scholar]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell. Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef]
- Bentley, E.; Abrams, G.A.; Covitz, D.; Cook, C.S.; Fischer, C.A.; Hacker, D.; Stuhr, C.M.; Reid, T.W.; Murphy, C.J. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2262–2269. [Google Scholar]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr. Eye Res. 1997, 16, 275–278. [Google Scholar] [CrossRef]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. The NK1 receptor and its participation in the synergistic enhancement of corneal epithelial migration by substance P and insulin-like growth factor-1. Br. J. Pharmacol. 1997, 120, 547–552. [Google Scholar] [CrossRef]
- Nakamura, M.; Chikama, T.; Nishida, T. Synergistic effect with Phe-Gly-Leu-Met-NH2 of the C-terminal of substance P and insulin-like growth factor-1 on epithelial wound healing of rabbit cornea. Br. J. Pharmacol. 1999, 127, 489–497. [Google Scholar] [CrossRef]
- Nishida, T.; Nakamura, M.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J. Cell. Physiol. 1996, 169, 159–166. [Google Scholar] [CrossRef]
- Ofuji, K.; Nakamura, M.; Nishida, T. Signaling regulation for synergistic effects of substance P and insulin-like growth factor-1 or epidermal growth factor on corneal epithelial migration. Jpn. J. Ophthalmol. 2000, 44, 1–8. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagano, T.; Chikama, T.; Nishida, T. Up-regulation of phosphorylation of focal adhesion kinase and paxillin by combination of substance P and IGF-1 in SV-40 transformed human corneal epithelial cells. Biochem. Biophys. Res. Commun. 1998, 242, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chikama, T.; Nishida, T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr. Eye Res. 2005, 30, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Aizawa, S.; Hiramoto, M.; Nakamura, M.; Iwase, O.; Nakata, K.; Sasaki, Y.; Mano, T.; Handa, H.; Tano, Y. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J. Cell. Physiol. 2000, 182, 189–195. [Google Scholar] [CrossRef]
- Ko, J.A.; Yanai, R.; Nishida, T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett. 2009, 583, 2148–2153. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Inui, M.; Nishida, T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Investig. Ophthalmol. Vis. Sci. 2005, 46, 833–839. [Google Scholar] [CrossRef]
- Kingsley, R.E.; Marfurt, C.F. Topical substance P and corneal epithelial wound closure in the rabbit. Investig. Ophthalmol. Vis. Sci. 1997, 38, 388–395. [Google Scholar]
- Chikama, T.; Fukuda, K.; Morishige, N.; Nishida, T. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet 1998, 351, 1783–1784. [Google Scholar] [CrossRef]
- Nagano, T.; Nakamura, M.; Nakata, K.; Yamaguchi, T.; Takase, K.; Okahara, A.; Ikuse, T.; Nishida, T. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3810–3815. [Google Scholar] [CrossRef]
- Chikamoto, N.; Chikama, T.; Yamada, N.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn. J. Ophthalmol. 2009, 53, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Matsuda, R.; Morishige, N.; Yanai, R.; Chikama, T.I.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br. J. Ophthalmol. 2008, 92, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Chikama, T.; Morishige, N.; Yanai, R.; Yamada, N.; Saito, J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn. J. Ophthalmol. 2007, 51, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kawahara, M.; Morishige, N.; Chikama, T.; Nakata, K.; Nishida, T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia 2003, 46, 839–842. [Google Scholar] [CrossRef]
- Lee, C.H.; Whiteman, A.L.; Murphy, C.J.; Barney, N.P.; Taylor, P.B.; Reid, T.W. Substance P, Insulinlike Growth Factor 1, and Surface Healing. Arch. Ophthalmol. 2002, 120, 215–217. [Google Scholar]
- Benitez-Del-Castillo, J.M.; Rodríguez-Bayo, S.; Fontan-Rivas, E.; Martinez-de-la-Casa, J.M.; Garcia-Sanchez, J. Treatment of recurrent corneal erosion with substance P-derived peptide and insulin-like growth factor I. Arch. Ophthalmol. 2005, 123, 1445–1447. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Yanai, R.; Nishida, T.; Hatano, M.; Uchi, S.H.; Yamada, N.; Kimura, K. Role of the Neurokinin-1 Receptor in the Promotion of Corneal Epithelial Wound Healing by the Peptides FGLM-NH2 and SSSR in Neurotrophic Keratopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Ko, J.A.; Murata, S.; Nishida, T. Up-regulation of the tight-junction protein ZO-1 by substance P and IGF-1 in A431 cells. Cell Biochem. Funct. 2009, 27, 388–394. [Google Scholar] [CrossRef]
- Brown, S.M.; Lamberts, D.W.; Reid, T.W.; Nishida, T.; Murphy, C.J. Neurotrophic and Anhidrotic Keratopathy Treated With Substance P and Insulinlike Growth Factor 1. Arch. Ophthalmol. 1997, 115, 926–927. [Google Scholar] [CrossRef]
- Nakamura, M.; Kawahara, M.; Nakata, K.; Nishida, T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2937–2940. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Kawamoto, K.; Nagano, T.; Nakamura, M.; Inui, M.; Nishida, T. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3286–3292. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Nakamura, M.; Inui, M.; Nishida, T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sui, W.; Li, Y.; Qi, X.; Wang, Y.; Zhou, Q.; Gao, H. Substance P Inhibits Hyperosmotic Stress-Induced Apoptosis in Corneal Epithelial Cells through the Mechanism of Akt Activation and Reactive Oxygen Species Scavenging via the Neurokinin-1 Receptor. PLoS ONE 2016, 11, e0149865. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yang, Z.J.; Zhang, Z.; Zhang, H. Aprepitant in the prevention of vomiting induced by moderately and highly emetogenic chemotherapy. Asian Pac. J. Cancer Prev. 2014, 15, 10045–10051. [Google Scholar] [CrossRef] [PubMed]
- Candelario, N.; Lu, M.L. Fosaprepitant dimeglumine for the management of chemotherapy-induced nausea and vomiting: Patient selection and perspectives. Cancer Manag. Res. 2016, 8, 77–82. [Google Scholar] [CrossRef]
- Jordan, K. Neurokinin-1-receptor antagonists: A new approach in antiemetic therapy. Onkologie 2006, 29, 39–43. [Google Scholar] [CrossRef]
- Keating, G.M. Netupitant/Palonosetron: A Review in the Prevention of Chemotherapy-Induced Nausea and Vomiting. Drugs 2015, 75, 2131–2141. [Google Scholar] [CrossRef]
- He, A.; Alhariri, J.M.; Sweren, R.J.; Kwatra, M.M.; Kwatra, S.G. Aprepitant for the Treatment of Chronic Refractory Pruritus. BioMed Res. Int. 2017, 2017, 4790810. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of depletion of substance P by capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar] [PubMed]
- Fernandes, E.S.; Cerqueira, A.R.; Soares, A.G.; Costa, S.K. Capsaicin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 91–125. [Google Scholar] [CrossRef]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, Cd007393. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Roy, S.; Thapaliya, M.; Ali, H. Roles of a Mast Cell-Specific Receptor MRGPRX2 in Host Defense and Inflammation. J. Dent. Res. 2020, 99, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Spellman, M.C.; Kwon, P.; Yosipovitch, G. The NK1 receptor antagonist serlopitant for treatment of chronic pruritus. Expert Opin. Investig. Drugs. 2019, 28, 659–666. [Google Scholar] [CrossRef]
- Bignami, F.; Giacomini, C.; Lorusso, A.; Aramini, A.; Rama, P.; Ferrari, G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6783–6794. [Google Scholar] [CrossRef]
- Bignami, F.; Lorusso, A.; Rama, P.; Ferrari, G. Growth inhibition of formed corneal neovascularization following Fosaprepitant treatment. Acta Ophthalmol. 2017, 95, e641–e648. [Google Scholar] [CrossRef]
- Cho, H.J.; Jeon, Y.J.; Yoon, W.; Yoon, J.; Kim, J.; Kim, J.W. Neovascular age-related macular degeneration without exudative recurrence over 24 months after initial remission. Sci. Rep. 2022, 12, 15662. [Google Scholar] [CrossRef]
- Foldenauer, M.E.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Substance P affects growth factors in Pseudomonas aeruginosa-infected mouse cornea. Cornea 2012, 31, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in pancreatic cancer. World J. Gastroenterol. 2014, 20, 2321–2334. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Bignami, F.; Rama, P.; Ferrari, G. Substance P and its Inhibition in Ocular Inflammation. Curr. Drug Targets 2016, 17, 1265–1274. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopel, J.; Keshvani, C.; Mitchell, K.; Reid, T. The Activity of Substance P (SP) on the Corneal Epithelium. J. Clin. Transl. Ophthalmol. 2023, 1, 35-51. https://doi.org/10.3390/jcto1020006
Kopel J, Keshvani C, Mitchell K, Reid T. The Activity of Substance P (SP) on the Corneal Epithelium. Journal of Clinical & Translational Ophthalmology. 2023; 1(2):35-51. https://doi.org/10.3390/jcto1020006
Chicago/Turabian StyleKopel, Jonathan, Caezaan Keshvani, Kelly Mitchell, and Ted Reid. 2023. "The Activity of Substance P (SP) on the Corneal Epithelium" Journal of Clinical & Translational Ophthalmology 1, no. 2: 35-51. https://doi.org/10.3390/jcto1020006
APA StyleKopel, J., Keshvani, C., Mitchell, K., & Reid, T. (2023). The Activity of Substance P (SP) on the Corneal Epithelium. Journal of Clinical & Translational Ophthalmology, 1(2), 35-51. https://doi.org/10.3390/jcto1020006





